SUMMARY
Pancreatic β-cell mass adapts to changing insulin demands in the body. One of the most amazing reversible β-cell adaptations occurs during pregnancy and postpartum conditions. During pregnancy, the increase in maternal insulin resistance is compensated by maternal β-cell hyperplasia and hyperfunctionality to maintain normal blood glucose. Although the cellular mechanisms involved in maternal β-cell expansion have been studied in detail in rodents, human studies are very sparse. A summary of these studies in rodents and humans is described below. Since β-cell mass expands during pregnancy, unraveling the endocrine/paracrine/autocrine molecular mechanisms responsible for these effects can be of great importance for predicting and treating gestational diabetes and for finding new cues that induce β-cell regeneration in diabetes. In addition to the well known implication of lactogens during maternal β-cell expansion, additional participants are being discovered such as serotonin and HGF. Transcription factors, such as hepatocyte nuclear factor-4α and the forkhead box protein-M1, and cell cycle regulators, such as menin, p27 and p18, are important intracellular signals responsible for these effects. In this article, we summarize and discuss novel studies uncovering molecular mechanisms involved in the maternal β-cell adaptive expansion during pregnancy.
Pancreatic β-cells & diabetes
Pancreatic β-cells produce and secrete the insulin needed for optimal glucose homeostasis. β-cell mass is determined by the number and size of β-cells in the pancreas, aspects that are regulated by cellular processes such as replication, death, hypertrophy and neogenesis. Both Type 1 and Type 2 diabetes occur when a decrease in β-cell mass and/or function negatively impacts the insulin requirements needed to control glucose homeostasis.
Adult β-cells proliferate at a very slow rate in basal physiologic conditions [1–4]; however, multiple studies have demonstrated the adaptive and dynamic plasticity of β-cells in response to a variety of physiological and pathophysiological situations. For example, studies have demonstrated that β-cell proliferation and β-cell mass are enhanced in situations in which there is an increase in metabolic demand in the body, such as during pregnancy, with attendant insulin resistance, glucose loading and obesity-induced insulin resistance [5–9]. On the other hand, a reduction in islet mass through increased β-cell death occurs in several physiological and pathological situations. It has been demonstrated that the normal low rates of apoptosis in β-cells are upregulated postpartum in the mother and in the neonate, and they are also increased after glucose deprivation [9–11]. In Type 1 diabetes, β-cell apoptosis is crucial at different stages during disease progression [12,13]. Significant β-cell death is also present during the islet isolation process and during islet engraftment following transplantation [14,15]. Immunosuppressants (cytotoxicity), absence of islet vascularization (i.e., hypoxia and nutrient deprivation) and hyperglycemia (glucotoxicity) are triggers known to induce islet β-cell death [16]. In addition, Type 2 diabetes is characterized by impaired insulin secretion and it has been suggested that a decrease in β-cell mass might also contribute to this pathology [17,18]. It has been shown that the increase in β-cell apoptosis in Type 2 diabetic patients may be responsible for this β-cell mass decrease since β-cell replication and islet neogenesis seem to be similar to those in nondiabetic subjects with a similar BMI [18]. Thus, determining the mechanisms involved in β-cell function, proliferation and survival can be critical in the prevention and treatment of diabetes.
Gestational diabetes
During pregnancy, physiologic insulin resistance occurs and leads to an adaptive increase in maternal insulin secretion [5,19–22]. This hyperinsulinism results from adaptive β-cell hyperplasia, hypertrophy and hyperfunction [5,19–26]. Several experimental models in rodents have shown that when β-cell expansion or function fails to compensate during pregnancy, hyperglycemia/diabetes occurs, suggesting that defective maternal β-cell adaptation can lead to gestational diabetes mellitus (GDM) [24–31]. In humans, GDM, defined as glucose intolerance first recognized during pregnancy, complicates 3–5% of all pregnancies in the USA, with prevalence rates varying between 1 and 14% depending on factors such as different diagnostic criteria or ethnic origin [32–35]. The consequences of GDM during pregnancy include both neonatal risks such as macrosomia, and maternal risks, such as an increased probability of cesarean delivery, and their corresponding co-morbidities [32–35]. Additionally, GDM markedly increases the rate of developing Type 2 diabetes after delivery, ranging from 2.6% to more than 70% in studies examining women at different postpartum times [36–38]. Therefore, pregnancy could be seen as a ‘stres-stest’ that reveals a woman’s predisposition to Type 2 diabetes. Predictors of postpartum diabetes in women are the presence of islet autoantibodies, the requirement of insulin treatment during pregnancy and a BMI greater than 30 [39]. However, the molecular determinants involved in the appearance of inadequate β-cell adaptation to pregnancy, GDM and consequent diabetes later in life have not been fully unraveled.
