Abstract
OLs (oligodendrocytes) are the myelinating cells of the CNS (central nervous system), wrapping axons in conductive sheathes to ensure effective transmission of neural signals. The regulation of OL development, from precursor to mature myelinating cell, is controlled by a variety of inhibitory and inductive signalling factors. The dorsal spinal cord contains signals that inhibit OL development, possibly to prevent premature and ectopic precursor differentiation. The Wnt and BMP (bone morphogenic protein) signalling pathways have been identified as dorsal spinal cord signals with overlapping temporal activity, and both have similar inhibitory effects on OL differentiation. Both these pathways feature prominently in many developmental processes and demyelinating events after injury, and they are known to interact in complex inductive, inhibitive and synergistic manners in many developing systems. The interaction between BMP and Wnt signalling in OL development, however, has not been extensively explored. In the present study, we examine the relationship between the canonical Wnt and BMP pathways. We use pharmacological and genetic paradigms to show that both Wnt3a and BMP4 will inhibit OL differentiation in vitro. We also show that when the canonical BMP signalling pathway is blocked, neither Wnt3a nor BMP4 have inhibitory effects on OL differentiation. In contrast, abrogating the Wnt signalling pathway does not alter the actions of BMP4 treatment. Our results indicate that the BMP signalling pathway is necessary for the canonical Wnt signalling pathway to exert its effects on OL development, but not vice versa, suggesting that Wnt signals upstream of BMP.
Keywords: bone morphogenic protein (BMP), development, glia, myelin, oligodendrocyte (OL), Wnt
Abbreviations: β-Cat-null, β-catenin loss of function mutant mice; Bmpr1 DKO, Bmpr1 double knockout; BMP, bone morphogenic protein; CNP, 2′,3′ cyclic nucleotide 3′-phosphodiesterase; CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; Dkk-1, Dikkopf-1; DKO, double knockout; DM, differentiation medium; GalC, galactocerebroside; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; HDAC, histone deacetylase; HRP, horseradish peroxidase; ID, inhibitor of DNA-binding protein; IHC, immunohistochemistry; KO, knockout; LEF, lymphoid enhancer factor; MBP, myelin basic protein; OL, oligodendrocyte; OPC, OL precursor cell; PLP, proteolipid protein; QPCR, quantitative real-time PCR; Shh, sonic hedgehog; TCF, T-cell factor
INTRODUCTION
Myelin is an essential physiological structure, allowing for the rapid and effective transmission of neural signals. Impaired myelination, prevalent in disorders such as multiple sclerosis, severely hampers the ability of neurons to communicate, resulting in functional deficits and axonal degeneration (Trapp et al., 1998; Lappe-Siefke et al., 2003; Edgar and Garbern, 2004). Factors involved in regulating myelination during development are often also involved in demyelinating disorders, and understanding their actions is crucial to designing treatments or therapies (Setoguchi et al., 2001; Armstrong et al., 2002; Liu et al., 2008b; Zhang et al., 2009; Cate et al., 2010).
OLs (oligodendrocytes) are the myelinating cells of the CNS (central nervous system). OLs are generated through a series of specific developmental stages (Pringle and Richardson, 1993; Ono et al., 1995), during which they are exposed to a range of signalling factors that can be inductive or inhibitory, extracellular or intracellular (Miller, 2002). OPCs (OL precursor cells) originate in ventricular zones at E12.5 (embryonic day 12.5) in the rodent CNS and migrate dorsally and radially, expressing markers such as A2B5, NG2 and PDGFr-α (platelet-derived growth factor receptor α). Once differentiation begins, OPCs progress to immature OLs, generating processes and expressing GalC (galactocerebroside). After contacting neurons, OPCs begin to extend processes, express myelin proteins, including PLP (proteolipid protein), MBP (myelin basic protein) and CNP (2′,3′ cyclic nucleotide 3′-phosphodiesterase), then ensheathe axons in protein and lipid heavy myelin (Grinspan, 2002; Miller, 2002). While the signals that influence this development are beginning to be characterized, the extent of their interactions remains to be fully explored.
Initially, signals in the ventral spinal cord, especially Shh (sonic hedgehog), induce expression of transcription factors essential for OL specification and development, including Olig1 and Olig2 (Lu et al., 2000; Zhou et al., 2000; Zhou and Anderson, 2002). In contrast, signals emanating from the roof plate in the dorsal spinal cord can inhibit this development (Wada et al., 2000), possibly to control the exact times when these cells reach and myelinate dorsal regions. There are, however, dorsal populations of OPCs whose generation is Shh independent, although their overall contributions appear to be limited and their distinct functions are unknown (Cai et al., 2005; Kasai et al., 2005; Vallstedt et al., 2005; Kessaris et al., 2006). Two families of dorsal signalling factors, the BMPs (bone morphogenic proteins) and the Wnts, have been shown to exert inhibitory effects on OPC differentiation.
BMPs are members of the TGFβ (transforming growth factor β) signalling family, and they have many roles in the developing nervous system involving embryonic patterning, cell proliferation, specification, differentiation and apoptosis (Liem et al., 1995, 2000; Mehler et al., 1997; Wine-Lee et al., 2004; See and Grinspan, 2009 for review). Of the Wnt and BMP families, BMP has been more extensively investigated with regard to OL development, exerting time- and stage-specific effects. BMPs drastically inhibit OPC differentiation into mature OLs, instead promoting astrogliogenesis (Grinspan et al., 2000; Miller et al., 2004; Samanta and Kessler, 2004; See et al., 2004; Cheng et al., 2007).
The Wnts are another family of dorsal-secreted factors involved in many of the same roles as BMPs in the nervous system, including embryonic patterning, cell specification, proliferation, migration and differentiation (Dorsky et al., 1998; Coyle-Rink et al., 2002; Braun et al., 2003; Hirabayashi et al., 2004; Karim et al., 2004; Kalani et al., 2008; Ulloa and Marti, 2010 for review). Recent studies have investigated the influence of canonical Wnt signalling on OL development, both in vitro and in vivo, indicating that the Wnts and BMPs have similar effects. In spinal cord explants and OPC cultures, ectopic Wnt3a application inhibits the differentiation of OPCs into mature cells (Shimizu et al., 2005; Kim et al., 2008; Fancy et al., 2009; Feigenson et al., 2009; Ye et al., 2009). Mutant mice in which Wnt signalling is constitutively activated in cells of OL lineage display an early developmental decrease in mature OLs. This effect diminishes as the mice age, however, suggesting that the importance of Wnt signalling may vary over the course of OL development (Fancy et al., 2009; Feigenson et al., 2009).
The interaction of BMPs and Wnts is well documented in a variety of different systems, their expression is spatially and temporally similar, and they are functionally involved in many of the same processes. Their relationship, however, varies based on tissue and developmental timeline: depending on context, they can directly or indirectly be inductive, antagonistic or synergistic (Soshnikova et al., 2003; Guo and Wang, 2009; Itasaki and Hoppler, 2010 for review). There is evidence that Wnt signalling prevents oligodendroglial specification at the neural stem cell stage by up-regulating BMPs through neurogenesis (Kasai et al., 2005), but how these two pathways may interact in OL development has not been explored in detail. An understanding of the interaction of these pathways is an important avenue for developing treatments and therapies for demyelinating events. It is well documented that BMPs are up-regulated in CNS injury and models of demyelinating disease (Setoguchi et al., 2001, 2004; See and Grinspan, 2009; Cate et al., 2010; Jablonska et al., 2010), and can play roles in preventing remyelination. In parallel, several recent studies have also found that members and effectors of the Wnt family are up-regulated in similar paradigms, and may have similar inhibitory effects on recovery (Liu et al., 2008b; Fancy et al., 2009; Miyashita et al., 2009; White et al., 2010).
