Abstract
Medication adherence studies increasingly collect data electronically, often using Medication Event Monitoring System (MEMS) caps. Analyses typically focus on summary adherence measures, although more complete analyses are possible using adaptive statistical methods. These methods were used to describe individual-subject adherence patterns for MEMS data from a clinical trial. Subjects were adaptively clustered into groups with similar adherence patterns and clusters were compared on a variety of subject characteristics. There were seven different adherence clusters: consistently high, consistently moderately high, consistently moderate, consistently moderately low, consistently low, deteriorating starting early, and deteriorating late. Compared to other subjects, subjects with consistently high and consistently moderately high adherence were more likely to be male, White, and older and to maintain during study participation a CD4 cell count over 500 and an HIV viral load of at most 400 copies/ml. These results demonstrate the effectiveness of adaptive methods for comprehensive analysis of MEMS data.
Keywords: Adaptive statistical methods, antiretroviral adherence, electronic monitoring, MEMS caps
Introduction
Medication adherence studies increasingly collect data electronically, often using Medication Event Monitoring System (MEMS) caps (AARDEX, Zurich, Switzerland). An advantage of electronic monitoring is that each subject's adherence can be analyzed separately. Analyses typically focus on summary measures of individual-subject adherence, e.g., percent prescribed doses taken (PDT), percent PDT at correct time interval, and therapeutic coverage (Sereika and Dunbar-Jacobs, 2001). Percent PDT is used most commonly, sometimes by itself (Holzemer et al., 2006), but often with other summary measures as well (Fletcher et al., 2005; Liu et al., 2006; Vrijens et al., 2005). Several studies have used MEMS-derived summary measures to demonstrate that moderate levels of adherence can result in virologic suppression with newer potent antiretroviral (ARV) regimens (Bangsberg, 2006; Shuter et al., 2007). MEMS data have also been useful for clarifying the possible relationship between viral load blips (i.e., intermittent low levels of plasma HIV-1 RNA) and ARV adherence (Podsadecki et al., 2007).
As an alternative to summary measures, adaptive statistical methods can be used to visualize individual-subject MEMS data grouped into cap opening counts and rates over separate time periods and to describe associated individual-subject adherence patterns (Knafl et al., 2004). Subjects can also be adaptively clustered into groups with similar adherence patterns (Delucchi et al., 2006) rather than using simple adherence ranges (e.g., designating adherence over 95% as high). In this paper, we used these adaptive methods to characterize MEMS-based patterns of ARV adherence for HIV-positive participants of a randomized clinical trial that evaluated the efficacy of a behavioral intervention to improve medication adherence (Williams et al., 2006).
Adaptive statistical methods are relatively new. Knafl et al. (2004) developed them specifically to model individual-subject MEMS adherence data using Poisson regression, commonly used with count/rate data, but they apply more generally (e.g. to linear and logistic regression). Knafl, Fennie, and OMalley (2006) extended these methods to handle repeated measurements and used them to model self-reported adherence over time.
In the context of MEMS data, these methods generate individual-subject adherence patterns corresponding to estimates of mean adherence as an arbitrary function of time. Mean adherence is treated as a general, possibly nonlinear function of time rather than as constant in time as is implicit for standard summary adherence measures, and so reflects a subject's actual adherence. Estimates are based on fractional polynomial models (Royston & Altman, 1994) in time. In other words, they are based on one or more transforms of time raised to possibly fractional powers rather than to only integer powers as in standard polynomials. The specific powers used in the model are selected through a heuristic search process (defined in Knafl et al., 2004) applied to individual-subject MEMS data, and so adapted to those data.
As part of the search process, models are evaluated using likelihood cross-validation (LCV) scores with larger values indicating better models (Knafl et al., 2004; Stone 1977). The advantage of LCV is that it adapts model evaluation to the distribution underlying an analysis. Knafl et al. (2004) used it with the Poisson distribution to model MEMS adherence. Delucchi et al. (2006) used it with mixtures of unstructured multivariate normal distributions (Symons, 1981) to compare the results of clustering algorithms applied to MEMS adherence patterns. Knafl, Fennie, & O'Malley (2006) used it with compound symmetric multivariate normal distributions to evaluate models for repeated measurements. Knafl and Grey (2007) used it with factor-analytic multivariate normal distributions to evaluate exploratory and confirmatory factor analysis models.
Our earlier work has focused on the development of adaptive statistical methods, reporting only limited analyses as examples of results possible for those developed methods. This paper demonstrates instead the effectiveness of previously developed adaptive methods for conducting comprehensive analyses of MEMS adherence data, the results of which are of importance in their own right in identifying ARV adherence pattern types and describing their relationships with subject characteristics and clinical outcomes.
Methods
The ATHENA Project
The Adherence through Home Education and Nursing Assessment (ATHENA) Project was a randomized clinical trial that tested a home-based nursing intervention to improve adherence to ARV medications (Williams et al., 2006). Subjects were recruited from HIV outpatient clinics and through flyers distributed at AIDS service organizations, support groups, and community centers. HIV-infected men and women were eligible for the study if they had been prescribed at least three ARV medications, spoke English or Spanish, had a home or place to receive the intervention and were not participating in another adherence study.
ATHENA subjects were given pill bottles equipped with MEMS caps containing an ARV medication randomly selected from among those prescribed at 2 doses per day. MEMS caps recorded the date and time of each cap opening and presumably of medication-taking. Subjects were informed about the purpose of the MEMS cap and were given verbal and written instructions on its use. All subjects had a 4-week lead-in time to use the MEMS cap before the baseline assessment. Face-to-face interview data, including self-reported adherence to all HIV medications, were also collected at baseline and up to 6 other times at 3 month intervals by trained interviewers blinded to the group assignment. Self-reported adherence was measured at each interview as the percent of prescribed ARV medications reported to have been taken over the three days prior to an interview. CD4 cell counts and HIV viral loads were obtained from subjects' medical records and matched in time to interview dates. Data were collected from 1999 through 2002. MEMS cap data were uploaded to the database at each study visit but were not reviewed with individual subjects. Subjects received $25 per study visit and an additional $10 if they brought the MEMS cap to the study visit. Subjects were also interviewed at 12 months on their experience with using MEMS caps (Bova et al., 2005).
Modeling of Individual-Subject Adherence Data
MEMS cap openings were grouped to generate opening counts and rates for disjoint intervals of subject participation in the study. Intervals over which subjects were not responsible for medication taking were removed prior to analysis. These included periods when subjects were off ARVs on provider order or continuing ARV therapy in a hospital, in-patient drug treatment facility, mental health facility, prison, or shelter. Subjects were asked to refill their pill bottles only after opening them to take a dose, so no adjustments were made for refill openings. Subjects were also asked not to pocket dose, but some subjects admitted pocket dosing in a special interview on their MEMS use (Bova et al. 2005). However, no adjustments were made for pocket dosing since there was no information in the interview data on when this occurred. MEMS data were not available for all eligible ATHENA subjects and so subjects with and without MEMS data were compared on a variety of categorical characteristics using χ2 tests unless any expected cell counts were less than 5 in which case Fisher's exact test (based on the statistic PFET estimating the population table probability) was used instead.
Adaptive Poisson regression modeling methods were used to analyze grouped MEMS data (Knafl et al., 2004). Poisson regression was used because that is an appropriate method for modeling event counts and rates. Each individual subject's grouped data were modeled separately, adaptively generating an estimated adherence pattern over time for that subject. These adherence patterns were allowed to have arbitrary nonlinear dependence on time, possibly quite different from the constant pattern at the prescribed rate. Adherence was also summarized using a measure of how consistent a subject's estimated adherence pattern was with presumed adherence at the prescribed rate. These percent consistency scores were compared to scores based on the standard summary measure of percent PDT and to average self-reported adherence during study participation.
Subjects were then adaptively clustered into groups with similarly shaped adherence patterns (Delucchi et al., 2006). Each subject's adherence pattern was represented by a vector of estimates of the mean cap opening rate at 20 equally spaced times (i.e., at 5%, 10%, …, 100% of total time of study participation). Subjects within adherence clusters were compared on a variety of categorical characteristics using χ2 or Fisher's exact tests as before and also on the basis of summary statistics for percent consistency, percent PDT, and average self-reported adherence.
