Abstract
Adipocyte differentiation in bone marrow is potentially deleterious to both bone integrity and lymphopoiesis. Here, we examine the hypothesis that organotins, common environmental contaminants that are dual ligands for peroxisome proliferator–activated receptor (PPAR) γ and its heterodimerization partner retinoid X receptor (RXR), are potent activators of bone marrow adipogenesis. A C57Bl/6-derived bone marrow multipotent mesenchymal stromal cell (MSC) line, BMS2, was treated with rosiglitazone, a PPARγ agonist, bexarotene, an RXR agonist, or a series of organotins. Rosiglitazone and bexarotene potently activated adipocyte differentiation; however, bexarotene had a maximal efficacy of only 20% of that induced by rosiglitazone. Organotins (tributyltin [TBT], triphenyltin, and dibutyltin) also stimulated adipocyte differentiation (EC50 of 10–20nM) but with submaximal, structure-dependent efficacy. In coexposures, both bexarotene and TBT enhanced rosiglitazone-induced adipogenesis. To investigate the contribution of PPARγ to TBT-induced adipogenesis, we examined expression of PPARγ2, as well as its transcriptional target FABP4. TBT-induced PPARγ2 and FABP4 protein expression with an efficacy intermediate between rosiglitazone and bexarotene, similar to lipid accumulation. A PPARγ antagonist and PPARγ-specific small hairpin RNA suppressed TBT-induced differentiation, although to a lesser extent than rosiglitazone-induced differentiation, suggesting that TBT may engage alternate pathways. TBT and bexarotene, but not rosiglitazone, also induced the expression of TGM2 (an RXR target) and ABCA1 (a liver X receptor target). The results show that an environmental contaminant, acting with the same potency as a therapeutic drug, induces PPARγ-dependent adipocyte differentiation in bone marrow MSCs. Activation of multiple nuclear receptor pathways by organotins may have significant implications for bone physiology.
Keywords: bone marrow, adipogenesis, organotin, PPARγ, RXR
Since the 1950s, organotins have been widely used as antifouling agents and have been implicated in detrimental effects on marine life and the marine environment. However, their use in agricultural pesticides, wood preservatives, and plastics manufacturing has resulted in significant land-based sources of these pollutants (Cornelissen et al., 2008). Organotins are readily measurable in house dust (Fromme et al., 2005; Kannan et al., 2010). Significant human exposure is indicated by the presence of organotins in liver and blood (0.05–450nM; Antizar-Ladislao, 2008).
A growing number of environmental contaminants, including organotins and phthalates, are being recognized for their ability to activate the master regulator of adipocyte differentiation peroxisome proliferator–activated receptor (PPAR) γ (Feige et al., 2007; Grun et al., 2006). PPARγ1 is widely expressed, particularly in adipose, liver heart, and spleen, whereas PPARγ2 expression is largely restricted to adipocytes. PPARγ forms a heterodimeric complex with retinoid X receptors (RXR) and binds to PPAR response elements (5′-AACTAGGNCA A AGGTCA-3′). Ligand binding initiates a conformational change that results in the dissociation of corepressors and the association of coactivators, allowing ligand-induced transactivation (as reviewed in Lefterova and Lazar, 2009). Intriguingly, organotins, including tributyltin (TBT) and triphenyltin (TPhT), bind and activate both PPARγ and RXRα (Grun et al., 2006; Hiromori et al., 2009; Kanayama et al., 2005; le Maire et al., 2009; Nakanishi et al., 2005). In fact, organotins activate RXRα with approximately 10-fold greater potency and efficacy than PPARγ in Gal4 ligand-binding domain reporter assays (Grun et al., 2006). This difference likely results from the ability of TBT to form a covalent bond with a cysteine residue within the RXRα ligand-binding domain, a bond which does not form in the PPARγ-binding site (le Maire et al., 2009).
RXRα, β, and γ heterodimerize with, and are essential for transcriptional activation by, multiple nuclear receptors; furthermore, they also may act as homodimers (as reviewed in (Lefebvre et al., 2010; Szeles et al., 2010). Nonpermissive heterodimer partners (e.g., vitamin D receptor and retinoic acid receptor) can only be activated by ligand binding to the partner and not ligand binding to RXR. This results from the fact that RXR is subordinate to the heterodimer partner and its activation is inhibited by that partner. Permissive heterodimer partners (e.g., liver X receptor [LXR] and PPARγ) may be activated by ligand binding to either the partner or to RXR. For example, RXR ligands cannot stimulate retinoic acid receptor-dependent leukemic cell differentiation (Lala et al., 1996) but can stimulate PPARγ-dependent adipocyte differentiation (Schulman et al., 1998). However, permissiveness can be limited, with efficacy dependent upon the heterodimerization partner, the cell type, and the gene target (Szeles et al., 2010). TBT has been shown to activate PPARδ, LXR, NURR1, all permissive RXR heterodimeric partners (Cui et al., 2010; Grun et al., 2006). It remains to be determined whether TBT exerts its biological effects through binding to RXR and RXR homodimer activation or RXR-driven permissive heterodimer activation, through binding and activation of PPARγ, or through recruitment of multiple pathways.
Therapeutic PPARγ agonists have been shown to induce adipogenesis in bone marrow–derived multipotent mesenchymal stromal cells (MSCs; Ali et al., 2005; Gimble et al., 1996). Exposure of rodents to thiazoladinediones, PPARγ agonists used in the treatment of type II diabetes, results in significant bone loss with a concomitant increase in fat content and expression of adipocyte-specific markers (Rzonca et al., 2004; Syversen et al., 2009), similar to changes that occur in aging bone (Lazarenko et al., 2007). Furthermore, human treatment with thiazoladinediones is associated with an increased risk of fracture and potentially increased adipogenesis in bone (Loke et al., 2009; McDonough et al., 2008). These results highlight the significance of PPARγ activation to bone physiology. On the other hand, the consequences of ligand-induced activation of RXRs in the bone marrow have not been investigated.
The studies described herein were designed to examine the hypothesis that organotins are highly potent activators of PPARγ and adipogenesis in bone marrow. Accordingly, we examined the potential for a PPARγ agonist (rosiglitazone) and a series of structurally diverse organotins to induce lipid accumulation and adipocyte maturation in a bone marrow MSC model, BMS2, and compared this with the effects of an RXR ligand (bexarotene). We tested the contribution of PPARγ using an antagonist and lentiviral delivery of small hairpin RNA (shRNA) vectors. Furthermore, we examined the expression of PPARγ target genes, as well as RXR and LXR target genes. The data are consistent with organotins being capable of activating multiple nuclear receptors and being significant modulators of bone marrow MSC physiology.
MATERIALS AND METHODS
Materials.
Rosiglitazone and T0070907 were from Cayman Chemical (Ann Arbor, MI). Antibodies specific for FABP4, PPARγ, and perilipin were from Cell Signaling Technology (Beverly, MA). Bexarotene was from LC Laboratories (Woburn, MA). The β-actin–specific antibody, hematoxylin, human insulin, lentiviral reagents, Nile Red, Oil Red O, tributyltin chloride, butyltin trichloride, triphenyltin chloride, dibutyltin dichloride, and trioctyltin chloride were from Sigma-Aldrich (St Louis, MO). All other reagents were from Thermo Fisher Scientific (Suwanee, GA).
Cell culture.