Because Wnts and BMPs have similar sites of origin and effects on OLs during development and demyelinating events, we hypothesized that these two signalling factors mutually regulate OL development. To explore this interaction, we have employed effectors and antagonists of both canonical Wnt and BMP signalling on both mutant and normal OPC cultures. We find that while both BMP4 and Wnt3a inhibit the differentiation of OPCs, when the BMP pathway is blocked – by either chemical or genetic means – the effects of both Wnt3a and BMP4 are abrogated. In contrast, the effect of BMP4 on OPC differentiation is not altered when the canonical Wnt signalling pathway is blocked. Our results indicate that the BMP pathway is necessary for canonical Wnt signalling to inhibit OPC differentiation.
EXPERIMENTAL
Cell culture generation and treatment
All experiments were performed in accordance with the guidelines set forth by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee. To generate purified OPC cultures from newborn Sprague–Dawley rats or mixed background mice, forebrain cells were harvested and seeded on 100 mm Petri dishes in serum containing medium as previously described (See et al., 2004; Feigenson et al., 2009). After 24 h, cultures were switched to serum-free growth medium containing Neurobasal medium (Invitrogen) with B27 supplement (1:50 Life technologies), 10 ng/ml basic fibroblast growth factor (R & D Systems, Minneapolis, MN, U.S.A.), 2 ng/ml platelet-derived growth factor (R & D), and 1 ng/ml neurotrophin-3 (Peprotech, Rocky Hill, NJ, U.S.A.).
Rat cultures were purified 6–8 days after plating by immunopanning as previously described (Grinspan et al., 2000). Cells were successively seeded on to two sets of dishes coated with Ran-2 antibody to bind type-1 astrocytes, meningeal cells and microglia. Cells were then put on dishes plated with A2B5 antibody (undiluted, hybridoma supernatant, ATCC, Rockville, MD, U.S.A.; Eisenbarth et al., 1979) to bind the OPCs. When cells reached confluency, they were sub-cultured in polylysine-coated flasks, 12 mm polylysine-coated coverslips, 100 mm polylysine-coated Petri dishes or 50 mm polylysine-coated Petri dishes. Cells could be passaged 3–4 times. Cultures were considered purified if they contained fewer than 5% astrocytes.
To culture mouse OPCs, cells were harvested from the forebrains of individual animals using the same methods and media as previously described. Individual mouse brains were cultured singularly until genotypes were identified by PCR of tail DNA. At this stage, identically genotyped cultures were combined. OPCs were purified using a gentle modified washdown procedure as previously described (Feigenson et al., 2009). Briefly, 4 ml of Hanks-buffered salt solution without Mg+ and Ca+ was drawn into a Pasteur pipette, and then forcefully released on the cell monolayer at an angle. OPCs would detach, leaving any astrocytes adherent to the plate. OPCs and the Hanks medium were then drawn up into the pipette and the procedure could be repeated several times until enough cells were collected. The detached OPCs were collected in Hanks medium, triturated several times with the Pasteur pipette and centrifuged at 100 g for 5 min. The pellet was then plated on to a polylysine-coated flask. Cultures were considered purified if they contained fewer than 5% astrocytes.
When OPCs were differentiated into mature OLs, growth medium was removed, and cell cultures were fed with DM (differentiation medium), consisting of 50% DMEM (Dulbecco's modified Eagle's medium) and 50% Ham's F12, with 50 μg/ml transferrin, 5 μg/ml putresine, 3 ng/ml progesterone, 2.5 ng/ml selenium, 12.5 μg/ml insulin, 0.4 μg/ml T4, 0.3% glucose, 2 mM glutamine and 10 ng/ml biotin. Cells were allowed to differentiate for 3 days to examine markers of immature OLs or 5 days to look at markers of mature OLs. Treatment conditions involved the application of signalling factors to some cultures; these were 50 ng/ml Wnt3a (R & D), 50 or 10 ng/ml BMP4 (R & D), 100 ng/ml Dkk-1 (Dikkopf-1) (R & D) and 500 ng/ml Noggin (R & D).
Immunofluorescence
Immunostaining and preparation of coverslip-plated cells were performed as previously described (Grinspan and Franceschini, 1995; Feigenson et al., 2009). Antibody pairs used to detect surface antibodies were anti-A2B5 with goat anti-mouse IgM and anti-galactocerebroside (RmAb GalC, undiluted, hybridoma supernatant; Ranscht et al., 1982) with goat-anti-mouse IgG3. The antibody pairs used to detect internal antibodies were GFAP (glial fibrillary acidic protein) and Olig2. Anti-GFAP (undiluted, hybridoma supernatant, gift of Dr Virginia Lee, University of Pennsylvania) was paired with goat anti-rat IgG. To label Olig2 (1:500, Millipore, paired with goat anti-rabbit IgG), cells were blocked for 10 min in 0.3% Triton X-100 after fixing with 4% (w/v) paraformaldehyde. To label TCF4 (TCF7L2, 1:100, Millipore, paired with goat anti-mouse IgG; Ye et al., 2009), cells were fixed with 4% paraformaldehyde and blocked for 30 min in block containing 0.1% Triton X-100, 2% BSA and 20% FCS (fetal calf serum). For all cell counts, positively labelled cells and DAPI+ (4', 6-diamidino-2-phenylindole) cells were counted in ten fields in each of two coverslips from each of at least three separate in vitro preparations, using a Leica DM6000B fluorescence microscope at ×63 magnification. Approx. 3000 cells were counted per experimental data point (1000 cells per condition per n).
Western-blot analysis
Rat cells were collected for Western-blot analysis as previously described (Feigenson et al., 2009). Cells were treated with 50 ng/ml BMP4 or Wnt3a and collected after 30 min, 3 or 48 h. Membranes were incubated with BMP4 antibody (1:500, R & D), HRP (horseradish peroxidase)-conjugated anti-mouse IgG secondary antibody (1:1000), or with Phosphorylated-Smad (1:3000, Cell Signaling) followed by HRP-conjugated anti-rat IgG secondary antibody (1:1000) and imaged using ECL® reagents (Amersham, Piscataway, NJ, U.S.A.) and hyperfilm (Amersham). Blots were stripped and reprobed with GAPDH (glyceraldehyde-3-phosphate dehydrogenase; 1:2,500, Chemicon International) as a loading control for protein quantification. Secondary antibodies were purchased from Jackson Laboratories.