Overview of Adaptive Modeling
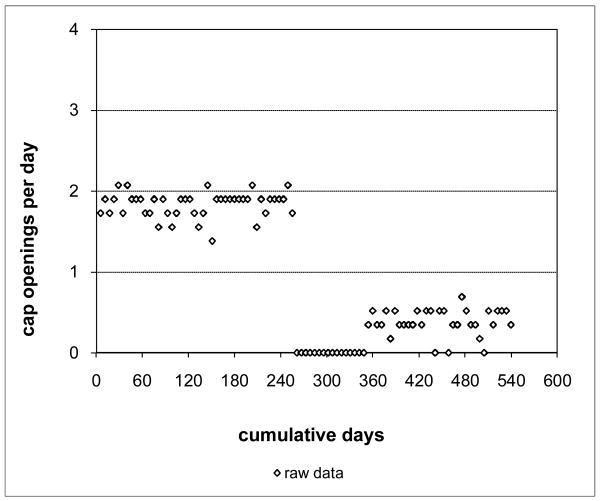
As an example of the adaptive modeling process, consider a hypothetical subject who used a single MEMS cap for 540 days or about 18 months of study participation. Suppose that the subject opened the cap during this period a total of 540 times for an overall cap opening rate of 1.00 per day. The corresponding standard summary measure of percent PDT equals 50% (obtained by dividing the overall cap opening rate by the prescribed rate of 2 doses per day and converting to a percentage). This suggests that the subject was adherent throughout study participation at one-half the prescribed rate, but that need not be the case.
A better assessment of how consistent this subject's adherence was can be obtained by grouping cap openings to generate counts and rates over disjoint periods of cap use. To do this, partition the 540 day participation period into 100 equal-sized intervals, so that each interval has length 5.4 days and corresponds to 1% of the subject's participation period at different points in time during that period. Then, count the number of openings in each interval and divide by 5.4 to get the associated opening rate for the interval. Suppose the results are as plotted in Figure 1. Time in days of study participation is plotted on the x-axis and the cap opening rate on the y-axis. This plot clearly indicates that the subject's adherence was close to the prescribed rate for about the first half of study participation, but then dropped to zero adherence for a while and then increased to around 0.5 medications per day for the rest of the time. A percent PDT of 50% provides a very misleading assessment of this subject's adherence. At no time during the study was the subject adherent at that rate. If actual adherence is incorrectly assessed in this way for too many subjects, adherence intervention studies relying on those adherence assessments may fail to demonstrate the efficacy of those interventions. When the medications of interest are ARV agents, undetected erratic or inconsistent adherence may explain the persistence of detectable virus, due to the development of ARV resistance. Thus, an accurate description of the actual pattern of medication adherence is of clinical significance.
Figure 1. Hypothetical Grouped Adherence Data.
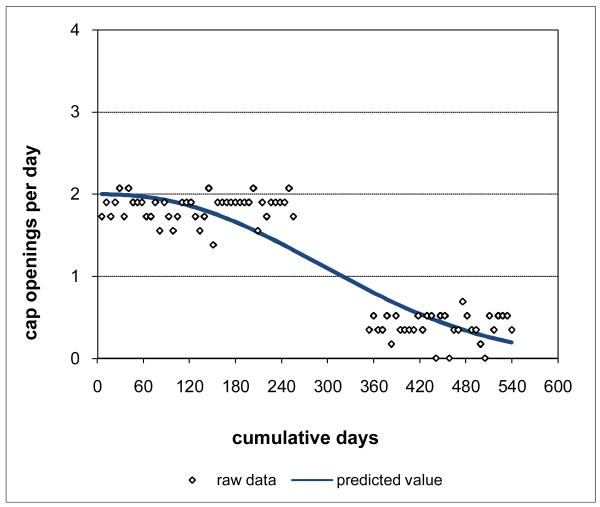
Assume further that the interview data indicated that the subject was in an in-patient drug treatment facility during the period of no cap openings in the middle of study participation. The data for that period do not reflect subject's actual adherence, and so are appropriately removed, leaving a gap in the data. The adaptive modeling process can be applied to these adjusted data generating the curve of Figure 2, representing the subject's adherence pattern over time during study participation. Since the curve is based on a fractional polynomial, it is possible to interpolate the adherence pattern to gaps in the data, representing estimated adherence for those gaps consistent with the subject's adherence when in control of medication-taking. Gaps can also occur because of lost caps and are handled in the same way.
Figure 2. Hypothetical Adjusted Grouped Adherence Data with Adaptively Selected Curve.
The curve of Figure 2 is generated through a search process that starts from the constant (intercept-only) model, systematically adds fractional power transforms of time to the model, and then contracts the model removing any extraneous transforms and adjusting the remaining transforms to improve the model. Models considered at the various stages of this selection process are compared using LCV scores, with larger scores indicating better models.
LCV scores can be used to obtain an alternative summary measure of how consistent observed adherence is with prescribed adherence, reflecting the pattern in the data rather than relying on an implicit assumption of a constant pattern. The LCV score for the model generated by the adaptive process reflects the observed adherence pattern. An LCV score can also be computed under the assumption that adherence was at the prescribed rate of 2 doses per day. When the observed adherence pattern is close to prescribed adherence, the ratio of these two LCV scores expressed as percent will be close to 100%. The further the observed adherence pattern is from prescribed adherence, the smaller this ratio will be. Using this approach, the adherence pattern of Figure 2 is assessed as only 10.0% consistent with the prescribed rate, much lower than the percent PDT score of 50%. For this subject, the standard summary measure percent PDT provides an inflated assessment of adherence in comparison to percent consistency.
We analyzed the MEMS adherence data for each subject one at a time in this way, generating individual-subject adherence patterns and percent consistency scores. Since these patterns span participation periods of different lengths, we matched them at relative times. For each subject, we computed the 20-vector of estimates of mean adherence at 5%, 10%, …, 100% of the subject's participation period. For example, for the hypothetical data of Figure 2, we would use estimated adherence rates at 27, 54, 81, …, 540 days. For a subject with a 480 day observation period, we would use estimated adherence rates at 24, 48, 72, …, 480 days instead. These two subjects would be assigned to the same cluster if their adherence was similar at corresponding proportional times into their observation periods (i.e., at multiples of 27 and 24 days, respectively). We clustered these 20-vectors into groups of subjects with similar adherence patterns. We considered 16 different clustering procedures (SAS Institute Inc., 2004), including 1- and 2-stage nearest neighbor procedures as well as the average, centroid, complete, EML, flexible, k-means, McQuitty, median, single, and Ward procedures, using for the average, centroid, median, and Ward procedures either Euclidean distance or the default of squared Euclidean distance, and each of these procedures with from 1 to 10 clusters. As described by Symons (1981), the mean vectors for the associated multivariate normal mixture model represent the clusters and so would always be treated as different across clusters while the covariance matrices may or may not be treated as different. We treated variance vectors as different across clusters, but used a common correlation matrix for all clusters in order to limit the number of parameters for the mixture model. We compared these clustering alternatives using LCV scores with likelihoods based on multivariate normal mixture models and then chose the alternative that generated the best LCV score.
Results
Characteristics of ATHENA Subjects
There were 172 eligible ATHENA subjects. Table I reports summary statistics for continuous characteristics for these subjects. ATHENA subjects represent wide ranges of HIV-positive patients as measured by these characteristics. Study participation ranged from 29 to 729 days with median of 476 days. Average self-reported adherence during study participation ranged from 13.3% to 100.0% with median of 97.4%. Age at baseline ranged from 22.3 to 63.2 years with median of 41.6 years. HIV duration at baseline ranged from 0.0 to 16.7 years with median of 8.1 years. Time on ARVs at baseline ranged from 0.0 to 15.2 years with median of 5.2 years. The minimum CD4 cell count during subjects' study participation ranged from 3 to 1,481 with median of 296. The maximum HIV viral load during subjects' study participation ranged from 20 to 850,000 copies/ml with median of 4,360 copies/ml. Undetectable HIV viral loads were set to associated detectable levels, which started at 400 copies/ml at the beginning of the study and decreased to 50 copies/ml during the study, with several detectable values recorded as less than 50 copies/ml. Means and standard deviations are not reported in Table I because distributions for some of the variables were distinctly skewed (e.g., the mean for the minimum CD4 cell count was 346 compared to a median of 296).