BMS2 cells are C57BL/6 mouse–derived bone marrow stromal cells (Pietrangeli et al., 1988; kindly provided by Dr P. Kincade, Oklahoma Medical Research Foundation). Stocks of BMS2 cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 5% bovine growth serum (Thermo Fisher Scientific, formerly Hyclone), 5 μg/ml plasmocin (Invivogen, San Diego, CA), and 20mM L-glutamine. Cultures were maintained at 37°C in a humidified, 5% CO2 atmosphere. Cell cultures were determined to be mycoplasma negative by PCR (Mycoplasma Detection Kit; ATCC, Manassas, VA). For experiments, BMS2 were plated at 40,000 cells/well (24-well plates) or 160,000 cells/well (6-well plates) in preadipocyte medium (DMEM with 5% fetal bovine serum [FBS]) and allowed to become confluent (3–4 days). Prior to dosing, the medium was replaced with preadipocyte medium supplemented with insulin (0.5 μg/ml). Cultures received no treatment (Naive) or were treated with Vh (dimethyl sulfoxide [DMSO], 0.1–0.2%), rosiglitazone (0.1nM–10μM), bexarotene (0.1–200nM), organotins (0.1–100nM), or combinations of ligands. Where applicable, cells were pretreated with Vh (DMSO, 0.1%) or the PPARγ antagonist T0070907 (20μM) for 24 h and were retreated with antagonist on day 1 and day 4. Following treatment, cells were cultured for 12 h (messenger RNA [mRNA] expression), 4 days (PPARγ protein expression and activation), or 6–7 days (lipid accumulation and perilipin expression). Medium was changed and the cultures were redosed two times for Oil Red O staining experiments. All other experiments received a single treatment.
Primary bone marrow cultures were prepared from wild-type C57BL/6 mice (male, 8–12 weeks of age). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Boston University. After euthanasia, limbs were aseptically dissected, and soft tissue was removed from the bone. Bone marrow was flushed from the femur, tibia, and humerus bones, strained through a 70-μm cell strainer, diluted in MSC media (α-minimal essential medium containing 10% FBS and 100 U/ml penicillin, 100 μg/ml streptomyocin, 0.25 μg/ml Amphotericin B), and seeded at 6 × 106/ml in 2 ml per well in a 6-well plate. Half of the medium was replaced 4 days after plating and the cultures continued for three more days before an experiment was started. Prior to dosing, the medium was replaced with MSC medium supplemented with insulin (0.5 μg/ml). Cultures received no treatment (Naive) or were treated with Vh (DMSO, 0.1%), dibutyltin (DBT), TBT, or TPhT (50–100nM). Following treatment, the cells were cultured for 14 days. Medium was changed, and the cultures were redosed five times.
Lipid accumulation.
Lipid accumulation was visualized by Oil Red O staining. Cultures were fixed with 2% paraformaldehyde. Cells were stained with an aqueous solution of Oil Red O for 30 min, counterstained with hematoxylin for 30 min, and rinsed. Cultures were magnified ×20 via light microscopy and photographed. Lipid accumulation was quantified by Nile Red staining. Cells were stained with an aqueous solution of Nile Red (1 ug/ml in phosphate buffered saline) for 10 min, and fluorescence (excitation 485 [20 nm bandwidth], emission 530 nm [25 nm bandwidth]) was measured using a Synergy2 multifunction plate reader (BioTek, Winooski, WT). For experiments with BMS2 cells, the fluorescence in all experimental wells was normalized by subtracting the fluorescence measured in wells with Naive cells. For experiments with primary bone marrow, the fluorescence in all experimental wells was normalized by dividing by the fluorescence measured in Naive cells and reported as “Fold Change from Naive.”
Immunoblotting.
BMS2 cells were washed once in cold PBS. Cells were collected and lysed in Cell Lysis Buffer (Cell Signaling Technology) followed by sonication. The lysates were cleared by centrifugation, and the supernatants were used for protein expression analyses. Protein concentrations were determined by the Bradford method. For positive controls, mouse PPARγ1 (plasmid 8886; Addgene, Inc., Cambridge, MA) and mouse PPARγ2 (plasmid 8862; Addgene; Tontonoz et al., 1994) were translated in vitro (TnT SP6 Coupled Reticulocyte Lysate System; Promega Corp., Madison, WI).
Total proteins (10–40 μg) were resolved on 10% (PPARγ and perilipin) or 15% (FABP4) gels, transferred to a 0.2-μm nitrocellulose membrane, and incubated with primary antibody. Primary antibodies included monoclonal rabbit anti-PPARγ (2443), polyclonal rabbit anti-perilipin (3470), and monoclonal rabbit anti-FABP4 (3544). Immunoreactive bands were detected using HRP-conjugated secondary antibodies (Biorad, Hercules, CA) followed by enhanced chemiluminescence. To control for equal protein loading, blots were reprobed with a β-actin–specific antibody (A5441) and analyzed as above.
mRNA expression.
BMS2 cells were washed once in cold PBS and frozen at −80°C. Total RNA was extracted and genomic DNA was removed using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was prepared from 1.3 μg of total RNA using the GoScript Reverse Transcription System (Promega), with a 1:1 mixture of random and Oligo (dT)15 primers. The cDNA was diluted 1:20 in RNase-free water prior to quantitative polymerase chain reaction (qPCR). All qPCR reactions were performed using the GoTaq qPCR Master Mix System (Promega), with reactions scaled to 25 μl. Validated primers were purchased from Qiagen, Inc. (ABCA1: NM_013454; FABP4: NM_024406; TGM2: NM_009373: CYP26A1; NM_007811: ANGPTL4; NM_020581). The β-actin (NM_007393) primer sequences (forward: 5′-ATT GCT GAC AGG ATG CAG AA-3′ and reverse: 5′-CAG GAG GAG CAA TGA TCT TGA-3′) were from Xu and Miller (2004) and were synthesized by Integrated DNA Technologies (Coralville, IA). qPCR reactions (in duplicate) were performed using a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA): Hot-Start activation at 95°C for 2 min, 40 cycles of denaturation (95°C for 15 s), and annealing/extension (55°C for 60 s), followed by melting curve analysis. Relative gene expression was determined using the Comparative CT method, using the threshold value for β-actin for normalization. The CT value for untreated samples was used as the reference point, and data were normalized by the fold change in expression in the Vh-treated samples.
Transfection and transduction.
Cos-7 cells were transiently transfected with vectors containing human PPARγ1, human PPARγ-dominant negative (DN; kindly provided by V. K. Chatterjee, University of Cambridge, Cambridge, UK; Gurnell et al., 2000), human RXRα (plasmid 8882; Addgene; Tontonoz et al., 1994), and/or pcDNA3 (Invitrogen, Carlsbad, CA), with PPRE x3-TK-luc (plasmid 1015; Addgene; Kim et al., 1998) and CMV-eGFP reporter constructs using Lipofectamine 2000 (Invitrogen). Transfected cultures were incubated for 3 h. Prior to dosing, the medium was replaced with antibiotic-free DMEM with 5% FBS. Cultures received no treatment (Naive) or were treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100nM) and incubated for 24 h. Cells were lysed in Glo Lysis Buffer (Promega). Lysates were transferred to a 96-well plate, to which Bright Glo Reagent (Promega) was added. Luminescence and fluorescence were determined using a Synergy2 multifunction plate reader. Luminescence was normalized by the GFP fluorescence in the same well. The normalized luminescence for each well was then divided by the normalized luminescence measured in untreated, DN-PPARγ–transfected wells to determine the “Fold-Change from DN-Naive.”