Besides the immediate adverse neonatal outcomes associated with GDM, population-based studies have demonstrated long-term health consequences for the offspring of gestational diabetic mothers including: obesity, hypertension, dyslipidemia, glucose intolerance and Type 2 diabetes [40,41]. In conclusion, alterations in molecular regulators of β-cell mass and function during pregnancy, together with environmental factors, could potentially lead to defective β-cell adaptation in the mother and the offspring, resulting in a higher risk of developing diabetes.
β-cell mass expansion during pregnancy: rodent models
β-cell mass expansion in animals and humans slows considerably during adulthood owing to extremely low rates of β-cell replication and neogenesis [1–4]. However, alterations in insulin demand in physiologic situations such as pregnancy can lead to adaptive β-cell mass expansion and increased insulin secretion [5,19–26,34]. The regulatory changes that occur in the β-cell during pregnancy are becoming increasingly well documented in rodents. In this section, we will summarize the changes in β-cell mass and function that occur during pregnancy, as well as the currently known molecular mechanisms involved in these effects.
Cellular mechanisms
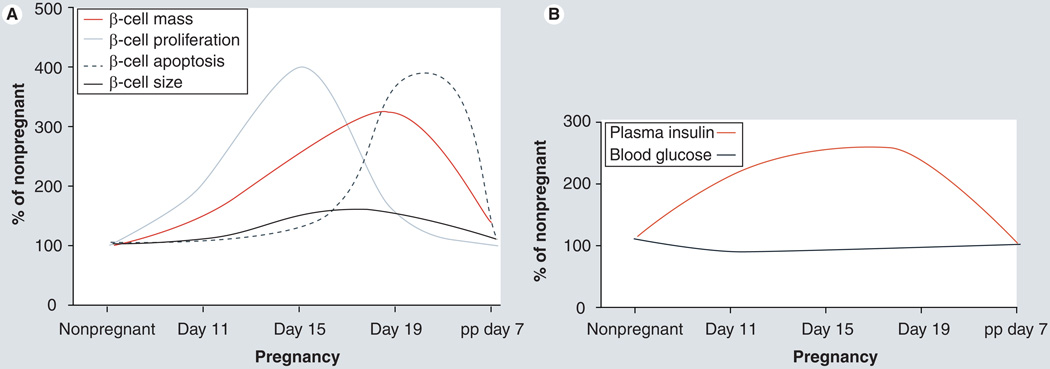
At the end of gestation, rodents display a two- to three-fold increase in β-cell mass [5,26,28–31,42]. Enhanced β-cell proliferation and hypertrophy are two of the main cellular processes involved in this increase in β-cell mass [5,26,31]. β-cell proliferation is maximally increased at gestational days 13–15 (two- to five-fold), declining thereafter to reach control levels at day 18–19 [5,26,28–31,42]. Whether β-cell neogenesis from non-β-cell progenitors is one of the processes involved in the maternal β-cell expansion during pregnancy has not yet been deciphered, but a recent study by Abouna et al. using lineage tracing analysis in pregnant mice marks the first step in this direction [43]. This study shows that following a labeling pulse before pregnancy, there is a decrease in the labeling index of β-cells during gestation, suggesting that conversion of non-β-cell progenitors into β-cells might also contribute to maternal β-cell expansion in pregnancy [43]. Return to normal β-cell homeostasis is achieved during the first week postpartum by means of decreased β-cell proliferation and size, and increased β-cell death (Figure 1A) [5,10,26,30]. Islet vascularization is also increased in pregnant rats following the same temporal pattern as β-cell replication [42]. β-cell functional changes also occur during normal pregnancy and these include the following:
-
▪
Increased glucose-stimulated insulin secretion with a decrease in the glucose stimulation threshold;
-
▪
Increased insulin synthesis;
-
▪
Increased glucose metabolism;
-
▪
Elevated levels of glucokinase and glucose transporters (Glut-2 and -5);
-
▪
Increased expression of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins [5,20,22,23,44,45].
