Abstract
Evidence is mounting to suggest that the transfer of carbon through roots of plants to the soil plays a primary role in regulating ecosystem responses to climate change and its mitigation. Future research is needed to improve understanding of the mechanisms involved in this phenomenon, its consequences for ecosystem carbon cycling, and the potential to exploit plant root traits and soil microbial processes that favor soil carbon sequestration.
Introduction
Human activities are rapidly changing the world’s ecosystems, with overall dire consequences for the Earth. The most obvious human impact is through the worldwide conversion of land for food production, but terrestrial ecosystems are also affected by climate change, invasion of alien species into new territories, and increasing rates of nitrogen deposition. This has led to a groundswell of research aimed at improving understanding of the impact of global changes on biodiversity and how ecosystems function, and also on management strategies to mitigate them.
Despite this topic receiving considerable attention, it is only recently that scientists have become aware that understanding the consequences of global change for ecosystem functioning requires consideration of linkages between plant and belowground microbial communities [1]. This is because the impact of human-induced global changes on the functioning of terrestrial ecosystems is often indirect: they operate via changes aboveground that cascade belowground to the hugely complex soil biological community, which drives biogeochemical processes and feedbacks to the Earth’s climate system. Here I highlight some recent developments in this area that illustrate how a combined aboveground–belowground approach can improve understanding of the consequences of global change for the Earth. In particular, recent studies have advanced our understanding of the role that plant–microbial–soil interactions, and specifically root carbon transfer to soil, play in governing the impact of climate change on ecosystem carbon cycling and climate mitigation.
Plant–soil feedbacks and climate change
The soil is the third largest global store of carbon and, together with plants, contains around 2.7 times more carbon than the atmosphere. As a result, there is much concern that climate change will enhance the decomposition of this carbon, potentially shifting soils from being carbon sinks to sources of atmospheric carbon dioxide and thereby accelerating climate change—the so-called carbon cycle feedback. Conversely, there is much current debate about the potential to increase the capacity of soils to sequester carbon from the atmosphere and hence mitigate climate change. Recent studies reveal that both of these processes, namely the loss and gain of carbon in soil, are strongly regulated by plant–microbial–soil interactions.
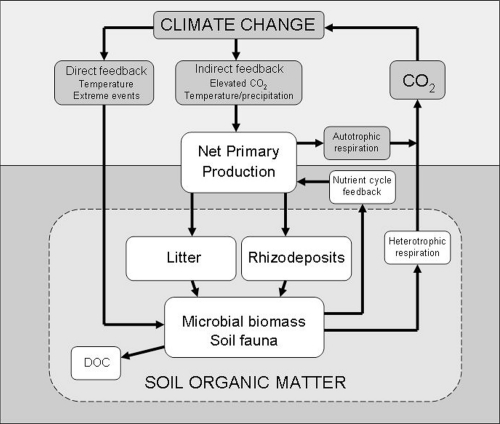
Climate change can affect soil carbon through a variety of routes, both directly and indirectly (Figure 1) [2]. With regard to direct effects, recent studies show that even subtle warming (by approximately 1°C) can directly stimulate microbial activity causing an increase in ecosystem respiration rates in subarctic peatland [3]. Likewise, it was recently shown that permafrost thaw over decades in an Alaskan tundra landscape has caused significant losses of soil carbon, despite increased plant growth and ecosystem carbon input [4]. Given that tundra soils are among the world’s largest carbon stores, these studies indicate that global warming may cause a large and long-lasting increase in atmospheric carbon dioxide in the global climate system. In general, there remains much uncertainty about how soil organisms directly respond to warming. For instance, it is unclear whether increases in microbial activity and carbon cycling in response to warming will be sustained due to short-term depletion of fast-cycling soil carbon pools, or whether soil communities will adapt to a warmer world [5,6]. Resolving this issue represents a major challenge.
Figure 1. Direct and indirect effects of climate change on soil microbial communities and feedback to the Earth’s carbon dioxide production.
Direct effects include the influence on soil microbes and greenhouse gas production of temperature, changing precipitation, and extreme climatic events. For example, increased temperature can stimulate microbial activity and carbon dioxide production. Indirect effects result from climate-driven changes in plant productivity and vegetation structure, which alter soil physicochemical conditions, the supply of carbon to soil in the form of root exudates and litter, and the structure and activity of microbial communities involved in carbon cycling . Autotrophs, such as plants, can convert carbon dioxide into organic carbon, whereas heterotrophs do the opposite. DOC, dissolved organic carbon. Adapted from Bardgett et al., 2008 [2].
