Abstract
Neurons born in the postnatal SVZ (subventricular zone) must migrate a great distance before becoming mature interneurons of the OB (olfactory bulb). During migration immature OB neurons maintain an immature morphology until they reach their destination. While the morphological development of these cells must be tightly regulated, the cellular pathways responsible are still largely unknown. Our results show that the non-canonical Wnt pathway induced by Wnt5a is important for the morphological development of OB interneurons both in vitro and in vivo. Additionally, we demonstrate that non-canonical Wnt signalling works in opposition to canonical Wnt signalling in neural precursors from the SVZ in vitro. This represents a novel role for Wnt5a in the development of OB interneurons and suggests that canonical and non-canonical Wnt pathways dynamically oppose each other in the regulation of dendrite maturation.
Keywords: dendrites, dkk1, non-canonical, sfrp1, subventricular zone (SVZ), wnt signalling
Abbreviations: BMP, bone morphogenic protein; CB, calbindin; CSF, cerebrospinal fluid; DAPI, 4′,6-diamidino-2-phenylindole; DKK-1, Dickkopf-related protein 1; JNK, c-Jun N-terminal kinase; LEF, lymphoid enhancer factor; LRP5/6, LDL (low-density lipoprotein)-receptor-related protein 5/6; NPC, neural progenitor cell; OB, olfactory bulb; OSN, olfactory sensory axon; PFA, paraformaldehyde; RMS, rostral migratory stream; sFRP-1, secreted frizzled-related protein 1; SVZ, subventricular zone; TDBTN, total dendritic branch tip number; TH, tyrosine hydroxylase; VGAT, vesicular γ-aminobutyric acid transporter
INTRODUCTION
The SVZ (subventricular zone) surrounding the lateral ventricles is one of the two neurogenic niches in the postnatal mouse brain, the other being the subgranular zone of the dentate gyrus. Neurons born in the SVZ migrate along the RMS (rostral migratory stream) to become OB (olfactory bulb) interneurons. While migrating, immature interneurons maintain a simplified morphology with a single leading process as they migrate in chains (Lois et al., 1996). OB interneurons do not attain full morphological maturity until they reach their appropriate destination within the OB. This entire process from specification to functional integration takes 5–9 days in the adult mouse (Petreanu and Alvarez-Buylla, 2002). As OB interneuron specification and complete morphological maturity are separated substantially in both space and time, this is an ideal system for studying the morphological development of neurons in the postnatal brain.
Wnt signalling is integral to both neuronal specification and development. In the postnatal brain, Wnts are important in stem cell proliferation (Adachi et al., 2007) and have been shown to be involved in both differentiation (Lie et al., 2005) and neurite development of hippocampal granule cells (Rosso et al., 2005; Yu and Malenka, 2003). However, it is unclear as to what roles specific Wnt signalling pathways play in these cells. Increased dendritic complexity was shown to be dependent on β-catenin; however, this effect did not require LEF (lymphoid enhancer factor)/TCF (T-cell factor)-mediated transcriptional activation (Yu and Malenka, 2003). Moreover, Yu and Malenka demonstrate that activated LEF-1 has the opposite effect of activated β-catenin, and suppressed neurite outgrowth in hippocampal cultures. It is therefore likely that β-catenin influences dendritic growth through its role in modulating cell adhesion and the actin cytoskeleton, independent of Wnt signalling (Yu and Malenka, 2003; Gavard and Mege, 2005).
Wnt7b has been shown to increase the complexity of hippocampal neurons in culture via the non-canonical JNK (c-Jun N-terminal kinase) Wnt pathway (Rosso et al., 2005). The addition of Wnt inhibitor sFRP-1 (secreted frizzled-related protein 1) to these cultures not only blocked the effect but also simplified the morphology of the neurons beyond that of control cultures, indicating an endogenous role for the non-canonical Wnt pathway in the morphological development of hippocampal cells. The JNK pathway was also shown to increase neurite outgrowth in ESFT (Ewing sarcoma family tumour) cells, which can be stimulated by Wnt3a or canonical inhibitor DKK-1 (Dickkopf-related protein 1) (Endo et al., 2008). However, sFRP-1 has also been shown to increase dendritic outgrowth in a non-Wnt-dependent manner in retinal ganglion cells (Rodriguez et al., 2005). Recently, Wnt5a signalling via the Wnt/Ca+ pathway was shown to stimulate dendritic spine morphogenesis in hippocampal neurons (Varela-Nallar et al., 2010). These findings suggest that specific Wnt molecules may play different roles depending on cellular context. It is still unknown as to whether Wnts play a role in the morphological development of SVZ-derived OB interneurons; although it does appear that the Wnt/β-catenin pathway is important for the proliferation of progenitor cells in the SVZ (Adachi et al., 2007). Interestingly, traditionally non-canonical Wnt ligands Wnt5a and Wnt7b, but not canonical Wnts, are expressed by OB interneurons themselves (Shimogori et al., 2004).
