Abstract
Circadian clocks exist in the heart tissue and modulate multiple physiological events, from cardiac metabolism to contractile function and expression of circadian oscillator and metabolic-related genes. Ample evidence has demonstrated that there are endogenous circadian oscillators in adult mammalian cardiomyocytes. However, mammalian embryos cannot be entrained independently to light-dark (LD) cycles in vivo without any maternal influence, but circadian genes are well expressed and able to oscillate in embryonic stages. The authors took advantage of using chick embryos that are independent of maternal influences to investigate whether embryonic hearts could be entrained under LD cycles in ovo. The authors found circadian regulation of L-type voltage-gated calcium channels (L-VGCCs), the ion channels responsible for the production of cardiac muscle contraction in embryonic chick hearts. The mRNA levels and protein expression of VGCCα1C and VGCCα1D are under circadian control, and the average L-VGCC current density is significantly larger when cardiomyocytes are recorded during the night than day. The phosphorylation states of several kinases involved in insulin signaling and cardiac metabolism, including extracellular signal-regulated kinase (Erk), stress-activated protein kinase (p38), protein kinase B (Akt), and glycogen synthase kinase-3β (GSK-3β), are also under circadian control. Both Erk and p38 have been implicated in regulating cardiac contractility and in the development of various pathological states, such as cardiac hypertrophy and heart failure. Even though both Erk and phosphoinositide 3-kinase (PI3K)-Akt signaling pathways participate in complex cellular processes regarding physiological or pathological states of cardiomyocytes, the circadian oscillators in the heart regulate these pathways independently, and both pathways contribute to the circadian regulation of L-VGCCs.
Keywords: Cardiomyocyte, Chick heart embryo, Circadian L-type voltage-gated calcium channel, Signal transduction
INTRODUCTION
Although the suprachiasmic nucleus (SCN) in the hypothalamus is known as the “master clock” in mammals, organs outside the brain also contain circadian oscillators, since certain gene expressions in cultured peripheral tissues such as retina, heart, lung, intestine, liver, and muscle display circadian rhythms under constant conditions (Duguay & Cermakian, 2009; Yamazaki et al., 2000). The cardiovascular system is known to be under circadian control (Young et al., 2001b), and genes responsible for circadian oscillation have been identified in various components of the cardiovascular system, including cardiomyocytes and vascular smooth muscle cells (Durgan et al., 2005; McNamara et al., 2001). Many cardiovascular diseases show characteristic daily variations in their occurrences. The circadian clocks, therefore, have the potential to modulate multiple physiological and pathophysiological cardiovascular parameters (Young, 2006). Circadian rhythms in the cardiovascular system are mediated by both endogenous (circadian oscillators in the cardiomyocytes) and exogenous (neurohumoral) influences (Young, 2006). In isolated adult rat cardiomyocytes, clock and several metabolic genes are expressed with circadian oscillations. Cardiomyocyte responses to starvation or fatty acid overload are also under the control of intrinsic circadian oscillators (Bray et al., 2008; Durgan et al., 2006a, 2006b, 2007; Stavinoha et al., 2004a, 2004b; Young et al., 2001a). Hence, the intrinsic clocks in cardiomyocytes play critical roles in preparing the heart to anticipate daily workload and synchronizing cardiac metabolism to the environment (Durgan et al., 2005; Young, 2006).
The L-type voltage-gated calcium channel (L-VGCC) consists of a pore-forming α1 subunit and regulatory β and α2δ subunits (Takahashi et al., 1987; Wang et al., 2004). Among major L-VGCCα1 subunits, VGCCα1C (CaV1.2) and α1D (CaV1.3) are the most prevalent in the heart and play important roles in cardiac physiology (Acosta et al., 2004; Seisenberger et al., 2000; Takamatsu et al., 2003; Zhang et al., 2002, 2005). In cardiomyocytes, L-VGCCs contribute to the maintenance of the action potential plateau that produces muscle contraction (Fauconnier et al., 2003; Kubalova 2003) and the rate of recovery from inactivation at the resting membrane potential (Gudzenko et al., 2007). Calcium that enters the cardiomyocyte via the L-VGCCs triggers a more substantial calcium release from the sarcoplasmic reticulum (Altamirano & Bers, 2007). This calcium-induced calcium release in cardiomyocytes underlies the control of cardiac contraction force during excitation-contraction coupling (Altamirano & Bers, 2007; Kranias & Bers, 2007). Therefore, L-VGCCs affect the heart rate, contractile force, and cardiac output in adult animals (Fauconnier et al., 2003; Kubalova, 2003). Clinical trails showed differential effects between morning and evening administration of L-VGCC blockers to patients with hypertension and arrhythmias (Kawano et al., 2003; Kitahara et al., 2004; Qiu et al., 2003), and down-regulation or mutations of L-VGCCs contributes to human heart failure (Boixel et al., 2001; Hullin et al., 2003) and arrhythmia (Splawski et al., 2004, 2005). Yet, it is not known if any oscillation in L-VGCC function in cardiomyocytes contributes to the daily variations of heart physiology.
Both phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) and Erk pathways participate in complex cellular processes regarding the physiological or pathological states of cardiomyocytes. Activation of these pathways has been linked to protective mechanisms against cardiac ischemia (Hu et al., 2008) and hypoxia (Mudalagiri et al., 2008), as well as normal cardiac growth during development (Shioi et al., 2000; Teos et al., 2008). In the hearts of heart-failure hypertensive patients, the activities of PI3K-Akt and Erk are significantly reduced (Gonzalez et al., 2007). The PI3K-Akt pathway is also involved in the regulation of excitation-contraction coupling in cardiomyocytes, which is in part through the regulation of L-VGCCs (McDowell et al., 2004).
Here, we show that there is a circadian regulation of L-VGCCs in chick hearts. The mRNA levels and protein expression of VGCCα1C and VGCCα1D are rhythmic, and the average L-VGCC current density is significantly larger when cardiomyocytes are recorded during the night than during the day. Further, we show that the phosphorylation states of extracellular signal-regulated kinase (Erk), stress-activated protein kinase (p38), protein kinase B (Akt), and glycogen synthase kinase-3β (GSK-3β) are under circadian control. Both Erk and p38 have been implicated in regulating cardiac contractility and development of various pathological states, such as cardiac hypertrophy and heart failure, since they control cell growth and proliferation (Olson & Molkentin, 1999; Sugden, 1999; Szokodi et al., 2008). In human failing hearts, the activity of GSK-3β is greatly inhibited (Haq et al., 2001). We demonstrate that whereas the phosphorylation states of Erk and p38 peak during the day, the phosphorylation of GSK-3β peaks late at night. We also show that the circadian oscillators in the heart independently regulate the Erk and PI3K-Akt signaling pathways, both of which participate in the circadian regulation of L-VGCCs. Knowledge of the circadian profiles of signaling molecules and L-VGCCs in cardiomyocytes may shed light on the cellular mechanisms underlying the circadian rhythms in cardiovascular physiology.
MATERIALS AND METHODS
Cardiomyocyte Cultures and Circadian Entrainment
Endogenous circadian rhythms in adult cardiomyocytes exist in both healthy and disease states (Bray et al., 2008; Durgan et al., 2005, 2006a, 2006b, 2007, 2010; Young, 2003, 2006; Young & Bray 2007; Young et al., 2001a, 2001b). However, in mammals during embryonic development, prenatal entrainment of the fetal biological clock is the result of communication between the mother and fetus in the uterus (Ohta et al., 2008; Parraguez et al., 1996; Reppert & Schwartz, 1983). Since mammalian embryos cannot be entrained independently to light-dark (LD) cycles without any maternal influence, we took advantage of using chick embryos to investigate whether the circadian oscillators in chick embryonic hearts could be entrained under LD cycles in ovo.