Figure 1. β-cell and glucose homeostasis changes during pregnancy in rodents.
Approximate changes in (A) cellular mechanisms (proliferation, death and size) involved in adaptive maternal β-cell mass expansion during pregnancy and (B) their correlation with blood glucose and plasma insulin levels during pregnancy in rodents are shown.
pp: Postpartum.
Data taken from [Demirci C, Ernst S, Garcia-Ocana A, Unpublished Data; 5,26,28–31,42].
During pregnancy in rodents, the enhancement in islet function occurs on days 11–19, returning to control levels by delivery (Figure 1B).
Hormones, neurotransmitters & growth factors during pregnancy: placental lactogen, serotonin, HGF & steroid hormones
Changes in maternal islet growth and function correlate with increased serum placental lactogen (PL) levels during gestation [21]. In addition, expression of prolactin (PRL) receptor (PRLR), the receptor that binds both PRL and PL, is also increased in maternal islets during pregnancy [5,46]. Multiple in vitro and in vivo studies examining the effects of homologous PL and PRL on islets have clearly shown that lactogens increase insulin secretion and β-cell proliferation, survival and mass, and lower the threshold for glucose-stimulated insulin secretion [5,21,22,47–53]. More recently, using heterozygous PRLR-knockout mice, Huang et al. reported that lactogens are required for normal glucose homeostasis and modulation of β-cell mass during pregnancy [30,54]. These studies, although performed with whole body heterozygote PRLR-knockout mice, constitute the first direct in vivo effort to determine the exact role of lactogens in pregnancy.
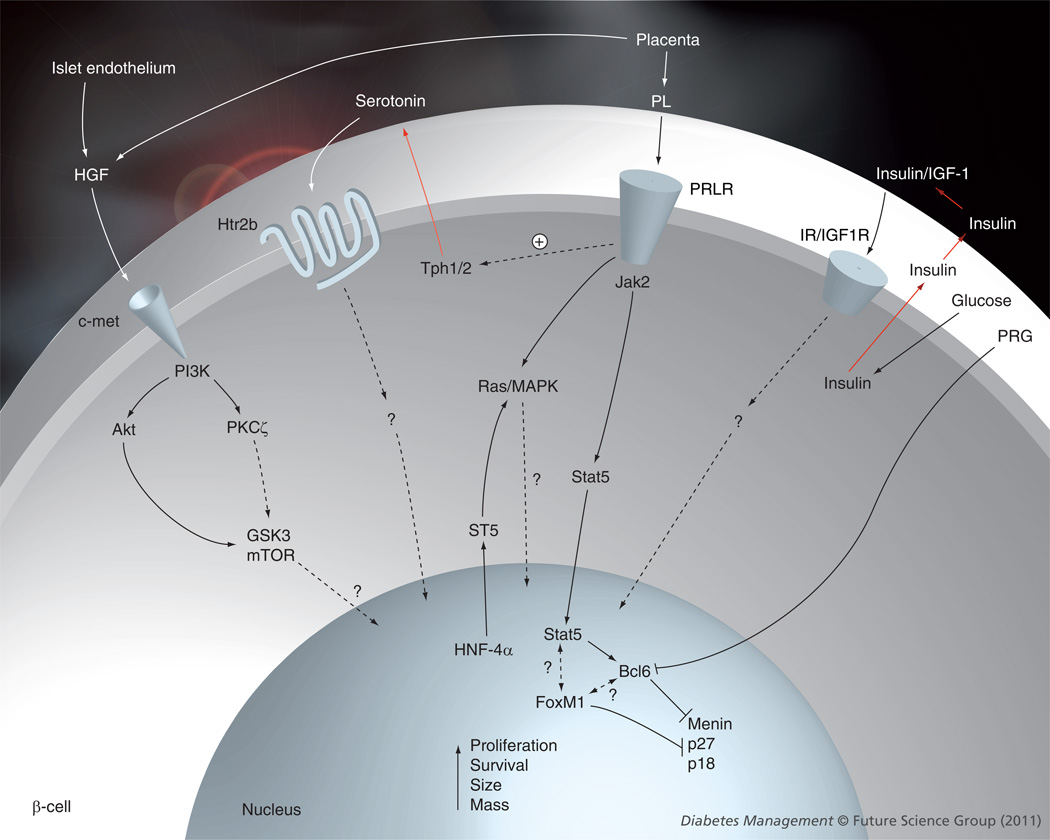
Three studies have recently analyzed the gene-expression pattern in the islet during pregnancy in mice, focusing on gene changes around gestational days 13–15 when maximal maternal β-cell proliferation occurs [55–57]. Hundreds of genes were altered in islets from pregnant mice, covering a wide spectrum of cellular processes such as metabolism, growth, death, insulin secretion, development and cell interaction. Among them, tryptophan hydroxylase 1 (Tph1), a serotonin synthetic enzyme, was highly upregulated in islets from pregnant mice [56]. Elegant studies by Michael German’s group have recently shown that inhibition of serotonin synthesis by dietary tryptophan restriction or Tph inhibition blocked β-cell proliferation and expansion, leading to