To prepare lentiviral particles, HEK293T/17 cells were transfected with MISSION Lentiviral Packaging Mix and the MISSION Non-target shRNA control vector (SHC002) or a MISSION PPARγ-shRNA vector (TRCN0000001658 or TRCN0000001660; all from Sigma-Aldrich) with Fugene 6 reagent (Roche Applied Science, Indianapolis, IN). Supernatant was collected from 48- to 96-h posttransfection. Viral particles were concentrated by ultracentrifugation at 18,500 revolutions per minute for 1 h 30 min with Beckman ultracentrifuge using SW28 rotor. Particles were resuspended in PBS and stored at −80°C until use. The viral titer was determined using a HIV-1 p24 Antigen ELISA Kit (Thermo Fisher Scientific). BMS2 were plated at 40,000 cells/well (24-well plates) or 1,60,000 cells/well (6-well plates) in preadipocyte medium and incubated overnight. Medium was replaced with preadipocyte medium containing polybrene (8 μg/ml). Cultures were not transduced or were transduced with either the nontarget (NT) lentivirus (multiplicity of infection of 100:1) or with PPARγ-shRNA lentivirus (multiplicity of infection of 50:1 of each virus) and incubated overnight. The medium was changed and the incubation continued overnight. Prior to dosing, the medium was replaced with preadipocyte medium supplemented with insulin (0.5 μg/ml). Cultures received no treatment (Naive) or were treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100nM). Following treatment, cells were cultured for 4 (PPARγ expression and activation) or 6–7 (lipid accumulation) days.
Statistics.
Statistical analyses were performed with Statview (SAS Institute, Cary, NC). Data are presented as mean ± SE. A two-factor ANOVA was used to analyze data from transfection, transduction, and antagonist experiments. A one-factor ANOVA, in conjunction with the Dunnett’s or Tukey-Kramer multiple comparisons tests, was used to analyze data from all other experiments. Dose-response curves were fit with the sigmoid 4-parameter Hill function in Sigma Plot (Systat Software, San Jose, CA).
RESULTS
A PPARγ Agonist (rosiglitazone) and an RXR Agonist (bexarotene) Stimulate Adipocyte Differentiation in a Bone Marrow MSC Model
Previous studies have demonstrated that rosiglitazone is a potent inducer of adipocyte differentiation in the C57BL/6-derived bone marrow MSC line BMS2 (Gimble et al., 1996) and other bone marrow MSC models (Lazarenko et al., 2007; Lecka-Czernik et al., 2007). PPARγ forms a permissive heterodimer with RXRs such that PPARγ-mediated gene transcription can be activated by ligands for either PPARγ or RXR; thus, we hypothesized that an RXR ligand would induce adipocyte differentiation in our bone marrow MSC model. The effects of the bexarotene, a therapeutic RXR ligand, were determined in the presence of low-level insulin supplementation, without other components of the traditional adipogenesis-inducing hormonal cocktail (e.g., dexamethasone, indomethacin or 3-isobutyl-1-methylxanthine).
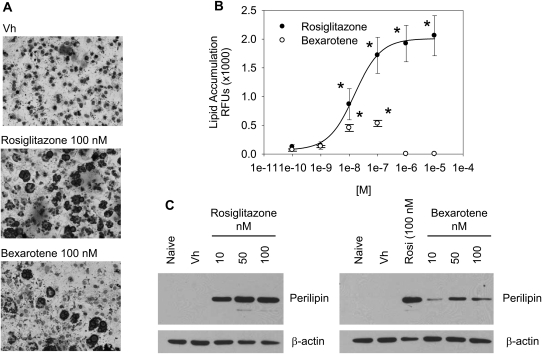
To analyze the effect of PPARγ and RXR ligand treatment on bone marrow MSC differentiation, BMS2 cells were allowed to grow to confluence and then were treated with Vh (DMSO, 0.1% final concentration), rosiglitazone, or bexarotene (0.1–10μM) in the presence of insulin (0.5 μg/ml) for 7 days. Lipid accumulation was visualized by Oil Red O staining. In Vh-treated cultures, no lipid vacuoles were evident; however, both rosiglitazone and bexarotene treatment resulted in formation of lipid vacuoles (Fig. 1A). In order to quantify lipid accumulation, cultures were stained with the fluorescent, lipophilic dye Nile Red. Both rosiglitazone and bexarotene were highly potent at stimulating adipocyte differentiation, with significant lipid accumulation following exposure to a 10nM concentration (Fig. 1B). Interestingly, lipid accumulation did not occur at concentrations of bexarotene greater than 100nM, despite the fact that no significant toxicity was observed (Fig. 1B, data not shown). Although both rosiglitazone and bexarotene were similarly potent at inducing lipid accumulation, rosiglitazone induced lipid accumulation 25-fold above Vh, whereas bexarotene-induced lipid accumulation by only 5-fold. In order to confirm that rosiglitazone and bexarotene stimulated terminal adipocyte differentiation, BMS2 cells were assessed for expression of the adipocyte-specific protein perilipin (Ducharme and Bickel, 2008). Treatment with either rosiglitazone or bexarotene resulted in increased expression of perilipin (Fig. 1D). Consistent with the lower efficacy of bexarotene, the extent of perilipin upregulation was less in bexarotene-treated cells than rosiglitazone-treated cells. The results indicate that activation of RXR is sufficient to stimulate adipogenesis in a bone marrow MSC model, without additional hormonal support; yet, differentiation is stimulated more efficaciously by ligand binding directly to PPARγ.
FIG. 1.
A PPARγ ligand has greater efficacy than an RXR ligand in stimulating adipocyte differentiation in a bone marrow MSC model. BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone (1nM–10μM), or bexarotene (1nM–10μM) in the presence of insulin (0.5 μg/ml) for 7 days. (A) To assess the potential for adipocyte differentiation, cultures were stained with Oil Red O and photographed (original magnification ×20). (B) To quantify lipid accumulation, the cultures were stained with Nile Red and assessed for fluorescence. (C) To determine if mature adipocytes were formed, cell lysates were prepared and analyzed for perilipin expression by immunoblotting. β-actin expression was determined to assure equal protein loading. Data are representative of at least three independent experiments or are presented as means ± SE from at least three independent experiments. *Statistically different from Vh treated (p < 0.05, ANOVA, Dunnett’s).
Structure-Dependent Activation of Adipocyte Differentiation by Organotin Compounds
Given that TBT is a dual PPAR/RXR ligand, we hypothesized that TBT would be a potent and efficacious activator of adipocyte differentiation in our bone marrow MSC model. Although attention has focused on TBT, organotin compounds substituted with a variety of functional groups (i.e., methyl, butyl, phenyl groups) are in common commercial use, and a number of these compounds have been shown to bind PPARγ and/or RXRα (Hiromori et al., 2009; Nakanishi et al., 2005). Thus, in addition to TBT, we tested the ability of monobutyltin, DBT, TPhT, and trioctyltin for their ability to stimulate adipocyte differentiation in our bone marrow MSC model and in primary bone marrow cultures.