All of the experimental procedures complied with the regulations of Texas A&M University and adhered to the guidelines of the journal for biological rhythm research (Portaluppi et al., 2008). Fertilized eggs (Gallus gallus, Single Comb White Leghorns) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX, USA). Chick embryos from embryonic day 12 (E12) or E13 were entrained to 12 h:12 h LD cycles for 6 days in ovo then kept in constant darkness (DD) for 2 days. All chick embryos were maintained at 39°C ± 0.5°C. Zeitgeber time (ZT) 0 was designated as lights-on, and ZT 12 as lights-off. On the second day of DD (E19 or E20), hearts were collected at six different circadian times (CTs), i.e., CT 0, 4, 8, 12, 16, and 20, for biochemical and molecular biological assays. In preparation for electrophysiological recordings, on the last day of LD, chick ventricular cardiomyocytes were dissociated and cultured on poly-d-lysine/collagen double-coated coverslips overnight. Electrophysiological recordings were done the next day. For some Western blotting experiments, on the last day of LD, chick hearts were dissected and cut into 1–2-mm3 pieces and cultured for 1 day in DD. On the second day of DD at different timepoints (as indicated in the results), cultures were treated with inhibitors for 2 h and then harvested for Western blotting. The culture medium contained Dulbecco’s modified Eagle’s medium (DMEM; Biowhittaker, Walkersville, MD, USA), 5% fetal bovine serum (Hyclone, Logan, UT, USA), 10% heat-inactivated horse serum (Biowhittaker), 2 mM GlutaMAX (Gibco/Invitrogen, Carlsbad, CA, USA), 50 U/mL penicillin (Sigma, St. Louis, MO, USA), 50 µg/mL streptomycin (Sigma), and 5 µg/mL retinol (Sigma). All cultures were maintained at 39°C ± 0.5°C.
Immunoblot Analysis
Samples were prepared and collected in a similar fashion as previous studies (Ko et al., 2001, 2007). Briefly, intact hearts were homogenized in RIPA buffer. The samples were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes. The primary antibodies used in this study were anti-L-VGCCα1C (Alomone, Jerusalem, Israel), anti-L-VGCCα1D (Alomone), anti-phospho308 Akt (pAktthr308; Cell Signaling Technology, Danvers, MA, USA), anti-phospho473 Akt (pAktser473; Cell Signaling Technology), anti-phosphoGSK-3β at ser9 (pGSK; Cell Signaling Technology), anti-phospho-stress activated protein kinase p38 (p38; Cell Signaling Technology), a monoclonal antibody specific for diphospho-extracellular signal-related kinase (pErk; Sigma), and a polyclonal antibody insensitive to the phosphorylation state of Erk (total Erk, used for internal and loading control; Santa Cruz Biochemicals, Santa Cruz, CA, USA). Blots were visualized using appropriate secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology) and an enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL, USA). Relative protein expressions for all proteins involved in this study are reported as a ratio to total Erk (total Erk remains constant throughout the day). Quantification was determined by densitometry using Scion Image (National Institutes of Health, Bethesda, MD, USA). All measurements were repeated at least four times. The mitogen-activated kinase kinase (MEK) inhibitor PD98059 was obtained from A. G. Scientific (San Diego, CA, USA), the Akt inhibitor 1-l-6-hydroxymethyl-chiroinositol-2-(R)-2-O-methyl-3-O-octadecylcarbonate was obtained from Calbiochem/EMD (San Diego, CA, USA), and the PI3K inhibitor LY294002 was also obtained from Calbiochem/EMD.
Quantitative Real-Time RT-PCR
The method used for quantitative real-time reverse transcriptase–polymerase chain (RT-PCR) reaction (Q-PCR) analysis was described previously (Ko et al., 2004, 2007). Intact hearts collected at different circadian timepoints were homogenized, and total RNA was collected using a commercially available kit (Qiagen, Valencia, CA, USA). Three hundred nanograms of total RNA were used to quantify VGCCα1C, VGCCα1D, Bmal1, and β-actin (loading control) mRNA by Q-PCR using the TaqMan one-step RT-PCR kit and an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA). Forward and reverse primers for chick β-actin, L-VGCCα1C, and L-VGCCα1D were previously listed (Ko et al., 2004, 2007). The forward and reverse primers for chick Bmal1 were 5′ATACAGAAGCCAACTATAAGCCTAGCT3′ and 5′CTGTAGTTGAGGATCTTGAAGACAGA3′, respectively. The Bmal1 TaqMan MGB probe sequence was 5′FAM-AAATCCATCTGCTGCCCTGAG-QFR3′. All measurements were repeated at least six times. For each individual experiment, a standard curve was generated with known quantities of β-actin mRNA loaded in curved quantities, e.g., 0.5 ×, 1 ×, 2 ×, 4 ×, 8 ×, 16 ×. The cycle values, corresponding to the log values of the standard curve quantities, were used to generate a linear regression formula. The cycle values from the sample RNAs were fit into the formula, and the mRNA quantities of the samples were obtained. The mRNA values of VGCCα1C, VGCCα1D, and Bmal1 were then divided by the value of β-actin mRNA (loading control), and for each set of experiments, the final value of RNAs at CT 0 was arbitrarily set at 1.
Electrophysiological Recordings and Statistical Analysis
Hearts were harvested from E19 embryos after LD entrainment at various time periods (ZT 1–4, 6–9, 14–17, 17–20, 21–24), and ventricular cardiomyocytes were cultured and maintained in DD for about 22–24 h. The next day at the corresponding time periods, as indicated in the results, whole-cell patch-clamp configuration of L-type Ca2+ channels (L-VGCCs) was recorded from spontaneously pulsing cardiomyocytes carried out using either suction-formed whole-cell or β-escin–based perforated-patch recording methods (Fan & Palade, 1998; Ko et al., 2007). The recording solutions were modified from Tohse et al. (1992). The external solution was (in mM): 145 TEACl, 9 BaCl2, 0.5 MgCl2, 5.5 glucose, 0.1 NiCl2, and 5 HEPES, pH 7.4, with CsOH or TEAOH. The pipette solution was (in mM): 125 Cs acetate, 20 CsCl, 3 MgCl2, 10 EGTA, and 5 HEPES, pH 7.4, adjusted with CsOH. Currents were recorded at room temperature using an A–M Systems model 2400 patch-clamp amplifier (A–M Systems, Sequim, WA, USA). Signals were low-pass filtered at 2 kHz and digitized at 5 kHz with Digidata 1440A interface and pCLAMP 10.0 software (Axon Instruments/Molecular Devices, Sunnyvale, CA, USA). Currents were leak subtracted. After gigaohm seals formed, the electrode capacitance was compensated. Cardiomyocytes were held at −40 mV, and Ba2+ currents were recorded immediately after whole-cell patches were formed by gentle suction or by β-escin perforation (within 6–10 min after gigaohm seals formed). β-Escin was prepared as a 25 mM stock solution in water and added to the pipette solution to yield a final concentration 30 µM. Current-voltage (I-V) relations were elicited from a holding potential at −40 mV using 200 ms steps (5 s between steps) over a range from −80 to +60 mV in 10-mV increments. In some experiments, cardiomyocytes were held at −40 mV, and ramp voltage commands (−80 to +60 mV in 500 ms) were used to evoke Ba2+ currents. At the end of the experiments, 10 µM nitrendipine (Sigma) dissolved in 0.05% DMSO was perfused extracellularly to inhibit L-VGCCs. The current densities (Idensity) were calculated from dividing the current amplitudes (pA) by membrane capacitances (pF).
All data are presented as mean ± SEM (standard error of mean). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for unbalanced n was used for statistical analyses of biochemical and molecular biological assays. Student’s t test was used for statistical analysis of electrophysiological recordings. Throughout, p < .05 was regarded as significant. Any defined rhythmic expression of mRNA, protein, or phosphorylation levels had to exhibit at least a 1.5-fold change in rhythmic amplitudes (Karaganis et al., 2008).
