Abstract
Helper-dependent adenoviral (HDAd) vectors are devoid of all viral genes and result in long-term transgene expression in the absence of chronic toxicity. Because of their ability to infect post-mitotic cells, including cells of the central nervous system, HDAd vectors are particularly attractive for brain-directed gene therapy. In this study, we show that intrathecal injection of HDAd results in extensive transduction of ependymal cells and sustained expression of the transgene up to 1 year post-administration. We also demonstrate, for the first time, the ability of HDAd injected by this route of delivery to transduce neuronal cells. The transduced neuroepithelial cells can be potentially used to secrete therapeutic proteins into the cerebrospinal fluid and provide them via cross-correction to nontransduced cells. Targeting of neuronal cells and long-term transgene expression make this approach attractive for the treatment of several neurologic diseases.
In this study, Dindot et al. show that intrathecal injection of helper-dependent adenovirus vector results in extensive transduction of ependymal and neuronal cells and sustained expression of the transgene up to 1 year post administration in mice.
Introduction
Adenoviral (Ad) vectors for delivering genes to the central nervous system (CNS) hold great promise for therapeutic applications. Because of their ability to infect post-mitotic cells, including cells of the CNS (Persson et al., 2006) and to mediate long-term transgene expression, Ad-based vectors are very attractive for brain-directed gene therapy. Unlike the rapid decline observed in transgene expression in peripheral organs following intravenous administration, first generation Ad (FGAd)-mediated transduction of adult brain cells results in long-term transgene expression (Davidson et al., 1993; Le Gal La Salle et al., 1993). It is thought that FGAd-mediated long-term transgene expression occurs because the brain is relatively protected from the effects of the immune response. Therefore, Ad injection into the brain results in an ineffective T-cell response against brain-transduced cells (Byrnes et al., 1996). However, the immune system can respond to antigenic stimuli in the brain (Perry et al., 1993), and if a peripheral immune response against Ad is elicited after natural infection or vector readministration, loss of transgene expression and chronic inflammation are observed (Thomas et al., 2000). Interestingly, these problems are not seen with helper-dependent adenoviral (HDAd) vectors (Thomas et al., 2000; Xiong et al., 2006). For example, in naïve animals, FGAd- and HDAd-mediated expression of β-galactosidase in the brain is long term, but in animals immunized prior to vector delivery, transgene expression is abolished in FGAd-injected mice but not in the mice injected with HDAd (Thomas et al., 2000; Xiong et al., 2006). These studies indicate that long-term HDAd-mediated transgene expression in the brain occurs even in animals that had been immunized systemically against Ad before the delivery of HDAd into the brain (Thomas et al., 2000; Xiong et al., 2006). Therefore, HDAd vectors are superior to FGAd vectors for gene therapy of brain disorders and are potentially useful even in patients pre-exposed to Ad (Barcia et al., 2007).
A major issue for brain directed Ad-mediated gene therapy is the route of administration. The blood–brain barrier limits transduction of brain cells by Ad vectors administered intravenously. For brain-directed gene therapy, it is necessary for the vector to either cross or circumvent the blood–brain barrier. Injecting vector directly into the brain parenchyma has been one of the most common approaches resulting in localized transduction of the cerebral parenchyma because this route of vector delivery has limited transduction not extending beyond a few millimeters from the needle track. While this approach could still result in clinical benefit in disorders such as Parkinson disease, in which a discrete set of neurons is affected, correction of brain disorders with diffuse involvement is far more complicated. Further problems with intracerebral injections are the invasiveness of the procedure, the small volume of vector that can be delivered, and the possible need for multiple sites of injection.
Injection of viral vectors into the cerebrospinal fluid (CSF) through injection in the cisterna magna or through lumbar puncture might represent an alternative approach for widespread CNS correction. Vector administration into the CSF circulation may allow viral vector-mediated transduction of neuroepithelial cells and the delivery of transgene products to the whole CNS through the ventricular circulation (Butti et al., 2008a). Therefore, this approach may have potential for several clinical applications that can benefit from the expression in the CSF of a bioactive molecule (Elliger et al., 1999, 2002; Watson et al., 2006). Intrathecal administration of HDAd resulted in transgene expression in neuroepithelial cells for about 3 months without systemic or local toxicity in nonhuman primates with pre-existing anti-adenoviral immunity (Butti et al., 2008b).