reduced glucose tolerance in pregnant mice without affecting insulin sensitivity [56]. Blocking the signaling of the Gαq-linked serotonin receptor 5-hydroxytryptamine receptor-2b (Htr2b) in maternal islets also blunted β-cell expansion and caused glucose intolerance, indicating that serotonin signaling is important for maternal β-cell expansion. PRL increased Tph1 expression and serotonin production in mouse islets in vitro, suggesting that serotonin acts downstream of lactogens/PRLR signaling to enhance maternal β-cell proliferation. These studies also suggest that interfering with serotonin signaling through pharmacological agents and diets can increase the risk of developing GDM.
Over the years, researchers analyzing β-cell adaptation during pregnancy have focused their attention mostly on the role of circulating lactogenic hormones. No studies have addressed the role of other hormones/growth factors that are known to be β-cell mitogens and prosurvival factors produced by, and/or with receptors in, the islet, including HGF, insulin, IGF, glucagon-like peptide-1, β-cellulin and parathyroid hormone-related protein [58]. Importantly, circulating HGF levels are markedly and significantly increased during pregnancy [59]. In vitro and in vivo studies using continuous β-cell lines, primary rodent islet cell cultures, genetically modified mice or adenoviral delivery have shown that HGF is an insulinotropic factor, a β-cell mitogen and a regulator of β-cell survival [42,58,60–66]. These effects of HGF in the β-cell are mainly mediated by activation of the PI3K, atypical PKC ζ and PKB signaling pathways [62,67]. Furthermore, a recent study has shown that HGF expression is upregulated in the rat islet endothelium at gestational day 15 when maximal β-cell proliferation and islet vascularization is observed [42]. Therefore, since the HGF receptor, c-Met, is expressed in the β-cell and HGF is a mitogen and an insulinotropic agent for the β-cell, it is likely that circulating and/or locally secreted HGF participates in the β-cell adaptation process during pregnancy. However, no attempt has been made to decipher the role of HGF/c-Met signaling in the β-cell during pregnancy. Preliminary evidence from our group using mice that lack c-Met expression in the pancreas [68] indicates that HGF/c-Met signaling might be essential for β-cell expansion during pregnancy [Demirci C, Ernst S, Garcia-Ocana A, Unpublished Data].