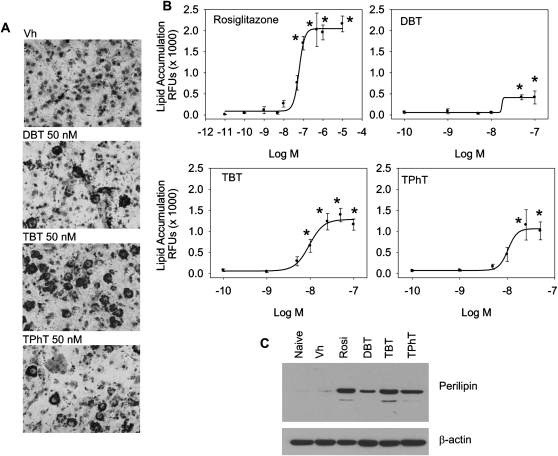
First, BMS2 cells were allowed to grow to confluence and then were treated with Vh (DMSO, 0.1% final concentration), rosiglitazone (0.1nM–10μM) or organotins (0.1–100nM) in the presence of insulin (0.5 μg/ml) for 7 days. It should be noted that concentrations above 100nM TBT and TPhT induced significant toxicity; thus, 100nM was used as a maximal dose (data not shown). Rosiglitazone did not cause toxicity at concentrations up to 10μM. Lipid accumulation was visualized by Oil Red O staining and quantified by Nile Red staining. No lipid accumulation was observed following treatment with monobutyltin or trioctyltin (data not shown). DBT, TBT, and TPhT all significantly stimulated lipid accumulation in BMS2 cells (Figs. 2A and 2B), with a potency similar to rosiglitazone (Table 1). However, the organotins had reduced efficacy in stimulating lipid accumulation (Rosiglitazone > TBT > TPhT > DBT; Table 1). Expression of perilipin following organotin treatment supports the conclusion that TBT, TPhT, and DBT all induce terminal adipocyte differentiation (Fig. 2C).
FIG. 2.
DBT, TBT, and TPhT potently stimulate adipocyte differentiation in a bone marrow MSC model. BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone (0.1nM–10μM), monobutyltin, DBT, TBT, trioctyltin, or TPhT (1–100nM), in the presence of insulin (0.5 μg/ml) for 7 days. Lipid accumulation was visualized (A) and quantified (B) as described in Figure 1. (C) Perilipin expression was determined as described in Figure 1. β-actin expression was determined to assure equal protein loading. Data are representative of at least three independent experiments or are presented as means ± SE from at least three independent experiments. *Statistically different from Vh treated (p < 0.05, ANOVA, Dunnett’s).
TABLE 1.
Comparison of Organotin Potency and Efficacy in Inducing Lipid Accumulation
| Chemical | EC50 (M)a | Hill coefficient | Maximum RFUs |
| Rosiglitazone | 6 × 10−8 | 3.0 | 1960 |
| DBT | 2 × 10−8 | 3.6 | 356 |
| TBT | 1 × 10−8 | 2.6 | 1225 |
| TPhT | 1 × 10−8 | 4.6 | 992 |
Derived from data shown in Figure 2B. Data were fitted with a four-paramater Hill function.
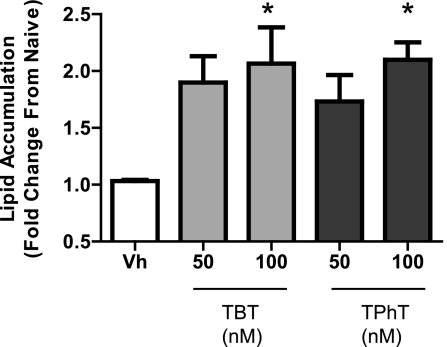
In order to confirm that organotin-induced adipocyte differentiation was a general feature of bone marrow–derived MSCs, primary bone marrow cultures were prepared from C57BL/6 mice, treated with Vh, DBT, TBT, or TPhT (10–100nM) in the presence of insulin (0.5 μg/ml) for 14 days and assessed for lipid accumulation. DBT did not induce lipid accumulation in primary MSC cultures (data not shown); however, both TBT and TPhT induced significant lipid accumulation at a concentration of 100nM (Fig. 3). The results are consistent with the conclusion that multiple organotins (TBT, TPhT, and to a lesser extent DBT) potently stimulate lipid accumulation and terminal adipocyte differentiation in bone marrow–derived MSCs.
FIG. 3.
TBT and TPhT induce adipocyte differentiation in a primary bone marrow cultures. Primary bone marrow cells were isolated from 8- to 10-week-old C57BL/6 mice, plated, and allowed to adhere for 7 days. The medium was changed to include insulin (0.5 μg/ml), except for Naive wells. Cells were treated with Vh (DMSO, 0.1%), DBT, TBT, or TPhT (50–100nM). Medium was changed and the cultures were redosed five times. Lipid accumulation was quantified by Nile Red staining on day 15. Data are presented as means ± SE from three independent bone marrow preparations. *Statistically different from Vh treated (p < 0.05, ANOVA, Dunnett’s).
TBT and Bexarotene Enhance Rosiglitazone-Induced Adipocyte Differentiation
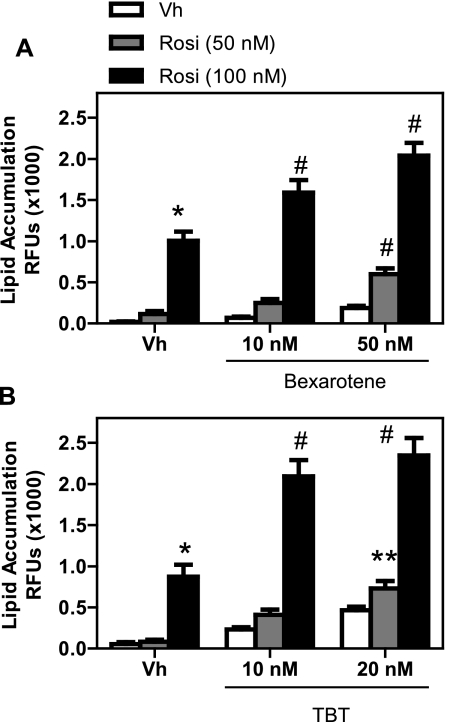
Coexposure to an RXR ligand can either enhance or inhibit PPARγ-mediated signaling, depending upon the cell type (Schulman et al., 1998; Szeles et al., 2010). Given that both bexarotene and TBT are ligands for RXRs and that RXR activation was sufficient to induce adipogenesis in BMS2 cells, we tested the ability of these compounds to enhance rosiglitazone-induced adipogenesis. BMS2 cells were allowed to grow to confluence and then were treated with Vh (DMSO, 0.2% final concentration), rosiglitazone, bexarotene, and/or TBT (1–100nM) in the presence of insulin (0.5 μg/ml) for 7 days and assessed for lipid accumulation. Bexarotene (Fig. 4A) and TBT (Fig. 4B) enhanced rosiglitazone-induced adipogenesis at concentrations as low as 10nM, a concentration at which bexarotene and TBT alone did not stimulate significant adipogenesis.
FIG. 4.
Cotreatment with bexarotene (A) or TBT (B) enhances rosiglitazone-induced adipocyte differentiation. BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone (10–100nM), bexarotene (10–50nM), and/or TBT (10–20nM) in the presence of insulin (0.5 μg/ml) for 7 days. Lipid accumulation was quantified as described in Figure 1. Data are presented as means ± SE from at least five independent experiments. *Statistically different from Vh treated. **Statistically different from Vh treated and Rosi treated at the same dose. #Statistically different from Vh, Rosi, Bex, or TBT treated at the same dose (p < 0.05, ANOVA, Tukey-Kramer).