Of more relevance to the thesis of this paper are studies that reveal the potential for climate change to have a strong indirect effect on soil microbes and carbon cycling, by affecting plants. Two main mechanisms operate here. First, rising atmospheric concentrations of carbon dioxide indirectly stimulate soil microbes via increased plant photosynthesis and transfer of photosynthetic carbon to soil; and second, long-term, climate change-induced changes in the composition and diversity of vegetation alter the amount and quality of organic matter entering soil, as well as other soil properties, affecting the activity and structure of belowground communities.
Recent studies reveal that both of these mechanisms have significant consequences for the carbon cycle of terrestrial ecosystems under climate change. For example, a study by Drake et al. [7] showed that elevated carbon dioxide concentrations caused an increase in carbon flow through the plant root–microbial–soil system of a low fertility pine forest in North Carolina, USA. Specifically, they found that elevated carbon dioxide increased the flux of carbon to plant roots, which in turn stimulated microbial activity and the breakdown of organic matter in the soil. Because this process also stimulated the release of nitrogen in the soil and its uptake by trees, it set in motion a positive feedback mechanism that increased rates of tree production, and hence carbon storage in tree biomass, but not in soil. A related study by Philips et al. [8] investigated the mechanisms behind this carbon cascade: over a period of 3 years, elevated carbon dioxide concentrations increased the rate of exudation from tree roots by 55%, leading to a 50% annual increase in soluble organic inputs to soil. Moreover, this increase in root-derived carbon in the soil was strongly linked to an increased microbial release of extracellular enzymes involved in breakdown of organic nitrogen. This suggests that plant root exudates stimulated microbial activity and accelerated the rate of soil organic nitrogen turnover. However, under different conditions, especially when nitrogen is strongly limiting, it is also possible for enhanced plant root exudation to stimulate microbial immobilization of nitrogen, which in turn limits nitrogen availability to plants [9], plant growth, and ultimately carbon transfer to soil. Importantly, enhanced root exudation, in response to elevated carbon dioxide, can also stimulate mineralization of both recent and old soil organic carbon (i.e., priming), thereby leading to carbon loss from soil [10,11], and can increase the growth of mycorrhizal fungi [12,13]. Changes in mycorrhizal fungi growth can in turn alter the release of carbon to the soil microbial community [14], and enhance the stabilization of soil organic carbon through promotion of soil aggregation [15,16]. Collectively, these studies show that long-term responses of forests to elevated carbon dioxide are strongly influenced by plant–microbial–soil feedbacks that are fuelled by plant root exudate-derived inputs of carbon to soil. Moreover, they bolster the growing view that transfers of recently fixed carbon from roots to the belowground subsystem are a major influence on soil food webs, and that this has major consequences for the functioning of terrestrial ecosystems in response to climate change.
There has also been progress in understanding the second long-term mechanism of climate change modulation of plant–soil feedbacks. Evidence is emerging that climate change can cause both local and regional shifts in vegetation composition through altered precipitation patterns, temperature regimes, and elevated carbon dioxide concentrations [1]. Recent studies indicate that such shifts in vegetation composition can in turn have implications for the transfer of recent photosynthetic carbon to soil and thus affect ecosystem carbon dynamics. For example, in situ 13CO2 stable isotope labeling approaches [17] showed that selective removal of key components of the vegetation of a dwarf-shrub heath strongly affected belowground transfer and respiration of recently photosynthetic carbon. In particular, the removal of dwarf shrubs greatly increased community-level rates of photosynthesis, the transfer of this recently assimilated carbon to soil, and its loss to the atmosphere through soil respiration, thereby speeding up rates of carbon cycling. There is growing evidence from a variety of ecosystem types that plant functional groups and species differentially influence the uptake and transfer of carbon to soil via their exudates, suggesting that changes in vegetation composition resulting from global change have the potential to alter short-term patterns of carbon exchange [17-19]. In general, however, there are more questions than answers on this topic, and an important challenge for the future will be to improve our understanding of the role of such plant–soil feedbacks in modifying ecosystem carbon dynamics, especially given the extent that climate-mediated changes in vegetation are occurring worldwide.