Table I. Summary Statistics for Continuous Characteristics of ATHENA Subjects.
| characteristic | na | min | percentiles | max | ||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| age (years) | 172 | 22.3 | 36.9 | 41.6 | 47.0 | 63.2 |
| study participation (days) | 172 | 29 | 389 | 476 | 581 | 729 |
| average SRAb | 171 | 13.0% | 84.7% | 97.4% | 100.0% | 100.0% |
| HIV duration (years) | 167 | 0.0 | 4.7 | 8.1 | 11.3 | 16.7 |
| time on ARVs (years) | 164 | 0.0 | 3.2 | 5.2 | 9.0 | 15.2 |
| min CD4 cell countc | 171 | 3 | 151 | 296 | 477 | 1,481 |
| max HIV VL (copies/ml)d | 171 | 20 | 400 | 4,360 | 57,100 | 850,000 |
ATHENA = Adherence through Home Education and Nursing Assessment; ARV = antiretroviral; VL = viral load; SRA = self-reported adherence
Out of 172 eligible subjects.
The average over all available interviews of self-reported adherence to all antiretroviral medications for the three days prior to an interview.
Minimum CD4 cell count over up to 7 times at 3 month intervals during study participation.
Maximum HIV viral load over up to 7 times at 3 month intervals during study participation. Undetectable viral loads were set to associated detectable limits of either 50 or 400 copies/ml.
Table II reports on a variety of categorical characteristics for ATHENA subjects. Of the 172 eligible subjects, 11.6% dropped out (due to death, withdrawal, or loss to follow-up), 50.6% were randomized to the intervention group, 51.7% were male, 42.1% White, 18.7% Latino, 47.7% over 42.0 years old, 65.7% educated with a high school degree or less, 75.6% participated for over 365 days, and 45.0% had an average self-reported adherence of 100%. At some time during their study participation, 14.5% of the subjects were off ARVs on provider order, 23.3% were in the hospital, 7.6% in an inpatient drug treatment facility, 1.7% in a mental health facility, 4.7% in prison, and 2.9% in a shelter. Subjects used from 1-3 MEMS caps during study participation, for a total of 200 caps, with 14.5% of the subjects using more than 1 cap and 1.7% using 3 caps. The ARV controlled by the MEMS cap at baseline was a nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) for 64.0% of the subjects, a non-NRTI (NNRTI) for 11.6% of the subjects, a protease inhibitor (PI) for 23.3% of the subjects, and hydroxyurea for 1.2% of the subjects. With regard to HIV disease, 49.7% had been HIV-positive for 8.0 years or less, 44.5% on ARVs for 5.0 years or less, 21.6% had a minimum CD4 cell count of over 500 during study participation, and 29.2% had a maximum HIV viral load of 400 copies/ml or less during study participation.
Table II. Comparison of ATHENA Subjects with and without MEMS Dataa.
| characteristic | all subjects | with MEMS | without MEMS |
|---|---|---|---|
| % (n/total) | % (n/total) | % (n/total) | |
| all subjects | 93.6 (161/172) | 6.4 (11/172) | |
| dropoutb | 11.6 (20/172) | 8.7 (14/161) | 54.5 (6/11)** |
| intervention group | 50.6 (87/172) | 50.3 (81/161) | 54.5 (6/11) |
| male | 51.7 (89/172) | 52.2 (84/161) | 45.5 (5/11) |
| White | 42.1 (72/171) | 42.5 (68/160) | 36.4 (4/11) |
| Latino | 18.7 (32/171) | 16.9 (27/160) | 45.5 (5/11)* |
| education ≤ HS degree | 65.7 (113/172) | 66.5 (107/161) | 54.5 (6/11) |
| age > 42.0 years | 47.7 (82/172) | 47.8 (77/161) | 45.5 (5/11) |
| participation > 365 days | 75.6 (130/172) | 78.9 (127/161) | 27.3 (3/11)** |
| average SRA = 100.0%c | 45.0 (77/171) | 45.0 (72/160) | 45.5 (5/11) |
| off ARVs on provider order | 14.5 (25/172) | 14.3 (23/161) | 18.2 92/11) |
| hospitald | 23.3 (40/172) | 21.7 (35/161) | 45.5 (5/11) |
| inpatient drug treatmentd | 7.6 (13/172) | 6.2 (10/161) | 27.3 (3/11)* |
| mental health facilityd | 1.7 (3/172) | 1.9 (3/161) | 0.0 (0/11) |
| prisond | 4.7(8/172) | 4.4 (7/161) | 9.1 (1/11) |
| shelterd | 2.9 (5/172) | 2.5 (4/161) | 9.1 (1/11) |
| multiple MEMS capse | 14.5 (25/175) | 14.3 (23/161) | 18.2 (2/11) |
| NRTI for MEMSf | 64.0 (110/172) | 65.2 (105/161) | 45.5 (5/11) |
| NNRTI for MEMSf | 11.6 (20/172) | 11.8 (19/161) | 9.1 (1/11) |
| PI for MEMSf | 23.3 (40/172) | 22.4 (36/161) | 36.4 (4/11) |
| ARV changed in MEMSf | 11.2 (18/161) | ||
| type changed in MEMSf | 5.6 (9/161) | ||
| HIV duration ≤ 8.0 years | 49.7 (83/167) | 48.7 (76/156) | 63.6 (7/11) |
| time on ARVs ≤ 5.0 years | 45.5 (73/164) | 44.4 (68/153) | 45.5 (5/11) |
| min CD4 > 500g | 21.6 (37/171) | 21.3 (34/160) | 27.3 (3/11) |
| max VL ≤ 400 copies/mlh | 29.2 (50/171) | 30.6 (49/160) | 9.1 (1/11) |
ATHENA = Adherence through Home Education and Nursing Assessment; MEMS = Medication Event Monitoring System; HS = high school; SRA = self-reported adherence; ARV = antiretroviral; NRTI = nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI = non-NRTI; PI = protease inhibitor; VL = viral load;
P< .05;
P < .01
Using a χ2 test for differences in occurrence with and without MEMS data, unless any expected cell counts were less than 5 in which case Fisher's exact test with statistic PFET was used instead.
Of the 172 eligible subjects, 4 (2.3%) died, 4 (2.3%) withdrew, and 12 (7.0%) were lost to follow-up for a total of 20 (11.6%) dropouts. Of the 11 subjects without MEMS data, 0 (0.0%) died, 1 (9.1%) withdrew, and 5 (45.5%) were lost to follow-up for a total of 6 (54.5%) dropouts.
The average over all available interviews of self-reported adherence to all antiretroviral medications for the three days prior to an interview.
Ever in the hospital, an inpatient drug treatment facility, a mental health facility, prison, or a shelter during study participation.
Subjects used from 1-3 MEMS caps during study participation for a total of 200 caps. Data were available for analysis for 187 of the caps. Three (1.7%) subjects used 3 caps, all with available data.
For 170 subjects, the pill bottle controlled by the MEMS cap contained at baseline either an NRTI, NNRTI, or PI. It contained hydroxyurea for the other 2 (1.2%) subjects, 1 with and 1 without MEMS data. Information on changes to the ARV controlled by the MEMS cap was only available for subjects with MEMS data. This ARV changed 2 times for 2 (1.2%) subjects, otherwise it changed at most 1 time.
Minimum CD4 cell count over up to 7 times at 3 month intervals during study participation.
Maximum HIV viral load over up to 7 times at 3 month intervals during study participation. Undetectable viral loads were set to associated detectable limits of either 50 or 400 copies/ml.