TBT Is a Unique Activator of Bone Marrow MSC Differentiation, in Comparison with Rosiglitazone and Bexarotene.
The biology of activation of PPAR/RXR by TBT may be unique, given the fact that TBT binds and activates both PPARγ and RXR. Activation of RXR opens the possibility that multiple pathways may be activated by TBT, including RXR homodimer– and LXR-driven pathways.
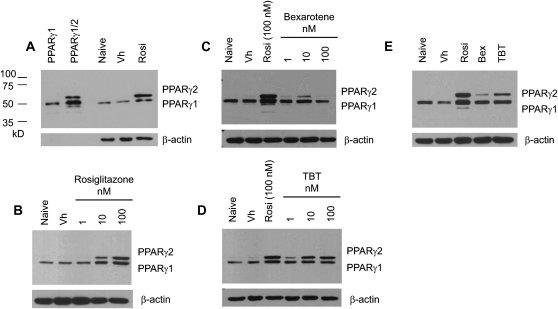
Results in experiments with either rosiglitazone or bexarotene showed that RXR activation was sufficient to stimulate bone marrow MSC differentiation (Fig. 1) but was limited in its ability to do so. If TBT acts through binding to both PPARγ and RXR, we would expect that TBT would be more efficacious than an RXR ligand at activating PPARγ and inducing adipocyte differentiation in bone marrow MSCs. To test this prediction, we examined upregulation of PPARγ2, a critical event in adipocyte differentiation (Lefterova and Lazar, 2009). BMS2 cells were allowed to grow to confluence and then were treated with Vh (DMSO, 0.1% final concentration), rosiglitazone, bexarotene, or TBT (1–100nM) in the presence of insulin (0.5 μg/ml) for 4 days. Expression of the PPARγ isoforms was determined in whole cell lysates by immunoblotting; the specificity of the bands was confirmed by comparison to in vitro translated mouse PPARγ1 and PPARγ1/2 (Fig. 5A). BMS2 cells constitutively express a significant level of PPARγ1, whereas PPARγ2 is minimally expressed (Fig. 5B). Rosiglitazone, bexarotene, and TBT all modestly increased the expression of PPARγ1 and significantly increased the expression of PPARγ2 at a concentration of 10nM (Figs. 5B–D). This increase in expression was maintained at 100nM for rosiglitazone and TBT (Figs. 5B and 5D). Although PPARγ2 expression is still induced by 100nM bexarotene, the level of expression is below that induced by 50nM bexarotene (Fig. 5B). To compare efficacy, BMS2 cells were treated with the same concentration of the three ligands (50nM), as above. Again, rosiglitazone had the greatest efficacy in inducing PPARγ expression; however, TBT had a distinctly greater capacity to induce PPARγ expression than bexarotene (Fig. 5E).
FIG. 5.
TBT induces expression of PPARγ in a bone marrow MSC model. BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100 nM) (A–D) or rosiglitazone, bexarotene, and TBT (50nM) (E) in the presence of insulin (0.5 μg/ml) for 4 days. Whole cell lysates were prepared and analyzed for PPARγ expression by immunoblotting. Identity of the bands was confirmed by comparison with in vitro translated mouse PPARγ1 and PPARγ2 (A). β-actin expression was determined to assure equal protein loading. Data are representative of three independent experiments.
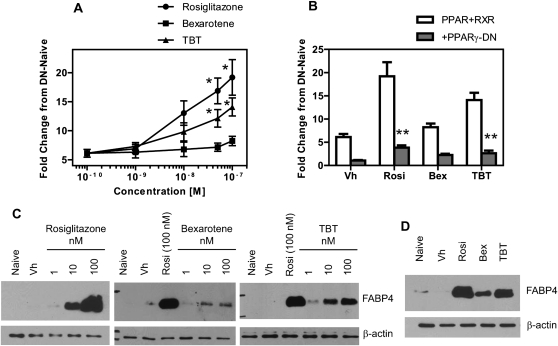
In order to investigate the activation of PPARγ-mediated gene transcription, two approaches were taken. Cos7 cells were used as a model to quantitatively examine the potency and efficacy of PPARγ transactivation by rosiglitazone, bexarotene, and TBT as BMS2 cells are difficult to transfect. Endogenous FABP4 expression, a PPARγ target gene, was then examined in BMS2 cells for comparison. Cos7 cells were transfected with human PPARγ1 and RXRα expression vectors and a PPRE-driven reporter construct. The cells were cotransfected either with a pcDNA3 vector or a PPARγ-DN expression vector. Transfected cultures received no treatment (Naive) or were treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100nM). Reporter expression was assessed after 24 h of exposure. Rosiglitazone and TBT significantly induced PPARγ-driven reporter activity at concentrations ≥ 50nM. Bexarotene increased reporter activity, but the increase was not statistically significant (Fig. 6A). A two-factor ANOVA analysis demonstrated that cotransfection of a dominant-negative form of PPARγ significantly reduced reporter activity induced by either rosiglitazone or TBT (p < 0.001, Fig. 6B). Second, we examined the expression of PPARγ-dependent protein expression in our bone marrow MSC model. BMS2 cells were allowed to grow to confluence and then were treated as described above. Expression of FABP4 was determined in whole cell lysates by immunoblotting. Rosiglitazone and TBT strongly induced the expression of FABP4 at a concentration as low as 10nM (Fig. 6C). Bexarotene also induced FABP4 expression at 10 and 100nM, although to a lesser extent (Fig. 6C). To compare efficacy, BMS2 cells were treated with the same concentration of the three ligands (50nM), as above. Again, rosiglitazone had the greatest efficacy in inducing FABP4 expression, followed by TBT and bexarotene (Fig. 6D).
FIG. 6.
TBT activates PPARγ more efficiently than an RXR agonist. (A, B) Cos-7 cells were transiently transfected with human PPARγ1 and RXRα vectors, with either pcDNA3 or PPARγ-DN vectors and with PPRE x3-TK-luc and CMV-eGFP reporter constructs. Transfected cultures received no treatment (Naive) or were treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100nM) and incubated for 24 h. Luminescence and fluorescence were determined, and the data were normalized as described in the methods (C–D) BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100nM) (C) or rosiglitazone, bexarotene, and TBT (50nM) (D) in the presence of insulin (0.5 μg/ml) for 4 days. Whole cell lysates were prepared and analyzed for FABP4 expression by immunoblotting. β-actin expression was determined to assure equal protein loading. Data are representative of at least three independent experiments or are presented as means ± SE from at least three independent experiments. *Statistically different from Vh treated. **Statistically different from pcDNA transfected at that dose (p < 0.05, ANOVA, Tukey-Kramer).