Generation of mutant mice
The Bmpr1 DKO (BMPR1 double-knockout) mice were generated as previously described (Wine-Lee et al., 2004; See et al., 2007). Lines include a classical BMPr1a KO (Mishina et al., 1995), a BMPR1a conditional KO mouse (Ahn et al., 2001; Mishina et al., 2002), a BMPR1b classical KO mouse (gift from Dr Karen Lyons, UCLA; Yi et al., 2000) and a Bcre-32 transgenic mouse. The generation of these mice has been previously described (Wine-Lee et al., 2004; See et al., 2007). Normal littermates have one functional allele of both BMPR1 receptors and do not exhibit any phenotype. All mice were killed at P1 and OPC cultures were successfully harvested. β-Cat-null (β-catenin loss-of-function mutant) mice were made available by crossing β-cateninfloxedexon3−6/floxedexon3−6 mice (made available by Dr Walter Birchmeier, Max Delbruck Centrum, Germany) with Cnp-Cre mice, obtained from Dr Klaus Nave, Max Planck Institute, Germany. Generation of β-cateninfloxedexon3−6/floxedexon3−6 mice has been described previously (Huelsken et al., 2001), as has the generation of Cnp-Cre mice (Lappe-Siefke et al., 2003). Offspring were established by mating β-cateninfloxedexon3−6/floxedexon3−6; +/+ mice with β-cateninfloxedexon3−6/+; CNP/+ mice. β-catenin loss of function mice had the genotype β-cateninfloxedexon3−6/floxedexon3−6; CNP/+.
Real-time PCR
OPC cultures were grown on 50 mm dishes and harvested at 6 or 48 h post-treatment with 50 ng/ml Wnt3a or 50 ng/ml BMP4, with or without 500 ng/ml Noggin. Cells were extracted with TRIzol® (Molecular Research Center, Cincinnati, OH, U.S.A.). cDNA was reverse transcribed from 2 to 5 μg RNA per treatment group using Superscript III First Strand Synthesis Kit for RT–PCR (reverse transcription–PCR; Invitrogen no. 18080044). Primers used were GAPDH, MBP and ID2 (IDT Technologies). QPCR was performed using SYBR Green (Applied Biosystems) with the MXPro 3000P. Quantification was determined using the SYBR Green with the Dissociation Curve method, as per the protocol of the manufacturer. Threshold values of fluorescence, Ct values, were calculated at least three times per cycle, and values relative to control conditions were used to determine treatment differences. Samples were measured in triplicate for each experiment.
Image acquisition
All images were acquired using a Leica DM6000B fluorescence microscope. All images were mounted using Vectashield (Vector). Images were acquired with a Leica DFC360 Fx camera, using Leica Application Suite 2.1.0 software. Images were evenly enhanced using Adobe Photoshop (Adobe Systems, Mountain View, CA, U.S.A.).
RESULTS
Wnt and BMP inhibit OL differentiation
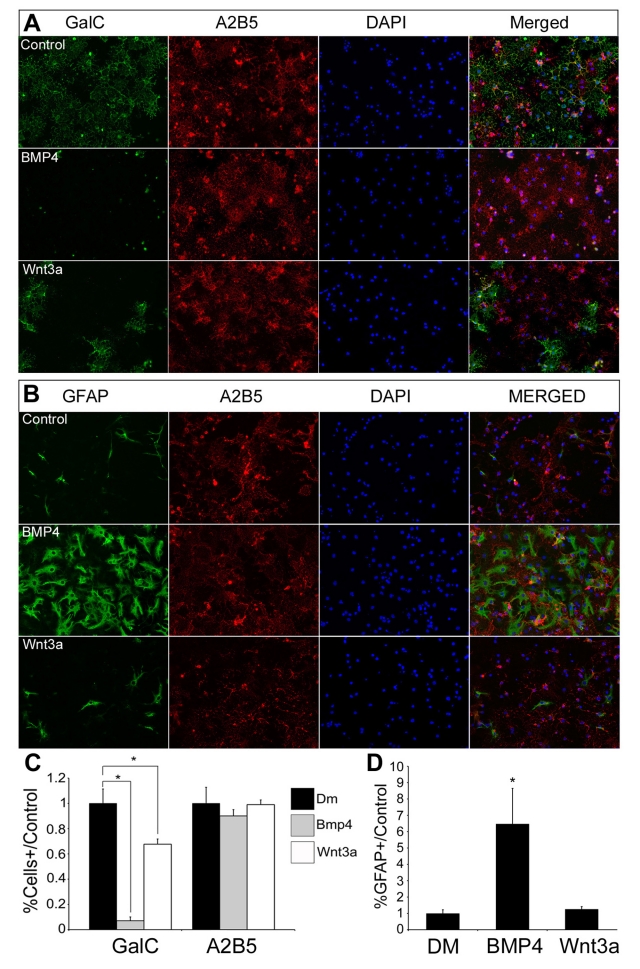
To observe the effects of Wnt and BMP on OL development in vitro, we established primary cultures of OPCs from mouse or rat brain and purified them to remove astrocytes and other non-OL lineage cells. Cells were grown to confluency on coverslips or dishes, subsequently growth factors were removed and DM was added with or without BMP4 (50 ng/ml) or Wnt3a (50 ng/ml). We selected BMP4 because, of all the BMP signalling effectors, it is the most intimately involved and most abundant during OL development (Ara et al., 2008). We chose 50 ng/ml BMP4 because it had the maximal effect in our previous studies (Grinspan et al., 2000; See et al., 2004). We selected Wnt3a as an effector of canonical Wnt signalling based on its location in developing dorsal spinal cord and its effects on OPCs from previous studies (Shimizu et al., 2005; Feigenson et al., 2009). A dose−response curve showed that 50 ng/ml Wnt3a most effectively inhibited OPCs from differentiating (K. Feigenson and J.B. Grinspan, results not shown). After 3 days in DM, the treated samples were processed for IHC (immunohistochemistry), and labelled with antibodies directed against GalC, a marker of early differentiation, and A2B5, which labels OPCs. In control conditions, 26% of rat cells expressed GalC, and 67% were labelled with A2B5, as observed in previous studies (Feigenson et al., 2009). The overlap in labelling between A2B5 and GalC is temporary and the A2B5 labelling will slowly disappear as the OLs mature (Crang et al., 2004; Feigenson et al., 2009). In contrast, GalC labelling was almost completely absent in BMP4 treatment conditions, while A2B5 labelling was unchanged. Wnt3a treatment reduced GalC labelling by 33% (P<0.05), in keeping with the 30–40% decrease we have observed previously, and also did not significantly affect the amount of cells being labelled with A2B5 or alter their morphology (Figures 1A and 1C).
Figure 1. BMP4 and Wnt3a treatment inhibit OL differentiation.
Cultures of rat OPCs were grown to confluence, switched to DM, alone or with BMP4 or Wnt3a, and then immunostained for GalC, GFAP and A2B5. (A) After 3 days in DM, many control OPCs expressed GalC and A2B5. BMP4-treated OPCs expressed A2B5 at comparable levels to controls conditions, but almost no cells expressed GalC (P<0.01). Wnt3a-treated cells had comparable levels of A2B5 expressing cells relative to control conditions, but a 33% reduction in the number of cells expressing GalC (P<0.05). (B) Control cultures had occasional labelling of GFAP+ cells, whereas 6.5 times as many cells in the BMP4 treatment condition were positively labelled with GFAP antibody (P<0.01). Wnt3a treatment did not noticeably change the number of GFAP+ cells relative to control conditions. (C, D) Bar graphs quantify the number of cells labelled with specific antibodies, measured relative to control conditions, n = 9. Images shown were taken at ×20 magnification.
To observe the effects of BMP4 and Wnt3a on the number of astrocytes in culture, we also immunolabelled cells with antibodies directed against GFAP. Control cultures and cultures treated with Wnt3a contained fewer than 5% astrocytes, while in BMP4-treated cultures the number of astrocytes increased 6.5-fold (P<0.05, Figures 1B and 1D). We have previously shown that neither signalling factor had any significant impact on the amount of cells undergoing apoptosis, proliferation or differentiation into neurons (Grinspan et al., 2000; Feigenson et al., 2009). These results indicate that BMP4 can completely inhibit OPC differentiation and induce astrogliogenesis, while Wnt3a can partially inhibit OPC differentiation without having an impact on astrocyte cell fate.