RESULTS
The mRNA Levels of Bmal1, VGCCα1C, and VGCCα1D Are Under Circadian Control in Chick Embryonic Hearts
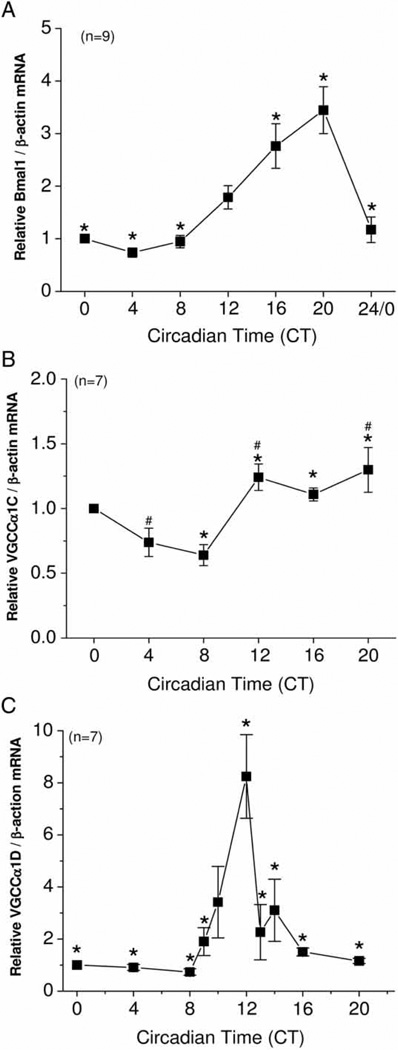
Two circadian oscillators, the retina and pineal gland, can be entrained to LD cycles in ovo as well as in vitro, since both are photosensitive in avian species (Iuvone, 1990; Iuvone et al., 1990; Ko et al., 2001, Nagy et al., 2009; Pierce et al., 1993). However, it was not known whether other peripheral organs without classical light-sensing mechanisms could also be light-entrained in embryonic stages. Chick embryos were entrained in 12:12-h LD cycles and kept in DD for 2 days. On the second day of DD, hearts were dissected at different circadian timepoints. Messenger RNA from the circadian oscillator gene Bmal1 peaked during the late night (CT 20) and was at its lowest in the early day (CT 4; Figure 1A). mRNA levels of both VGCCα1C and VGCCα1D also displayed circadian rhythms. In spite of the fact that the rhythmic amplitude of VGCCα1C mRNA was low, with ~2-fold difference between peak (CT 20) and trough (CT 8) values, VGCCα1C mRNA levels were significantly higher during the subjective night (CT 12, 16, 20) than subjective day (CT 8; Figure 1B). VGCCα1D mRNA peaked at CT 12, with a near 9-fold difference between the peak and trough values (CT 8; Figure 1C). Hence, chick hearts could be entrained to light cycles at embryonic stages in ovo. Since the mRNA of L-VGCCα1C and L-VGCCα1D were rhythmic, we further explored whether the functional expression of these channels are under circadian control.
FIGURE 1.
The mRNA levels of Bmal1, VGCCα1C, and VGCCα1D are under circadian control in chick hearts. (A) The circadian rhythm of Bmal1 mRNA reached high values during the subjective night and low ones during the subjective day. *: Bmal1 levels were significantly higher at CT 16 and CT 20 compared to CT 0, 4, 8, and 24, *p < .05; n = 9/timepoint. (B) *: The mRNA levels of VGCCα1C were significantly higher at CT 12, 16, and 20 than CT 8, *p < .05; #: VGCCα1C mRNA levels were significantly higher at CT 12 and CT 20 than CT 4, #p < .05; n = 7/timepoint. (C) The mRNA level of VGCCα1D peaked at CT 12 and was significantly higher than the levels at CT 0, 4, 8, 9, 13, 14, 16, and 20, *p < 0.05; n = 7/timepoint. Comparisons were made using ANOVA with Tukey’s post hoc test across different circadian timepoints.
The Functional Expression of L-Type Voltage-Gated Calcium Channels Is Under Circadian Control
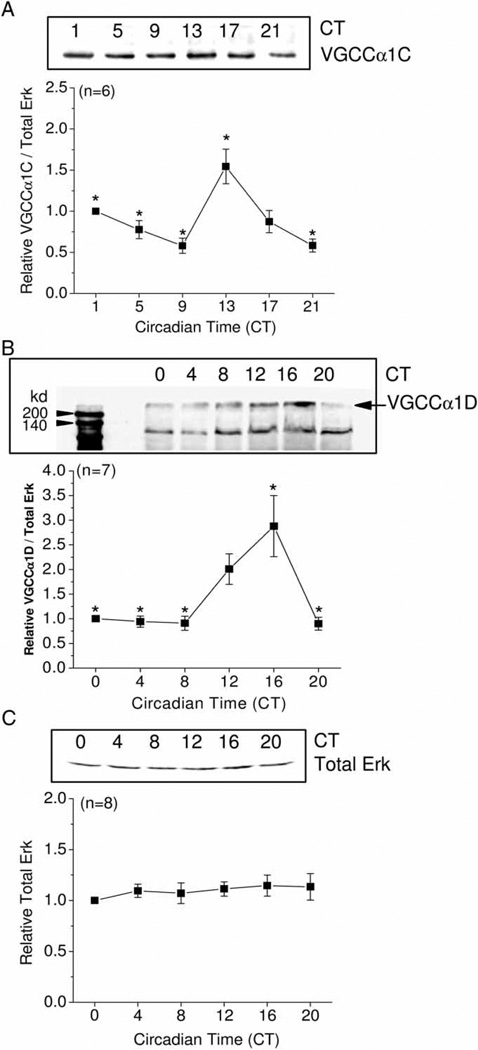
Since the α1 subunits are the pore-forming subunits for L-type VGCCs (Takahashi et al., 1987; Wang et al., 2004), and since VGCCα1C and VGCCα1D subunits are the most abundant in the heart (Acosta et al., 2004; Seisenberger et al., 2000; Takamatsu et al., 2003; Zhang et al., 2002, 2005), we examined the protein expressions of these channels. Similar to the oscillations seen in its mRNA levels, the rhythmic amplitude in VGCCα1C protein expression was also shallow, but significant, with a peak at CT 13, which was significantly different from values at CT 1, 5, 9, and 21 and with a near 2-fold difference between peak and trough levels (Figure 2A). VGCCα1D protein expression reached its peak at CT 16, which was significantly different from that at CT 0, 4, 8, and 20 (Figure 2B). The total amount of extracellular signal-regulated kinase (Erk, active and inactive forms) remained constant throughout the day (Figure 2C) and therefore Erk was used as our loading control. Hence, the overall expression of L-VGCCα1 subunits was high during the subjective night and lower during the subjective day.
FIGURE 2.
Protein expression of chick cardiac VGCCα1C and VGCCα1D are under circadian control. (A) The protein expression of VGCCα1C peaked at CT 13, which was significantly higher than the protein expression at CT 0, 5, 9, and 21, *p < .05; n = 6. (B) The protein expression of VGCCα1D peaked at CT 16, which was significantly higher than the protein expression at CT 0, 4, 8, and 20, *p < .05; n = 7. (C) Total Erk (phosphorylated and nonphosphorylated forms) remained constant throughout the day; n = 8. Comparisons were made using ANOVA with Tukey’s post hoc test across different circadian timepoints.
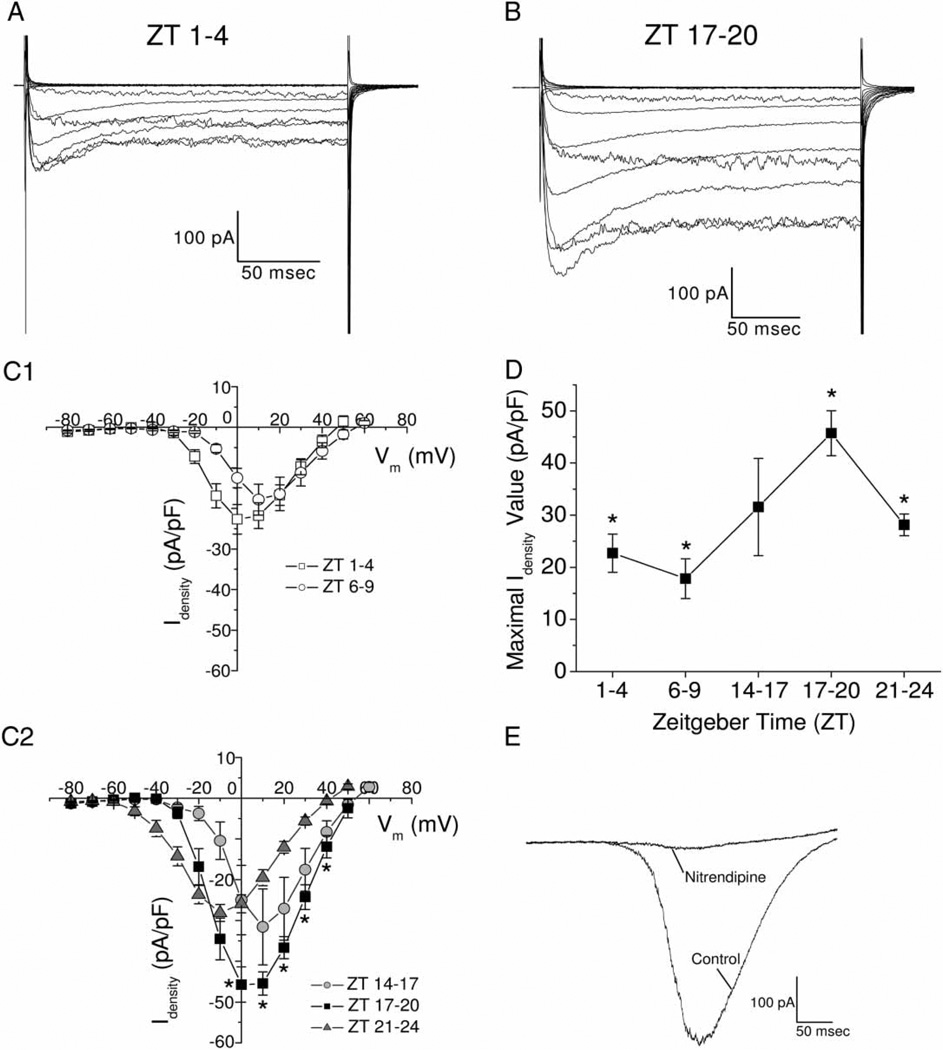
From these results, we further investigated L-VGCC currents from cultured cardiomyocytes. After chick embryos were entrained in LD cycles, ventricular cardiomyocytes were dissociated and cultured overnight in DD, and whole-cell patch-clamp recordings were carried out the next day at different time periods (ZT 1–4, 6–9, 14–17, 17–20, and 21–24). Average maximal L-VGCC current densities carried by Ba2+ were significantly greater in cardiomyocytes recorded during the night (ZT 17–20) than day (ZT 1–4 and ZT 6–9) and late night (ZT 21–24; Figure 3A–D). L-VGCC currents carried by Ba2+ were blocked by extracellular perfusion of 10 µM nitrendipine, regardless of when the cardiomyocytes were recorded (Figure 3E). Thus, there was a circadian regulation of L-VGCCs from the mRNA level to functional protein expression. The average values (± SEM) of maximal L-VGCC current densities were ZT 1–4: 22.7 ± 3.67 pA/pF, n = 23; ZT 6–9: 17.8 ± 3.82 pA/pF, n = 8; ZT 14–17: 31.6 ± 9.32 pA/pF, n = 8; ZT 17–20: 45.7 ± 4.33 pA/pF, n = 24; ZT 21–24: 28.1 ± 2.07 pA/pF, n = 27; after perfusion with nitrendipine: 3.43 ± 0.25 pA/pF. The value of L-VGCC current density after superperfusion of nitrendipine was smaller than most of the standard errors obtained from the average of current densities at the various time periods.