In addition to lactogens, circulating levels of other maternal hormones such as progesterone (PRG) and estradiol continuously rise during gestation [69–71]. Steroid hormones are known inhibitors of lactogen-induced β-cell proliferation, survival and function in vitro [53,72]. In addition, low-dose dexamethasone administration during late pregnancy impairs glucose-stimulated insulin secretion [73]. These observations have led to the hypothesis that upregulation of steroid hormone levels might be responsible for the increase in β-cell death, the decrease in β-cell proliferation and the normalization of β-cell mass and insulin secretion at the end of pregnancy and in early postpartum periods. However, studies by Nieuwenhuizen et al. have shown that PRG treatment increases β-cell proliferation in pregnant and nonpregnant rats, but not in gonadectomized female rats or in gonadectomized female rats supplemented with estradiol [74,75]. This suggests that PRG might stimulate β-cell proliferation indirectly through gonadal factors but not estradiol, and that other maternal hormones might be involved in β-cell mass regression to normal values at term. On the other hand, PRG receptor-null female mice display increased β-cell proliferation, mass and insulin secretion [76], highlighting that the exact role of PRG in the β-cell in the hormonal milleu during pregnancy is still unclear. Taken together, these studies indicate the need for further in vivo studies analyzing the involvement of steroid hormones in β-cell adaptation during pregnancy.
Intracellular mechanisms
Increased activation of insulin receptor substrates-1 and -2, PI3K, PKB, p70S6 kinase, ERK1/2, JAK2 and STAT5 have been observed in the islets of pregnant rats [77]. However, whether these signaling pathways are important for β-cell adaptation during pregnancy is still unknown. The mTOR signaling pathway is a critical regulator of β-cell proliferation, mass and d-cyclin expression [78,79]. Interestingly, a recent report observed that administration of rapamycin, an inhibitor of mTOR, impairs β-cell proliferation and expansion during pregnancy in mice [80]. However, this decrease in maternal β-cell expansion did not affect blood glucose levels or glucose tolerance in pregnant mice. The authors suggest that rapamycin could improve peripheral insulin sensitivity, compensating for the decrease in β-cell expansion, leading to comparable glucose clearance in both rapamycin-treated and -untreated pregnant mice. However, insulin sensitivity was not assessed in this study. Nevertheless, these studies point towards a potential role for mTOR activation in β-cell expansion during pregnancy.
The importance of transcription factors in the regulation of maternal β-cell growth and function has been recently addressed in two exciting publications investigating the role of the orphan nuclear receptor hepatocyte nuclear factor-4α (HNF-4α) and the forkhead box protein FoxM1 [29,31]. Mutations in HNF-4α cause maturity-onset diabetes of the young 1 (MODY1) [81], and elimination of HNF-4α from β-cells in mice using the Cre-lox system leads to decreased maternal β-cell proliferation and mass at gestational day 14.5 [29]. This decrease is due, at least in part, to reduced activation of the Ras/MAPK signaling cascade through downregulation of suppression of tumorigenicity 5 (ST5), a regulator of this signaling pathway and a direct transcriptional target of HNF-4α in β-cells [29]. In addition, although virgin female mice with HNF-4α deficiency in β-cells were already glucose intolerant, pregnancy further impaired their response to a glucose challenge. Thus, although HNF-4α is seen as a MODY gene involved in regulating insulin secretion, these new data from pregnant mice also point out that HNF-4α is required for the physiological expansion of maternal β-cell mass in mice. Several mutations in MODY genes have been identified in women with gestational diabetes including glucokinase (MODY2), HNF-1α (MODY3) and Pdx-1 (MODY4) [82]. Therefore, it could be possible that loss-of-function mutations in HNF-4α may also be associated with an increased risk for gestational diabetes in humans.
FoxM1 is a transcription factor that is highly upregulated in proliferating cells [83], embryonic and neonatal pancreatic endocrine cells [84], and is also required for liver regeneration [85]. Mice lacking FoxM1 in their pancreas display a striking reduction in β-cell mass in basal conditions leading to frank diabetes in male mice but normal glucose homeostasis in female mice [84]. FoxM1 mRNA expression is increased at gestational day 14.5 in mice, returning to normal levels postpartum [31]. Importantly, pregnant mice with pancreatic deletion of FoxM1 display decreased β-cell replication and β-cell mass compared with control mice, leading to profound glucose intolerance at gestational day 15.5 [31]. Since lactogens also induce FoxM1 expression in isolated islets in vitro and inactivation of this transcription factor prevents PL-mediated β-cell proliferation, the authors conclude that FoxM1 is a downstream regulator of lactogens and plays a critical role in β-cell adaptation to pregnancy [31]. However, since female knockout mice display a profound decrease in β-cell mass before pregnancy, it is difficult to assess the impact of this pre-existing deficiency in β-cell mass on the observed effects during gestation.