A BMP inhibitor blocks the effects of both BMP4 and Wnt3a signalling but a Wnt inhibitor blocks only Wnt3a signalling
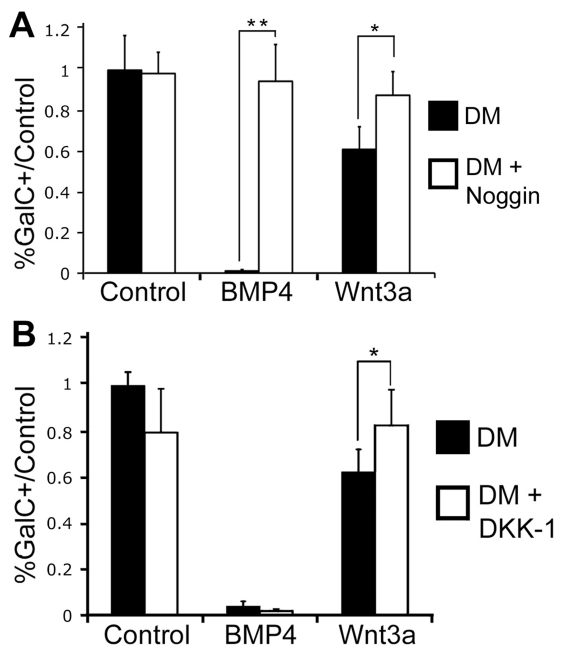
To determine whether there is a relationship between the Wnt and BMP signalling pathways, pharmacological inhibitors of canonical Wnt signalling were added concurrently with BMP4 and pharmacological inhibitors of BMP signalling were added concurrently with Wnt3a. Noggin (500 ng/ml), an inhibitor of canonical BMP signalling, was added to confluent cell cultures along with DM, 50 ng/ml BMP4 or 50 ng/ml Wnt3a. Noggin treatment alone did not alter the number of GalC+ cells relative to control conditions (Figure 2A). Although OPCs secrete both BMP and noggin, the secreted noggin normally limits the endogenous effects of BMP (Kondo and Raff, 2004). Noggin added with BMP4 completely blocked the effect of BMP4 and the number of GalC+ cells was not significantly different from the untreated control condition (Figure 2A). Similarly, when noggin was added along with Wnt3a, the number of GalC+ cells was the same as in the untreated control condition, indicating that blocking the BMP signalling pathway blocks the inhibitory actions of BMP4 and Wnt3a on OPC differentiation in vitro (Figure 2A).
Figure 2. Pharmacological inhibition of the BMP pathway blocks the effects of Wnt3a and BMP4 on OL differentiation.
OPC cultures were placed in DM for 3 days, alone or with BMP4 or Wnt3. Noggin or DKK-1 was added to similarly treated cells. (A) BMP4 treatment eliminated GalC expression (P<0.01), but the addition of noggin concurrently with BMP4 rescued this effect. Similarly, Wnt3a treatment reduced the number of GalC+ cells by 39% relative to controls (P<0.05), but concurrent noggin treatment rescued this effect (n = 7). (B) Dkk-1 addition abrogated the effect of Wnt3a on the number of GalC+ cells (P<0.01), and did not alter the BMP4 mediated elimination of GalC expressing cells (P<0.05, n = 3).
To determine whether blocking the canonical Wnt signalling pathway could block BMP signalling, we treated OPC cultures with Dkk-1, an inhibitor of canonical Wnt signalling. Dkk-1 treatment (100 ng/ml) alone did not alter the number of GalC+ cells relative to control conditions. When used concurrently with Wnt3a, however, Dkk-1 blocked the inhibitory effect of Wnt3a. In contrast, BMP4 completely inhibited GalC expression in cells even when treated concurrently with Dkk-1 (Figure 2B), indicating that blocking the canonical Wnt signalling pathway does not block the BMP signalling pathway in vitro. These results suggest that BMP4 does not signal upstream of the canonical Wnt pathway, and that the BMP pathway is not dependent on the Wnt signalling pathway.
Genetic ablation of the BMP signalling pathway blocks the effects of BMP4 and Wnt3a
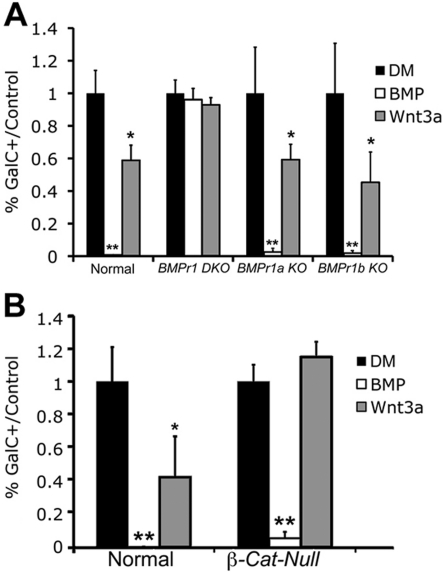
We next used a genetic model to help determine the relationship between BMP4 and Wnt3a and confirm our pharmacological results. We obtained Bmpr1 DKO mice in which both BMPR1A and BMPR1B have been functionally inactivated in the neural tube by E10.5 using the Bcre-32 transgenic allele. These mice have been characterized previously (Wine-Lee et al., 2004; See et al., 2007). OPCs cultured from the animals are able to differentiate, do not translocate Smad (a downstream effector of BMP signalling) to the nucleus, and do not respond to BMP treatment (See et al., 2007). We generated cultures from these DKO (double-knockout) animals as well as from single KOs (knockouts; Bmpr1b KO, Bmpr1a KO) and normal littermates. We then treated these cultures with DM alone or DM containing BMP4 or Wnt3a.
BMP4 completely eliminated GalC expression in normal mouse OPC cultures after 3 days in DM, and Wnt3a reduced the number of GalC+ cells by 41%. The number of A2B5+ cells did not vary from control conditions, replicating our previous findings in control cultures. These results were further replicated in OPC cultures from Bmpr1a KO and Bmpr1b KO mice (Figure 3A). In the Bmpr1 DKO OPC cultures that were untreated or treated with BMP4, the cells differentiated to the extent of the normal cells, as we have previously shown (See et al., 2007). This indicates that the OPCs from animals without BMP signalling are able to differentiate normally but do not respond to BMP4. Similarly, when the Bmpr1 DKO OPC cultures were treated with Wnt3a, the number of GalC+ cells was not significantly different from control conditions or Bmpr1 DKO cells treated with BMP4 (Figure 3A). These results indicate that when the BMP signalling pathway is completely abrogated, the canonical Wnt signalling pathway is similarly prevented from exerting an inhibitory effect on OPC differentiation. Further, when either Bmpr1a or Bmpr1b is individually deleted, the other type I receptor can compensate to maintain the integrity of the BMP signalling pathway and allow it to influence OL development. Our combined pharmacological and genetic results suggest that we can successfully block the effects of Wnt3a on OL differentiation by abrogating the BMP signalling pathway via two independent methods.
Figure 3. OPCs from mice lacking the BMP type I receptor do not respond to BMP4 or Wnt3a treatment.