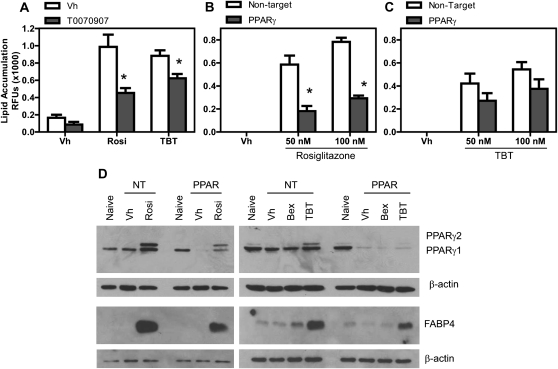
Next, we tested the contribution of PPARγ to TBT-induced bone marrow MSC differentiation. First, BMS2 cells were grown to confluence, pretreated with either Vh or T0090709 (20μM) and then treated with Vh, rosiglitazone, or TBT (50–100nM) for 7 days. Lipid accumulation was quantified by Nile Red staining. T0090709 significantly reduced lipid accumulation induced by both rosiglitazone and TBT (Fig. 7A), although the effect of TBT was reduced to a lesser extent presumably because of its ability to bind and activate RXR (Lee et al., 2002). Second, to test the effect of specifically knocking down expression of PPARγ, we analyzed BMS2 cells transduced with either a lentivirus carrying a NT shRNA vector or lentivirus carrying a PPARγ-shRNA vector. BMS2 cells were not transduced or were transduced with NT or PPARγ-shRNA vectors, treated, and analyzed for lipid accumulation, as above. Transduction of the BMS2 cells with NT shRNA alone resulted in lower responsiveness to ligand-driven differentiation that we assume is a result of the transduction process (20–25% decrease in lipid accumulation in NT shRNA-transduced vs. nontransduced cells, data not shown). Two-factor ANOVA analysis showed that knockdown of PPARγ significantly reduced adipogenesis (lipid accumulation) induced by either rosiglitazone or TBT, although the effect of TBT was reduced to a lesser extent (rosiglitazone: p < 0.001 [Fig. 7B], TBT: p = 0.054 [Fig. 7C]). Basal expression of PPARγ1 was significantly reduced by the PPARγ-specific shRNA (compare Naive, untransduced cells, and Vh-treated, NT shRNA–transduced cells to Vh-treated, PPAR shRNA–transduced cells, Fig. 7D). Further, the induction of both PPARγ2 and FABP4 by rosiglitazone and TBT was suppressed, although not eliminated (Fig. 7D). Bexarotene was completely ineffective at inducing PPARγ2 expression, FABP4 expression, or lipid accumulation even in cells transduced with the NT shRNA vector (Fig. 7D, data not shown). As we predicted, TBT had greater efficiency than bexarotene in inducing adipocyte differentiation, suggesting that direct activation of PPARγ enhanced initiation of the adipogenic program.
FIG. 7.
Bone marrow adipogenesis mediated by rosiglitazone and TBT requires PPARγ. (A) BMS2 cells were allowed to become confluent and then pretreated with Vh (DMSO, 0.1%) or T0070907 (20μM) in the presence of insulin (0.5 μg/ml) and incubated overnight. The cultures were retreated with Vh or T0070907 and then treated with Vh (DMSO, 0.1%), rosiglitazone (100nM), or TBT (50nM). The cultures were redosed with Vh and T0070907 after 3 days, and lipid accumulation was quantified by Nile Red staining on day 7. (B and C) BMS2 cells were allowed to become confluent and then transduced with either control lentivirus or PPARγ-shRNA lentivirus overnight. Cultures then were treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (1–100 nM) for 6–7 days in the presence of insulin (0.5 μg/ml). Lipid accumulation was quantified by Nile Red staining. (D) BMS2 cells were cultured, transduced, and treated as above. After 4 days, whole cell lysates were prepared and analyzed for PPARγ and FABP4 expression by immunoblotting. β-actin expression was determined to assure equal protein loading. Data are representative of at least three independent experiments or are presented as means ± SE from at least three independent experiments. *Statistically different from non-antagonist treated or NT transduced at that dose (p < 0.05, ANOVA, Tukey-Kramer).
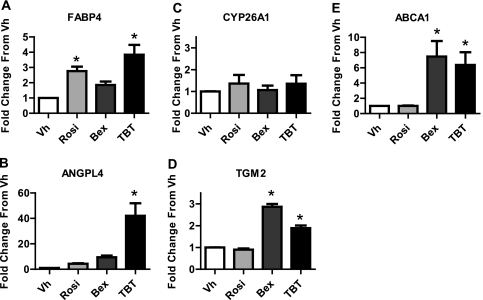
The data suggest that TBT induced adipocyte differentiation through activation of PPARγ, but the possibility remained that TBT could activate other nuclear receptors that may contribute to this process. To begin to examine the complement of nuclear receptors activated by TBT in our bone marrow MSC model, we determined the expression of PPARγ, RXR, and LXR gene targets. BMS2 cells were grown to confluence and treated with Vh, rosiglitazone, or TBT (100nM) for 12 h. A short time point was chosen to better assess early, receptor-dependent gene expression. We measured expression of FABP4 and ANGPLT4 (PPARγ targets [Kaddatz et al., 2010; Tontonoz et al., 1994]), CYP26A1 and TGM2 (RAR/RXR targets [Idres et al., 2005; Szeles et al., 2010]), and ABCA1 (LXR target [Venkateswaran et al., 2000]). As expected, given the increased expression of FABP4 protein, rosiglitazone and TBT significantly induced the expression of FABP4 mRNA (Fig. 8A). Surprisingly, TBT also very strongly induced the expression of ANGPTL4 (∼40-fold over Vh-treated cells); rosiglitazone and bexarotene induced the expression of ANGPLT4 less than 10-fold (Fig. 8B). Although CYP26A1 was not induced by any chemical, the RXR homodimer target TGM2 was significantly induced by both bexarotene and TBT (Figs. 8C and 8D). Similarly, bexarotene and TBT, but not rosiglitazone, significantly induced the expression of ABCA1 (Fig. 8E). For comparison, the LXR agonist T0901317 induced ABCA1 expression 11-fold in BMS2 cells (data not shown). The data indicate that in addition to activation of PPARγ-dependent adipogenesis, TBT can activate the expression of RXR homodimer and LXR gene targets in our bone marrow MSC model.
FIG. 8.
TBT induces a unique gene expression pattern. BMS2 cells were allowed to become confluent and then treated with Vh (DMSO, 0.1%), rosiglitazone, bexarotene, or TBT (100nM) in the presence of insulin (0.5 μg/ml) for 12 h. RNA was isolated and reverse transcribed. Gene expression was measured by qPCR. Relative gene expression was determined using the Comparative CT method, using the threshold value for β-actin for normalization. The CT value for untreated samples was used as the reference point, and data were normalized by the expression in the Vh-treated samples. Data are presented as means ± SE from four independent experiments. *Statistically different from Vh treated (p < 0.05, ANOVA, Dunnett’s).
DISCUSSION
Bone marrow is a source of MSCs capable of differentiating into osteoblasts and adipocytes, and data suggest that PPARγ is a crucial mediator of differentiation in this organ (Akune et al., 2004; Cock et al., 2004; Moerman et al., 2004). Alteration in the balance between osteogenesis and adipogenesis by exposure to an exogenous PPARγ ligand may have significant consequences for bone health, as evidenced by the association of thiazoladinedione treatment with increased fracture risk (Loke et al., 2009; McDonough et al., 2008). That PPARγ forms a permissive heterodimer with RXR raises the possibility that exposure to RXR ligands, in addition to PPARγ ligands, could modulate bone marrow physiology. TBT and structurally similar organotins represent a unique class of nuclear receptor ligands in that they are dual PPAR/RXR ligands (Grun et al., 2006). Here, we examine the hypothesis that organotins are significant modulators of bone marrow physiology and investigate the contribution of PPAR and RXR to their effects.