Using soluble Wnt ligands Wnt5a and Wnt3a, and inhibitors DKK-1 and sFRP-1 on SVZ progenitor cell cultures, we show that non-canonical Wnt signalling is necessary for normal morphological maturation of OB interneurons. In our culture system the activation of the non-canonical Wnt pathway acts in opposition to canonical Wnt/β-catenin signalling. Moreover, evidence from the Wnt5a knockout mouse indicates that normal morphological development of OB interneurons is disrupted in the absence of endogenous Wnt5a.
MATERIALS AND METHODS
Animals
All animal protocols were approved by the UCSF IACUC. P5 CD1s (Charles River) were used for cultures. Wnt5a knockout mice (Yamaguchi et al., 1999) were backcrossed more than 7 generations with C57BL/6. Pregnant mice were killed at E18.5 (embryonic day 18.5) via cervical dislocation after being anaesthetized with isofluorane. Mutants were identified visually using the very distinctive Wnt5a phenotype. Males and females were used for all experiments.
In situ hybridization
P1 brain tissues were collected after intracardiac perfusion with 4% (w/v) PFA (paraformaldehyde) and post-fixed with 4% PFA overnight at 4°C. Fixed tissues were cryoprotected in 20% sucrose before embedding in OCT (optimal cutting temperature) compound (Sakura Finetek, Torrance, CA, U.S.A.). Tissues were cut on a cryostat at 20 μm and directly mounted on to Colorfrost slides (Fisher Scientific, Houston, TX, U.S.A.). The slides were stored at −80°C before use. For in situ hybridization, slides were warmed to room temperature and treated with proteinase K (50 μg/ml) for 2 min, and fixed with 4% PFA for 10 min. Acetylation was performed using 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0, for 10 min, followed by three PBS washes. Slides were incubated with hybridization buffer (50% formamide, 5×SSC, 0.3 mg/ml yeast tRNA, 100 mg/ml heparin, 1×Denhart's, 0.1% Tween 20, 0.1% CHAPS, 5 mM EDTA) for 10 min at 65°C, followed by overnight incubation with a digoxigenin-labelled probe (300 ng/ml). Five high-stringency washes were performed with 0.2×SSC at 65°C. Slides were then incubated with horseradish AP (alkaline phosphatase)-conjugated anti-digoxigenin and NBT (nitroblue tetrazolium)/BCIP (5-bromo-4-chloro-indolyl phosphate) (Roche) for signal detection. The non-radioactive probes were made from EST (expressed sequence tag) clones for Wnt2b and Wnt5a, which were purchased from Open Biosystems, and the Wnt3a cDNA plasmid was a gift from Roel Nusse (Roelink and Nusse, 1991).
Immunohistochemistry
Brains were processed using standard methods. Cells and tissue sections were stained using standard protocols with: mouse anti-Tuj1 (Covance, 1:3000 for cells), rabbit anti-MAP2 (Chemicon, 1:500 for cells), rabbit anti-Pax6 (Covance, 1:150 for tissue), rabbit anti-CB (Swant, 1:1500 for tissue), rabbit anti-TH (tyrosine hydroxylase) (Chemicon, 1:400 for tissue) and rabbit anti-VGAT (vasicular γ-aminobutyric acid transporter) (Synaptic Systems, 1:200 for cells). Fluorescent secondary antibodies (1:500, Invitrogen) and DAPI (4′,6-diamidino-2-phenylindole) (1:1000, Sigma) were used.