FIGURE 3.
There is a circadian regulation of L-type VGCC current density in chick cardiomyocytes. The L-type VGCC current amplitudes and densities recorded during the night (ZT 17–20) were larger than those recorded during the day (ZT 1–4, 6–9). Chick embryos were entrained in LD cycles in ovo, and on the last day of LD cardiomyocytes were dissociated, cultured overnight, and patch-clamp recordings were executed the next day. (A) An example of L-VGCC currents recorded during the day (ZT 1–4). (B) An example of L-VGCC currents recorded at night (ZT 17–20). (C) Average current-voltage (I–V) relationships were obtained from the step command as in current density. Cardiomyocytes were recorded at five different time periods: ZT 1–4 (C1), ZT 6–9 (C1), ZT 14–17 (C2), ZT 17–20 (C2), and ZT 21–24 (C2). *: The current densities were significantly higher when cardiomyocytes were recorded at ZT 17–20 than ZT 1–4 and ZT 6–9, at multiple voltage commends. (D) The maximal average current density was significantly higher in cardiomyocytes recorded at ZT 17–20, compared with cardiomyocytes recorded at ZT 1–4, 6–9, and 21–24. (E) Two representative traces recorded from a single cardiomyocyte using a ramp voltage command (−80 to +60 mV in 500 ms) showed L-VGCC current (control) was inhibited by superfusion of 10 µM nitrendipine. Comparisons were made using one-way ANOVA at different activation voltages, *p < .05.
The Phosphorylation States of Several Signaling Molecules Are Under Circadian Control
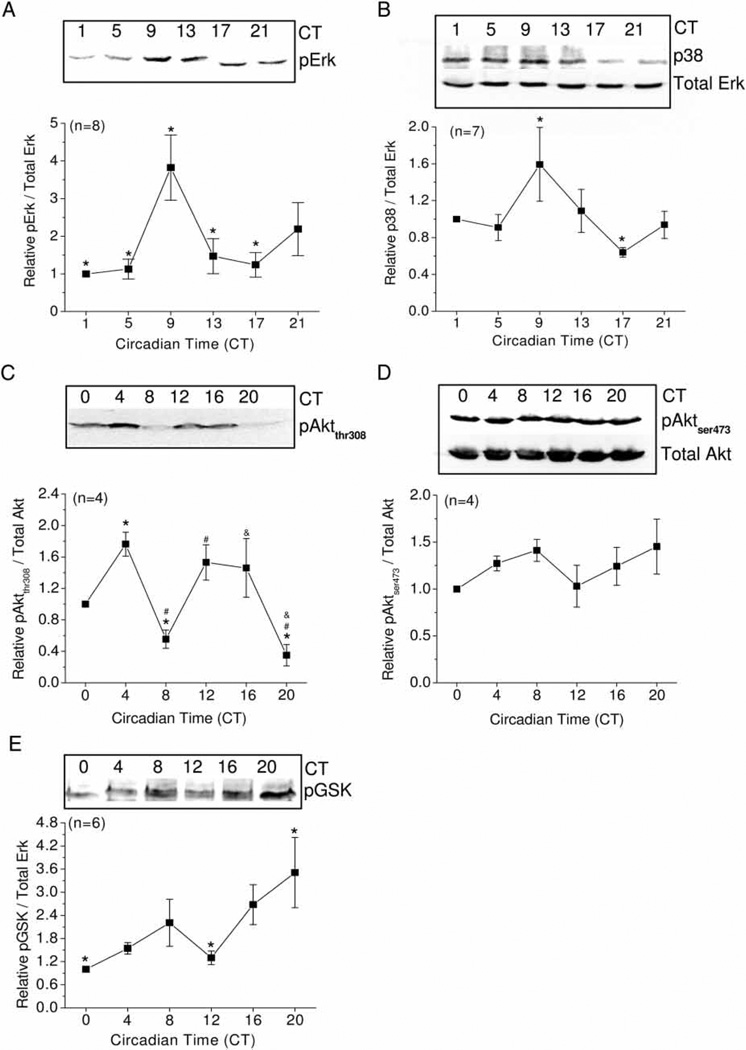
The extracellular signal-regulated (Erk) and stress-activated protein (p38) kinases have been shown to serve in dual capacities as circadian inputs to entrain circadian oscillators (Butcher et al., 2002, 2003; Dziema et al., 2003; Hasegawa & Cahill, 2004), as well as circadian outputs to regulate downstream targets (Ko et al., 2001, 2007; Vitalini et al., 2007). Both Erk and p38 mediate factor-induced gene expression and metabolic changes in cardiomyocytes (Ballard-Croft et al., 2008; Dhingra et al., 2007; Sharma et al., 2007; Sun et al., 2008; Yan et al., 2008; Yang et al., 2008). We found that the phosphorylation of Erk (measured as diphosphorylated Erk; pErk) is under circadian control with its peak at CT 9 (significantly different from CT 1, 5, 13, 17; Figure 4A), whereas total Erk remains constant throughout the day (Figures 2C and 4B). The phosphorylation of p38 (shown as p38) is also under circadian control with the peak at CT 9 and the trough at CT17, with a lower rhythmic amplitude, amounting to a ~2.5-fold difference between the peak and trough values (Figures 3B).
FIGURE 4.
Activities of Erk, p38, pAktthr308, and GSK3β are under circadian regulation. Immunoblot analysis showing phosphorylation of Erk (pErk), p38 (pp38), Aktthr308 (pAktthr308), Aktser473 (pAktser473), and GSK-3β (pGSK) in whole hearts harvested at different circadian times on the second day of DD. (A) The protein level of pErk showed a circadian rhythm with its peak at CT 9 that differed significantly from CT 1, 5, 13, and 17 (n = 8/timepoint). (B) The protein level of phosphorylated p38 (p38) showed a circadian rhythm with its peak at CT 9 that differed significantly from CT 17 (n = 7/timepoint). (C) The protein level of pAktthr308 displayed an ultradian rhythm with peaks at CT 4 and CT 12–16 (n = 4/timepoint). *: Significant differences between CT 4 and CT 8, CT 4, and CT 20; #: CT 12 is significantly different from CT 8 and CT 20; &: CT 16 is significantly different from CT 20. (D) The protein level of pAktser473 did not display any circadian rhythm (n = 4/timepoint). (E) The protein level of pGSK showed a circadian rhythm with its peak at CT 20 that was significantly different from CT 0 and CT 12 (n = 6/timepoint). All comparisons were made using ANOVA with Tukey’s post hoc test across different circadian timepoints, p < .05.
The phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) signaling pathway plays an essential role in L-VGCC ion channel trafficking and insertion into the cell membrane (Le Blanc et al., 2004; Macrez et al., 2001; Quignard et al., 2001; Viard et al., 2004). This pathway is also involved in the regulation of excitation-contraction coupling in cardiomyocytes through the regulation of L-VGCCs (McDowell et al., 2004). Activation of Akt requires a multistep process that includes phosphorylation of threonine 308 (Thr308) in the kinase domain and serine 473 (Ser473) within the regulatory domain (Fayard et al., 2005). In chick hearts, phosphorylation of Akt at Thr308 (pAktthr308) displayed an ultradian rhythm, which peaked at CT 4 and CT 12–16 (Figure 4C), whereas phosphorylation of Akt at Ser473 (pAktser473) did not show significant circadian rhythmicity (Figure 4D). One of the downstream targets of the PI3K-Akt pathway is glycogen synthase kinase-3β, which is a key regulator of growth and death of cardiomyocytes (Matsuda et al., 2008). There was also a circadian regulation of phosphorylated GSK-3β (pGSK) that peaked at CT 20 (Figure 4E), and the circadian phase of pGSK lagged the phase of pAktthr308 by ~4 h. Hence, both Erk and PI3K-Akt-GSK signaling pathways are under circadian control in chick hearts.
Both Erk and PI3K-Akt Signaling Pathways Are Part of the Circadian Output to Regulate L-VGCCs in Chick Hearts
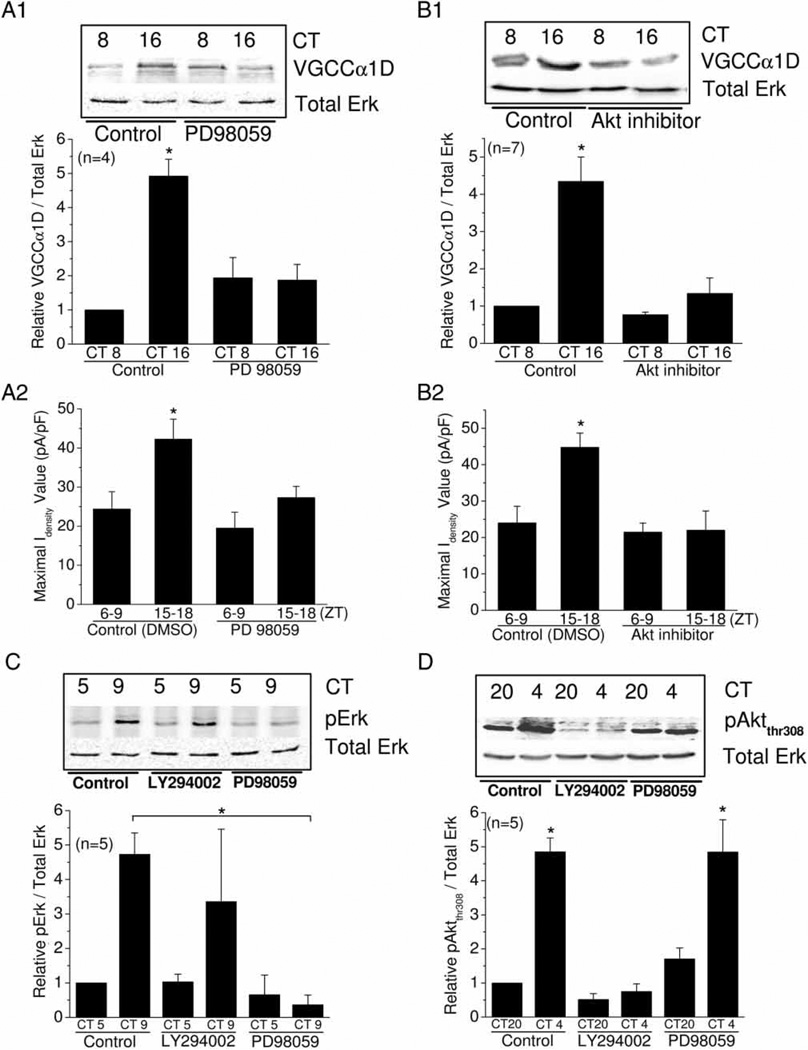
In chick hearts, we found that the phosphorylation of Erk (pErk) peaked at CT 9, a few hours ahead of the peaks of L-VGCCα1C and L-VGCCα1D (Figures 2A, B and 4A). The phosphorylation of Akt at thr 308 (pAktthr308) had a peak at CT 12–16, which was in synch with the peak expression of L-VGCCα1C (at CT 13) and α1D (at CT 16; Figures 2A, B and 4C). Since both Erk and PI3K-Akt signaling pathways play important roles in the metabolism and function of cardiomyocytes, we next investigated whether these two pathways might be involved in the circadian regulation of L-VGCCs. Because the circadian rhythmic amplitude of VGCCα1D was larger than that of VGCCα1C, we chose to investigate the circadian output regulation of L-VGCCs through this subtype, as these channels would give us a more sensitive assessment. Chick embryos were entrained in LD cycles in ovo for several days. On the last day of LD, hearts were collected, cut into 1–2-mm3 pieces, and cultured in DD. On the second day of DD at two different circadian timepoints, heart tissue cultures were treated with the MEK1 inhibitor PD98059 (50 µM), Akt inhibitor (10 µM), or control (DMSO, 0.1%) for 2 h prior to harvest for western blotting. Since the peak and trough of VGCCα1D are at CT 16 and CT 8, respectively, the heart tissue cultures were treated with PD98059, Akt inhibitor, or DMSO at CT 14 and CT 6 for 2 h. Inhibition of either Erk signaling by PD98059 or PI3K-Akt signaling by Akt inhibitor dampened the circadian rhythmicity of L-VGCCα1D (Figure 5A1 and B1), as well as the L-VGCC current densities in whole-cell patch recordings (Figure 5A2 and B2). Therefore, both Erk and PI3K-Akt pathways participate in the circadian regulation of cardiac L-VGCCs. Figure 5A2 shows the average values ± SEM of maximal current densities were Control (ZT 6–9): 24.4 ± 4.41 (n = 6); Control (ZT 15–18): 42.3 ± 5.11 (n = 8); PD98059 (ZT 6–9): 19.5 ± 4.07 (n = 8); PD98059 (ZT 15–18): 27.3 ± 2.87 (n = 16). Figure 5B2 shows the average values ± SEM of maximal current densities were: Control (ZT 6–9): 24.0 ± 4.53 (n = 9); Control (ZT 15–18): 44.8 ± 3.92 (n = 7); Akt inhibitor (ZT 6–9): 21.5 ± 2.51 (n = 7); Akt inhibitor (ZT 15–18): 22.0 ± 5.30 (n = 11).
FIGURE 5.
Circadian phase-dependent regulation of L-VGCCs is mediated by Erk and PI3K-Akt signaling pathways, and these pathways are independently regulated in chick hearts. (A1) The protein level of VGCCα1D was significantly higher at CT 16 than CT 8 (control). Treatment with 50 µM PD98059 (MEK 1 inhibitor) for 2 h dampened the VGCCα1D rhythm in heart tissue cultures. (A2) Treatment with PD98059 for 2 h also dampened the L-VGCC current density rhythms. (B1) Treatment with 10 µM of Akt inhibitor for 2 h significantly decreased the protein level of VGCCα1D during the night (CT 16) but not day (CT 8). (B2) Treatment with Akt inhibitor also dampened L-VGCC current density rhythms. (C) Activated Erk (pErk) levels were significantly higher at CT 9 than CT 5. Treatment with 50 µM LY294002 (PI3K inhibitor) for 2 h did not affect the pErk rhythm, whereas treatment with 50 µM PD98059 for 2 h abolished the pErk rhythm. (D) The pAktthr308 levels were significantly higher at CT 4 than CT 20. Treatment with 50 µM LY294002 for 2 h abolishes the pAktthr308 rhythm, whereas treatment with 50 µM PD98059 for 2 h did not have any effect on the pAktthr308 rhythm.