Plant–soil feedbacks and climate mitigation
A major global challenge is to increase the amount of carbon sequestered in soil in order to mitigate climate change. Much discussion in this area has focused on management of arable and degraded soils to increase carbon sequestration, and the potential for forest soils to sequester carbon. Recent evidence, however, suggests that modifying plant–soil feedbacks in grassland may be an obvious route to enhance soil carbon stocks, whilst reaping additional benefits for biodiversity conservation. For example, in model grassland systems it was shown that increasing grassland plant diversity enhances community-level carbon dioxide uptake and belowground allocation to roots and mycorrhizal fungi [20,21], which is a key mechanism governing carbon sequestration in soil [22]. These effects, however, were due to the presence of legumes in high diversity mixtures rather than diversity per se. Consistent with this finding, the introduction of legumes into long-term diversity restoration grasslands in northern England enhanced soil carbon sequestration, most likely because of increased input of carbon and nitrogen to soil and suppression of extracellular enzyme activities involved in organic matter breakdown [23]. Combined with results from other experimental grasslands [24-26], these findings point to potential benefits for soil carbon sequestration of restoring plant diversity. Further research is needed to exploit this approach, especially since restoration of high diversity grassland on degraded and ex-arable land is as key objective of policy for sustainable agriculture in many parts of the world.
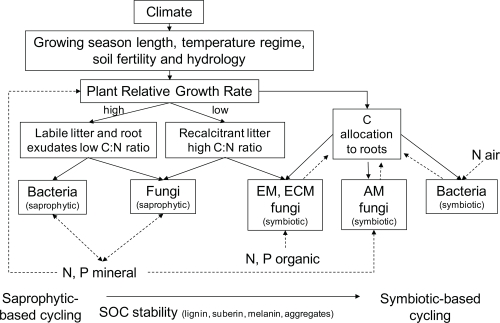
The mechanisms involved in plant modulation of soil carbon sequestration are complex and involve a myriad of biotic interactions between plants, their symbionts (i.e., mycorrhizal fungi and nitrogen-fixing bacteria), and decomposer organisms, whose activities determine the rate of decomposition of organic matter and hence carbon loss from soil. De Deyn et al. [22] proposed a simplified plant trait-based framework for understanding linkages between plant communities, the microbial community, and soil carbon sequestration (Figure 2). This framework is consistent with the growing recognition that plant traits are major influences on soil nutrient and carbon cycling, and that certain plant traits can select for particular groups of soil organisms that play key roles in these processes [1]. For instance, a recent study of a range of grassland plant species showed that certain root traits, such as root carbon and nitrogen content, were strongly correlated with several soil properties, especially the biomass and composition of the microbial community [27]. Also, in a study of 16 grassland plant species, it was shown that increased root nitrogen concentration slowed down the decomposition of recalcitrant soil organic matter, whereas increasing plant biomass enhanced rates of decomposition [28]. But, specific plant traits such as differences in the quantity and chemical composition of root exudates are also likely to affect the mineralization of soil carbon by altering microbial activity [28]. These studies suggest that plant traits, and especially those of roots, offer a potential tool for predicting how changes in plant species composition associated with global change will affect the Earth [1]. Moreover, given the importance of root traits for soil microbial carbon cycling, modification of root traits of crops, including the development of perennial grains with greater root mass and depth, may offer a way to maximize soil carbon sequestration whilst also producing food [29,30].
Figure 2. Plant-trait framework for understanding linkages between plant traits, resource inputs to soil, the functional composition of the soil microbial community, and soil carbon sequestration.
The schematic shows the interdependency of labile and recalcitrant litter inputs to soil, which drive abundances of different functional groups of the soil microbial community and their involvement in carbon sequestration. Labile inputs to soil of low carbon:nitrogen ratio from litter and root exudates favor saprophytic (feeding, absorbing or growing upon decaying matter) bacterial-dominated microbial communities that promote carbon mineralization, and hence carbon loss. In contrast, recalcitrant litter inputs with high carbon:nitrogen ratio and low nutrient availability favor saprophytic fungi and carbon allocation to symbiotic fungi and bacteria, which both promote carbon storage in soil. Solid lines indicate carbon and dotted lines indicate mineral nitrogen and phosphorus flow. Overall, the stability and storage of carbon in soil increases along the spectrum from saprophytic- to symbiotic-based cycling, as indicated on the lower arrow. AM, arbuscular mycorrhizal; ECM, ectomycorrhizal; EM, ericoid mycorrhizal; SOC, soil organic carbon. Adapted from De Deyn et al., 2008 [22].
