Abstract
Background
Taraxacum officinale (L.) Weber (Asteraceae) has been extensively employed as a diuretic in traditional folk medicine and in modern phytotherapy in Europe, Asia, and the Americas without prior clinical trial substantiation.
Objectives
In this pilot study, a high-quality fresh leaf hydroethanolic extract of the medicinal plant T. officinale (dandelion) was ingested by volunteers to investigate whether an increased urinary frequency and volume would result.
Design
Volume of urinary output and fluid intake were recorded by subjects. Baseline values for urinary frequency and excretion ratio (urination volume:fluid intake) were established 2 days prior to dandelion dosing (8 mL TID) and monitored throughout a 1-day dosing period and 24 hours postdosing.
Results
For the entire population (n = 17) there was a significant (p < 0.05) increase in the frequency of urination in the 5-hour period after the first dose. There was also a significant (p < 0.001) increase in the excretion ratio in the 5-hour period after the second dose of extract. The third dose failed to change any of the measured parameters.
Conclusions
Based on these first human data, T. officinale ethanolic extract shows promise as a diuretic in humans. Further studies are needed to establish the value of this herb for induction of diuresis in human subjects.
Introduction
Use of medicinal plants is increasingly popular in the United States and Europe, and out of economic necessity, continues to be so in developing countries. In some cases, extracts of medicinal plants are used by traditional healers as diuretics.1 A review by Dearing et al.2 reports that 85 species of plants from diverse families have demonstrated evidence of diuretic activity. After reviewing the available literature on medicinal plants used to induced diuresis, Yarnell1 suggests that a number of different botanical medicines show promise in the treatment of a variety of urologic disorders. Additionally, the Commission E approves at least 12 plant extracts as diuretics. Although there are human trials to support or refute the use of traditional remedies as diuretics, they are few and thus, there is a significant void in research to reveal the value of the majority of these remedies.
Species of Taraxacum have been employed as a diuretic for over 2000 years in both Traditional Chinese Medicine and in Ayurvedic medicine.3–5 The species found in most U.S. and European herbal remedies is Taraxacum officinale (L.) Weber (Asteraceae), commonly known as dandelion, Herba Taraxaci, or Taraxacum herba. It is used ubiquitously as a remedy in a variety of conditions. Even before publication in Elizabeth Blackwell's Curious Herbal in 1734, the use of dandelion leaf as a diuretic persisted across cultural and temporal barriers. In French, dandelion is known as pissenlit, a colorful description of its diuretic activity. These ethnobotanical and historical data suggest that various species of dandelion have been widely employed for urinary and renal diseases to enhance the renal elimination of fluids. As suggested by Mills* when societies socially and geographically distant from one another find common uses for the same genera, this provides empirical evidence of a medicinal plant's pharmacological activity.
Currently, clinicians using phytotherapy make use of dandelion leaf in various preparations including infusions, ethanolic extracts, or fresh expressed juice where “enhanced urinary output is desirable.”6 Supporting this indication the German Standard License for dandelion tea includes stimulation of diuresis, and the German Commission E approves the use of dandelion for diuresis.7,8 Rácz-Kotilla et al.9 quantified the diuretic activity in a murine model, reporting that the leaf of dandelion, a better diuretic and saluretic than the root, was comparable to furosemide (Lasix at 80 mg/kg). Despite consistent traditional use, a recent review article by Schutz et al.10 discusses the mixed results and scant data of the pharmacological research characterizing dandelion's utility as a diuretic.
Besides its diuretic utility, dandelion is added to salads perhaps due to its rich nutrient content.11 Dandelion is a significant source of potassium, as well as other vitamins and minerals.12 Reported potassium levels have ranged from 23.3 mg/g13 to 59.9 mg/g,14 with median figures of 42.5 mg/g9 and 45.1 mg/g15 of dried leaf. Rácz-Kotilla et al.9 conclude that dandelion contains three times the amount of potassium in other botanical diuretics and provides more potassium than that lost from diuresis induced by ingesting dandelion. Considering the requirement for potassium supplementation that typically accompanies the use of a pharmaceutical diuretic, dandelion could offer a therapeutically significant potassium contribution by replacing the potassium loss induced by most diuretics.