Subjects with and without MEMS Data
MEMS data were available for 161 (93.6%) of the eligible subjects from 187 (93.5%) of the caps. These consisted of over 75,000 cap openings for over 66,000 days of use within a period of about two and a half years from August, 1999 to March, 2002. Table II contains results of comparisons of subjects with and without MEMS data. For subjects with MEMS data, the ARV controlled by the MEMS cap changed for 11.2%, including 2 times for 0.6%, and 1 time otherwise. The type of ARV controlled by the MEMS cap (NRTI, NNRTI, PI, or hydroxyurea) changed for 5.6% of the subjects with MEMS data. No changes in ARVs were recorded for subjects without MEMS data. Subjects without MEMS data were significantly more likely than subjects with MEMS data to have dropped out (45.5% versus 8.7%; PFET < .01, P < 0.01), been Latino (45.5% versus 16.9%; PFET = .03, P < 0.05), and been in an inpatient drug treatment facility (27.3% versus 6.2%; PFET = .03, P < 0.05) as well as significantly less likely to have participated for over 365 days (27.3% versus 78.9%; PFET < .01, P < 0.01), but did not differ significantly on all the other characteristics of Table II.
Individual-Subject Adherence Patterns
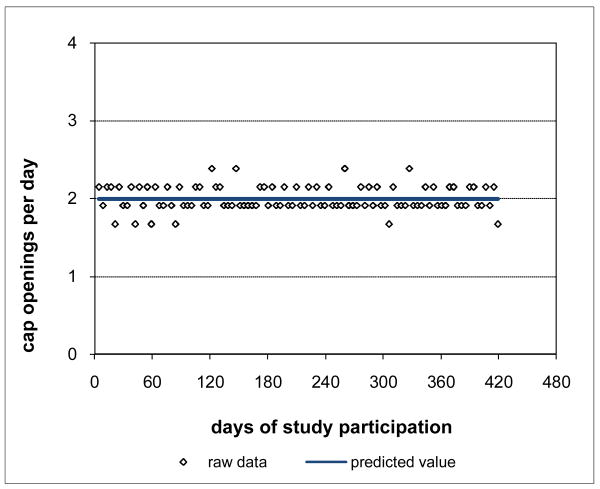
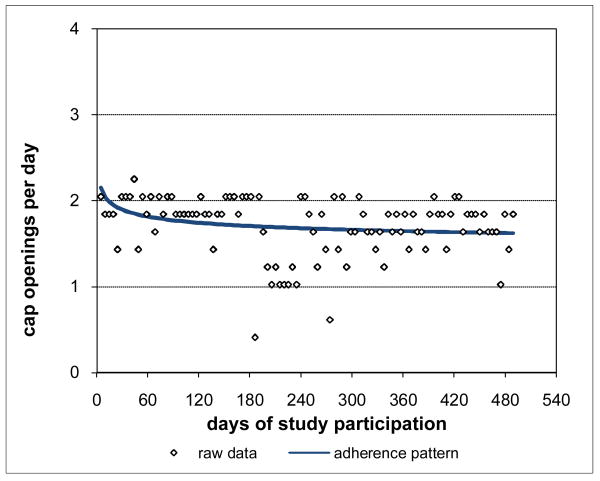
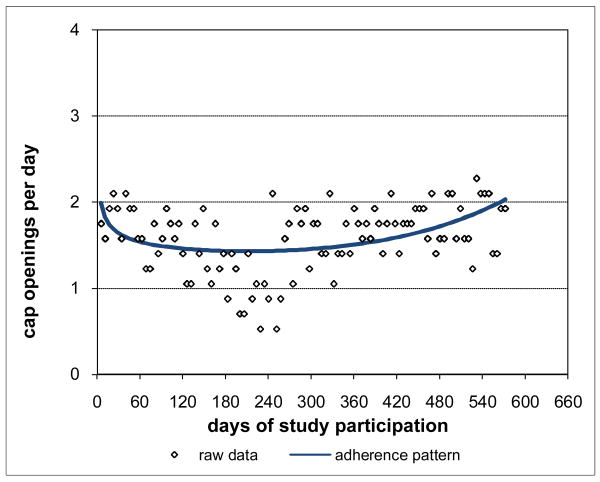
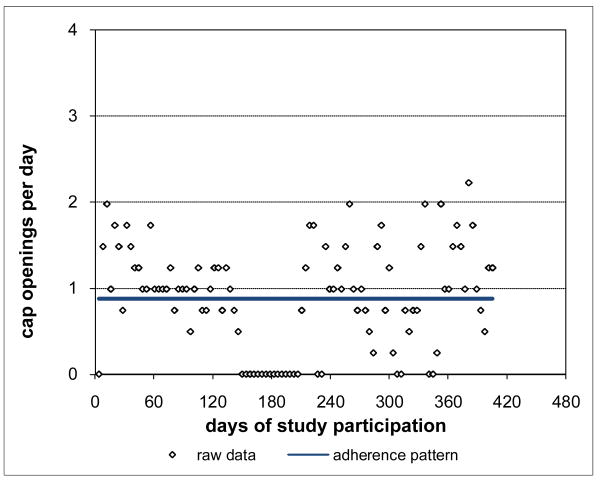
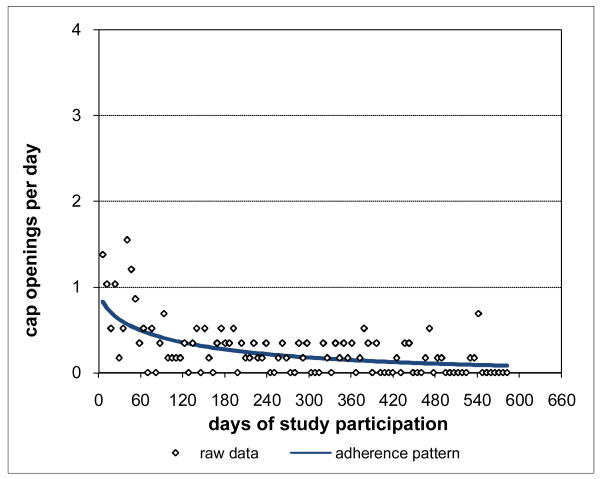
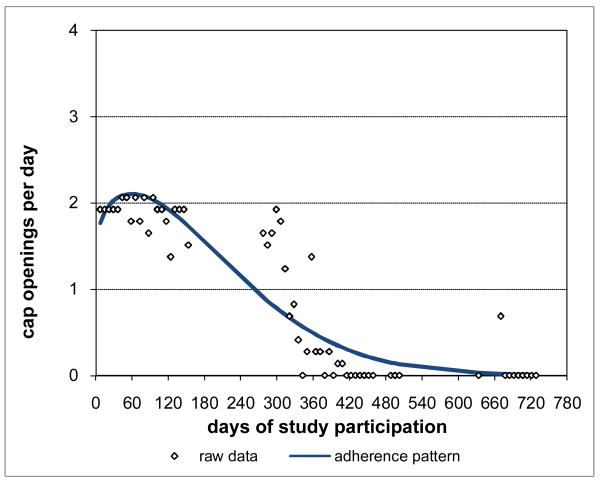
Adherence for Subject 1 is plotted in Figure 3 and was clearly consistently close to the prescribed rate of 2 doses per day throughout Subject 1's participation period. Not surprisingly, Subject 1's adherence pattern over time during study participation was a constant curve with value close to the prescribed rate and with associated percent consistency score of 100%. However, not all subjects were as highly adherent as Subject 1. Subject 2 had moderately high adherence (Figure 4), 88.8% consistent with prescribed adherence that decreased from about the prescribed rate at baseline and then settled into a nearly constant pattern somewhat below the prescribed rate. Subject 3 had moderate adherence (Figure 5), 72.0% consistent with prescribed adherence that decreased quickly from about the prescribed rate at baseline to a level around 1.5 openings per day and then eventually increased back to about the prescribed rate by the end of study participation. While 72.0% is a moderately high level of adherence, it would usually not be considered acceptable adherence for ARVs which require high levels to be effective (Low-Beer et al., 2000; Paterson et al., 2000). Moreover, less than high levels of adherence may put a subject at increased risk for developing drug resistant viral strains. Subject 4's adherence (Figure 6) was moderately low at a constant rate just below one-half the prescribed rate and only 17.5% consistent with prescribed adherence. Subject 5 had low adherence (Figure 7), only 0.1% consistent with prescribed adherence, decreasing nonlinearly from about one-half the prescribed rate at baseline to nearly zero adherence by the end of study participation.