In the present study, we analyzed in detail the cell types that are transduced by HDAd delivered through CSF injection, and we also investigated the duration of HDAd-mediated transgene expression.
Materials and Methods
Vectors
HDAd-CMV-LacZ bears a cytomegalovirus (CMV)-LacZ expression cassette and HDAd-CMV-GFP contains a CMV-GFP expression cassette. HDAd vectors were produced in 116 cells with the helper virus AdNG163 as described elsewhere (Palmer and Ng, 2003). Helper virus contamination levels were determined as described elsewhere and were found to be <0.05%. DNA analyses of HDAd genomic structure was confirmed as described elsewhere (Palmer and Ng, 2003). All vector preparations were tested using Multi-test Limulus Amebocyte Lysate (Pyrogent, Biowhittaker, Walkersville, MD) for the presence of endotoxin and were found to be below the limit of detection (endotoxin <0.5 EU/ml).
Mice and injections
Nine- to 12-week-old male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used for all the experiments. Intrathecal HDAd administrations were performed in saline solution by cisterna magna injections (Ueda et al., 1979) in a total volume of 10 μl injected in approximately 10 sec.
Analyses of brains
X-gal histochemistry was performed on brain tissues as previously described (Brunetti-Pierri et al., 2004). Total proteins were extracted from the brains and the β-galactosidase activity was determined using the β-Galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega, Madison, WI) following quantification using the Micro BCA Protein Assay Kit (Pierce, Rockford, IL).
Brain perfusion and immunofluorescence
Mice were perfused with ice-cold phosphate-buffered saline (PBS) and 4% paraformaldehyde. Dissected brains were post-fixed in 4% paraformaldehyde solution overnight and then cryoprotected in 30% sucrose solution. Forty-five-micrometer sections were cut on a cryostat and stored in PBS. Sections were washed in PBS and blocked in T-TBS (10 mM Tris-HCl, pH 7.5, 0.3% Triton-X100) plus 5% normal goat or donkey serum for 1–2 hr at 4°C in a humidified chamber with gentle agitation. Anti-GFP (NB 600-308; Novus Biologicals, Littleton, CO) was used a 1:2000 dilution, anti-Notch1 (05-557; Millipore, Billerica, MA) at 1:250, anti-Map2 (MAB3418; Millipore) at 1:250, anti-Doublecortin (SC-8066; Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:250, and anti-Calbindin (C9848; Sigma) at 1:250. Sections were washed three times in T-TBS for 10 min each and then incubated with fluorescently labeled secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 25 hr at 4°C in the dark. Anti-rabbit Alexa 488, anti-mouse Alexa 557, and anti-goat Alexa 647 were used at 1:200 dilutions. Sections were washed in T-TBS for 20 min each and mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA) mounting reagent. Confocal images were obtained using a LSM 510 META NLO multiphoton microscope (Zeiss, Oberkochen, Germany).
Vector genome copies
Total DNA was extracted from livers and brains from HDAd-injected mice (n = 3) using phenol–chloroform extraction and quantitated by absorbance at 260 nm. Quantitative real-time polymerase chain reaction (PCR) was performed using the LightCycler FastStart DNA Master SYBER Green I (Roche, Indianapolis, IN) in a total volume of 20 μl with 100 ng of template DNA, 1 μM of each HDAd-specific primers (5′-TCTGAATAATTTTGTGTTACTCATAGCGCG-3′ and 5′- CCCATAAGCTCCTTTTAACTTGTTAAAGTC-3′). Cycling conditions consisted of 95°C for 10 min followed by 45 cycles at 95°C for 10 sec, 60°C for 7 sec, and 72°C for 20 sec. Serial dilutions of a plasmid bearing the PCR target sequence were used as a control to determine the amounts of HDAd and results were analyzed with LightCycler software version 3.5 (Roche).