In spite of a high safety rating, to date there are no published human studies on extracts of T. officinale.16 Given the extensive traditional use of dandelion and its observed therapeutic activity, the objective of this pilot study was to investigate whether ingestion of a high-quality ethanolic extract of dandelion folium results in an increased urinary frequency and volume.
Methodology
Design
This study was conducted over a period of 4 days at the Tai Sophia Institute in Laurel, MD, with a group of healthy female subjects aged 18–65. Conducted under Tai Sophia Institute's IRB approval, it was performed according to the guidelines of the Declaration of Helsinki and Tokyo for humans, and was performed only after informed consent was obtained. Subjects were instructed to abstain from consumption of alcohol and to practice daily consistency with any medications and caffeinated beverages.
Materials
The dandelion ethanolic extract used in the interventions was from a single organically grown batch (Oregon Tilth) and extracted by Oregon's Wild Harvest (Sandy, OR). Botanical identity was confirmed by thin layer chromatography (Fig. 1) and a Certificate of Analysis, both provided by the manufacturer. The concentration of the tincture was 1 g to 1mL of menstruum (95% EtOH). The water content of the leaf, measured by weight of dehydrated herb, was 87%. The manufacturer reports a calculated ethanol content of 43.5% in the final extract.
FIG. 1.
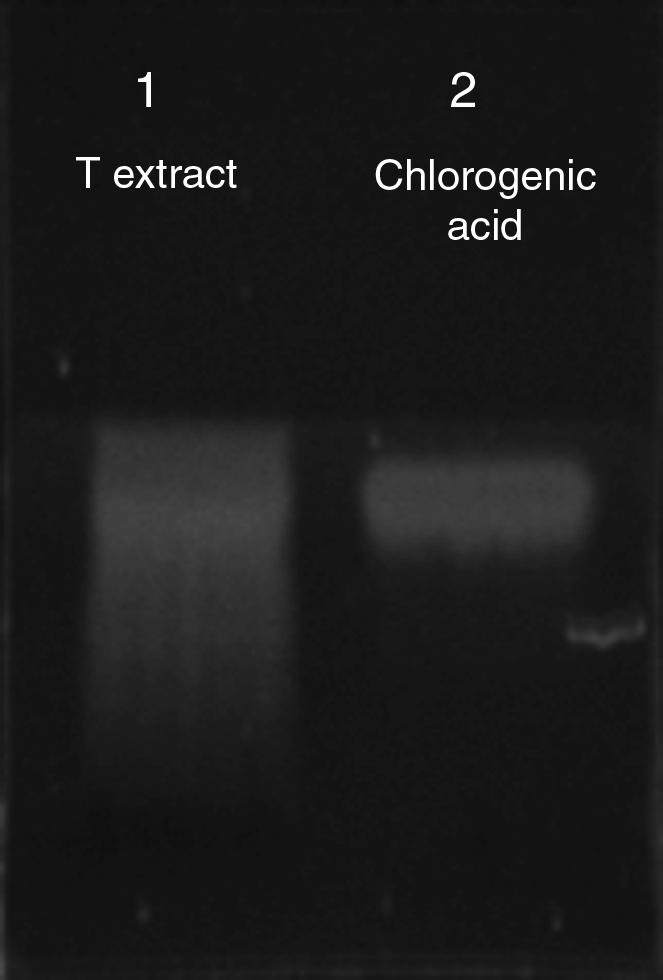
Confirmation of Taraxacum officinale ethanolic extract identity. A thin layer chromatography (TLC) plate of 10 mL of T. officinale leaf ethanolic extract DNL-03305E (lane 1) and 10 mL of chlorogenic acid (lane 2, 0.1mg/mL) TLC plate of 10 mL of T. officinale leaf ethanolic extract DNL-03305E developed with 100:11:11:27 ethyl acetate:formic acid:glacial acetic acid:water, viewed under 365 nm ultraviolet light.
Measuring devices
Participants were supplied and educated in the use of two measurement devices. For the collection and determination of urine, study participants were supplied with a single graduated 600-mL Disposa-Beaker. The participants also received a device for measuring their dosage of the dandelion extract, with graduations of 1 mL up to 10 mL.
Procedure
Participants attended an information session where they were informed of the details and potential adverse events of the study. Upon signing the consent form, they were given the materials for the study. Twenty-eight (28) people volunteered to participate in the study and signed the consent form. The mean age of participants was 37.9 years.