Figure 3. High Adherence for Subject 1 100% Consistent with Prescribed Adherence.
Figure 4. Moderately High Adherence for Subject 2 88.8% Consistent with Prescribed Adherence.
Figure 5. Moderate Adherence for Subject 3 72.0% Consistent with Prescribed Adherence.
Figure 6. Moderately Low Adherence for Subject 4 17.5% Consistent with Prescribed Adherence.
Figure 7. Low Adherence for Subject 5 0.1% Consistent with Prescribed Adherence.
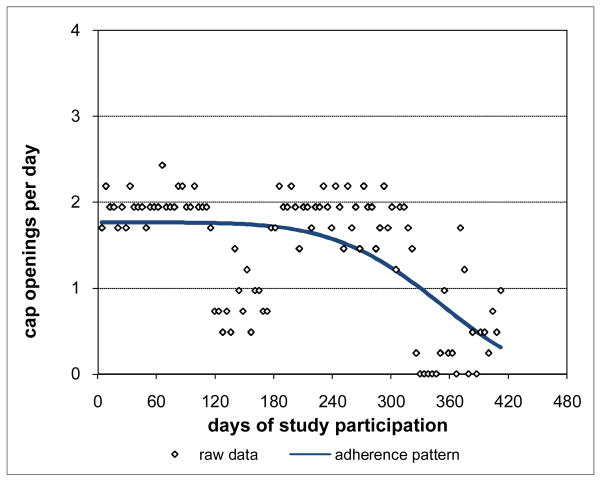
While the adherence patterns for Subjects 1-5 were not all constant, they were still relatively consistent over time. Other subjects had distinctly deteriorating adherence patterns. For example, Subject 6 had adherence (Figure 8) that was initially nearly constant at just below the prescribed rate but that steadily deteriorated late in study participation to a very low level. Subject 7's adherence (Figure 9) followed a distinctly nonlinear pattern over time, deteriorating early from around the prescribed rate to essentially zero adherence and remaining there for a long period at the end of study participation. Subject 7 was off medications on provider order once during study participation, in prison once, and in an inpatient drug treatment facility at three different times. MEMS data for these periods were removed leaving the gaps in Subject 7's raw data. However, the adherence pattern was interpolated across these gaps using the remaining MEMS data, providing an assessment of the adherence Subject 7 would be expected to have had during these periods if Subject 7 had been responsible for medication-taking.
Figure 8. Deteriorating Late Adherence for Subject 6 48.2% Consistent with Prescribed Adherence.
Figure 9. Deteriorating Early Adherence for Subject 7 0.5% Consistent with Prescribed Adherence.
Subject 7's actual adherence (as represented by the raw data of Figure 9) was between 1-2 openings per day for about the first half of study participation and then between 0-1 openings per day after that, producing an associated percent PDT of 45.4%. This suggests that Subject 7 had mid-range adherence for most of study participation when in fact Subject 7 rarely had mid-range adherence during that time. On the other hand, Subject 7's adherence pattern was only 0.5% consistent with prescribed adherence because that pattern is distinctly different from a constant pattern around the prescribed rate. The standard summary measure percent PDT inflates Subject 7's adherence in comparison to percent consistency. This also demonstrates that results like those of the hypothetical example of Figures 1-2 can occur in practice.
Distributions for Adherence Measures
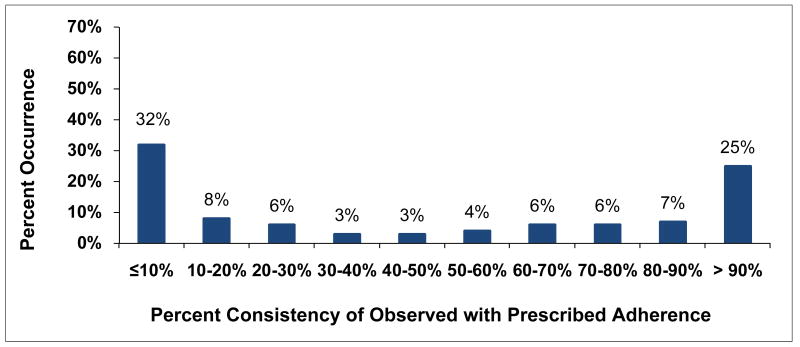
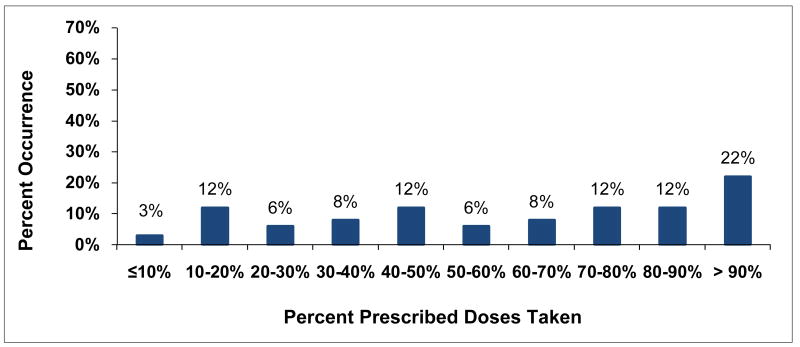
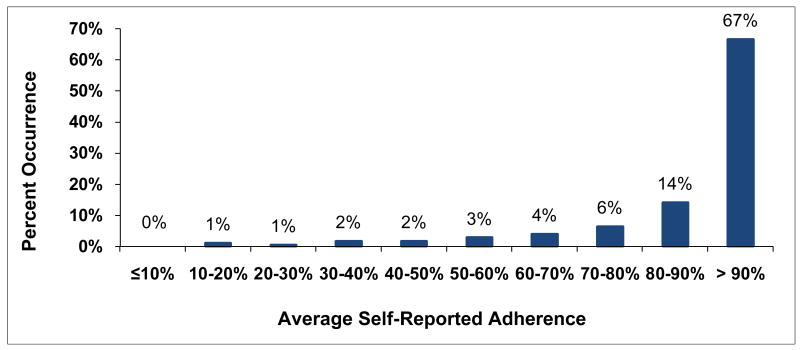
Individual-subject adherence patterns were generated for all 161 subjects with MEMS data. The distribution for associated percent consistency scores (Figure 10) was bimodal with peaks at low adherence levels of 10% consistency or less and at high adherence levels of more than 90% consistency. Adherence was almost evenly spread out between these two extremes with mid-range adherence the least likely level. On the other hand, percent PDT (Figure 11) was similar to percent consistency for high levels of adherence of over 90% PDT, perhaps because summary measures are likely to be close in value for high levels of adherence. However, low levels of percent PDT of 10% or less rarely occurred. In between, percent PDT was distinctly higher than percent consistency, an effect related to the inflation of adherence for percent PDT identified for Subject 7. Self-reported adherence was available for 171 of the eligible subjects at up to 7 time points. The distribution for average self-reported adherence over subject's study participation was highly skewed (Figure 12) with 67% of subjects self-reporting high levels of adherence over 90%. In comparison, only 25% of subjects with MEMS data had high percent consistency over 90% and only 22% had high percent PDT over 90%.
Figure 10. Distribution for Percent Consistency.
Figure 11. Distribution for Percent Prescribed Doses Taken (PDT).
Figure 12. Distribution for Average Self-Reported Adherence.