Since β-cells can proliferate under physiologic and pathophysiologic conditions such as obesity, glucose-loading, pregnancy and Type 1 diabetes [5–9,86], an increasing interest in defining the cell cycle machinery in the β-cell has recently emerged [87]. Among the information published thus far, Seung Kim’s group demonstrated that menin, an endocrine tumor suppressor and transcriptional regulator, negatively regulates β-cell proliferation and expansion in pregnant mice [28]. β-cell proliferation during pregnancy associates with reduced islet levels of menin and its cell cycle transcriptional targets p18 and p27, both of which are inhibitors of cell cycle progression [28]. Analysis of the mechanisms involved in this reduction of menin expression indicates that lactogenic hormones stimulate phosphorylation and nuclear accumulation of STAT5, which induces the expression of Bcl6, a transcriptional repressor of the Men1 gene, leading to lower levels of menin in islets during pregnancy [28]. Tetracycline-induced expression of menin in the β-cells of pregnant transgenic mice prevented islet expansion, induced hyperglycemia and impaired glucose tolerance during pregnancy, indicating that increased levels of menin in β-cells can lead to incomplete maternal β-cell expansion and GDM [28]. These pioneer studies indicate that a failure in β-cell cell cycle mechanisms can lead to the development of GDM. Whether other cell cycle molecules are also involved in adaptive β-cell proliferation during pregnancy is still unknown. A summary of the molecular mechanisms involved in maternal β-cell expansion during pregnancy is presented in Figure 2.
Figure 2. Extracellular and intracellular signaling mechanisms involved in maternal β-cell expansion during pregnancy.
Dotted arrows represent unknown mechanisms.
Bcl-6: β-cell lymphoma 6 protein; GSK3: Glycogen synthase kinase 3; IGF1R: IGF-1 receptor; IR: Insulin receptor; PL: Placental lactogen; PRG: Progesterone; PRLR: Prolactin receptor.
β-cell mass expansion during pregnancy in humans
As in rodents, insulin sensitivity also declines during the second half of gestation in humans, and an increase in insulin secretion occurs in order to maintain normal, or even lower, blood glucose levels [88]. Although multiple studies have addressed glucose homeostasis control during pregnancy, only two studies have analyzed potential changes in human β-cell mass adaptation during gestation [89,90]. Importantly, both studies report an increase in β-cell hyperplasia during pregnancy in humans. In the first study, Van Assche et al. compared the percentage of endocrine tissue and β-cells between five pregnant women who died either in the third trimester or on the day of delivery and five nonpregnant women of comparable ages and weights who died owing to car accidents [89]. They reported a twofold increase in endocrine/β-cell fractional area (a surrogate of β-cell mass) in the pregnant women, indicating for the first time that in humans, the endocrine pancreas is able to adapt to the metabolic changes of pregnancy by β-cell hyperplasia. Recently, Butler’s group analyzed the adaptive changes in β-cell fractional area and turnover in pancreatic biopsies from a larger cohort of women who died while pregnant (n = 18), postpartum (n = 6) or from nonpregnant controls (n = 20) [90]. A significant 1.4-fold increase in β-cell fractional area was found in pancreas samples from pregnant women compared with nonpregnant women, and this increase was lower and not significant in the postpartum samples, suggesting a return to normal β-cell homeostasis after delivery, although the number of postpartum samples was small. In this study, the magnitude of the expansion of β-cells in pregnant women was not as large as in rodents or in the previous human pregnancy study, but it is important to note that this number represented the average β-cell fractional area analyzed over a wide range of gestational ages (10–40 weeks). In rodent studies, the maximal increase (two- to three-fold) was observed at the end of the gestational period, and in the human study by Van Assche et al., most of the samples were from women at the end of pregnancy [89]. In addition, prepregnancy BMI was also variable in these women (18.3–34.1), which could also be a factor increasing variability in the data. Contrary to rodent studies, β-cell hypertrophy, augmented islet size, enhanced β-cell number per islet, increased β-cell replication and changes in β-cell apoptosis were not observed in pregnant women in this recent study [90]. However, the numbers of scattered β-cells and insulin-positive cells in ducts were increased in pregnant women, suggesting that the adaptive increase in fractional β-cell area during pregnancy in humans is mostly achieved through β-cell neogenesis rather than through the replication of existing β-cells [90]. However, the results regarding β-cell proliferation and apoptosis should be taken cautiously for two reasons. First, as mentioned earlier, all gestational ages were pooled together, and if proliferation and death occur in a narrow temporal gestational window (as in rodents), it is possible that increases in β-cell proliferation and apoptosis will be diluted with the wide range of gestational ages analyzed. Second, since β-cell proliferation and apoptosis rates were provided per 100 islets and the number of small islets is markedly increased in the pancreas from pregnant women, this would suggest that both rates expressed as percentages of total β-cells might be increased in pregnant women. Unfortunately, these percentages were not included in this report. Taken together, although this study clearly confirms that β-cell expansion occurs in humans during pregnancy to adapt to the increased insulin demand, the precise cellular mechanisms involved in this adaptive response still require further analysis.