Subjects monitored their fluid intake for 4 consecutive days. The subjects also recorded their urine output for 3 consecutive days starting on the second day (day −1). Subjects self-administered a measured 8-mL dose of dandelion leaf extract at 8:00 am, 1:00 pm, and 6:00 pm on the study day (day 0). After the follow-up day (day 1), participants returned the data sheets for analysis. Eleven (11) participants were unable to complete the study, citing difficulty collecting and measuring of fluid output and/or input. Concurrent medication use was limited to 3 subjects; 1 subject reported medication for asthma and 2 subjects reported oral contraceptive use.
Trial outline
The outline for the 4-day trial is summarized in Table 1, with the consumption of alcoholic and caffeinated beverages restricted throughout the trial.
Table 1.
Outline for the 4-Day Trial
| Day −2 | Restriction on type of fluid intake, record fluid intake |
| Day −1 | Restriction on type of fluid intake, record fluid intake, record urine output |
| Day 0 | Restriction on type of fluid intake, take Taraxacum extract at 8:00 am, 1:00 pm, and 6:00 pm, record fluid intake, record urine output |
| Day 1 | Restriction on type of fluid intake, record of fluid intake, record urine output |
Safety measures
Participants were given a phone number that had direct access to the principal investigator and were advised to contact this individual should any adverse events occur. No adverse events were recorded.
Statistics
All data were recorded on standardized forms. Data analysis was performed on both the frequency of urination and the ratio of fluid output to intake volume (excretion ratio). Data are expressed as the mean ± standard error of the mean and significance was determined using paired or unpaired t-tests as appropriate. The first day of full data collection (day −1) was used as the control day, and the subsequent treatment day (day 0) and the follow-up day (day 1) were analyzed in comparison to that day. Data were analyzed in 5-hour blocks, aligned with the administration times of 8:00 am, 1:00 pm, and 6:00 pm. Five (5)–hour time periods were chosen to resolve the purported fast onset of a diuretic effect without compromising the daily lifestyle of the subjects. The mean values were considered significantly different if p < 0.05.
Results
Frequency of urination
The mean daily frequency of urination for the entire population (n = 17) on the control day (day −1) was 8.0 ± 0.76. This increased to 9.0 ± 0.93 on the day of the trial (day 0) and decreased on the day after the trial to 8.1 ± 1.1 (day 1). By pairwise comparison, there is a significant (p < 0.05) increase in the frequency of urination for the study group on the day of the trial.
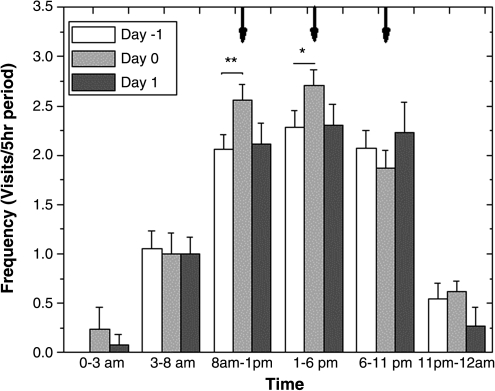
Figure 2 plots the average frequency of urination per subject broken down into 5-hour periods throughout the day, on the day before, the day of, and the day after the trial. There was a significant increase (p < 0.05) in the frequency on the day of the trial from 8:00 am to 1:00 pm, coinciding with the first dose. A smaller increase (p < 0.1) was observed coinciding with the second dose.
FIG. 2.
The mean frequency of urination for all subjects in 5-hour periods, aligned with the times of administration on day 0 (black arrows). A significant (p < 0.05) increase in frequency of urination was observed between 8:00 am and 1:00 pm on day 0. *p < 0.1, **p < 0.05.
Excretion ratio
For the study group, the mean fluid intake (output) per subject on the control day was 2125 ± 188 mL (1874 ± 221 mL), increasing to 2313 ± 249 mL (2148 ± 212 mL) on the day of the trial and decreasing to 1998 ± 153 mL (1988 ± 205 mL) on day 1. From these statistics, there was no significant difference in either the volume of fluids consumed or excreted on a daily basis during the trial.