Results
Delivery of HDAd via intrathecal injection
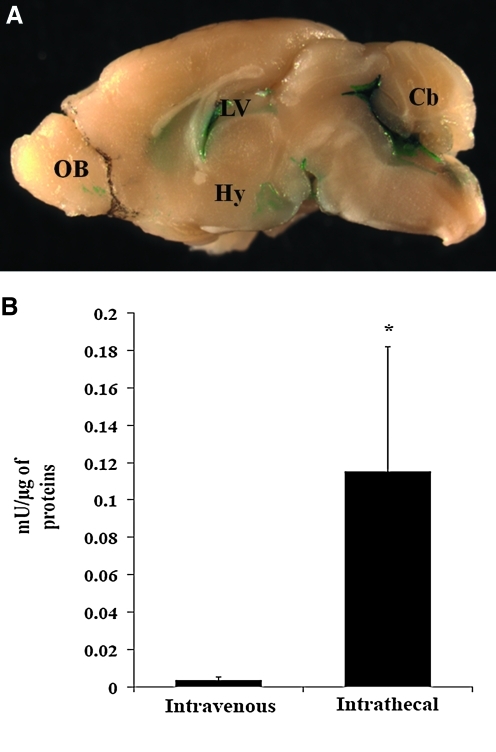
To investigate the efficacy of transgene expression following intrathecal delivery of HDAd vectors, we injected C57BL/6 mice via cisterna magna (Ueda et al., 1979) with 1 × 1012 viral particles (vp)/kg of HDAd-CMV-LacZ vector (Brunetti-Pierri et al., 2004) (n = 4). Mice were sacrificed 48 hr post-injection and stained with X-gal and each mouse stained positive for LacZ expression. Macroscopic examination of injected brains indicated the transgene was expressed in cells lining the ventricles, the spinal cord, and the cerebellum. Expression was also detected in the hypothalamus and in the olfactory bulbs (Fig. 1A). As shown by β-galactosidase activity, administration of HDAd by intrathecal injection resulted in significant cerebral transduction as compared with the brain transduction achieved by intravenous administration of the same vector dose (Fig. 1B).
FIG. 1.
Gross X-gal staining of helper-dependent adenoviral (HDAd)-injected brains. (A) X-gal staining of HDAd-CMV-LacZ injected brains. CMV, cytomegalovirus; Cb, cerebellum; LV, lateral ventricle; Hy, hypothalamus; OB, olfactory bulb. (B) β-galactosidase in brains harvested 48 hr after the intravenous or intrathecal injection of HDAd-CMV-LacZ at the dose of 1 × 1012 vp/kg. *p <0.05 (t-test).
Transgene expression in the CNS
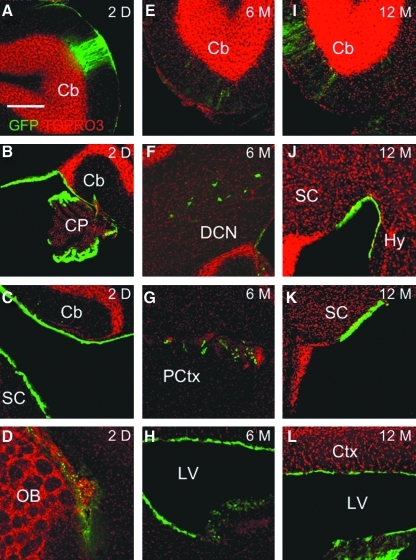
To evaluate the duration of transgene expression, we injected C57BL/6 mice with either saline or with an HDAd vector expressing green fluorescent protein (GFP) under the control of the ubiquitous CMV promoter (HDAd-CMV-GFP) at the dose of 1 × 1012 vp/kg. Mice were sacrificed at 2 days (n = 4), 6 months (n = 5), and 12 months (n = 9) post-injection, and the brains were harvested for immunofluorescence detection of GFP (Mullen et al., 1992). At 2 days post-injection, four mice (100%) were positive for GFP expression and high levels were detected in the molecular layer of the cerebellum (Fig. 2A), choroid plexus (Fig. 2B), ependymal cells lining the spinal cord and cerebellum (Fig. 2C), and in cells within the olfactory bulb (Fig. 2D). At 6 months, four mice (80%) were positive for GFP expression, which was detected in the molecular layers of the cerebellum (Fig. 2E), deep cerebellar nuclei (Fig. 2F), prefrontal cortex (Fig. 2G), and in ependymal cells lining the lateral ventricles (Fig. 2H). At 12 months, seven mice (78%) were positive for GFP expression, although it appeared that there was a reduction in the amount of GFP expression in the molecular and granular layer of the cerebellum (Fig. 2I), hypothalamus (Fig. 2J), spinal cord (Fig. 2K), and in ependymal cells lining the lateral ventricles (Fig. 2L). Collectively, these data indicate long-term expression of the HDAd vector in various regions and cell types within the CNS and demonstrate a minimal reduction of expression at 12 months post-injection.