Conclusions and future challenges
I have used a selection of examples, but their central message is similar: understanding ecosystem function and response to global change requires consideration of feedbacks between plants, microbes, and soil processes. In particular, it is clear that plant root carbon transfer to the soil and resulting carbon cascades through the plant–microbial–soil system play a primary role in driving carbon-cycle feedbacks and in regulating ecosystem responses to climate change. Moreover, recent studies identify the potential to apply such understanding to improve land management, such as enhancing soil carbon sequestration in grassland and degraded farming systems, which also has potential benefits for food production and biodiversity conservation. Research effort is also required in order to realize the potential for targeted crop improvement strategies based on root traits that favor carbon sequestration in soil whilst also producing food. A new age of research and funding is needed to meet these scientific challenges and to integrate such understanding into future land management and climate mitigation strategies.
Acknowledgments
RDB is grateful to Dr. Franciska De Vries and two anonymous referees who provided helpful comments on a previous version of this paper. RDB was supported by the Natural Environment Research Council (NERC) as part of the BiodivERsA funded project, “Ecosystem service provision from coupled plant and microbial functional diversity in managed grasslands (VITAL),” an ERA-net project within the European Union’s 7th Framework Programme for Research.
Competing interests
The author declares that he has no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/3/16
References
- 1.Bardgett RD, Wardle DA. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 2.Bardgett RD, Freeman C, Ostle NJ. Microbial contributions to climate change through carbon-cycle feedbacks. ISME J. 2008;2:805–14. doi: 10.1038/ismej.2008.58. [DOI] [PubMed] [Google Scholar]
- 3.Dorrepaal ES, Toet S, van Logtestijn RS, Swart E, van de Weg MJ, Callaghan TV, Aerts R. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature. 2009;460:616–9. doi: 10.1038/nature08216. [DOI] [Google Scholar]; F1000 Factor 8Evaluated by Jennifer Dungan 01 Jun 2010
- 4.Schuur EAG, Vogel JG, Crummer KG, Lee H, Sickman JO, Osterkamp TE. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 2009;459:556–9. doi: 10.1038/nature08031. [DOI] [PubMed] [Google Scholar]; F1000 Factor 7Evaluated by Evan DeLucia 29 Jun 2009, Richard Bardgett 28 Jul 2011
- 5.Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11:1316–27. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 6.Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA. Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett. 2008;11:1092–100. doi: 10.1111/j.1461-0248.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 7.Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJ, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, Delucia EH, Finzi AC. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett. 2011;14:349–57. doi: 10.1111/j.1461-0248.2011.01593.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 7Evaluated by George Hurtt 15 Apr 2011, Richard Bardgett 28 Jul 2011
- 8.Philips RP, Finzi AC, Bernhardt ES. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett. 2011;14:187–94. doi: 10.1111/j.1461-0248.2010.01570.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 9Evaluated by Noah Fierer 07 Feb 2011, Christian Koerner 17 May 2011
- 9.De Graaff MA, Six J, van Kessel C. Elevated CO2 increases nitrogen rhizodeposition and microbial immobilization of root-derived nitrogen. New Phytol. 2007;173:778–86. doi: 10.1111/j.1469-8137.2006.01974.x. [DOI] [PubMed] [Google Scholar]
- 10.Heath J, Ayres E, Possell M, Bardgett RD, Black HI, Grant H, Ineson P, Kerstiens G. Rising atmospheric CO2 reduces soil carbon sequestration. Science. 2005;309:1711–13. doi: 10.1126/science.1110700. [DOI] [PubMed] [Google Scholar]
- 11.Dijkstra FA, Cheng WX. Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol Lett. 2007;10:1046–53. doi: 10.1111/j.1461-0248.2007.01095.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Richard Bardgett 28 Jul 2011