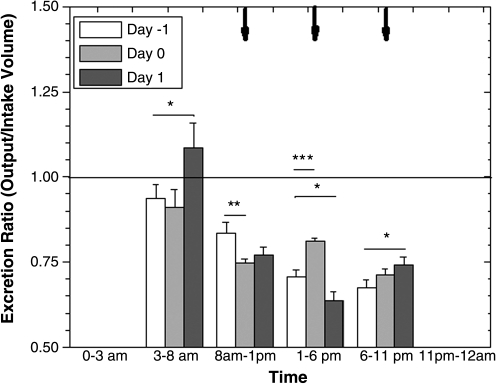
Figure 3 shows the average excretion ratio for all subjects per 5-hour period. Between 1:00 pm and 6:00 pm on the study day (day 0), there was a significant increase (p < 0.001) in the average excretion ratio compared to the control day, primarily due to both an increase in the urination volume per visit (p < 0.1), decrease in fluid intake (p < 0.1) and increase in frequency (p < 0.1).
FIG. 3.
The mean excretion ratio (output/intake volume) of all subjects in 5-hour periods, aligned with the times of administration on day 0 (black arrows). The 0–3:00 am and 11:00 pm-12:00 am has been omitted due to the large variance. A significant (p < 0.001) increase in excretion ratio was observed between 1:00 pm and 6:00 pm on day 0. *p < 0.1, **p < 0.05, ***p < 0.001.
Discussion
The limitations of this study design include lack of blinding, small numbers of subjects, self-monitoring for fluid input/output, no correction for water content of food consumed, and limited baseline values of urine output. A double-blind placebo-controlled trial with larger numbers of subjects, coupled with more objective measurements of the active diuretic compounds in dandelion and fluid output and input, would facilitate further evaluation of the diuretic effects of dandelion leaf extracts and is planned for future investigations. Despite these limitations, the data presented in this pilot study, which mimics actual use of dandelion by those using herbal remedies, offers insight into the diuretic activity of a dandelion ethanolic extract.
There was a significant increase in urination frequency (p < 0.05) demonstrated by trial subjects after administration of the extract. The excretion ratio also increased (p < 0.001). In particular, the 5-hour period after the first dose had a significant increase in frequency and following a second dose, in the second 5-hour period, there was a significant increase in the excretion ratio. However, after a third dose (evening dose), there were no significant changes in fluid intake, urination, or frequency.
The lack of effect observed in the last dose of the day may relate to circadian shifts in kidney function. Due to decrease of solute diuresis (e.g., urea, sodium, and potassium) and nocturnal decrease in fluid intake, nocturnal urinary volume is lower than that of daytime hours.17 Another factor limiting nocturnal urinary volume is the increase in urine concentration due to increased vasopressin secretion/activity.18 Healthy subjects demonstrate a urinary output that is three times the nocturnal output18 and show the average rate of urinary production at night is less than half of that produced during daytime hours.19
These results suggest that dandelion leaf may be fast acting and rapidly cleared. However, these results do not rule out that dandelion leaf acts as a bladder irritant due to the increase in the daily frequency but not the daily urination volume. Other investigations utilizing murine models have failed to find increases in daily urination volume. Tita et al.20 observed no diuresis over 2 hours after a single unspecified dose. Hook et al.,15 also using a single dose over a few hours, observed a saluresis, but not diuresis. In contrast, the results from Rácz-Kotilla et al.9 demonstrate a diuretic effect at the human equivalent dose (HED) of 640mg/kg. It should be noted that the dosing used in the present study was over 22 times lower than the optimal diuretic dose used in Rácz-Kotilla's investigation and over 82 times lower than the dose inducing acute toxicity (HED = 2.3 g/kg).9 Considering the strength of these data, a higher dose in capsule form would facilitate a placebo control and perhaps more clearly elucidate the effect of dandelion on diuresis. An unfortunate consequence of pharmaceutical diuretic therapies may be oxidative damage to the kidneys resulting from glucose intolerance2 and electrolyte and acid–base disorders.21 Diuretics such as furosemide and hydrochlorothiazide with choline substituents in their molecular structure, may also lead to free-radical reactions within multiple tissue compartments.22 A substantial portion of these detrimental effects are the result of hypokalemia, as well as hypomagnesemia. Although magnesium depletion is a less recognized effect of pharmaceutical diuretics, as many as 40% of those on diuretic therapy who develop hypokalemia may also have hypomagnesemia.23 In addition, hypokalemia may be refractory to potassium supplementation unless magnesium is adequately repleted.24 As a result of these detrimental effects, many diuretics may be harmful with long-term use.25–28