Cell Culture
NPCs (neural progenitor cells) were harvested from lateral ventricle SVZ tissue of P5 CD1s. Tissue was dissociated with 0.1% Trypsin (Worthington) and 0.1% DNAse1 (Roche). DMEM (Dulbecco's modified Eagle's medium)/F12 (50:50; Gibco) was supplemented with 10×hormone mix (40 mg transferrin, 10 mg insulin, 3.86 mg putrescine, 4.0 ml 3 mM selenium, 4.0 ml 2 mM progesterone, 10 ml 2 mg/ml Heparin [all from Sigma]), 0.8 ml 30% glucose, 0.6 ml 7.5% NaHCO3, 10 ml 30% glucose, 7.5 ml 7.5% NaHCO3, 2.5 ml 1 M Hepes, 5 ml 200 mM glutamine, 5 ml Pencillin–Streptomycin and 2 ml Fungizone). Medium containing EGF (epidermal growth factor; Sigma, 10 ng/ml), bFGF (basic fibroblast growth factor; Sigma, 20 ng/ml) and B27 (Invitrogen) was added. Cells were plated at 80000 cells per 25 cm2 flask (Corning), grown in 5 ml of complete medium plus growth factors and B27. Passage 2 or 3 NPCs were used.
Cell culture-Wnt treatments
NPCs were dissociated with 0.05% trypsin-EDTA, washed with PBS, pelleted and resuspended in complete medium with 2% FBS (fetal bovine serum) (Hyclone). The cells were plated on poly-l-lysine 0.01% (Sigma, MW 70000–150000 kDa) and laminin-coated (1 mg/ml, Invitrogen) chamber slides (Nunc) at 37500 cells/well. Plated cells were left to differentiate for 24 h (Figure 1) or 96 h (Figure 2) before treatment with Wnt factors. At the appropriate time mouse Wnt5a (0.375 ng/μl final working concentration), Wnt3a (0.15 ng/μl final working concentration), sFRP-1 (5.0 ng/μl final working concentration), DKK-1 (0.06 ng/μl final working concentration) (all from R&D Systems) or an equivalent volume of vehicle were added to each well. One-half of the treated medium was replaced each day. The cultures were analysed after 4 days of treatment.
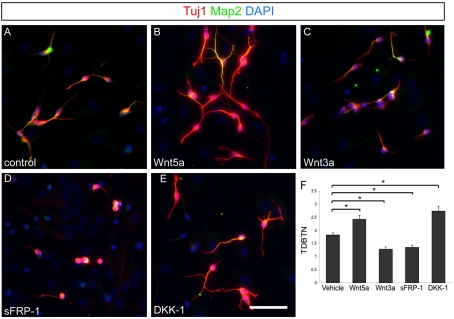
Figure 1. Wnt5a and Wnt3a have opposite effects on neurite development.
Neurons are identified by staining with Tuj1 (red) and MAP2 (green) antibodies. DAPI counterstain is used to show the density of other cells in the culture. (A) Neurons treated with vehicle for 3 days as a control. (B) Cultured neurons treated with soluble Wnt5a. (C) Neurons treated with Wnt3a. (D) Neurons treated with non-specific Wnt inhibitor sFRP-1. (E) Neurons treated with canonical inhibitor DKK-1. (F) Quantification of TDBTN for each condition. All P values <0.001. Scale bar = 50 μm.
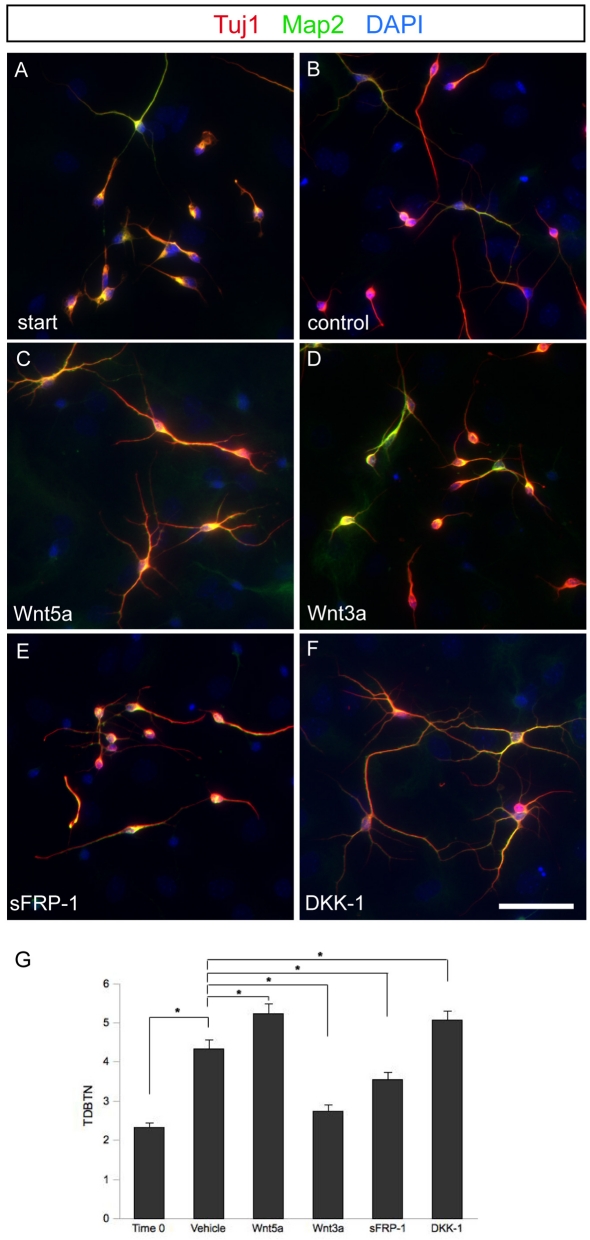
Figure 2. Effects of Wnts signalling on growth of neurites in later cultures.