OPC cultures were generated from Bmpr1 DKO, Bmpr1a KO, Bmpr1b KO, and normal mice, grown, and placed in DM. (A) Three days after DM treatment, OPCs from all three types of mutant mice exhibited similar numbers of GalC+ cells relative to controls. BMP4 treatment almost completely eliminated GalC+ cells in Bmpr1a KO, Bmpr1b KO, and normal mouse OPCs (P<0.01), but not in OPC cultures from Bmpr1 DKO mice. Similarly, Wnt3a expression decreased GalC+ cells in cultures from Bmpr1a KO, Bmpr1b KO and normal mice (P<0.05), but not in those from Bmpr1 DKO mice. (B) OPC cultures generated from β-Cat-null mice and normal littermates were treated with BMP4 or Wnt3a. BMP4 almost completely reduced the number of GalC+ cells in both types of cultures (P<0.01). Wnt3a reduced the number of GalC+ cells by 57% in control cultures, but this effect was not observed in the mutant cultures (P<0.05, n = 3).
Genetic inhibition of the canonical Wnt signalling pathway blocks the effect of Wnt3a, but not that of BMP4, on OL differentiation
To observe the effectiveness of canonical Wnt signalling and canonical BMP signalling on OL development when the Wnt signalling pathway has been effectively eliminated, we employed mutant mice with a conditional KO of the nuclear effector of canonical Wnt signalling, β-catenin [β-Cat-null (β-catenin loss of function mutant mice)]. Mice expressing a CNP-Cre transgene have been described previously (Lappe-Siefke et al., 2003; Feigenson et al., 2009). These mice are bred to a line of mice containing floxed sites surrounding exons 3–6 on the β-catenin gene (Huelsken et al., 2001). Upon Cre-mediated recombination, these exons are excised from the transcribed product and no functional β-catenin protein is produced. Wnt signalling is normally activated when β-catenin translocates to the nucleus, where it can activate TCF (T-cell factor)/LEF-1 (lymphoid enhancer factor-1) transcription factors. In these mutant mice, the Wnt signalling pathway is inhibited in all cells of OL lineage by preventing active β-catenin from being produced.
Mice were viable at P1 and OPC cultures were generated from mutants and normal littermates as previously described. Cultures were treated with DM with or without BMP4 or Wnt3a. Treatment with Wnt3a did not change the number of GalC+ cells relative to DM in the β-Cat-null OPC cultures, indicating that canonical Wnt signalling was effectively eliminated from OPCs (Figure 3B). In contrast, BMP4 completely eliminated the expression of GalC in treated cells. These results indicate that blocking the canonical Wnt signalling pathway prevents the inhibitory effect of Wnt3a on OL development, but not that of BMP4, and that the BMP signalling pathway is not upstream of the Wnt signalling pathway or dependent on the Wnt signalling pathway in this system.
Wnt3a does not up-regulate the canonical BMP signalling pathway
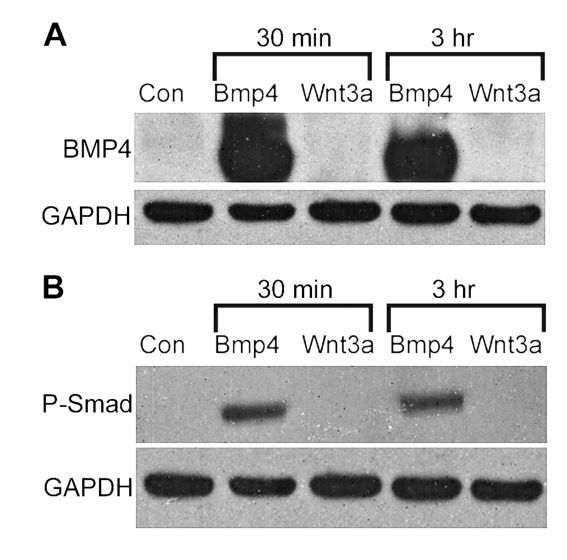
To identify if Wnt signalling directly increases the BMP ligand, we treated cultured OPC cultures with Wnt3a and looked for changes in BMP4 protein by Western-blot analysis. Wnt3a treatment did not result in an increase in BMP4 protein after 30 min or 3 h, when compared with untreated conditions or BMP4-positive control conditions (Figure 4A). Later time points at 2 and 5 days post-treatment also showed no sign of BMP4 up-regulation (results not shown). To see if Wnt3a could up-regulate the canonical BMP signalling pathway independently from increasing the amount of BMP ligand, we treated cultured OPCs with BMP4 and Wnt3a and looked for increases of phosphorylated-Smad 1/5/8. When cultures were treated with BMP4, there were clear increases in phosphorylated-Smad levels observed by Western blots by 30 min post-treatment (Figure 4B). In contrast, Wnt3a did not noticeably increase Smad levels after the same amount of time. Longer time points, up to 48 h, also did not show increased levels of phosphorylated-Smad (results not shown). These results indicate that Wnt3a does not directly up-regulate canonical BMP signalling.
Figure 4. Wnt3a does not directly up-regulate the canonical BMP signalling pathway.
Rat cells were treated with Wnt3a or BMP4, cells were harvested for protein at 30 min and 3 h post treatment, and Western blots were performed. (A) At both time points, BMP4 bands are visible in the positive control conditions, as compared with untreated and Wnt3a-treated conditions. (B) At both time points, phosphorylated-Smad bands are visible in BMP4-treated conditions but not in untreated or Wnt3a-treated conditions. GAPDH was used as a loading control for both analyses.
BMP4 and Wnt3a do not decrease OPC differentiation in a combinatorial manner
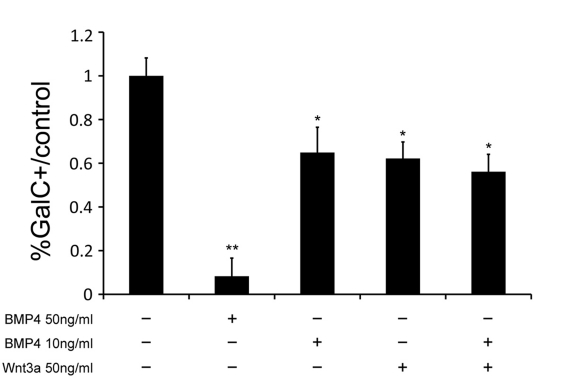
To determine whether the effects of BMP4 and Wnt3a were additive, we simultaneously treated OPCs with DM containing 50 ng/ml Wnt3a and 10 ng/ml BMP4, both of which separately reduce the number of GalC+ cells by approx. 40% relative to control conditions. We also treated cells with each growth factor individually and with 50 ng/ml BMP4 as a positive control. If BMP and Wnt signalling reduce the percentage of GalC+ cells independently, we could expect to see additional decreases in the number of mature OLs with combined treatment. There were no significant decreases in GalC expression, however, in the combined treatment condition when compared with conditions when either 10 ng/ml BMP4 or 50 ng/ml Wnt3a were added separately (Figure 5).
Figure 5. Collective treatment with BMP4 and Wnt3a does not inhibit precursor differentiation greater than individual treatment with BMP4 or Wnt3a.