Considering the mineral content and the antioxidant activity of dandelion, an interesting possibility is utilizing dandelion as an adjuvant with pharmaceutical diuretics. Given that the combination of potassium and magnesium in supplement form have been shown to be equally as effective as KCl,29 it may be that the mineral content of dandelion leaf with potassium at ≈42.5 mg/g9 and magnesium at ≈2.5 mg/g13,14 may mitigate some of the electrolyte imbalance resulting from pharmaceutical diuretic use. Animal models have demonstrated that supplementation of dandelion leaf results in elimination of less potassium and magnesium than that contained in extracts of dandelion.30
In considering the tissue damage induced by oxidative stress from pharmaceutical diuretic use, adjuvant use of dandelion extracts, shown to have substantial antioxidant activity, could be advantageous. Antioxidant activity has been observed in a number of different models.3,31,32 Sumanth and Rana33 reported increases in the levels of superoxide dismutase, catalase, and glutathione using an ethanolic preparation of dandelion. Zhu et al,32 also using an ethanolic extract, confirmed Rana's results finding superoxide dismutase, catalase, and glutathione levels increased along with peroxidase levels, and also found reduced lipid peroxidation. Hu and Kitts3 demonstrated protection of cells from peroxyl-radical-induced intracellular oxidation, speculating that the protection may have been the result of scavenging of intercellular and intracellular peroxyl radicals by a phenolic-rich extract from dandelion flowers. This group also showed a synergic effect with α-tocopherol and a 40% ascorbate equivalence, which they believed was responsible for regenerating the α-tocopherol.
Many of the complications of pharmaceutical diuretics may be mitigated by proper dosage and replacement of electrolyte losses.2 Accordingly, combination of a dandelion extract with a pharmaceutical diuretic should be investigated. Possible outcomes include reduced dosage requirements of the pharmaceutical diuretic, improved diuresis, decreased oxidative kidney damage and decreased need for potassium and magnesium supplementation.
Of the half dozen modes of activity through which diuretics increase the flow of urine, the most efficacious pharmaceutical diuretics act through a single route referred to as “loop diuresis”—affecting sodium reabsorption in the loop of Henle.34 Of the eight drugs classified as loop diuretics, there is little chemical structural similarity or functional group commonalities between them. Similarly, diuretic compounds found in plants may be due to several constituent groups of secondary metabolites such as terpenes, phenolics, or alkaloids.34 Of interest is the number of secondary metabolites in dandelion that are diuretic. According to Duke's USDA database,35 dandelion has up to nine compounds that are diuretic (Table 2).Given that the saluretic effects of dandelion leaf have been shown to be due to multiple fractions of the extract,15 the diuretic activity of dandelion may be due to several compounds via different diuretic and saluretic pathways. Considering that these active constituents have many other beneficial effects (Table 2), including favorable cardiovascular activity, further research is needed on dandelion leaf's potential therapeutic role.
Table 2.
Diuretic Compounds in Taraxacum officinale herba and their additional properties
| Compound | Other activities of diuretic compounds |
|---|---|
| Ascorbic acid | Nutrient |
| Caffeic acid | Antiaggregant, anti-inflammatory, antioxidant, anxiolytic |
| Calcium | Nutrient |
| Chlorogenic acid | Anti-inflammatory, antioxidant, cardioprotective |
| Isoquercitrin | Anti-inflammatory, antioxidant, hypotensive |
| Luteolin | Anti-inflammatory, antioxidant, hypocholesterolemic, vasodilator |
| Magnesium | Nutrient |
| Mannitol | Anti-inflammatory, antioxidant |
| Potassium | Nutrient |
From ref. 35.
Conclusions
The data from this human trial demonstrate that an ethanolic extract of T. officinale fresh leaf (1 g:1 mL), increases the frequency and excretion ratio of fluids in healthy human subjects. These results suggest further detailed investigations are warranted.
Footnotes
Mills S. Uncovering meaning in a fragmentary evidence base: A Rosetta stone from traditional herb use? Unpublished manuscript.