Wnts and Wnt inhibitors affect neuron development up to 96 h after specification. (A) Untreated cells after 96 h in vitro fixed to serve as T = 0 for normalization of neurite outgrowth. (B) Control neurons treated with vehicle beginning 96 h after specification for another 72 h. (C) Wnt5a continues to enhance morphologic development when applied 96 h after specification. (D) Wnt3a inhibits morphological development when applied 96 h after cell specification. (E) Neurons treated with Wnt inhibitor sFRP-1 96 h after specification continue to develop, although not as rapidly as neurons treated with vehicle alone. (F) Canonical Wnt inhibitor DKK-1 enhances morphological development when applied 96 h after cell specification. (G) Quantification of TDBTN of treated cells. All P-values <0.05. Scale bar = 50 μm.
Image analysis and quantification
Three biological replicates of each experiment were performed. For in vitro experiments, cells from 3 to 5 ×20 magnification images were quantified for each replicate of each culture condition, including at least 100 cells from each condition. Cells were excluded from the analysis if all dendrite tips could not be clearly visualized.
One mutant and one littermate control animal were analysed from three separate litters. Cell counts from standardized counting boxes in three coronal sections of each brain were averaged for both the Pax6 and CB staining. DAPI counterstains were used to confirm the presence of cell bodies. PGL width was measured in four places for each TH and VGAT stained section and averaged. These values for each animal were then pooled and compared across genotypes.
Statistical analysis
Results are expressed as means±S.E.M. Results were analysed using two-tailed Student's t test with unequal variance. Any value of P≤0.05 was considered significant.
RESULTS
Wnt5a and non-canonical Wnt signalling increase neurite complexity
To study the role of Wnt ligands in regulating the neuronal differentiation of SVZ-derived OB interneurons, we took advantage of the ease of generating these cells in vitro via differentiation from neural precursors. We cultured neural precursor cells from the SVZ of P5 mice, then placed them in serum-containing medium for 24 h to induce differentiation. We then treated the cultures with soluble Wnt ligands and inhibitors for 4 days, and then analysed the neuritic complexity of the resulting neurons. This experiment is designed to examine the effects of Wnt ligands on differentiation rather than neuronal specification since the ligands were not added until well after the global differentiation stimulus of serum was added to the cells. The largely non-canonical Wnt ligand, Wnt5a, and the largely canonical Wnt ligand, Wnt3a, had markedly different effects on neurite development in these cells (Figures 1A–1C). Wnt5a treatment led to increased neurite growth, whereas Wnt3a inhibited neurite outgrowth. We used a simple measure of neurite complexity, TDBTN (total dendritic branch tip number) (Rosso et al., 2005), to assess these findings in a quantitative way. We found that TDBTN was significantly higher in cultures treated with Wnt5a compared with controls, and cultures treated with Wnt3a significantly decreased TDBTN compared with control cultures treated with vehicle only (Figure 1F).
Our results imply that Wnt5a leads to distinct effects on neuritic maturation in these cultures compared with Wnt3a, possibly due to differences in Wnt signalling pathway activation (Wnt/β-catenin signalling versus non-canonical signalling). To tease apart the differences in these effects, we treated the cultures with different families of extracellular Wnt inhibitors to determine the pathway specificity. When sFRP-1 was applied to cultures, an inhibitor that blocks both canonical and non-canonical Wnt signalling by sequestering Wnt ligands and so they cannot bind their cognate Frizzled receptors (Rattner et al., 1997), the neurite complexity was decreased (Figure 1D) and TDBTN was significantly lower than in control cultures (Figure 1F). In contrast, cultures treated with DKK-1, an inhibitor that specifically blocks canonical Wnt signalling by interfering with LRP5/6 [LDL (low-density lipoprotein)-receptor-related protein 5/6] co-receptors (Mao et al., 2001), showed an increase in neurite complexity (Figure 1E) reflected by a significant increase in TDBTN (Figure 1E). Our results imply that canonical and non-canonical Wnt signalling have markedly different effects on the morphological maturation of neurons derived from the mouse SVZ. In addition, the effects of inhibitors on cultures without the addition of exogenous Wnts indicate that under normal culture conditions Wnt signalling is probably a potent regulator of neurite outgrowth, both positively and negatively.