FIG. 2.
Expression patterns of HdAd-CMV-GFP in the brain. At 2 days (2D) post-injection, GFP expression was detected in the (A) cerebellum, (B) choroid plexus, (C) ependymal cells lining the spinal cord and cerebellum, and (D) olfactory bulb. At 6 months (6M), GFP expression was detected in the (E) cerebellum, (F) deep cerebellar nuclei, (G) prefrontal cortex, and (H) lateral ventricles. At 12 months (12M), GFP expression was detected in the (I) cerebellum, (J) hypothalamus, (K) spinal cord, and (L) lateral ventricles. GFP, green fluorescent protein; D, day; M, month; Cb, cerebellum; CP, choroid plexus; SC, spinal cord; OB, olfactory bulb; DCN, deep cerebellar nuclei; PCtx, prefrontal cortex; Ctx, cortex; LV, lateral ventricle. TOPRO3 stains nuclei. Scale bar represents 300 μm.
Lower doses of HDAd results in reduced cellular transduction and expression of the transgene
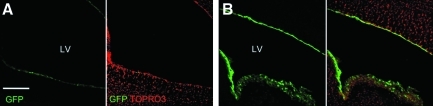
To examine the effects of lower vector doses on the cellular transduction and expression of HDAd-CMV-GFP, we injected mice with 1 × 1010 vp/kg and 1 × 1011 vp/kg of HDAd-CMV-GFP vector. Mice were sacrificed 2 days post-injection, and the brains were harvested for immunofluorescence detection of GFP. Analysis of lateral ventricles indicated that the dose of 1 × 1010 vp/kg resulted in less cellular transduction and expression of GFP expression, whereas the dose of 1 × 1011 vp/kg resulted in more cellular transduction and high levels of GFP expression, although not equivalent to the 1 × 1012 vp/kg (Fig. 3A, B). Collectively, these data indicate that the level of transduction and expression of the HDAd-CMV-GFP virus is dose dependent.
FIG. 3.
Injections of lower doses of HDAd-CMV-GFP. (A) Injections of 1 × 1010 vp/kg resulted in reduced cellular transduction and GFP expression in cells lining lateral ventricles. (B) Injections of 1 × 1011 vp/kg resulted in increased transduction and expression in cells lining the lateral ventricles. TOPRO3 stains nuclei. Scale bar represents 300 μm.
Ciliated ependymal cells and subpopulations of neurons and neuronal precursors are transduced by intrathecal HDAd injections
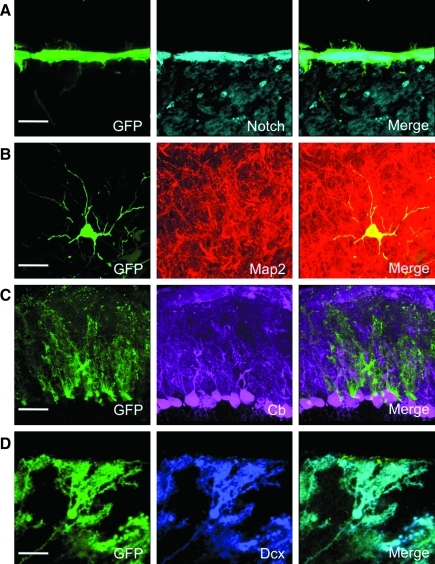
To determine the cell types transduced by intrathecal injection of HDAd vectors, we performed double fluorescent staining of HDAd-injected brains (Izant and McIntosh, 1980). We detected GFP expression in Notch1-expressing (Carlen et al., 2009) ciliated cells lining the lateral ventricles (Fig. 4A). Additionally, we detected GFP expression in neuronal cells in the hypothalamus expressing Map2, which is a marker of mature, differentiated neurons (Menezes and Luskin, 1994) (Fig. 4B). In the cerebellum, we detected GFP expression in cells located adjacent to calbindin-expressing Purkinje cells (Fig. 4C). Interestingly, these cells expressed doublecortin, which is a marker of neuronal lineage commitment (Walker et al., 2007) (Fig. 4D), and the doublecortin staining was exclusive to these GFP-expressing cells. Transduction of neuronal cells was only observed at 6 months post-injection, whereas transduction of ependymal cells and cerebellar cells were observed at each time point examined.