Adherence Clusters
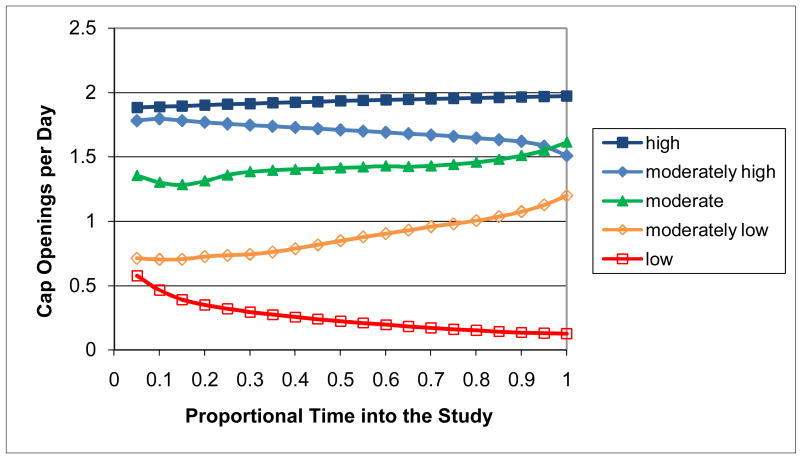
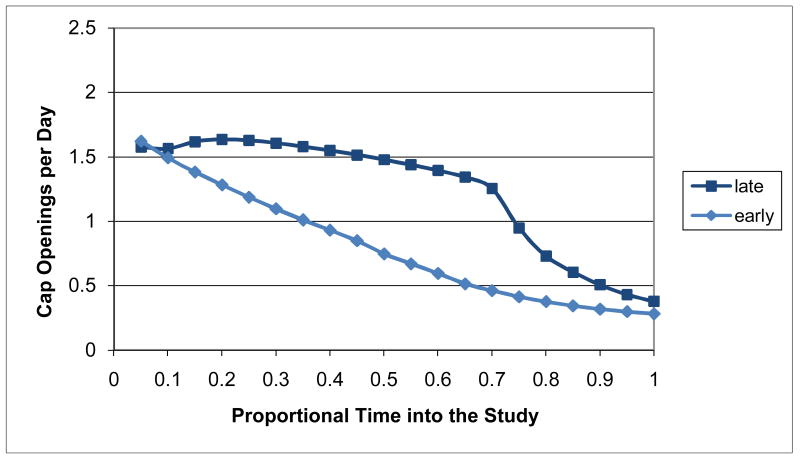
When the individual-subject adherence patterns for all 161 subjects with MEMS data were adaptively clustered, the best LCV score occurred for the Ward clustering procedure using (unsquared) Euclidean distance and 7 clusters with 5 correponding to relatively consistent patterns (Figure 13) and 2 others to distinctly deteriorating patterns (Figure 14). Clusters 1-5 represent consistent adherence types with, respectively, 35 (21.7%) of the subjects having consistently high adherence, 23 (14.3%) consistently moderately high adherence, 22 (13.7%) consistently moderate adherence, 25 (15.5%) consistently moderately low adherence, and 27 (16.8%) consistently low adherence. Clusters 6-7 represent deteriorating adherence types with, respectively, 8 (5.0%) of the subjects having deteriorating late adherence and 21 (13.0%) having deteriorating early adherence. The adherence patterns for Subjects 1-7 were in Clusters 1-7, respectively, and so are representative of patterns within the clusters. There were no sparse clusters with all containing patterns for at least 5% of the subjects. Subjects tended to be allocated to clusters in comparable-sized sets with the exception of the consistently high adherence cluster with the largest number of subjects and the deteriorating late cluster with the smallest number of subjects. There was one other clustering alternative that generated a competitive LCV score within 1% of the best score: the reduced 4-cluster solution with the consistently moderately high and consistently moderate clusters combined together, the consistently moderately low and the deteriorating early and late clusters combined together, and the consistently high and consistently low clusters uncombined. This reduced 4-cluster solution could have been used instead as a parsimonious alternative to the 7-cluster solution.
Figure 13. Relatively Consistent Adherence Clusters.
Figure 14. Distinctly Deteriorating Adherence Clusters.
Comparison of Adherence Types
Since high levels of adherence are especially important for ARVs (Low-Beer et al., 2000; Paterson et al., 2000), characterization of the consistently high and consistently moderately high adherence levels of Clusters 1 and 2 in comparison to the combined moderate to low adherence levels of Clusters 3-7 is of clinical relevance. Table III contains results for a comparison of subjects across these three adherence types. Compared to subjects with moderate to low adherence, subjects with consistently high adherence and consistently moderately high adherence were significantly more likely to have been male (65.7% and 73.9%, respectively, versus 42.7%; χ2 = 10.62, df = 2, P < 0.01), White (71.4% and 52.2%, respectively, versus 30.1%; χ2 = 19.38, df = 2, P < 0.01), older than 42.0 years (54.3% and 69.6%, respectively, versus 40.8%; χ2 = 6.99, df = 2, P < 0.05), have a minimum CD4 cell count during study participation of over 500 (34.3% and 34.8%, respectively, versus 13.7%; χ2 = 9.52, df = 2, P < 0.01), and a maximum HIV viral load during study participation of 400 copies/ml or less (62.9% and 47.8%, respectively, versus 15.7%; χ2 = 31.03, df = 2, P < 0.01). They were also significantly less likely to have the ARV controlled by the MEMS cap change (5.7% and 0.0%, respectively, versus 15.5%; PFET <01, P < 0.05). On the other hand, subjects with consistently high adherence and consistently moderately high adherence had similar chances to subjects with moderate to low adherence of dropping out of the study, being in the adherence intervention group, being Latino, being educated with a high school degree or less, participating for over 365 days, ever being off ARVs on provider order, ever being in a hospital, inpatient drug treatment facility, mental health facility, prison, or shelter, having used multiple MEMS caps during study participation, having an NRTI, NNRTI, or PI controlled by the MEMS cap at baseline, having the type of this ARV change during study participation, being HIV-positive for 8.0 years or less, or being on ARVs for 5.0 years or less. Also, when similar analyses were conducted comparing subjects of Clusters 3, 4, 5, and 6-7 with consistently moderate, consistently moderately low, consistently low, and deteriorating (i.e., deteriorating early and deteriorating late combined) adherence, respectively, on the characteristics of Table III, all comparisons were statistically non-significant indicating that subjects in the moderate to low clusters were quite homogeneous.
Table III. Comparison of ATHENA Subjects with MEMS Data by Type of Adherence.
| characteristic | type of adherence | ||
|---|---|---|---|
| consistently high | consistently moderately high | moderate to low | |
| % (n/total) | % (n/total) | % (n/total) | |
| all subjects | 21.7 (35/161) | 14.3 (23/161) | 64.0 (103/161) |
| dropoutb | 11.4 (4/35) | 4.4 (1/23) | 8.7 (9/103) |
| intervention group | 60.0 (21/35) | 43.5 (10/23) | 48.5 (50/103) |
| male | 65.7 (23/35) | 73.9 (17/23) | 42.7 (44/103)** |
| White | 71.4 (25/35) | 52.2 (12/23) | 30.1 (31/103)** |
| Latino | 5.7 (2/35) | 17.4 (4/23) | 20.4 (21/103) |
| age > 42.0 years | 54.3 (19/35) | 69.6 (16/23) | 40.8 (42/103)* |
| education ≤ HS degree | 71.4 (25/35) | 65.2 (15/23) | 65.1 (67/103) |
| participation > 365 days | 82.9 (29/35) | 82.6 (19/23) | 76.7 (79/103) |
| off ARVs on provider order | 8.6 (3/35) | 1.9 (3/23) | 16.5 (17/103) |
| hospitalc | 17.1 (6/35) | 30.4 (7/23) | 21.4 (22/103) |
| inpatient drug treatmentc | 2.9 (1/35) | 4.4 (1/23) | 7.8 (8/103) |
| mental health facilityc | 0.0 (0/35) | 0.0 (0/23) | 2.9 (3/103) |
| prisonc | 0.0 (0/35) | 8.7 (2/23) | 4.9 (5/103) |
| shelterc | 2.9 (1/35) | 4.4 (1/23) | 1.9 (2/103) |
| multiple MEMS caps | 14.5 (25/175) | 14.3 (23/161) | 18.2 (2/11) |
| NRTI in MEMSd | 74.3 (26/35) | 60.9 (14/23) | 63.1 (65/103) |
| NNRTI in MEMSd | 8.6 (3/35) | 4.4 (1/23) | 14.6 (15/103) |
| PI in MEMSd | 17.1 (6/35) | 34.8 (8/23) | 21.4 (22/103) |
| ARV changed in MEMS | 5.7 (2/35) | 0.0 (0/23) | 15.5 (16/103)** |
| type changed in MEMS | 2.9 (1/35) | 0.0 (0/23) | 7.8 (8/103) |
| HIV duration ≤ 8.0 years | 47.1 (16/34) | 47.8 (11/23) | 49.5 (49/99) |
| time on ARVs ≤ 5.0 years | 45.7 (16/35)) | 45.0 (9/20) | 43.9 (43/98) |
| min CD4 > 500e | 34.3 (12/35) | 34.8 (8/23) | 13.7 (14/102)* |
| max VL ≤ 400 copies/mlf | 62.9 (22/35) | 47.8 (11/23) | 15.7 (16/102)** |
ATHENA = Adherence through Home Education and Nursing Assessment; MEMS = Medication Event Monitoring System; HS = high school; ARV = antiretroviral; NRTI = nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI = non-NRTI; PI = protease inhibitor; VL = viral load;
P< .05;
P < .01
Using a χ2 test for differences in occurrence across adherence types, unless any expected cell counts were less than 5 in which case Fisher's exact test was used instead.