Wnt signalling plays a sustained role in neurite differentiation in culture
Wnt signalling appears to be a significant regulator of neurite development in neural precursor cells during the first few days in culture. However, given the profound roles of canonical Wnt signalling in neural precursor proliferation and cell fate acquisition, we wanted to determine whether Wnt signalling plays an ongoing role in the growth of neurites or whether the Wnts predominantly act at the stage of initial neurite outgrowth, perhaps by influencing the cell fate acquisition process itself or initial stages of neurite polarization. In addition, if Wnt signalling plays a role in neurite maturation when the interneurons reach the OB, then the effects of Wnts should be ongoing even after several days.
To address this, we used neurons differentiated from SVZ cultures that were allowed to grow in serum without additives for 72 h. We then treated the cells with Wnts and Wnt inhibitors starting at this later time point. It was apparent that neurites continued to extend during this growth period in control cultures (Figures 2A and 2B) and this was reflected in the TDBTN (Figure 2G). Like at the earlier stage of neurite outgrowth, we found that Wnt5a increased the complexity of neurite outgrowth significantly over what we observed in controls and Wnt3a inhibited the complexity compared with control situations (Figures 2C, 2D and 2G). The normal increase in neurite complexity was also blocked by sFRP-1 (Figures 2E and 2G) and significantly increased when the selective canonical Wnt inhibitor DKK-1 was added to cultures (Figures 2F and 2G).
Again the significant effects seen with the inhibitors, particularly the DKK-1 blockade of endogenous canonical Wnt ligand signalling, indicate that this pathway is an ongoing regulator of neurite maturation in these cultures. The retardation of outgrowth in the sFRP-1 condition compared with the acceleration in the DKK-1 condition suggests an ongoing balance of endogenous non-canonical and canonical Wnt signalling that acts to regulate the rate and extent of neurite growth and complexity throughout the culture period.
Cellular organization of the OB is intact in Wnt5a mutant mice
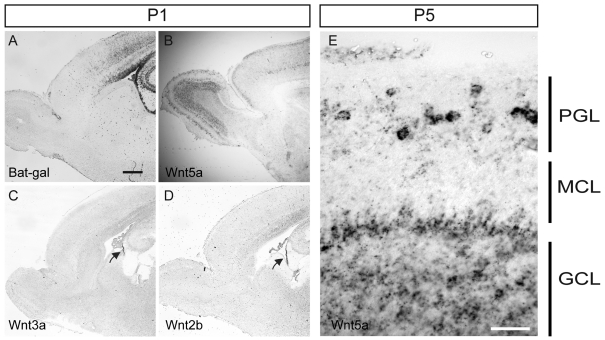
Since there are many Wnt ligands in the genome and there is extensive redundancy, examples of clear loss-of-function phenotypes for Wnt ligands are not that common. However, we wanted to determine whether there is a role for Wnt5a in regulating olfactory interneuron neurite development in vivo. Previous reports showed that Wnt5a is expressed in the early postnatal mouse OB periglomerular (PGC) and granule (GC) cells (Shimogori et al., 2004). First though we examined the distribution of canonical Wnt signalling during the early postnatal phase. Interestingly we found using the Bat-gal mice (Maretto et al., 2003) that P1 mice have ongoing signalling in the SVZ but little evidence of this in the RMS or within the OB (Figure 3A). This may be indicative of our model that non-canonical Wnt ligands may have a more prominent effect on the OB interneurons as they arrive in the OB and begin to mature. Indeed, we found that Wnt5a is expressed in GCs, PGCs, and at the base of the RMS at both P1 and P5 (Figures 3B and 3E). Therefore, as immature interneurons reach the OB after migrating through the RMS, they begin to express Wnt5a and also encounter already-established neurons expressing Wnt5a as they seek to integrate themselves into the local network. To determine the expression of potentially relevant canonical Wnt ligands, we examined the expression of Wnt3a, Wnt2b and Wnt1, all previously examined by others in these structures as well (Shimogori et al., 2004). We found both Wnt3a and Wnt2b to be expressed at high levels primarily in the fimbrial neuroepithelium at P1 (Figures 3C and 3D) and were unable to see convincing evidence for Wnt1 expression at this age (results not shown). Given the recent evidence that there is extensive exposure of the ventricular zone and SVZ to ligands secreted into the CSF (cerebrospinal fluid; Lehtinen et al., 2011), and our finding that canonical Wnt signalling is abundant in the SVZ (Figure 3A), it seems likely that the fimbrial neuroepithelium may be a relevant physiological source of Wnt ligands in the CSF.