To determine whether rexinoids are capable of engaging PPAR/RXR in bone marrow MSCs and inducing differentiation, we examined the ability of rosiglitazone, a PPARγ agonist, and bexarotene, an RXR agonist, to induce bone marrow MSC adipocyte differentiation. In accordance with previous findings (Gimble et al., 1996), our results demonstrate that rosiglitazone is highly potent and efficacious at stimulating adipocyte differentiation in BMS2 cells, with significant increases in PPARγ and FABP4 expression occurring at a concentration as low as 10nM in BMS2. As far as we can determine, this is the first study to investigate the potential of an RXR agonist to alter the differentiation of bone marrow–derived MSCs. We show that bexarotene induced lipid accumulation and perlipin expression, indicative of adipocyte terminal differentiation, concurrently with upregulation of PPARγ2 and FABP4. Bexarotene-induced differentiation occurred in medium supplemented with only a low level of insulin and did not require coadministration of a hormonal induction cocktail. The results are consistent with a recent study of human adipose-derived MSCs showing that the RXR agonist AGN195203 can stimulate hormone-driven adipocyte differentiation in human adipose-derived MSC (Kirchner et al., 2010). In each assessment of adipocyte differentiation, bexarotene was less efficacious at stimulating bone marrow MSC differentiation than rosiglitazone.
A recent study investigated the RXR-dependent transcriptome in monocyte-derived dendritic cells (Szeles et al., 2010). The results revealed that RXR ligands induced a transcriptome dependent upon RXR, PPARγ, and LXR activation, with little contribution by retinoic acid receptor α or vitamin D receptor (nonpermissive heterodimer partners). However, the RXR ligands did not upregulate all PPARγ and LXR-dependent genes or upregulated genes to a lesser extent than the receptor-specific ligands. Upregulation of genes uniquely by RXR ligands also suggested the participation of RXR homodimers. The authors suggested that permissivity was partially impaired in monocyte-derived dendritic cells. Similarly, our results with bexarotene suggest that PPAR/RXR is suboptimally activated through ligation of RXR alone in a bone marrow MSC model.
Although TBT binds and activates both PPARγ and RXR, it has been suggested that TBT exerts its physiological effects via activation of RXR, rather than PPARγ, because of the greater efficacy with which it activates RXRs (Grun et al., 2006; le Maire et al., 2009; Nakanishi et al., 2005). Given the limited efficacy of an RXR ligand to activate adipogenesis in BMS2 cells, it was important to determine the potential for TBT to drive adipocyte differentiation. TBT was highly potent at inducing the expression and activation of PPARγ, resulting in lipid accumulation and terminal adipocyte differentiation, at concentrations on par with a therapeutic ligand. The efficacy of TBT was greater than that of bexarotene, suggesting the contribution of a mechanism in addition to RXR activation to TBT-induced effects. Both antagonism and knockdown of PPARγ reduced the response to TBT, indicating the important role of PPARγ in the TBT-induced effects.
An intriguing aspect of PPAR/RXR physiology is the potential for synergistic transactivation in the presence of chemical mixtures as both PPARγ and RXR can bind distinct ligands (Gampe et al., 2000). Cotreatment with agonists for both PPARγ and RXR results in an enhanced PPARγ-dependent transactivation of reporter genes, adipocyte differentiation in cultured cells (Sato et al., 2001; Schulman et al., 1998), lipid metabolism in skeletal muscle (Cha et al., 2001), and differentiation of liposarcomas in vivo (Tontonoz et al., 1997). Here we observed that both bexarotene and TBT enhance rosiglitazone-induced adipocyte differentiation in bone marrow MSCs. A more detailed and extensive combination dose-response, in concert with isobole analysis, will be required to determine the nature of the enhancement (i.e., additive or synergistic).
Given the potential for TBT to activate RXR and thus the possibility that multiple nuclear receptor pathways may be engaged (Szeles et al., 2010), we examined the expression of PPARγ, RXR, and LXR targets in BMS2 cells. As expected, rosiglitazone and TBT significantly induced expression of the PPARγ target, FABP4; however, only TBT significantly induced ANGPTL4. ANGPLT4 expression is strongly activated by PPARβ/δ (Kaddatz et al., 2010), a receptor also activated by TBT (Grun et al., 2006), thus recruitment of multiple PPARs may be supporting induction of ANGPTL4 expression by TBT. The induction of TGM2 expression, an RXR homodimer target (Szeles et al., 2010), by TBT and bexarotene suggests that TBT does interact with RXR directly in BMS2 cells. Futhermore, the induction of ABCA1 expression, a target of LXR, indicates that TBT is capable of activating permissive RXR heterodimers in this bone marrow MSC model. Activation of LXR by TBT also has been observed in macrophages (Cui et al., 2010).
Contaminant-stimulated adipocyte differentiation in bone has significant implications for bone marrow physiology. Osteoblasts and adipocytes share a common precursor, and there is a largely reciprocal relationship between differentiation of these cells types (Chan and Duque, 2002; Lazarenko et al., 2007; Moerman et al., 2004). A consequence of loss of osteoblast differentiation is loss of bone integrity. Indeed, osteoporosis has been referred to as “obesity of the bone” (Rosen and Bouxsein, 2006). However, loss of osteoblasts could influence broader end points, including energy metabolism and immune system function. Bone has recently been recognized as an endocrine organ. Osteoblasts produce the hormone osteocalcin, which increases both insulin expression by the pancreas, as well as adiponectin expression by adipocytes, thereby increasing both insulin excretion and insulin sensitivity (Lee et al., 2007). Thus, skewing bone marrow MSC differentiation away from osteogenesis may compromise the role of bone in maintaining energy homeostasis. An altered adipocyte:osteoblast ratio also has significant implications for the immune system. Considerable evidence supports the conclusion that osteoblasts support hematopoiesis, and lymphopoiesis in particular (Wu et al., 2008). Furthermore, adipocytes not only displace lymphopoiesis-supporting osteoblasts, but they also produce negative regulatory factors (Naveiras et al., 2009). Thus, we hypothesize that stimulation of bone marrow adipocyte differentiation by environmental PPARγ agonists, potentially in concert with therapeutic PPARγ agonists, will have deleterious effects on multiple aspects of bone-supported physiological processes.
There is growing concern about exposure to environmental contaminants that are ligands for PPARγ, including organotins. Importantly, humans are exposed to multiple organotins (Antizar-Ladislao, 2008), and the data suggest that organotins structurally related to TBT, such as TPhT, also are PPAR/RXR dual agonists (Hiromori et al., 2009; Kanayama et al., 2005; Nakanishi et al., 2005). Here, we show that TBT and TPhT potently stimulate adipocyte differentiation in BMS2 cells; these results are in line with those previously found for TBT (Carfi et al., 2008; Kirchner et al., 2010). DBT has been shown to bind to PPARγ but not to RXR, although the physiological significance of DBT PPARγ binding was unclear (Hiromori et al., 2009; Kanayama et al., 2005; Nakanishi et al., 2005). In our bone marrow MSC model, DBT induced adipocyte differentiation at concentrations of 50–100nM but was not nearly as efficacious as TBT or TPhT. The potency of organotins to induce adipogenesis in bone marrow MSCs, either a cultured model or primary cells, suggests that even very low-level exposures to organotins may result in a significant response. Coupled with the fact that phthalates, contaminants of plastics to which humans are highly exposed, also are PPARγ agonists capable of inducing adipocyte differentiation (Feige et al., 2007), environmental exposures could contribute significantly to the pathology of osteoporosis.