Either died, withdrew, or lost to follow-up.
Ever in the hospital, an inpatient drug treatment facility, a mental health facility, prison, or a shelter during study participation.
Based on the ARV controlled by the MEMS cap at baseline.
Minimum CD4 cell count over up to 7 times at 3 month intervals during study participation.
Maximum HIV viral load over up to 7 times at 3 month intervals during study participation. Undetectable viral loads were set to associated detectable limits of either 50 or 400 copies/ml.
Table IV presents summary statistics for percent consistency, percent PDT, and average self-reported adherence for the consistently high, consistently moderately high, and moderate to low adherence types. For the consistently high and consistently moderately high adherence types, mean percent consistency was close to mean percent PDT and their medians were close to their means, so that subjects within these two adherence types have similar properties based on these summary adherence measures. However, there was a lot of overlap between ranges of percent consistency and percent PDT scores for the three adherence types (e.g., percent consistency scores can be as low as 78.4% for consistently high adherence and as high as 96.4% for consistently moderately high adherence, overlapping on an interval of length 18.0%), and so these adherence types cannot be simply determined through the conventional use of ranges for summary adherence measures (e.g., over 95%). Similar results held for average self-reported adherence but with values for the moderate to low adherence types much larger than for percent consistency and percent PDT. Also, there were subjects with average self-reported adherence of 100% in the consistently moderately high and moderate to low adherence types, not just the consistently high adherence type.
Table IV. Summary Statistics for Adherence Measures by Type of Adherencea.
| summary measure | type of adherence | ||
|---|---|---|---|
| consistently high | consistently moderately high | moderate to low | |
| percent consistency | |||
| meanb | 97.7% | 84.1% | 21.6% |
| median | 97.1% | 86.8% | 10.1% |
| minimum | 78.4% | 59.4% | 0.0% |
| maximum | 100.0% | 96.4% | 89.0% |
| percent PDT | |||
| meanb | 96.4% | 85.0% | 42.5% |
| median | 97.1% | 85.9% | 42.2% |
| minimum | 86.3% | 76.9% | 1.6% |
| maximum | 100.0% | 91.9% | 83.8% |
| average self-reported adherence | |||
| meanc | 98.4% | 96.3% | 85.6% |
| median | 100.0% | 100.0% | 90.8% |
| minimum | 87.0% | 70.0% | 25.0% |
| maximum | 100.0% | 100.0% | 100.0% |
PDT = percent doses taken
Percent consistency and percent PDT statistics computed for the 161 subjects with Medication Event Monitoring System (MEMS) data. Average self-reported adherence computed for the 160 of these subjects with self-reported adherence data.
Significantly different by type of adherence at P < 0.01 for the F test of equal means with all three means distinctly different using Scheffé's multiple comparisons procedure.
Significantly different by type of adherence at P < 0.01 for the F test of equal means with the mean for moderate to low adherence differing from the means for high and moderately high adherence which did not differ from each other using Scheffé's multiple comparisons procedure.
Discussion
The present study provides an important contribution to the literature on ARV adherence. It demonstrates that adaptive statistical methods provide a more comprehensive analysis of adherence compared to the conventional use of summary measures like percent PDT and to self-reported adherence. These methods provide for visualization of individual-subject adherence patterns along with measures of how consistent those patterns are with presumed adherence at the prescribed rate. Describing adherence data in this way may improve the understanding of adherence for individual patients over time.
These methods also provide characterizations of typical adherence patterns. Studies of other chronic conditions have demonstrated the usefulness of identifying adherence pattern types as an important first step towards developing tailored adherence interventions (Ahn et al., 2008; Gerbino and Shoheiber, 2007; Russell et al., 2006). ARV adherence pattern types could support the choice of regimens that match HIV-disease treatment strategies with individual adherence behavior and guide behavioral interventions (Bangsberg, 2008). For example, it would be possible to study whether certain lower cost interventions (e.g. pill boxes, brief health care provider counseling) worked best in patients with more easily remedied adherence patterns (e.g. late deteriorating) as opposed to costly, intensive adherence interventions (e.g., medication managers, home-based nursing interventions) which might work best for patients with consistently poor adherence.Tailored adherence interventions coupled with better regimen choice could be an important advancement in long-term ARV management.
Recognizing adherence types may be especially important as studies focus greater attention on forgiveness of poor adherence (Shuter, 2008). The concept of forgiveness has been used to describe the degree to which ARV dosing events can be missed and still maintain adequate HIV viral suppression. Recent studies suggest that viral suppression is possible with more modest adherence rates when contemporary regimens are used (e.g., boosted PI or a potent NNRTI) (Bangsberg, 2006; Shuter et al., 2007). Therefore, it may be possible to match adherence pattern types with regimen types having specific forgiveness profiles to optimize individual regimen recommendations, e.g., patients with low adherence patterns could be prescribed ARV regimens with high forgiveness profiles.
The deteriorating early and deteriorating late patterns may be of particular interest to clinicians and researchers. It is important to note that both of these deteriorating adherence patterns began with suboptimal adherence and that subjects with deteriorating adherence were not significantly different from the other moderate to low adherers on any major characteristics. Qualitative methods may be needed to help uncover the hidden dimensions related to deteriorating adherence compared to other types of moderate to low adherence.
Usable MEMS data were not available for all eligible ATHENA subjects. This is analogous to non-response to a mailed questionnaire. The “non-response” rate in this case was quite low (6.4% or 11/172 subjects), but the reported results may not apply generally if the non-responders differed from responders on subject characteristics. Not surprisingly, ATHENA subjects without MEMS data were more likely than subjects with MEMS data to have dropped out of the study and to have been in an inpatient drug treatment facility during the study as well as less likely to have participated longer in the study. However, they were also more likely to be Latino. Consequently, reported results may not fully reflect adherence for Latinos. On the other hand, there were no significant differences on a wide variety of other subject characteristics, suggesting that the results may apply to a wide range of HIV-positive subjects. In particular, subjects without MEMS data were not less likely to self-report having high levels of adherence, suggesting that they were not more likely to be non-adherers.
The MEMS data analyzed here were collected as part of an adherence intervention study. While 60% of the subjects in the consistently high adherence group were also in the intervention group compared to 43.5% of subjects in the consistently moderately high adherence group and 48.5% in the moderate to low adherence group, these differences were not statistically significant. When the consistently moderately high and moderate to low groups were combined, the P-value improved but was still not significant. Larger sample sizes may be needed to identify an effect to an intervention on having consistently high adherence, and reported results could be used to power future studies.