FIG. 4.
Characterization of cell types expressing HDAd-CMV-GFP. Double immunofluorescence staining of GFP and cell type specific markers in the CNS revealed the HDAd vector transduced: (A) ciliated ependymal cells lining the lateral ventricles expressing Notch1, (B) mature neurons expressing Map2 in the hypothalamus, (C) cells adjacent to Purkinje cells in the cerebellum, and (D) doublecortin-expressing cells in the molecular cell layer of the cerebellum. GFP, green fluorescent protein; Map2, microtubule-associated protein 2; Cb, calbindin; Dcx, doublecortin. Scale bar represents 25 μm for (A) and (D), and 50 μm for (B) and (C).
Inflammatory responses are dose dependent in HDAd-injected mice
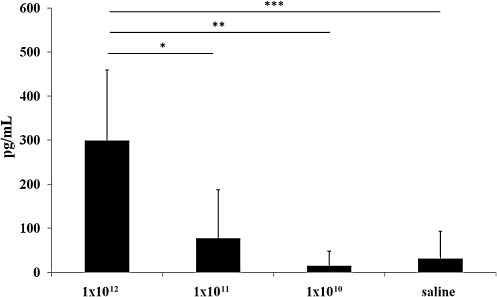
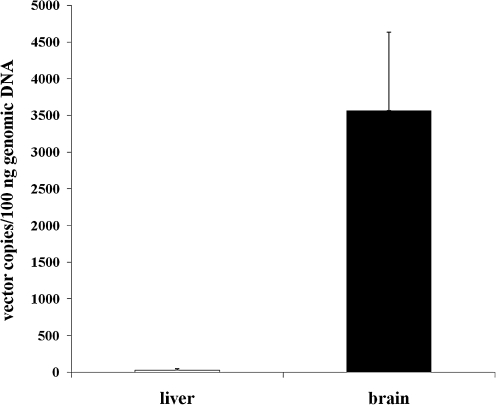
To determine the degree of activation of the inflammatory response, we analyzed serum samples collected from mice at different time points (6, 24, 48 hr) post-injection to measure interleukin (IL)-6 levels. Similarly to the intravascular delivery (Zhang et al., 2001), intrathecal delivery of HDAd results in a dose-dependent increase in serum IL-6 at 6 hr post-injection (Fig. 5); however, serum IL-6 was undetectable at the later time points (data not shown). To evaluate whether intrathecal injections of HDAd result in vector systemic dissemination, we determined by real-time PCR the amount of HDAd vector genomes in livers and brains of animals 48 hr post-intrathecal injections of 1 × 1012 vp/kg of HDAd vector. The real-time PCR showed that the amount of vector genomes detected in the livers was below the limit of detection (10 copies of vector genome) and the vector genomes were only detected in brains of vector-injected mice (n = 3) (Fig. 6).
FIG. 5.
Serum interleukin (IL)-6 at 6 hr after the injection of various doses expressed in vp/kg of HDAd-CMV-GFP. Intrathecal delivery of HDAd results in a dose-dependent and transient increase in serum IL-6. *p <0.05; **p <0.005; ***p <0.01 (t-test).
FIG. 6.
Vector genome copies in brain and liver tissues in animals injected at the dose of 1 × 1012 vp/kg of HDAd-CMV-LacZ.
Discussion
In this study, we show for the first time that HDAd vectors delivered by intrathecal injection result in transduction and long-term transgene expression from neuroependymal and neuronal cells. Intravenous administration of the HDAd-CMV-LacZ vector indicated that the vector is not able to cross the blood–brain barrier in significant amounts, as shown by the minimal increase in β-galactoidase activity in the brain (Fig. 1), whereas other tissues (particularly the liver) had substantial activity (data not shown). Conversely, in intrathecal injected animals β-galactosidase activity was restricted to the brain.