Acknowledgments
R.C. acknowledges the support of a National Research Council Research Associateship Award, held at National Institute of Neurological Disorders and Stroke. K.S. acknowledges educational support from Tai Sophia Institute. Special thanks to Oregon Wild Harvest for providing the Taraxacum extract and Matthew Creswell for providing the TLC data. In addition, special thanks are owed to Jerry Cott, Kimberly Duncan, Steven Dentali, Simon Mills, Dorena Rode, and James Snow for editorial commentary.
Disclosure Statement
The Taraxacum extract used for this study was supplied at no cost by Oregon's Wild Harvest. Tai Sophia Institute currently carries products from this company in their clinic and has a long-term relationship with this supplier. The authors have no financial interest in Oregon Wild Harvest.
References
- 1.Yarnell E. Botanical medicines for the urinary tract. World J Urol. 2002;20:285–293. doi: 10.1007/s00345-002-0293-0. [DOI] [PubMed] [Google Scholar]
- 2.Dearing MD. Mangione AM. Karasov WH. Plant secondary compounds as diuretics: An overlooked consequence. Am Zool. 2001;41:890–901. [Google Scholar]
- 3.Hu C. Kitts DD. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomed. 2005;12:588–597. doi: 10.1016/j.phymed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Bensky D. Gamble A. Kaptchuk TJ. Chinese Herbal Medicine: Materia Medica. Rev. ed. Seattle, WA: Eastland Press; 1992. [Google Scholar]
- 5.Nadkarni KM. Nadkarni AK. Indian Materia Medica. 3rd. Bombay: Popular Book Depot; 1955. [Google Scholar]
- 6.European Scientific Cooperative on Phytotherapy. ESCOP Monographs, The Scientific Foundation for Herbal Medicinal Products. 2nd. Exeter, UK: European Scientific Cooperative on Phytotherapy; 2003. [Google Scholar]
- 7.Blumenthal M. Busse WR. Federal Institute for Drugs and Medical Devices; (Germany). The Complete German Commission E Monographs, Therapeutic Guide to Herbal Medicines. Austin, TX: American Botanical Council; Integrative Medicine Communications; 1998. [Google Scholar]
- 8.Wichtl M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis. 3rd expanded and completely rev. ed. Stuttgart/Boca Raton, FL: Medpharm, CRC Press; 2004. [Google Scholar]
- 9.Rácz-Kotilla E. Rácz G. Solomon A. Action of Taraxacum Officinale extracts on body-weight and diuresis of laboratory-animals. Planta Med. 1974;26:262–217. doi: 10.1055/s-0028-1099379. [DOI] [PubMed] [Google Scholar]
- 10.Schutz K. Carle R. Schieber A. Taraxacum: A review on its phytochemical and pharmacological profile. J Ethnopharmacol. 2006;107:313–323. doi: 10.1016/j.jep.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture (USDA) Agricultural Research Service. Release 18. USDA National Nutrient Database for Standard Reference. www.nal.usda.gov/fnic/food.comp/Data/ [May 17;2006 ]. www.nal.usda.gov/fnic/food.comp/Data/
- 12.U.S. Department of Agriculture (USDA) Agricultural Research Service. Release 19. USDA National Nutrient Database for Standard Reference. www.nal.usda.gov/fnic/food.comp/Data/SR19/nutrlist/sr19a430.pdf. [May 17;2006 ]. www.nal.usda.gov/fnic/food.comp/Data/SR19/nutrlist/sr19a430.pdf
- 13.Rozycki VR. Baigorria CM. Freyre M, et al. Nutrient content in vegetable species from the Argentine Chaco [in Spanish] Arch Latinoam Nutr. 1997;47:265–270. [PubMed] [Google Scholar]
- 14.Müller HL. Kirchgessner M. Quantity and trace element contents of dandelions and their dependence on growth [in German] Das Wirtshaftseigene Fuller. 1972;18:213–221. [Google Scholar]
- 15.Hook I. McGee A. Henman M. Evaluation of dandelion for diuretic activity and variation in potassium content. Int J Pharmacog. 1993;31:29–34. [Google Scholar]