Rat OPCs were cultured and then placed in DM with 50 ng/ml BMP4, 10 ng/ml BMP4, 50 ng/ml Wnt3a or 50 ng/ml Wnt3a and 10 ng/ml BMP4. After 3 days, 50 ng/ml BMP4 decreased GalC+ cells by 92% relative to control conditions (P<0.05), 10 ng/ml BMP4 decreased OPC differentiation by 35% (P<0.05), 50 ng/ml Wnt3a decreased OPC differentiation by 38% (P<0.05) and 50 ng/ml Wnt3a combined with 10 ng/ml BMP4 decreased OPC differentiation by 44% (P<0.05). The difference between the combined addition of BMP4 and Wnt3a was not significantly different from the application of either treatment factor individually (n = 3).
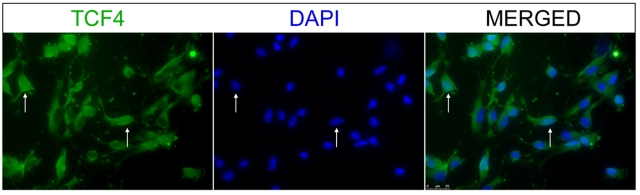
To determine what could restrict the effect of Wnt3a to a subset of cells, we labelled OPC cultures with antibody to TCF4, the downstream transcriptional effector of canonical Wnt signalling. Nuclear TCF4 labelling was observed in 46.7% of cultured OPCs (Figure 6), suggesting that it is the nuclear expression of TCF4 that facilitates the effects of Wnt3a on OL differentiation. Taken together, these results are consistent with the interpretation that the same downstream pathway is used by both signalling factors to inhibit OL maturation. It is also consistent with our data that Wnt only affects a subset of TCF4 expressing cells that are targeted in parallel by BMP4.
Figure 6. TCF4 is expressed in 46.7% of OPCs in vitro.
Cultures of OPCs were harvested and grown to confluency, then labelled with antibody to TCF4. Photomicrographs depict a portion of cells co-labelled with DAPI and TCF4, indicating nuclear expression. Arrows indicate examples of double-labelled cells. Quantification of the total number of nuclear expressing TCF cells showed the proportion of positively labelled cells was 0.467±0.071 (n = 3).
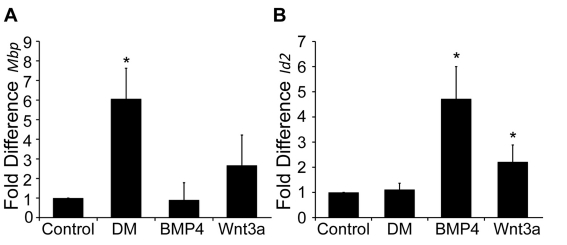
Wnt3a and BMP4 increase expression of Id2 mRNA
We used QPCR (quantitative real-time PCR) to determine what downstream effectors are influenced by both Wnt and BMP treatment. OPC cultures were obtained from normal rats as previously described. Cells were grown on 50 mm dishes and treated with Wnt3a or BMP4 for 6 and 48 h. We observed a decrease in Mbp transcripts at 48 h after treatment with Wnt3a and BMP4, mirroring our IHC results (Figure 7A). When assaying for Id2 mRNA, however, both Wnt3a and BMP4 significantly increased transcript levels (Figure 7B). IDs (inhibitor of DNA-binding proteins) are known inhibitors of differentiation, and previous studies have revealed that they are targets of BMP and Wnt signalling (Samanta and Kessler, 2004; Ye et al., 2009). These results indicate that Wnt3a and BMP4 can target the same effectors of OL differentiation.
Figure 7. BMP4 and Wnt3a regulate Id2 and Mbp transcript levels.
Rat OPCs were cultured and treated with Wnt3a or BMP4 and assayed for QPCR. (A) After treatment for 48 h, levels of Mbp mRNA were calculated compared with untreated control OPCs. DM-treated cells had a 7-fold increase in Mbp expression over that of untreated cells and BMP4-treated cells (P<0.05). This expression was also 2.33-fold more than that of Wnt3a-treated cells (P<0.05, n = 4). (B) After treatment for 6 h, levels of Id2 mRNA were calculated compared with untreated control OPCs. BMP4 and Wnt3a treatment increased Id2 transcript expression 4.7- and 2.2-fold, respectively (P<0.05, n = 7).
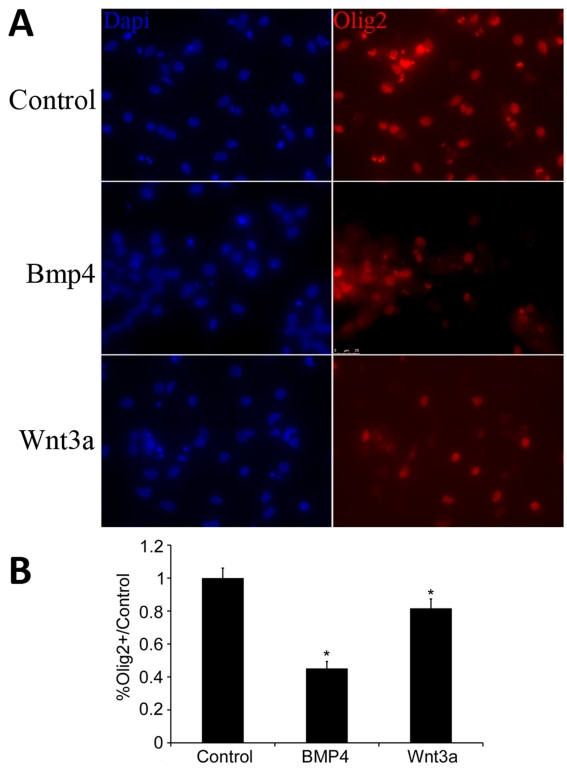
Wnt3a and BMP4 reduce levels of nuclear Olig2 in OPCs
ID2 prevents the nuclear localization of Olig2 after stimulation with BMP4, thus inhibiting BMP signalling (Samanta and Kessler, 2004). We used IHC to examine the localization of Olig2 after treatment with either BMP4 or Wnt3a. After 2 days of treatment, we observed a significant decrease in the total number of cells in which Olig2 was clearly visible co-localized with cell nuclei in both BMP4- and Wnt3a-treated cultures. There was a 55% reduction in Olig2 and DAPI co-labelling in BMP4-treated cultures and a 19% reduction in Wnt3a-treated cultures relative to controls (Figures 8A and 8B). These results further indicate that BMP4 and Wnt3a are targeting common factors in OPC differentiation.
Figure 8. BMP4 and Wnt3a decrease levels of Olig2.
Mouse OPCs were cultured, placed in DM, and treated with Wnt3a or BMP4. (A) Two days after treatment, cells were labelled for Olig2 and A2B5. (B) Bar graphs quantify the number of Olig2+ cells in each condition relative to control. BMP4 decreased the number of nuclei co-labelled with Olig2 by 55% (P<0.01, n = 5). Wnt3a decreased the number of nuclei co-labelled with Olig2 by 19% (P<0.05, n = 5). Images shown were taken at ×40 magnification.
DISCUSSION
We have analysed the relationship between canonical Wnt and BMP signalling in OL development, and our results indicate that both pathways rely on a fully functional BMP signalling pathway. In primary rodent OPC cultures, BMP4 by itself almost completely eliminates OPC differentiation and significantly increases the number of astrocytes. Wnt3a by itself decreases OPC differentiation by 30–40% without a corresponding increase in astrocyte generation. Blocking the BMP signalling pathway with pharmacological or genetic techniques at different points on the BMP pathway prevents the effects of both BMP4 and Wnt3a. In contrast, blocking the canonical Wnt signalling pathway was able to counter the effect of Wnt3a on OPC differentiation, but not that of BMP4.