Figure 3. Expression of Wnt ligands and Wnt signalling in the SVZ and OB.
(A) Bat-gal reporter mice show that canonical Wnt signalling is prominent in the SVZ at P1 but much less apparent in the RMS and OB. (B) Wnt5a is expressed in cortical neurons, hippocampal neurons and OB neurons at this age. (C, D) The two canonical Wnt ligands Wnt3a and Wnt2b are expressed at significant levels only in the fimbrial neuroepithelium (see arrows) at this age, in contact with the CSF space. (E) High-magnification image of the OB showing that Wnt5a is expressed in granule cells and periglomerular cells in the OB. Scale bar in (A) is 400 μm and applies to (A–D), scale bar in (E) is 50 μm.
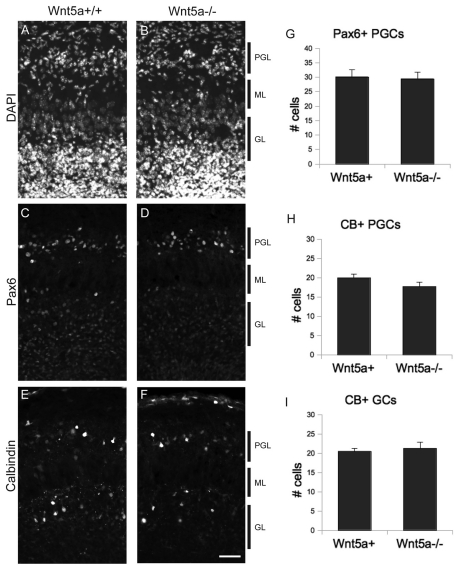
We turned to the previously characterized Wnt5a null mutant mice (Yamaguchi et al., 1999) to determine whether Wnt5a plays a role in OB development. These mice do not survive past birth, and so we confined our analysis to late embryonic stages (E18.5). Since the role of Wnt5a in olfactory interneurons has not been previously examined, we first looked for defects in cell number and organization of PGCs and GCs in the OB of Wnt5a knockout mice. A nuclear DAPI stain of Wnt5a−/− OB shows no obvious difference from littermate control animals (Figures 4A and 4B). To examine specific populations of OB interneurons, we turned to antibodies that are specific for subpopulations of PGCs and GCs. Pax6 is a nuclear marker that labels a subset of both GCs and PGCs in the OB (Kohwi et al., 2005) and allows for a straightforward and sensitive measure of the cell density of these groups of neurons. There was no obvious difference between Wnt5a−/− mice and littermate controls in the PGC layer (Figures 4C and 4D). CB (calbindin) is another useful marker expressed in the cytoplasm of subsets of OB interneurons (Kohwi et al., 2007), and we found no clear difference in the numbers of CB+ cells in the OB of mutants and control mice (Figures 4E and 4F). To quantify the numbers of Pax6+ and CB+ interneurons in these mice, we counted them in standardized counting boxes. For Pax6, we restricted our cell counts to PGCs because of the substantial number of neural progenitor cells that express Pax6 deep in the core of the OB near GCs. This analysis revealed no difference in the number of these cells between mutant and control mice (Figure 4G). We also examined CB+ PGCs and GCs and found no difference between mutants and controls (Figures 4H and 4I).
Figure 4. Interneuron organization and numbers are normal in Wnt5a mutants.
(A) Wnt5a+/+ and (B) Wnt5a−/− OB stained with DAPI. (C) Wnt5a+/+ and (D) Wnt5a−/− OB stained for Pax6. (E) Wnt5a+/+ and (F) Wnt5a−/− OB stained for CB. (G) Number of Pax6+ periglomerular cells is unchanged in mice lacking Wnt5a. (H) Number of CB+ periglomerular cells and (I) granule cells are unchanged in mice lacking Wnt5a. Scale bar = 50 μm.