An earlier analysis (Williams et al., 2006) of ATHENA Project MEMS data identified an intervention effect on the occurrence over time of high levels of adherence measured by a percent PDT greater than 90%. However, this did not translate into a significant effect of the intervention on improved CD4 cell counts and HIV viral loads. This earlier analysis addressed whether or not intervention group subjects had a greater chance of high levels of the summary measure percent PDT at any given time during their study participation. The analyses reported here used different methods, addressed the different issue of whether intervention group subjects had a greater chance of consistently high adherence throughout their study participation, and found that such adherence was associated with improved levels of CD4 cell counts and HIV viral loads. The results of these two kinds of analyses suggest that measuring high adherence as a high value for a summary measure separately at each point in time during a study may identify an intervention effect on adherence, but measuring adherence as consistently high over time appears to be needed to also identify an intervention effect on improved clinical outcomes like CD4 cell counts and HIV viral loads. Consequently, interventions that effectively promote consistently high adherence over time may result in better clinical outcomes.
A limitation of this study, and of MEMS caps in general, is that cap openings may over-estimate adherence since caps can be opened without taking medications, for example, due to refills. They may also under-estimate adherence, for example, due to pocket dosing. We can only presume that the cap opening process provides a reasonably accurate assessment of actual adherence. Another limitation of this study is the use of older ARV medications that included higher pill burden and more frequent dosing than most contemporary regimens. However, our analyses were conducted using only one ARV agent (taken twice daily), which is similar to many current regimens. A more important problem is the inability to calculate differential adherence among ARV agents used in combination. Gardner and colleagues (2008) found that differential adherence was present for 29% of subjects over a 60 month period and that differential adherence preceded virologic failure in 36% of subjects. Therefore, further work is needed to examine differential adherence to newer regimens using electronic monitoring and adaptive statistical methods.
The adherence pattern types identified here were for subjects of a single study. It is important to replicate this work using data from other studies of adherence to ARVs as well as to other kinds of medications. Other adherence pattern types may be identifiable, for example, distinctly improving adherence types.
Adaptive statistical methods have only recently been developed and so are not supported as standard options in popular statistical tools. To support their use, we have developed SAS macros automating their computation. These macros are available from the corresponding author.
In summary, the use of adaptive statistical methods can identify variations in individual-subject adherence that are masked by summary adherence measures and by self-report. The adherence types identified by these methods represent “signature adherence patterns” (Bangsberg, 2008) characterizing patterns in adherence over time subjects are likely to have. These adherence types may be important for predicting virologic failure and the development of ARV resistance. Also, the availability of adherence types can support the matching of subjects to adherence interventions tailored to their special needs.
Acknowledgments
This work was supported in part by grant R01 AI57043 from the NIAID of the NIH, Oregon Health & Science University's Oregon Clinical and Translational Research Institute (OCTRI) through grant UL1 RR024140 from the NCRR of the NIH and the NIH Roadmap for Medical Research, Yale University's Center for Interdisciplinary Research on AIDS (CIRA) through grant P30 MH62294 from the NIMH of the NIH. Collection of the data used in analyses was supported in part by grant R01 NR04744 from the NINR of the NIH, Yale University's GCRC Program through grant M01 RR00125 from the NCRR of the NIH, and the University of Connecticut Health Center's GCRC Program through grant M01 RR06192 from the NCRR of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, the NCRR, the NIMH, the NINR, or the NIH.
References
- Ahn J, McCombs JS, Jung C, Croudace TJ, McDonnell D, Ascher-Svanum A, Edgell ET, Shi L. Classifying patients by antipsychotic adherence patterns using latent class analysis: Characteristics of nonadherent groups in the California Medicaid Program. Value in Health. 2008;11:48–56. doi: 10.1111/j.1524-4733.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. Journal of Infectious Diseases. 2008;197:S272–S278. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- Bova C, Fennie KP, Dieckhaus K, Knafl GJ, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: Practical considerations. AIDS and Behavior. 2005;9:103–110. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- Delucchi K, Knafl G, Haug N, Sorensen J. 2006 proceedings of the section on statistics in epidemiology. Alexandria, VA: American Statistical Association; 2006. Adaptive Poisson modeling of medication adherence among HIV-positive methadone patients; pp. 2525–2527. [Google Scholar]
- Fletcher CV, Testa MA, Brundage RC, Chesney MA, Haubrich R, Acosta EP, Martinez A, Jiang H, Gulick RM. Four measures of antiretroviral medication adherence and virologic response in AIDS Clinical Trials Group Study 359. Journal of Acquired Immune Deficiency Syndromes. 2005;40:301–306. doi: 10.1097/01.qai.0000180078.53321.6a. [DOI] [PubMed] [Google Scholar]
- Gardner EM, Sharma S, Hullsiek KH, Burman WJ, MacArthur RD, Chesney M, Telzak EE, Friedland G, Mannheimer SB. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22:75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agents. American Journal of Health-System Pharmacy. 2007;64:1279–1283. doi: 10.2146/ajhp060434. [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J, Wantland S, Mullan JT. Testing a nurse-tailored HIV medication adherence intervention. Nursing Research. 2006;55:189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Knafl GJ, Fennie KP, Bova C, Dieckhaus K, Williams AB. Electronic monitoring device event modelling on an individual-subject basis using adaptive Poisson regression. Statistics in Medicine. 2004;23:783–801. doi: 10.1002/sim.1624. [DOI] [PubMed] [Google Scholar]
- Knafl GJ, Fennie KP, O'Malley JP. Adaptive repeated measures modeling using likelihood cross-validation. In: Bovaruchuk B, editor. Proceedings second IASTED international conference on computational intelligence. Anaheim, CA: ACTA Press; 2006. pp. 422–427. [Google Scholar]
- Knafl GJ, Grey M. Factor analysis model evaluation through likelihood cross-validation. Statistical Methods in Medical Research. 2007;16:77–102. doi: 10.1177/0962280206070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, Kaplan AH. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. Journal of Acquired Immune Deficiency Syndromes. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JSG. Adherence to triple therapy and viral load response. Journal of Acquired Immune Deficiency Syndromes. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. Journal of Infectious Diseases. 2007;196:1773–1778. doi: 10.1086/523704. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. Applied Statistics. 1994;43:429–467. [Google Scholar]
- Russell CL, Conn VS, Ashbaugh C, Madsen R, Hayes K, Ross G. Medication adherence patterns in adult renal transplant recipients. Research in Nursing and Health. 2006;29:521–532. doi: 10.1002/nur.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.1 user's guide. Cary, NC: SAS Institute; 2004. [Google Scholar]
- Sereika SM, Dunbar-Jacob J. Analysis of electronic event monitored adherence. In: Burke LE, Ockene IS, editors. Compliance in healthcare and research. Armonk, NY: Futura Publishing; 2001. pp. 139–162. [Google Scholar]
- Shuter J. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. Journal of Antimicrobial Chemotherapy. 2008;61:769–773. doi: 10.1093/jac/dkn020. [DOI] [PubMed] [Google Scholar]
- Shuter J, Salro JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% Journal of Acquired Immune Deficiency Syndromes. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- Stone M. An asymptotic equivalence of choice of model by cross-validation and Akaike's criterion. (Series B).Journal of the Royal Statistical Society. 1977;39:44–47. [Google Scholar]
- Symons MJ. Clustering criteria and multivariate normal mixtures. Biometrics. 1981;37:35–43. [Google Scholar]
- Vrijens B, Goetghebeur E, de Klerk E, Rode R, Mayer S, Urquhart J. Modelling the association between adherence and viral load in HIV-infected patients. Statistics in Medicine. 2005;24:2719–2731. doi: 10.1002/sim.2130. [DOI] [PubMed] [Google Scholar]
- Williams AB, Fennie KP, Bova CA, Burgess JD, Danvers KA, Dieckhaus KD. Home visits to improve adherence to highly active antiretroviral therapy: A randomized clinical trial. Journal of Acquired Immune Deficiency Syndromes. 2006;42:314–321. doi: 10.1097/01.qai.0000221681.60187.88. [DOI] [PubMed] [Google Scholar]