- 12.Rillig MC, Hernández GY, Newton PCD. Arbuscular mycorrhizae respond to elevated atmospheric CO2 after long-term exposure: evidence from a CO2 spring in New Zealand supports the resource balance model. Ecol Lett. 2000;3:475–8. doi: 10.1111/j.1461-0248.2000.00178.x. [DOI] [Google Scholar]
- 13.Staddon PL, Jakobsen I, Blum H. Nitrogen input mediates the effects of free-air CO2 enrichment on mycorrhizal fungal abundance. Glob Change Biol. 2004;10:1687–8. doi: 10.1111/j.1365-2486.2004.00853.x. [DOI] [Google Scholar]
- 14.Högberg P, Read DJ. Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol. 2006;21:548–54. doi: 10.1016/j.tree.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Rillig MC, Mummey DL. Mycorrhizas and soil structure. New Phytol. 2006;171:41–53. doi: 10.1111/j.1469-8137.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett. 2009;12:452–61. doi: 10.1111/j.1461-0248.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 17.Ward SE, Bardgett RD, McNamara NP, Ostle NJ. Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct Ecol. 2009;23:454–62. doi: 10.1111/j.1365-2435.2008.01521.x. [DOI] [Google Scholar]
- 18.Woodin SJ, van der Wal R, Sommerkorn M, Gornall JL. Differential allocation of carbon in mosses and grasses governs ecosystem sequestration: a 13C tracer study in the high Arctic. New Phytol. 2009;184:944–9. doi: 10.1111/j.1469-8137.2009.03022.x. [DOI] [PubMed] [Google Scholar]
- 19.De Deyn GB, Quirk H, Oakley S, Ostle N, Bardgett RD. Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed species-rich grasslands. Biogeosciences. 2011;8:1131–9. doi: 10.5194/bg-8-1131-2011. [DOI] [Google Scholar]
- 20.De Deyn GB, Quirk H, Yi Z, Oakley S, Ostle NJ, Bardgett RD. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J Ecol. 2009;97:864–75. doi: 10.1111/j.1365-2745.2009.01536.x. [DOI] [Google Scholar]
- 21.De Deyn GB, Quirk H, Bardgett RD. Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility. Biol Lett. 2011;7:75–8. doi: 10.1098/rsbl.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Deyn GB, Cornelissen JH, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett. 2008;11:516–31. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 23.De Deyn GB, Shiel RS, Ostle NJ, McNamara NP, Oakley S, Young I, Freeman C, Fenner N, Quirk H, Bardgett RD. Additional carbon sequestration benefits of grassland diversity restoration. J Appl Ecol. 2011;48:600–8. doi: 10.1111/j.1365-2664.2010.01925.x. [DOI] [Google Scholar]
- 24.Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–600. doi: 10.1126/science.1133306. [DOI] [PubMed] [Google Scholar]; F1000 Factor 15Evaluated by Evan DeLucia 10 Jan 2007, Joy Ward 11 Jan 2007, J Emmett Duffy 17 Jan 2007, Andrew Hector 20 Feb 2007, Judy Wall 28 Mar 2007
- 25.Fornara DA, Tilman D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol. 2008;96:314–22. doi: 10.1111/j.1365-2745.2007.01345.x. [DOI] [Google Scholar]
- 26.Steinbeiss S, Beßler H, Engels C, Temperton VM, Buchmann N, Roscher C, Kreutziger Y, Baade J, Habekost M, Gleixner G. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob Change Biol. 2008;14:2937–49. doi: 10.1111/j.1365-2486.2008.01697.x. [DOI] [Google Scholar]
- 27.Orwin KA, Buckland SM, Johnson D, Turner BL, Smart S, Oakley S, Bardgett RD. Linkages between plant traits and soil properties related to the functioning of temperate grassland. J Ecol. 2010;98:1074–83. doi: 10.1111/j.1365-2745.2010.01679.x. [DOI] [Google Scholar]
- 28.Dijkstra FA, Hobbie SE, Reich PB. Soil processes affected by sixteen grassland species grown under different environmental conditions. Soil Sci Soc Am J. 2006;70:770–7. doi: 10.2136/sssaj2005.0088. [DOI] [Google Scholar]
- 29.Glover JD, Reganold JP, Bell LW, Borevitz J, Brummer EC, Buckler ES, Cox CM, Cox TS, Crews TE, Culman SW, DeHaan LR, Eriksson D, Gill BS, Holland J, Hu F, Hulke BS, Ibrahim AM, Jackson W, Jones SS, Murray SC, Paterson AH, Ploschuk E, Sacks EJ, Snapp S, Tao D, Van Tassel DL, Wade LJ, Wyse DL, Xu Y. Agriculture. Increased for and ecosystem security via perennial grains. Science. 2010;328:1638–9. doi: 10.1126/science.1188761. [DOI] [PubMed] [Google Scholar]
- 30.To JPC, Zhu J, Benfey PN, Elich T. Optimizing root system architecture in biofuel crops for sustainable energy production and soil carbon sequestration. F1000 Biol Rep. 2010;2:65. doi: 10.3410/B2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]