The circadian rhythms of pErk and pAktthr308 seemed to cycle in antiphase to one another (Figure 4A and C). Phosphorylated Erk (pErk) peaked at CT 9 with significantly lower phosphorylation at CT 5 (Figures 4A and 5C), whereas pAktthr308 peaked at CT 4 and 12 with significantly lower phosphorylation at CT 20 (Figures 4C and 5D). We postulated whether there was an interaction between the Erk and PI3K-Akt signaling pathways. Heart tissue cultures as described previously were treated with PD98059 (50 µM), PI3K inhibitor LY294002 (10 µM), or control (DMSO, 0.1%) for 2 h prior to harvesting for Western blotting. Treatment with PD98059 at CT 3 and CT 7 for 2 h completely inhibited the phosphorylation of Erk (Figure 5C), whereas treatment with the PI3K inhibitor LY294002 had no effect on pErk (Figure 5C). Conversely, LY294002 completely abolished the pAktthr308 rhythm, but PD98059 did not (Figure 5D). Hence, Erk and PI3K-Akt are two signaling pathways that are under independent circadian control in chick hearts.
DISCUSSION
Even though the SCN serves as the master clock to coordinate overall circadian rhythm in higher organisms, circadian oscillators also exist in peripheral tissues (Davidson et al., 2003; Yamazaki et al., 2000). The general consensus is that circadian variations in cardiac physiology are due to physiological responses to autonomic stimuli (Yamashita et al., 2003). Nevertheless, there are intrinsic circadian changes in cardiac metabolism (Bray et al., 2007; Durgan et al., 2005, 2006b, 2007; Stavinoha et al., 2004b; Young & Bray, 2007; Young et al., 2001a, 2003), contractile function (Young et al., 2001a), and expression of circadian clock genes (Davidson et al., 2005; Storch et al., 2002; Young, 2003; Young et al., 2001b) independent of autonomic inputs. We found that mRNA levels to functional expression of L-VGCCs are under circadian control in chick embryonic hearts (Figures 1 and 2). Specifically, the protein expression of both VGCCα1C and VGCCα1D are high during the subjective night and low during the subjective day (Figure 2). This finding is echoed by the significantly higher L-VGCC current densities recorded during the night than day (Figure 3). Phosphorylation of signaling molecules, including Erk, p38, Akt, and GSK, are also under circadian control (Figure 4). Although both Erk and PI3K-Akt signaling pathways serve as part of the circadian output pathway to regulate L-VGCCs, there is no circadian interaction between these pathways (Figure 5), even though pErk and pAktthr308 seemed to cycle in anti-phase to one other.
There are technical limitations of this study. Whereas the mRNA and protein expression/phosphorylation profiles were derived from whole hearts rapidly excised from chick embryos (Figures 1, 2, and 4) or from minced heart tissue cultured in DD for 2 days (Figure 5A1, B1, C, and D), the electrophysiological recordings were obtained from dissociated cardiomyocytes that were cultured overnight (Figures 3, 5A2 and B2). We were unable to record from acutely dissociated chick cardiomyocytes that would exactly match the circadian profiles from the whole hearts. For rodent cardiomyocytes studies, acutely isolated cardiomyocytes are often used as in Collins and Rodrigo’s study (Collins & Rodrigo, 2010), since they are uniformly rod shaped and easy to patch on for recordings. However, acutely isolated chick embryonic cardiomyocytes are rounded with no spontaneous pulsing. As a result, we could not record L-VGCC currents from them. In order to maintain healthy dissociated cardiomyocytes, cardiac tissue was gently triturated without complete dissociation, leaving tissue chunks mixed with dissociated cardiomyocytes in our cultures. After 22–24 h in culture, some chicken embryonic cardiomyocytes displayed a triangular or rectangular shape similar to that described by Carroll and Horowits (2000), and ~20–30% of these cardiomyocytes had spontaneous pulsing. Hence, we only recorded from pulsing cardiomyocytes, which all had recordable L-VGCCs. As a result, the circadian phases from whole hearts might not be exactly the same as reflected in electrophysiological recordings, even though in our pharmacological studies (Figure 5) data from the minced heart tissue cultures matched well with the electrophysiological recordings.
In addition, we observed that dissociated cardiomyocytes remained rhythmic in cultures under DD only for 1 day after dissection from circadian entrained embryos, so the results in Figure 3 were denoted in zeitgeber times (ZT). After 48~72 h in DD, dissociated cardiomyocytes still appeared healthy, retained their ability to spontaneously pulse, and generated recordable L-VGCC currents, but circadian rhythms in L-VGCC current density dissipated during this time. Thus, the loss of L-VGCC current density rhythm after 1 day in DD does not appear to be the result of failing cardiomyocyte health. An alternative explanation is that the circadian rhythm of the whole heart requires synchronization among the cardiomyocytes. Mechanical dissociation used to achieve monolayer cell cultures for patch-clamp recordings may have disrupted the synchronization between cells and caused individual cardiomyocytes to fall out of phase with each other after 24 h in culture, thereby causing the loss of circadian rhythms in L-VGCC current density. Another possibility is that the maintenance of the heart circadian rhythm requires cardiomyocytes to be in three-dimensional states, so when cardiomyocytes were cultured as a monolayer for patch-clamp recordings, they lost their circadian rhythms. We observed that in heart tissue culture preparation, in which the heart was minced into 1–2-mm3 pieces without vigorous dissociation, the circadian expression of proteins was retained (Figure 5). The circadian rhythm of cultured heart tissue explants from rats was also demonstrated previously (Davidson et al., 2005). Hence, it is possible to entrain heart tissue or cardiomyocytes in vitro for future mechanistic investigation once culture and entrainment conditions are optimized, which is currently on going in our research group.
Circadian regulation of L-VGCC current amplitudes or densities has been observed in gold fish retinal bipolar cells (Hull et al., 2006), chick cone photoreceptors (Ko et al., 2007), SCN neurons (Pennartz et al., 2002), and ventricular cardiomyocytes (Collins & Rodrigo, 2010). The activation voltages that elicit L-VGCC currents (i.e., current-voltage relationship) and channel gating kinetics did not change throughout the day in these four studies. In mature mammalian hearts, the L-VGCC α1C and α1D subunits have different roles in cardiac function with different distributions. Whereas α1C is important in cardiomyocyte contraction, α1D is important in cardiac pacemaker activity (Mangoni et al., 2003). The α1C subunit is more prevalent and is present throughout the heart, whereas the distribution of α1D varies in a species-dependent manner (Gaborit et al., 2007; Qu et al., 2005a; Zhang et al., 2005). In humans, L-VGCCα1D is expressed in both ventricles and atria, but at lower quantities compared to L-VGCCα1C (Gaborit et al., 2007), whereas in rabbits and rats, it is only present in the atria (Qu et al., 2005b). In mice, the L-VGCCα1D subtype is highly expressed in the atria, but much less so in the ventricles (Zhang et al., 2005). Currently, there is no report on the atrial/ventricular distribution of the L-VGCC α1C and α1D subunits in chickens. Since we could detect circadian rhythms of both subunits in whole chicken hearts, we suspect that the distribution of these subunits in chick hearts could be similar to that in humans.
One major question yet to be fully addressed is the physiological meaning and function of the circadian rhythms of L-VGCCs in the heart. In adult cardiomyocytes, L-VGCCs contribute to cardiac action potentials and calcium-induced calcium release from the sarcoplasmic reticulum (SR, Altamirano & Bers, 2007), and, therefore, L-VGCCs affect heart rate, contractile force, excitation-contraction (E-C) coupling, and cardiac output (Fauconnier et al., 2003; Kubalova, 2003). Collins and Rodrigo (2010) found that E-C coupling, basal diastolic and systolic [Ca2+]i, sarcoplasmic reticulum (SR) Ca2+ content, and contraction strengths were significantly higher during the resting period. The response of L-VGCCs to β-adrenergic stimulation was also greater during the resting period. Interestingly, they showed a reverse diurnal variation of peak L-VGCC current densities in ventricular cardiomyocytes that were higher during the active period, whereas gene expression of VGCCα1C was not expressed in a diurnal fashion (Collins & Rodrigo, 2010). Since L-VGCCs directly link to E-C coupling in cardiomyocytes, it is puzzling why L-VGCCs have an oppositely staged 24-h rhythm from other parameters that are responsible for E-C coupling. Maybe L-VGCCs play a more significant role in contraction frequency than E-C coupling in adult cardiomyocytes.