To address why Wnt3a only partially inhibits OL differentiation, in comparison with the complete inhibitory activity of BMP4, we examined downstream expression of Wnt effectors. We observed that 46.7% of OPCs in culture express TCF4, which corresponds to the decrease in differentiation after Wnt3a treatment. This suggests two possibilities: TCF4 is expressed only in a subset of OPCs or TCF4 is only transiently expressed in all OPCs and 46.7% of these cells expressed TCF4 at the time of treatment. Both possibilities indicate that the nuclear expression of TCF4 appears to control the ability of Wnt3a to affect OL differentiation. This is consistent with the conclusions drawn by Fancy et al. (2009) concerning TCF4 expression in vivo.
To find at what level BMP4 and Wnt3a may be inhibiting OPC differentiation, we performed QPCR and assayed for Id2 transcript levels. Both BMP4 and Wnt3a consistently reduced transcript levels of Mbp, complementing our IHC results, and also increased levels of Id2, in keeping with earlier studies (Samanta and Kessler, 2004; Ye et al., 2009). IDs are actively involved in preventing OPC differentiation and OL myelination, largely by preventing nuclear localization of Olig proteins to keep the timing of differentiation precise and regulated (Kondo and Raff, 2000; Wang et al., 2001; Samanta and Kessler, 2004; Gokhan et al., 2005; Marin-Husstege et al., 2006; Cheng et al., 2007). We found that both Wnt3a and BMP4 reduced the number of cells co-labelled with Olig2 in the nucleus, indicating that both factors were actively preventing the nuclear activity and differentiating capabilities of Olig2, possibly via ID2 induction. There was a larger effect of BMP4 treatment on both Olig2 and ID2 treatment when compared with Wnt3a treatment, consistent with our differentiation results.
The BMP and Wnt pathways have many contextually and temporally dependent interactions throughout development (Itasaki and Hoppler, 2010). In the developing spinal cord, overexpression of either signalling factor alone induces a dorsalized phenotype, whereas their removal produces ventralized phenotypes, indicating that both signals may have functionally similar, interacting or redundant effects (Nguyen et al., 2000; Muroyama et al., 2002; Timmer et al., 2002; Zechner et al., 2007; Alvarez-Medina et al., 2008). In some developmental systems, the BMP pathway up-regulates or acts upstream of Wnt signalling, such as in neural crest delamination (Burstyn-Cohen et al., 2004), keratinocyte development (Yang et al., 2006) and dorsal/ventral patterning (Zechner et al., 2007). Conversely, Wnt signalling can act upstream or up-regulate the BMP pathway, such as during neurogenesis and astrogliogenesis (Kasai et al., 2005), limb mesenchyme development (Hill et al., 2006) and tooth development (Liu et al., 2008a). The downstream effectors of both signalling pathways can also interact in synergistic manners to regulate transcription (Labbe et al., 2000; Nishita et al., 2000; Letamendia et al., 2001; Theil et al., 2002; Hussein et al., 2003), and canonical Wnt signalling can prolong the activity of the BMP pathway (Fuentealba et al., 2007). In other contexts, Wnt and BMP signalling can be directly antagonistic, such as in neuroepithelial cell development (Ille et al., 2007), aspects of neural development (Gomez-Skarmeta et al., 2001), muscle positioning (Bonafede et al., 2006) and osteoblast development (Kamiya et al., 2008a, 2008b; Honda et al., 2010). In some systems, such as during aspects of limb bud and apical ectodermal ridge formation, the BMP and Wnt pathways can complexly regulate each other through parallel signalling and feedback systems (Soshnikova et al., 2003; Villacorte et al., 2010).
Our results clearly demonstrate that the BMP pathway is essential for the Wnt pathway to inhibit OPC differentiation, as eliminating BMP signalling effectively blocks the effects of Wnt3a. This is not just a result of the BMP antagonist simultaneously blocking Wnt signalling, because genetic removal of BMP receptors also prevented Wnt3a from inhibiting differentiation.
There are three potential mechanisms through which the BMP and Wnt pathways could interact. The most direct identifiable interaction would be through the canonical Wnt signalling pathway up-regulating phosphorylated-Smad, the major effector of canonical BMP signalling. However, we did not detect increases in phosphorylated-Smad levels on Wnt3a treatment, although we were able to consistently detect phosphorylated-Smad increases after standard BMP4 treatment. It is possible that Wnt signalling may directly activate the BMP signalling pathway at levels below detection in Smad assays. We also did not detect up-regulation of BMP protein after Wnt3a treatment using Western-blot analysis.
The second interaction could involve activation of non-canonical BMP pathways, including kinase mTOR (mammalian target of rapamycin)/FRAP via STAT3 (signal transducer and activator of transcription), activated mitogen kinases and LIM kinase (Chen and Panchision, 2007 for review). In other systems, such as multipotent neural precursor cells, BMP4 activates both canonical and non-canonical pathways for separate effects (Rajan et al., 2003). It is possible that Wnts and BMPs interact through one of the non-canonical routes. The possibility of signalling through multiple, alternative pathways could also explain why both BMP4 and Wnt3a treatment inhibit OPC differentiation and maintain similar levels of A2B5+ cells, despite only BMP4 directly increasing the number of astrocytes.
Finally, If Wnt signalling does not up-regulate BMP signalling, downstream mechanisms could be evoked. Some common transcriptional targets of both pathways have Smad and TCF/LEF-binding sites in close proximity, indicating that the two pathways have synergistic activity among their downstream effectors, including Emx2 (Theil et al., 2002), Msx2 (Willert et al., 2002; Hussein et al., 2003), c-myc (Hu and Rosenblum, 2005) and Xtwin (Labbe et al., 2000; Nishita et al., 2000; Letamendia et al., 2001). The BMP and Wnt signalling pathways may also interact during epigenetic regulation of OL differentiation. Recent studies have shown that HDACs (histone deacetylases) compete with activated β-catenin to bind with TCF4. Bound to β-catenin, TCF4 acts as a transcriptional repressor of OL differentiation by up-regulating factors such as ID2/4. Bound to HDACs, TCF4 acts as a transcriptional repressor, inhibiting the activity of ID2/4 and up-regulating myelin-promoting genes such as Mbp (Marin-Husstege et al., 2002; Shen et al., 2005; He et al., 2007; Ye et al., 2009). Our experiments indicate that canonical Wnt signalling depends on the BMP pathway to inhibit OL development; it is possible that the TCF transcriptional complex requires inherent Smad elements or other downstream effectors of BMP signalling to interact with IDs or other inhibitory elements. The transcriptional activity of the Wnt signalling pathway may then rely on endogenous BMP functioning, and will therefore be inhibited when BMP signalling is abrogated.
Understanding what signals regulate the timing mechanisms of OL maturation will facilitate the development of therapies for myelin disorders. BMP up-regulation has been well documented in many types of CNS disease and demyelinating paradigms (See and Grinspan, 2009). Furthermore, several recent studies demonstrate that Wnt signalling is active during white matter injury as well, including aspects of axonal regeneration (Liu et al., 2008b; Miyashita et al., 2009), NG2+ cell proliferation (Orre et al., 2009; White et al., 2010) and remyelination (Fancy et al., 2009). BMPs and Wnts interact in many intricate ways during development, and it is likely that they have similar interactions during CNS injury and recovery. It is, therefore, important to understand how these pathways function independently and in relationship to one another. We have shown that the BMP pathway is a competence factor for canonical Wnt signalling to inhibit OPC differentiation in cell culture paradigms. Further experiments should investigate this interaction during remyelination events and in CNS injury models.