At the microscopic level, intrathecal injection of HDAd vectors resulted primarily in transduction of ependymal cells lining the ventricles, which form the blood–CSF barrier surrounding both the brain and the spinal cord (Fig. 2). Interestingly, we have also shown that HDAd delivered by this route results in transduction of neurons located deep within the cerebral parenchyma at different time points post-vector injection. We reasoned that HDAd delivered by intrathecal injection infects the cells lining the ventricular system, which include a single layer of ependymal cells facing the lumen and the cells of the subventricular zone (SVZ) lying underneath the ependymal layer. It is well established that the SVZ of the lateral ventricles is a source of adult neuronal stem cells (NSCs). NSCs in the SVZ can differentiate into neurons in the olfactory bulbs and in the corpus callosum, as well as in fimbria and striatum oligodendrocytes. Although the ability of the ependyma to give rise to NSCs is still controversial (Ma et al., 2009), it is becoming clear that NSCs directly face the lateral ventricles through small apical processes (Mirzadeh et al., 2008), from where they likely come in contact with viral particles injected into the CSF space. Recently, also leptomeningeal compartment has been suggested to host a NSC niche (Bifari et al., 2009). Therefore, it is possible that HDAd delivered by intrathecal injection may allow transduction of NSCs that are subsequently found deep in the cerebral parenchyma. The detection of GFP+/Dcx+ cells in the cerebellum supports this hypothesis. In fact, Dcx expression in the adult brain was earlier considered to be restricted to the neuronal precursor phase of the neuronal lineage (Brown et al., 2003), while more recently Dcx+ neurosphere-forming cells were identified in the cerebellum of adult mouse (Walker et al., 2007). However whether Dcx expression represent just a stage before final commitment of stem cells to the neuronal lineage or whether this represents an entirely separate precursor population remains controversial.
Dcx is not expressed during gliogenesis or regenerative axonal growth (Couillard-Despres et al., 2005). For these reasons, it is unlikely that GFP+/Dcx+ cells result from the damage induced by the injection.
Intra-CSF injection (both intraventricular and lumbar puncture) of FGAd vectors expressing LacZ in nonhuman primates resulted in high transduction efficiency of leptomeningeal cells as shown by tissue staining at 72 hr post-injection (Driesse et al., 1999). Microscopic examination revealed transduction of arachnoid cells and to a lesser extent the cells of the pia mater. Ependymal and choroid plexus cells were also transduced (Driesse et al., 1999). Duration of transgene expression up to 3 months was observed in rhesus macaques injected with HDAd expressing GFP by lumbar puncture without signs of systemic or local toxicity or evidence of CNS-specific immune reaction (Butti et al., 2008b; Terashima et al., 2009). However, in these studies transduction of neurons in the cerebral parenchyma has not been investigated. Our study also support that, similarly to intravascular delivery (Schnell et al., 2001; Brunetti-Pierri et al., 2004), intrathecal injection of HDAd results in a rapid dose-dependent acute inflammatory response (Fig. 5). This acute response is resolved by 48 hr post-injection and is likely the result of capsid-mediated activation of the innate immunity. Studies in large animal models are required to establish whether this response would be clinically acceptable.
An important finding of our study is that transgene expression is long term following intrathecal injection of HDAd vectors, at least in mice. Moreover, it indicates for the first time that this route of delivery results in transduction of neuronal cells. This finding is significant because targeting of neuronal cells is important for correction of several neurologic diseases and long-term expression is required for the treatment of genetic diseases affecting the CNS. In contrast to the limited vector distribution achieved by intracerebral injection, administration of HDAd vectors into the CSF has the potential advantage of widespread transduction along the cells lining the CSF space. Moreover, intrathecal injection is a far less invasive procedure than intracerebral injection and therefore is attractive for clinical applications.
The transduced ependymal cells could be potentially used to secrete therapeutic proteins into the CSF. As previously shown this route of administration resulted in the production of significant amounts of bioactive proteins which can be exploited for multiple therapeutic purposes (Betz et al., 1995; Furlan et al., 1998). Transduced cells could secrete the therapeutic protein via the CSF for cross-correction of nontransduced cells. Thus, this approach may be particularly attractive for lysosomal storage diseases in which cross-correction is mediated by the mannose-6-phosphate receptor. It remains to be seen whether intrathecal administration of an HDAd vector is equally effective in larger mammals where greater diffusion distances may limit effective distribution of the vector and/or its therapeutic product.