- 16.McGuffin M. American Herbal Products Association. American Herbal Products Association's Botanical Safety Handbook. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 17.Robertson GL. Norgaard JP. Renal regulation of urine volume: Potential implications for nocturia. BJU Int. 2002;90(s3):7–10. doi: 10.1046/j.1464-410x.90.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 18.Moon DG. Jin MH. Lee JG, et al. Antidiuretic hormone in elderly male patients with severe nocturia: A circadian study. BJU Int. 2004;94:571–575. doi: 10.1111/j.1464-410X.2004.05003.x. [DOI] [PubMed] [Google Scholar]
- 19.Robertson G. Rittig S. Kovacs L, et al. Pathophysiology and treatment of enuresis in adults. Scand J Urol Nephrol. 1999;33:36–39. doi: 10.1080/003655999750169420. [DOI] [PubMed] [Google Scholar]
- 20.Tita B. Bello U. Faccendini P, et al. Taraxacum officinale W.: Pharmacological effect of ethanol extract. Pharmacol Res. 1993;27(suppl 1):23–24. [Google Scholar]
- 21.Greenberg A. Diuretic complications. Am J Med Sci. 2000;319:10–24. [PubMed] [Google Scholar]
- 22.Moore DE. Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention and management. Drug Safety. 2002;25:345–372. doi: 10.2165/00002018-200225050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Odvina CV. Mason RP. Pak CY. Prevention of thiazide-induced hypokalemia without magnesium depletion by potassium-magnesium-citrate. Am J Ther. 2006;13:101–108. doi: 10.1097/01.mjt.0000149922.16098.c0. [DOI] [PubMed] [Google Scholar]
- 24.Whang R. Whang DD. Ryan MP. Refractory potassium repletion: A consequence of magnesium deficiency. Arch Intern Med. 1992;152:40–45. [PubMed] [Google Scholar]
- 25.Wang J-L. Cheng H-F. Shappell S. Harris RC. A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int. 2000;57:2334. doi: 10.1046/j.1523-1755.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 26.Ono Y. Ono H. Frohlich ED. Hydrochlorothiazide exacerbates nitric oxide-blockade nephrosclerosis with glomerular hypertension in spontaneously hypertensive rats. J Hypertens. 1996;14:823. doi: 10.1097/00004872-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Francischetti A. Ono H. Frohlich ED. Renoprotective effects of felodipine and/or enalapril in spontaneously hypertensive rats with and without L-NAME. Hypertension. 1998;31:795. doi: 10.1161/01.hyp.31.3.795. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y. Ono H. Zhou X. Frohlich ED. Angiotensin type 1 receptor antagonism and ACE inhibition produce similar renoprotection in N-nitro-L-arginine methyl ester/spontaneously hypertensive rats. Hypertension. 2001;37:1262. doi: 10.1161/01.hyp.37.5.1262. [DOI] [PubMed] [Google Scholar]
- 29.Wuermser LA. Reilly C. Poindexter JR, et al. Potassium-magnesium citrate versus potassium chloride in thiazide-induced hypokalemia. Kidney Int. 2000;57:607–612. doi: 10.1046/j.1523-1755.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- 30.Raczkoti E. Racz G. Solomon A. Action of Taraxacum Officinale extracts on body-weight and diuresis of laboratory-animals. Planta Med. 1974;26:262–217. doi: 10.1055/s-0028-1099379. [DOI] [PubMed] [Google Scholar]
- 31.Sumanth M. Rana A. In vivo antioxidant activity of hydro-alcoholic extract of Taraxacum officinale roots in rats. Indian J Pharmacol. 2006;38 pNA. [Google Scholar]
- 32.Zhu M. Wong PY. Li RC. Effects of Taraxacum mongolicum on the bioavailability and disposition of ciprofloxacin in rats. J Pharm Sci. 1999;88:632–634. doi: 10.1021/js980367q. [DOI] [PubMed] [Google Scholar]
- 33.Moerman DE. Native American ethnobotany. Portland, OR: Timber Press; 1998. [Google Scholar]
- 34.Goodman LS. Hardman JG. Limbird LE. Gilman AG. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 10th. New York: McGraw-Hill; 2001. [Google Scholar]
- 35.Duke J. Dr. Duke's Phytochemical and Ethnobotanical Databases. Apr 10, 2005. www.ars-grin.gov/duke. [Jul 15;2006 ]. www.ars-grin.gov/duke