However, in embryonic cardiomyocytes, L-VGCCs might not play a significant role in the regulation of contraction frequency, but, instead, the major function of L-VGCCs is for refilling of intracellular Ca2+ stores (Sauer et al., 2001). There are stable spontaneous oscillations of cytosolic Ca2+ concentration ([Ca2+]i) from the SR in embryonic cardiomyocytes that are able to evoke contractions without action potentials (Sasse et al., 2007). The spontaneous oscillations of [Ca2+]i through inositol 1,4,5-trisphosphate (IP3) and ryanodine-dependent intracellular stores in the SR subsequently elicit the activation of Na+-Ca2+ exchangers (NCXs). Activation of NCXs leads to membrane depolarization, and when membrane potentials reach threshold, it further evokes action potentials that appear to synchronize contractions among cardiomyocytes and the coordinated pumping of blood (Korhonen et al., 2008; Rapila et al., 2008; Sasse et al., 2007). Hence, the major player regulating contraction frequency in embryonic cardiomyocytes is the spontaneous oscillations of [Ca2+]i from the SR, whereas L-VGCCs contribute to the refilling of intracellular Ca2+ stores (Sauer et al., 2001). We postulate that one of the important functions for the circadian regulation of L-VGCCs could be the refilling of intracellular Ca2+ stores, which could be under circadian control in embryonic as well as adult cardiomyocytes (Collins & Rodrigo, 2010).
Previously, Durgan et al. (2010) showed that the phosphorylation of Akt and GSK-3β are under circadian control in mouse cardiomyocytes, but it was not clear whether the phosphorylation of Akt was at Thr308 or Ser473. We found that Akt phosphorylation on Thr308 (pAktthr308) was rhythmic, and the circadian phase of pGSK lagged by ~4 h. The circadian pattern of GSK-3β phosphorylation (pGSK) followed the pattern of Aktser473 phosphorylation, even though the rhythmic amplitude of pAktser473 was not large enough to be considered statistically significant. It is possible that the small oscillation of pAktser473 might be sufficient to drive the circadian rhythm of its downstream target pGSK. The PI3K-Akt and Erk pathways are independent in various cell types, and what we observed in this study is in agreement with the notion that circadian oscillators in the heart independently regulate these signaling pathways. Whereas endothelin-induced phosphorylation of GSK3 is through Erk, insulin-induced phosphorylation of GSK is through PI3K-Akt (Gonzalez et al., 2007). Since in chick hearts, both pAktthr308 and pGSK-3β are under circadian control, it may be that insulin-mediated metabolic changes could be under circadian control in the cardiovascular system. In fact, the responsiveness of the myocardium to insulin, fatty acids, and workload, as well as triglyceride and glycogen metabolism of cardiomyocytes, are under circadian control in rodents (Bray & Young, 2008; Bray et al., 2008).
In summary, we found that there is a circadian regulation of the functional expression of L-VGCCs from mRNA levels to current density in cardiomyocytes, and activities of PI3K-Akt and Erk signaling pathways are also under circadian control in chick hearts. Circadian regulation of signaling pathways and ion channels in cardiomyocytes are essential for allowing the heart to anticipate environmental stimuli (such as changes in workload, feeding status) prior to their onset. Loss of cardiac circadian rhythmicity may contribute to metabolic inadequacies that might lead to myocardial contractile dysfunction (Bray & Young, 2008).
ACKNOWLEDGMENTS
We thank Ms. Darya Vernikovskaya’s technical assistance. We are grateful for the comments from Drs. Paul Hardin, Deborah Bell-Pedersen, and Terry Thomas.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Acosta L, Haase H, Morano I, Moorman AF, Franco D. Regional expression of L-type calcium channel subunits during cardiac development. Dev. Dyn. 2004;230:131–136. doi: 10.1002/dvdy.20023. [DOI] [PubMed] [Google Scholar]
- Altamirano J, Bers DM. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ. Res. 2007;101:590–597. doi: 10.1161/CIRCRESAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- Ballard-Croft C, Locklar AC, Keith BJ, Mentzer RM, Jr, Lasley RD. Oxidative stress and adenosine A1 receptor activation differentially modulate subcellular cardiomyocyte MAPKs. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H263–H271. doi: 10.1152/ajpheart.01067.2007. [DOI] [PubMed] [Google Scholar]
- Boixel C, Gonzalez W, Louedec L, Hatem SN. Mechanisms of L-type Ca(2+) current downregulation in rat atrial myocytes during heart failure. Circ. Res. 2001;89:607–613. doi: 10.1161/hh1901.096702. [DOI] [PubMed] [Google Scholar]
- Bray MS, Young ME. Diurnal variations in myocardial metabolism. Cardiovasc. Res. 2008;79:228–237. doi: 10.1093/cvr/cvn054. [DOI] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Doner J, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J. Biol. Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Obrietan K. Temporal regulation of light-induced extracellular signal-regulated kinase activation in the suprachiasmatic nucleus. J. Neurophysiol. 2003;90:3854–3863. doi: 10.1152/jn.00524.2003. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Horowits R. Myofibrillogenesis and formation of cell contacts mediate the localization of N-RAP in cultured chick cardiomyocytes. Cell. Motil. Cytoskeleton. 2000;47:63–76. doi: 10.1002/1097-0169(200009)47:1<63::AID-CM6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Collins HE, Rodrigo GC. Inotropic response of cardiac ventricular myocytes to beta-adrenergic stimulation with isoproterenol exhibits diurnal variation: involvement of nitric oxide. Circ. Res. 2010;106:1244–1252. doi: 10.1161/CIRCRESAHA.109.213942. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found. Symp. 2003;253:110–121. discussion 121–115, 281–114. [PubMed] [Google Scholar]
- Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin. Exp. Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am. J. Physiol Heart Circ. Physiol. 2007;293:H3524–H3531. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- Duguay D, Cermakina N. Crosstalk between physiology and circadian clock proteins. Chronobiol. Int. 2009;26:1479–1513. doi: 10.3109/07420520903497575. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am. J. Physiol Heart. Circ. Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Smith JK, Hotze MA, Egbejimi O, Cuthbert KD, Zaha VG, Dyck JR, Abel ED, Young ME. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am. J. Physiol. Heart Circ. Physiol. 2006a;290:H2480–H2497. doi: 10.1152/ajpheart.01344.2005. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J. Biol. Chem. 2006b;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Moore MW, Ha NP, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, Hickson-Bick DL, Heird WC, Dyck JR, Chandler MP, Young ME. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2385–H2393. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ. Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur. J. Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- Fan JS, Palade P. Perforated patch recording with beta-escin. Pflugers Arch. 1998;436:1021–1023. doi: 10.1007/pl00008086. [DOI] [PubMed] [Google Scholar]
- Fauconnier J, Bedut S, Le Guennec JY, Babuty D, Richard S. Ca2+ current-mediated regulation of action potential by pacing rate in rat ventricular myocytes. Cardiovasc. Res. 2003;57:670–680. doi: 10.1016/s0008-6363(02)00731-9. [DOI] [PubMed] [Google Scholar]
- Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J. Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Ravassa S, Loperena I, Lopez B, Beaumont J, Querejeta R, Larman M, Diez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J. Hypertens. 2007;25:2148–2157. doi: 10.1097/HJH.0b013e32828626e2. [DOI] [PubMed] [Google Scholar]
- Gudzenko V, Shiferaw Y, Savalli N, Vyas R, Weiss JN, Olcese R. Influence of channel subunit composition on L-type Ca2+ current kinetics and cardiac wave stability. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1805–H1815. doi: 10.1152/ajpheart.01160.2006. [DOI] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Regulation of the circadian oscillator in Xenopus retinal photoreceptors by protein kinases sensitive to the stress-activated protein kinase inhibitor, SB 203580. J. Biol. Chem. 2004;279:22738–22746. doi: 10.1074/jbc.M401389200. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J. Neurophysiol. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, Schwartz A, Herzig S. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J. Biol. Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- Iuvone PM. Development of melatonin synthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillators, and cyclic AMP. J. Neurochem. 1990;54:1562–1568. doi: 10.1111/j.1471-4159.1990.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Avendano G, Butler BJ, Adler R. Cyclic AMP-dependent induction of serotonin N-acetyltransferase activity in photoreceptor-enriched chick retinal cell cultures: characterization and inhibition by dopamine. J. Neurochem. 1990;55:673–682. doi: 10.1111/j.1471-4159.1990.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Karaganis SP, Kumar V, Beremand PD, Bailey MJ, Thomas TL, Cassone VM. Circadian genomics of the chick pineal gland in vitro. BMC Genomics. 2008;9:206. doi: 10.1186/1471-2164-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Ashizawa N, Toda G, Seto S, Yano K. Administration of nifedipine CR immediately after awakening prevents a morning surge in hypertensive patients. Case report of three cases. Blood Press. (Suppl.) 2003;1:44–48. doi: 10.1080/08038020310000113. [DOI] [PubMed] [Google Scholar]