Wnt5a mutant mice have reduced OB interneuron neuropil
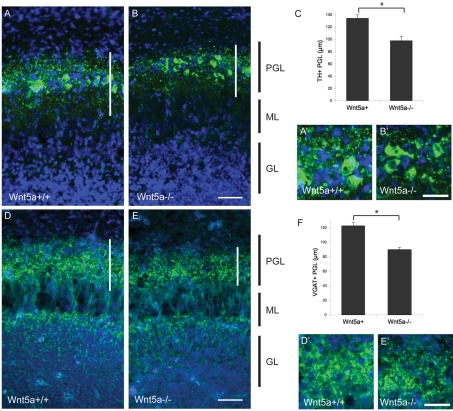
The results of our Pax6 and CB analyses indicate that overall organization and cell number of several groups of OB interneurons are preserved in the OB of Wnt5a−/− mice, but we wondered whether their morphology might be altered. We wanted to examine structural differences in the OB of Wnt5a−/− mice directly using more sensitive markers of neuropil morphology. TH is expressed in a subset of PGCs (Kohwi et al., 2007), staining both their cell bodies and dendrites, and gives a useful view of PGC morphology. Control mice had strong staining for TH in the PGC layer. This marker highlights the cell bodies of these cells and also stains a reticular network of processes that are distributed throughout the PGC layer forming a meshwork of dendritic processes around the glomeruli (Figure 5A). In Wnt5a−/− mice, the number of PGCs appeared unchanged (similar to our findings with Pax6 and CB), but the density of neuropil staining was dramatically decreased and the reticular network of dendrites was substantially less dense (Figure 5B). These differences were even more apparent in high-power images of the PGCs (Figures 5A' and 5B'). To quantify this difference, we measured the width of the TH+ neuropil in multiple places in the PGC layer to establish a measure of PGC layer neurite complexity and found that it was significantly reduced compared with littermate controls (Figure 5C).
Figure 5. Reduction of TH± and VGAT±neuropil in Wnt5a mutant mice.
(A) Wnt5a+/+ mice (high-power inset A') and (B) Wnt5a−/− (high-power inset B') mice stained for TH. (C) Width of TH+ PGL is reduced in mice lacking Wnt5a (P<0.01). (D) Wnt5a+/+ mice (high-power inset D') and (E) Wnt5a−/− mice (high-power inset E') OB stained for VGAT. (F) Width of VGAT+ PGL is reduced in mutants (P<0.01). (A, B, D, E) Scale bar = 50 μm. (A', B', D', E') Scale bar = 25 μm.
Since periglomerular interneurons are GABAergic, we also stained for the VGAT to further examine the neuropil in the PGC layer in these mutants. This showed that VGAT staining was markedly decreased in Wnt5a−/− mice (Figure 5E). At high power, much of the VGAT staining was punctate in appearance, presumably reflecting its localization to presynaptic GABAergic terminals, and the density of these puncta was apparently decreased in mutants (Figures 5D' and 5E'). In addition, as with TH, the thickness of the PGC layer was much reduced in mutants (Figure 5F).
DISCUSSION
Our results show that the morphological development of OB interneurons derived from the SVZ is dependent on Wnt5a, which likely acts via non-canonical Wnt signalling to increase neurite complexity. Blockade of Wnt signalling in SVZ-derived neural precursor cells in vitro using the non-specific Wnt inhibitor sFRP-1 resulted in a simplified neuronal morphology, indicating a role for endogenous Wnts in morphological development. In mice lacking Wnt5a, interneuron morphology is disrupted in the OB, while cell number and OB architecture remain intact. Our findings are reminiscent of those in rat hippocampal cells where sFRP-1 blocks neurite outgrowth and non-canonical Wnt ligand Wnt7b increases dendritic complexity (Rosso et al., 2005; Endo et al., 2008). Moreover our results support previous reports that Wnt/β-catenin signalling inhibits neurite outgrowth (Ouchi et al., 2005; Votin et al., 2005; Endo et al., 2008); although β-catenin seems to have the opposite effect when acting outside the Wnt pathway (Yu and Malenka, 2003). This implies that Wnt signalling may be important in regulating dendrite maturation elsewhere in the nervous system, although the role of specific Wnt molecules may vary in different systems.