Results from these studies show that organotins, with minimal hormonal support, initiate adipocyte differentiation of bone marrow MSCs, both in a cell line model and in primary bone marrow–derived MSCs. TBT-induced differentiation requires PPARγ, but TBT likely engages multiple nuclear receptor pathways, as evidenced by the increased expression of RXR homodimer and LXR gene targets. In future studies, analysis of the contribution of TBT binding to PPARγ, as compared with the contribution of RXR binding, to bone marrow MSC differentiation may reveal important information regarding the biology of RXR hetero- and homodimers, as well as the contribution of these receptors to bone physiology. Considering that aging-associated bone loss is caused, in part, by preferential differentiation of MSCs into adipocytes at the expense of osteoblast differentiation (Nishikawa et al., 2010), these results suggest that exposure to environmental PPARγ and RXR agonists may contribute to bone aging processes and resultant comorbidities such as loss of bone integrity and immune dysregulation.
FUNDING
Boston University School of Public Health; the Superfund Research Program at the National Institutes of Health (P42 ES007381).
Acknowledgments
The authors would like to thank Ms Faye Andrews and Mr Manuel Flores Molina for their superb technical assistance and Dr David Sherr for his helpful comments on the manuscript.
References
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ. Int. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Carfi M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, Gribaldo L. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology. 2008;249:11–18. doi: 10.1016/j.tox.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Cha BS, Ciaraldi TP, Carter L, Nikoulina SE, Mudaliar S, Mukherjee R, Paterniti JR, Jr, Henry RR. Peroxisome proliferator-activated receptor (PPAR) gamma and retinoid X receptor (RXR) agonists have complementary effects on glucose and lipid metabolism in human skeletal muscle. Diabetologia. 2001;44:444–452. doi: 10.1007/s001250051642. [DOI] [PubMed] [Google Scholar]
- Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology. 2002;48:62–71. doi: 10.1159/000048929. [DOI] [PubMed] [Google Scholar]
- Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO. Rep. 2004;5:1007–1012. doi: 10.1038/sj.embor.7400254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen G, Pettersen A, Nesse E, Eek E, Helland A, Breedveld GD. The contribution of urban runoff to organic contaminant levels in harbour sediments near two Norwegian cities. Mar. Pollut. Bull. 2008;56:565–573. doi: 10.1016/j.marpolbul.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Cui H, Okuhira K, Ohoka N, Naito M, Kagechika H, Hirose A, Nishimaki-Mogami T. Tributyltin chloride induces ABCA1 expression and apolipoprotein A-I-mediated cellular cholesterol efflux by activating LXRalpha/RXR. Biochem. Pharmacol. 2010;81:819–824. doi: 10.1016/j.bcp.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J. Biol. Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mattulat A, Lahrz T, Ruden H. Occurrence of organotin compounds in house dust in Berlin (Germany) Chemosphere. 2005;58:1377–1383. doi: 10.1016/j.chemosphere.2004.09.092. [DOI] [PubMed] [Google Scholar]
- Gampe RT, Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol. Cell. 2000;5:545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol. Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- Gurnell M, Wentworth JM, Agostini M, Adams M, Collingwood TN, Provenzano C, Browne PO, Rajanayagam O, Burris TP, Schwabe JW, et al. A dominant-negative peroxisome proliferator-activated receptor gamma (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma-mediated adipogenesis. J. Biol. Chem. 2000;275:5754–5759. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- Hiromori Y, Nishikawa J, Yoshida I, Nagase H, Nakanishi T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) gamma by organotin compounds. Chem. Biol. Interact. 2009;180:238–244. doi: 10.1016/j.cbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Idres N, Marill J, Chabot GG. Regulation of CYP26A1 expression by selective RAR and RXR agonists in human NB4 promyelocytic leukemia cells. Biochem. Pharmacol. 2005;69:1595–1601. doi: 10.1016/j.bcp.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Kaddatz K, Adhikary T, Finkernagel F, Meissner W, Muller-Brusselbach S, Muller R. Transcriptional profiling identifies functional interactions of TGF beta and PPAR beta/delta signaling: synergistic induction of ANGPTL4 transcription. J. Biol. Chem. 2010;285:29469–29479. doi: 10.1074/jbc.M110.142018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol. Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch. Environ. Contam. Toxicol. 2010;58:901–907. doi: 10.1007/s00244-010-9513-6. [DOI] [PubMed] [Google Scholar]
- Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala DS, Mukherjee R, Schulman IG, Koch SS, Dardashti LJ, Nadzan AM, Croston GE, Evans RM, Heyman RA. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature. 1996;383:450–453. doi: 10.1038/383450a0. [DOI] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009;10:367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007;148:903–911. doi: 10.1210/en.2006-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, Nakamura M, Miao S, Cao P, Learned RM, et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J. Biol. Chem. 2002;277:19649–19657. doi: 10.1074/jbc.M200743200. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends. Endocrinol. Metab. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Lazar MA. New developments in adipogenesis. Trends. Endocrinol. Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. Can. Med. Assoc. J. 2009;180:32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on BMD and osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:507–513. doi: 10.1038/ncpendmet0920. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Nishikawa J, Hiromori Y, Yokoyama H, Koyanagi M, Takasuga S, Ishizaki J, Watanabe M, Isa S, Utoguchi N, et al. Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol. Endocrinol. 2005;19:2502–2516. doi: 10.1210/me.2004-0397. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, Kodama T, Yamaguchi A, Owen MJ, Takahashi S, et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J. Clin. Invest. 2010;120:3455–3465. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangeli C, Hayashi S-I, Kincade P. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur. J. Immunol. 1988;18:863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Yajima Y, Kawashima S, Tanaka K, Kagechika H. Synergistic potentiation of thiazolidinedione-induced ST 13 preadipocyte differentiation by RAR synergists. Biochem. Biophys. Res. Commun. 2001;280:646–651. doi: 10.1006/bbrc.2000.4172. [DOI] [PubMed] [Google Scholar]
- Schulman IG, Shao G, Heyman RA. Transactivation by retinoid X receptor-peroxisome proliferators-activated receptor (PPARγ) heterodimers: intermolecular synergy requires only the PPARγ hormone-dependent activation function. Mol. Cell Biol. 1998;18:3483–3494. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr. Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeles L, Poliska S, Nagy G, Szatmari I, Szanto A, Pap A, Lindstedt M, Santegoets SJ, Ruhl R, Dezso B, et al. Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol. Endocrinol. 2010;24:2218–2231. doi: 10.1210/me.2010-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes. Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JS, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferators-activated receptor γ and the retinoid X receptor. Proc. Nat. Acad. Sci. U.S.A. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Miller MS. Determination of murine fetal Cyp1a1 and 1b1 expression by real-time fluorescence reverse transcription-polymerase chain reaction. Toxicol. Appl. Pharmacol. 2004;201:295–302. doi: 10.1016/j.taap.2004.05.011. [DOI] [PubMed] [Google Scholar]