Acknowledgments
We are grateful for financial support from the MPS Society to NB-P. Confocal microscopy was performed in the Texas A&M University College of Veterinary Medicine & Biomedical Sciences Image Analysis Laboratory, supported by NIH-NCRR (1 S10 RR22532-01).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Barcia C. Jimenez-Dalmaroni M. Kroeger K.M., et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol. Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A.L. Yang G.Y. Davidson B.L. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J. Cereb. Blood Flow Metab. 1995;15:547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- Bifari F. Decimo I. Chiamulera C., et al. Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J. Cell Mol. Med. 2009;13(9B):3195–3208. doi: 10.1111/j.1582-4934.2009.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.P. Couillard-Despres S. Cooper-Kuhn C.M., et al. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Palmer D.J. Beaudet A.L., et al. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Butti E. Bergami A. Recchia A., et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008a;15:504–515. doi: 10.1038/gt.2008.10. [DOI] [PubMed] [Google Scholar]
- Butti E. Bergami A. Recchia A., et al. Absence of an intrathecal immune reaction to a helper-dependent adenoviral vector delivered into the cerebrospinal fluid of non-human primates. Gene Ther. 2008b;15:233–238. doi: 10.1038/sj.gt.3303050. [DOI] [PubMed] [Google Scholar]
- Byrnes A.P. Wood M.J. Charlton H.M. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996;3:644–651. [PubMed] [Google Scholar]
- Carlen M. Meletis K. Goritz C., et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S. Winner B. Schaubeck S., et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Davidson B.L. Allen E.D. Kozarsky K.F., et al. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Driesse M.J. Kros J.M. Avezaat C.J., et al. Distribution of recombinant adenovirus in the cerebrospinal fluid of nonhuman primates. Hum. Gene Ther. 1999;10:2347–2354. doi: 10.1089/10430349950016997. [DOI] [PubMed] [Google Scholar]
- Elliger S.S. Elliger C.A. Aguilar C.P., et al. Elimination of lysosomal storage in brains of MPS VII mice treated by intrathecal administration of an adeno-associated virus vector. Gene Ther. 1999;6:1175–1178. doi: 10.1038/sj.gt.3300931. [DOI] [PubMed] [Google Scholar]
- Elliger S.S. Elliger C.A. Lang C. Watson G.L. Enhanced secretion and uptake of beta-glucuronidase improves adeno-associated viral-mediated gene therapy of mucopolysaccharidosis type VII mice. Mol. Ther. 2002;5:617–626. doi: 10.1006/mthe.2002.0594. [DOI] [PubMed] [Google Scholar]
- Furlan R. Poliani P.L. Galbiati F., et al. Central nervous system delivery of interleukin 4 by a nonreplicative herpes simplex type 1 viral vector ameliorates autoimmune demyelination. Hum. Gene Ther. 1998;9:2605–2617. doi: 10.1089/hum.1998.9.17-2605. [DOI] [PubMed] [Google Scholar]
- Izant J.G. McIntosh J.R. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc. Natl. Acad. Sci. U. S. A. 1980;77:4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal La Salle G. Robert J.J. Berrard S., et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Ma D.K. Bonaguidi M.A. Ming G.L. Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J.R. Luskin M.B. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J. Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z. Merkle F.T. Soriano-Navarro M., et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R.J. Buck C.R. Smith A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Palmer D. Ng P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Perry V.H. Andersson P.B. Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993;16:268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- Persson A. Fan X. Widegren B. Englund E. Cell type- and region-dependent coxsackie adenovirus receptor expression in the central nervous system. J. Neurooncol. 2006;78:1–6. doi: 10.1007/s11060-005-9055-3. [DOI] [PubMed] [Google Scholar]
- Schnell M.A. Zhang Y. Tazelaar J., et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Terashima T. Oka K. Kritz A.B., et al. DRG-targeted helper-dependent adenoviruses mediate selective gene delivery for therapeutic rescue of sensory neuronopathies in mice. J. Clin. Invest. 2009;119:2100–2112. doi: 10.1172/JCI39038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.E. Schiedner G. Kochanek S., et al. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H. Amano H. Shiomi H. Takagi H. Comparison of the analgesic effects of various opioid peptides by a newly devised intracisternal injection technique in conscious mice. Eur. J. Pharmacol. 1979;56:265–268. doi: 10.1016/0014-2999(79)90181-x. [DOI] [PubMed] [Google Scholar]
- Walker T.L. Yasuda T. Adams D.J. Bartlett P.F. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J. Neurosci. 2007;27:3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G. Bastacky J. Belichenko P., et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13:917–925. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- Xiong W. Goverdhana S. Sciascia S.A., et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Chirmule N. Gao G.P., et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]