Wnt5a is able to act as either a canonical or non-canonical Wnt ligand depending on receptor context (Mikels and Nusse, 2006), and previous studies have been ambiguous about which Wnt pathways are important for morphological development in postnatal neurogenesis (Yu and Malenka, 2003; Rosso et al., 2005; Endo et al., 2008; Varela-Nallar et al., 2010). In our cell culture experiments, DKK-1, a canonical Wnt inhibitor acts to increase neurite complexity, and the largely canonical Wnt ligand Wnt3a slows down the rate of morphological development. These findings suggest that Wnt5a acts on the non-canonical pathway in this system. It also indicates that canonical Wnt signalling directly suppresses dendrite maturation and acts in opposition to non-canonical signalling in SVZ-derived neurons.
Morphogenesis is a dynamic process in neural precursors from the SVZ, with migrating and dividing cells of the RMS continually protruding and retracting processes throughout the cell cycle (Coskun and Luskin, 2002). During migration, dividing cells predictably retract their processes immediately before cytokinesis (Coskun et al., 2007), indicating that cell cycle factors also impact morphogenesis. More specifically, a gradient of BMPs (bone morphogenic proteins) in the RMS has been shown to influence when SVZ-derived immature neurons become postmitotic (Coskun and Luskin, 2002), and may have some additional influence on neurite development in vivo. The presence of BMP or other factors in the RMS may therefore contribute additional regulation to neurite outgrowth, providing a bridge for controlling morphogenesis while immature neurons migrate from the SVZ to the OB.
Once in the OB, PGCs form synapses around the glomeruli where they come into contact with OSNs (olfactory sensory axons) and mitral cells. OSNs have been previously shown to express Wnt5a, and their axons are responsive to Wnt5a in vitro (Rodriguez-Gil and Greer, 2008). Additionally, exogenous application of Wnt5a on to OSNs in culture resulted in increased growth cone branching compared with control cultures (Rodriguez-Gil and Greer, 2008). These findings indicate that Wnt5a in the OB may affect multiple cell populations, including innervation of the glomeruli by OSN. It is therefore possible that the thinning of the glomerular layer we observed may reflect decreased innervation by the OSNs, and further studies should be conducted to test this possibility.
Previous studies have highlighted the role of canonical Wnt signalling in the regulation of neural precursor proliferation and neural cell fate (Yu and Malenka, 2003; Adachi et al., 2007). Our findings suggest that canonical Wnt signalling may have an additional role in maintaining already differentiated OB interneurons in a simplified morphology even after cell fate has been determined. In our model, neural precursor cells are exposed to canonical Wnt signalling in the SVZ, which along with other factors including BMPs help maintain cells in a simplified morphology. Once the migratory neurons reach the anterior RMS and OB and contact mature interneurons in the periglomerular layer and granule layer, they are exposed to Wnt5a via paracrine signalling and complete their morphologic differentiation. This model implicates a novel interaction between canonical and non-canonical Wnt signalling pathways in the regulation of neuronal morphology in OB interneurons that may be important elsewhere in the nervous system.
ACKNOWLEDGEMENTS
We thank the members of the Pleasure laboratory for discussion and scientific support during the course of this project.
Footnotes
This work was supported by the National Science Federation [a predoctoral fellowship (to D.P.) and grant numbers K02 MH074958 and R01 MH066084 (to S.J.P.)].
REFERENCES
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Coskun V, Luskin MB. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J Neurosci Res. 2002;69:795–802. doi: 10.1002/jnr.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun V, Falls DL, Lane R, Czirok A, Luskin MB. Subventricular zone neuronal progenitors undergo multiple divisions and retract their processes prior to each cytokinesis. Eur J Neurosci. 2007;26:593–604. doi: 10.1111/j.1460-9568.2007.05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, Rubin JS. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Mege RM. Once upon a time there was beta-catenin in cadherin-mediated signalling. Biol Cell. 2005;97:921–926. doi: 10.1042/BC20040531. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'Ercole AJ, Wong ET, Lamantia AS, Walsh CA. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Tabata Y, Arai K, Watanabe S. Negative regulation of retinal-neurite extension by beta-catenin signaling pathway. J Cell Sci. 2005;118:4473–4483. doi: 10.1242/jcs.02575. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Greer CA. Wnt/Frizzled family members mediate olfactory sensory neuron axon extension. J Comp Neurol. 2008;511:301–317. doi: 10.1002/cne.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Nusse R. Expression of two members of the Wnt family during mouse development-restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votin V, Nelson WJ, Barth AI. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci. 2005;118:5699–5708. doi: 10.1242/jcs.02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]