- Kitahara Y, Saito F, Akao M, Fujita H, Takahashi A, Taguchi H, Hino T, Otsuka Y, Kushiro T, Kanmatsuse K. Effect of morning and bedtime dosing with cilnidipine on blood pressure, heart rate, and sympathetic nervous activity in essential hypertensive patients. J. Cardiovasc. Pharmacol. 2004;43:68–73. doi: 10.1097/00005344-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J. Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J. Neurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Rapila R, Tavi P. Mathematical model of mouse embryonic cardiomyocyte excitation-contraction coupling. J. Gen. Physiol. 2008;132:407–419. doi: 10.1085/jgp.200809961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell. Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- Kubalova Z. Inactivation of L-type calcium channels in cardiomyocytes. Experimental and theoretical approaches. Gen. Physiol. Biophys. 2003;22:441–454. [PubMed] [Google Scholar]
- Le Blanc C, Mironneau C, Barbot C, Henaff M, Bondeva T, Wetzker R, Macrez N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ. Res. 2004;95:300–307. doi: 10.1161/01.RES.0000138017.76125.8b. [DOI] [PubMed] [Google Scholar]
- Macrez N, Mironneau C, Carricaburu V, Quignard JF, Babich A, Czupalla C, Nurnberg B, Mironneau J. Phosphoinositide 3-kinase isoforms selectively couple receptors to vascular L-type Ca(2+) channels. Circ. Res. 2001;89:692–699. doi: 10.1161/hh2001.097864. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20900–20905. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell SA, McCall E, Matter WF, Estridge TB, Vlahos CJ. Phosphoinositide 3-kinase regulates excitation-contraction coupling in neonatal cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H796–H805. doi: 10.1152/ajpheart.00546.2003. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Mudalagiri NR, Mocanu MM, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Yellon DM. Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol-3 kinase and ERK1/2 activation. Br. J. Pharmacol. 2008;153:50–56. doi: 10.1038/sj.bjp.0707461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy AD, Kommedal S, Seomangal K, Csernus VJ. Circadian expression of clock genes clock and Cry1 in the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2009;1163:484–487. doi: 10.1111/j.1749-6632.2008.03639.x. [DOI] [PubMed] [Google Scholar]
- Ohta H, Xu S, Moriya T, Iigo M, Watanabe T, Nakahata N, Chisaka H, Hanita T, Matsuda T, Ohura T, Kimura Y, Yaegashi N, Tsuchiya S, Tei H, Okamura K. Maternal feeding controls fetal biological clock. PLoS One. 2008;3:pe2601. doi: 10.1371/journal.pone.0002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Molkentin JD. Prevention of cardiac hypertrophy by calcineurin inhibition: hope or hype? Circ. Res. 1999;84:623–632. doi: 10.1161/01.res.84.6.623. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Valenzuela GJ, Vergara M, Ducsay CA, Yellon SM, Seron-Ferre M. Effect of constant light on fetal and maternal prolactin rhythms in sheep. Endocrinology. 1996;137:2355–2361. doi: 10.1210/endo.137.6.8641186. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Potraluppi F, Touitou Y, Smolensky MH. Ethical and methological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Qiu YG, Chen JZ, Zhu JH, Yao XY. Differential effects of morning or evening dosing of amlodipine on circadian blood pressure and heart rate. Cardiovasc. Drugs Ther. 2003;17:335–341. doi: 10.1023/a:1027347723347. [DOI] [PubMed] [Google Scholar]
- Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005a;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of {alpha}1D (Cav1.3) L-type Ca channel by protein kinase A. Am. J. Physiol. Heart Circ. Physiol. 2005b;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, Mironneau C, Macrez N. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J. Biol. Chem. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- Rapila R, Korhonen T, Tavi P. Excitation-contraction coupling of the mouse embryonic cardiomyocyte. J. Gen. Physiol. 2008;132:397–405. doi: 10.1085/jgp.200809960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. Maternal coordination of the fetal biological clock in utero. Science. 1983;220:969–971. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J. Gen. Physiol. 2007;130:133–144. doi: 10.1085/jgp.200609575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Theben T, Hescheler J, Lindner M, Brandt MC, Wartenberg M. Characteristics of calcium sparks in cardiomyocytes derived from embryonic stem cells. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kühbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Dhingra S, Khaper N, Singal PK. Activation of apoptotic processes during transition from hypertrophy to heart failure in guinea pigs. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1384–H1390. doi: 10.1152/ajpheart.00553.2007. [DOI] [PubMed] [Google Scholar]
- Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavinoha MA, RaySpellicy JW, Essop MF, Graveleau C, Abel ED, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Evidence for mitochondrial thioesterase 1 as a peroxisome proliferator-activated receptor-alpha-regulated gene in cardiac and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004a;287:E888–E895. doi: 10.1152/ajpendo.00190.2004. [DOI] [PubMed] [Google Scholar]
- Stavinoha MA, Rayspellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am. J. Physiol. Endocrinol. Metab. 2004b;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Sugden PH. Signaling in myocardial hypertrophy: life after calcineurin? Circ. Res. 1999;84:633–646. doi: 10.1161/01.res.84.6.633. [DOI] [PubMed] [Google Scholar]
- Sun H, Xu B, Inoue H, Chen QM. P38 MAPK mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell Signal. 2008;20:1952–1959. doi: 10.1016/j.cellsig.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Szokodi I, Kerkela R, Kubin AM, Sármán B, Pikkarainen S, Kónyi A, Horváth IG, Papp L, Tóth M, Skoumal R, Ruskoaho H. Functionally opposing roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the regulation of cardiac contractility. Circulation. 2008;118:1651–1658. doi: 10.1161/CIRCULATIONAHA.107.758623. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H, Nagao T, Ichijo H, Adachi-Akahane S. L-type Ca2+ channels serve as a sensor of the SR Ca2+ for tuning the efficacy of Ca2+-induced Ca2+ release in rat ventricular myocytes. J. Physiol. 2003;552:415–424. doi: 10.1113/jphysiol.2003.050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teos LY, Zhao A, Alvin Z, Laurence GG, Li C, Haddad GE. Basal and IGF-I-dependent regulation of potassium channels by MAP kinases and PI3-kinase during eccentric cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1834–H1845. doi: 10.1152/ajpheart.321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohse N, Meszaros J, Sperelakis N. Developmental changes in long-opening behavior of L-type Ca2+ channels in embryonic chick heart cells. Circ. Res. 1992;71:376–384. doi: 10.1161/01.res.71.2.376. [DOI] [PubMed] [Google Scholar]
- Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- Vitalini MW, de Paula RM, Goldsmith CS, Jones CA, Borkovich KA, Bell-Pedersen D. Circadian rhythmicity mediated by temporal regulation of the activity of p38 MAPK. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18223–18228. doi: 10.1073/pnas.0704900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Collins RF, Ford RC, Berrow NS, Dolphin AC, Kitmitto A. The three-dimensional structure of the cardiac L-type voltage-gated calcium channel: comparison with the skeletal muscle form reveals a common architectural motif. J. Biol. Chem. 2004;279:7159–7168. doi: 10.1074/jbc.M308057200. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H. Circadian variation of cardiac K+ channel gene expression. Circulation. 2003;107:1917–1922. doi: 10.1161/01.CIR.0000058752.79734.F0. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yan L, Tang Q, Shen D, Peng S, Zheng Q, Guo H, Jiang M, Deng W. SOCS-1 inhibits TNF-alpha-induced cardiomyocyte apoptosis via ERK1/2 pathway activation. Inflammation. 2008;31:180–188. doi: 10.1007/s10753-008-9063-5. [DOI] [PubMed] [Google Scholar]
- Yang M, Wu J, Martin CM, Kvietys PR, Rui T. Important role of p38 MAP kinase/NF-kappaB signaling pathway in the sepsis-induced conversion of cardiac myocytes to a proinflammatory phenotype. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H994–H1001. doi: 10.1152/ajpheart.01044.2007. [DOI] [PubMed] [Google Scholar]
- Young ME. Circadian rhythms in cardiac gene expression. Curr. Hypertens. Rep. 2003;5:445–453. doi: 10.1007/s11906-003-0051-8. [DOI] [PubMed] [Google Scholar]
- Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- Young ME, Bray MS. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007;8:656–667. doi: 10.1016/j.sleep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ. Res. 2001a;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ. Res. 2001b;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ. Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]