Footnotes
This work was supported by the National Institutes of Health [grant number P30 HD026979 and the National Multiple Sclerosis Society grant number RG4105A].
REFERENCES
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB III. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafede A, Kohler T, Rodriguez-Niedenfuhr M, Brand-Saberi B. BMPs restrict the position of premuscle masses in the limb buds by influencing Tcf4 expression. Dev Biol. 2006;299:330–344. doi: 10.1016/j.ydbio.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327–5339. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Cate HS, Sabo JK, Merlo D, Kemper D, Aumann TD, Robinson J, Merson TD, Emery B, Perreau VM, Kilpatrick TJ. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. [DOI] [PubMed] [Google Scholar]
- Chen HL, Panchision DM. Concise review: bone morphogenetic protein pleiotropism in neural stem cells and their derivatives–alternative pathways, convergent signals. Stem Cells. 2007;25:63–68. doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, Cao Q. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells. 2007;25:3204–3214. doi: 10.1634/stemcells.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Rink J, Del Valle L, Sweet T, Khalili K, Amini S. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol Ther. 2002;1:640–645. doi: 10.4161/cbt.313. [DOI] [PubMed] [Google Scholar]
- Crang AJ, Gilson JM, Li WW, Blakemore WF. The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. Eur J Neurosci. 2004;20:1445–460. doi: 10.1111/j.1460-9568.2004.03606.x. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Edgar JM, Garbern J. The myelinated axon is dependent on the myelinating cell for support and maintenance: molecules involved. J Neurosci Res. 2004;76:593–598. doi: 10.1002/jnr.20063. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G, Walsh F, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci USA. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw EB III, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhan S, Marin-Husstege M, Yung SY, Fontanez D, Casaccia-Bonnefil P, Mehler MF. Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. J Neurosci. 2005;25:8311–8321. doi: 10.1523/JNEUROSCI.1850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Skarmeta J, de La Calle-Mustienes E, Modolell J. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development. 2001;128:551–560. doi: 10.1242/dev.128.4.551. [DOI] [PubMed] [Google Scholar]
- Grinspan J. Cells and signaling in oligodendrocyte development. J Neuropathol Exp Neurol. 2002;61:297–306. doi: 10.1093/jnen/61.4.297. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Franceschini B. PDGF is a survival factor for PSA-NCAM+ oligodendroglial pre-progenitor cells. J Neurosci Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic protein on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133:1219–1229. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Honda T, Yamamoto H, Ishii A, Inui M. PDZRN3 negatively regulates BMP-2-induced osteoblast differentiation through inhibition of Wnt signaling. Mol Biol Cell. 2010;21:3269–3277. doi: 10.1091/mbc.E10-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Rosenblum ND. Smad1, beta-catenin and Tcf4 associate in a molecular complex with the Myc promoter in dysplastic renal tissue and cooperate to control Myc transcription. Development. 2005;132:215–225. doi: 10.1242/dev.01573. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–48814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- Ille F, Atanasoski S, Falk S, Ittner LM, Marki D, Buchmann-Moller S, Wurdak H, Suter U, Taketo MM, Sommer L. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007;304:394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Hoppler S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev Dyn. 2010;239:16–33. doi: 10.1002/dvdy.22009. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci USA. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008a;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008b;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Tse G, Putti T, Scolyer R, Lee S. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36:120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]
- Kasai M, Satoh K, Akiyama T. Wnt signaling regulates the sequential onset of neurogenesis and gliogenesis via induction of BMPs. Genes Cells. 2005;10:777–783. doi: 10.1111/j.1365-2443.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim SH, Kim H, Chung AY, Cha YI, Kim CH, Huh TL, Park HC. Frizzled 8a function is required for oligodendrocyte development in the zebrafish spinal cord. Dev Dyn. 2008;237:3324–3331. doi: 10.1002/dvdy.21739. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2000;19:1998–2007. doi: 10.1093/emboj/19.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. A role for noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004;267:242–251. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. [see comment]. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am. 2001;83((A Suppl 1)):S31–S39. [PubMed] [Google Scholar]
- Liem KF Jr, Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–4866. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- Liem KF Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008a;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008b;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Yuk DI, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, He Y, Li J, Kondo T, Sablitzky F, Casaccia-Bonnefil P. Multiple roles of Id4 in developmental myelination: predicted outcomes and unexpected findings. Glia. 2006;54:285–296. doi: 10.1002/glia.20385. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic protein in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. BMPr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, Yamashita T. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26:955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Ono K, Bansal R, Payne J, Rutishauser U, Miller RH. Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development. 1995;121:1743–1754. doi: 10.1242/dev.121.6.1743. [DOI] [PubMed] [Google Scholar]
- Orre K, Wennstrom M, Tingstrom A. Chronic lithium treatment decreases NG2 cell proliferation in rat dentate hilus, amygdala and corpus callosum. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:503–510. doi: 10.1016/j.pnpbp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. a singularity of PDGFalpha receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Rajan P, Panchision DM, Newell LF, McKay RD. BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol. 2003;161:911–921. doi: 10.1083/jcb.200211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B, Clapschaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- See J, Mamontov P, Ahn K, Wine-Lee L, Crenshaw EB III, Grinspan JB. BMP signaling mutant mice exhibit glial cell maturation defects. Mol Cell Neurosci. 2007;35:171–182. doi: 10.1016/j.mcn.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun SB, Mamontov P, Golden JA, Grinspan JB. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- See JM, Grinspan JB. Sending mixed signals: bone morphogenetic protein in myelination and demyelination. J Neuropathol Exp Neurol. 2009;68:595–604. doi: 10.1097/NEN.0b013e3181a66ad9. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP 7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursors cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB III, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal–ventral axis in the limb. Genes Dev. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transaction in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Marti E. Wnt won the war: antagonistic role of Wnt over Shh controls dorso–ventral patterning of the vertebrate neural tube. Dev Dyn. 2010;239:69–76. doi: 10.1002/dvdy.22058. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Villacorte M, Suzuki K, Hayashi K, de Sousa Lopes SC, Haraguchi R, Taketo MM, Nakagata N, Yamada G. Antagonistic crosstalk of Wnt/beta-catenin/Bmp signaling within the apical ectodermal ridge (AER) regulates interdigit formation. Biochem Biophys Res Commun. 2010;391:1653–1657. doi: 10.1016/j.bbrc.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kagawa T, Ivanova A, Zalc B, Shirasaki R, Murakami F, Iemura S, Ueno N, Ikenaka K. Dorsal spinal cord inhibits oligodendrocyte development. Dev Biol. 2000;227:42–55. doi: 10.1006/dbio.2000.9869. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603–614. doi: 10.1016/s0896-6273(01)00237-9. [DOI] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB III. Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–5403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- Yang L, Yamasaki K, Shirakata Y, Dai X, Tokumaru S, Yahata Y, Tohyama M, Hanakawa Y, Sayama K, Hashimoto K. Bone morphogenetic protein-2 modulates Wnt and frizzled expression and enhances the canonical pathway of Wnt signaling in normal keratinocytes. J Dermatol Sci. 2006;42:111–119. doi: 10.1016/j.jdermsci.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB III, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci USA. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Indentification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]