Future perspective
Several obvious reasons make the study of the molecular and cellular determinants involved in β-cell adaptation during pregnancy an exciting and important target for advancements in the treatment of diabetes. First, both human and rodent studies demonstrate similar β-cell adaptation during pregnancy, indicating that studying the mechanisms involved in β-cell expansion and hyperfunction in rodent models can have direct applicability in human disease. This is of particular importance for analyzing the cellular mechanisms involved in human maternal β-cell adaptive expansion, since studies in human pancreas samples are very difficult for evident reasons and are therefore limited. Second, these studies can lead to the discovery of associations of gene alterations/mutations/polymorphisms with GDM that could result in improvements in predicting and modifying therapies for the treatment of diabetes. Third, these studies could have a significant impact, not only on the wellbeing of the mother during and after pregnancy, but on the offspring of mothers with GDM who are known to have an increased risk of developing diabetes. Fourth, these studies uncovering molecular cues involved in β-cell expansion and hyperfunction during pregnancy can provide targets for future therapeutic drugs that can be used to enhance β-cell regeneration and function in diabetic situations other than GDM. Based on this reasoning, and taking advantage of the technical and intellectual progress made in recent decades (e.g., genetically modified mice, human islet biology, high-throughput screening and drug development), the next 5–10 years will see an in-depth analysis of the molecular determinants controlling the β-cell expansion and function machinery in one of the oldest known biological conditions, pregnancy.
Practice Points.
-
■
Maternal β-cell mass expands during pregnancy to respond to the increased insulin demand.
-
■
Return to normal β-cell mass levels occurs in the immediate postpartum period.
-
■
Alterations that affect β-cell mass expansion and function during pregnancy lead to deregulated glucose homeostasis and gestational diabetes mellitus.
-
■
Gestational diabetes mellitus markedly increases the risk of developing Type 2 diabetes after delivery and also leads to long-term health consequences for the offspring including obesity, hypertension, dyslipidemia, glucose intolerance and Type 2 diabetes.
-
■
Increased β-cell proliferation, size, function and potentially neogenesis are the cellular responses for the adaptive maternal response of the β-cell during gestation.
-
■
Lactogens and serotonin are extracellular factors that regulate β-cell expansion and function during pregnancy.
-
■
Intracellularly, HNF-4α, FoxM1, menin and mTOR are involved in the maternal β-cell adaptive expansion during gestation.
Acknowledgments
The authors are grateful to Rupangi Vasavada for her helpful comments on this article.
This work was supported by grants from the NIH (DK067351), the Juvenile Diabetes Research Foundation (1-2007-3) and the American Diabetes Association (1-10-BS-59) to Adolfo Garcia-Ocaña. Cem Demirci was the recipient of a research fellowship from the Lawson Wilkins Pediatric Endocrine Society.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 2.Dhawan S, Georgia S, Bhushan A. Formation and regeneration of the endocrine pancreas. Curr. Opin. Cell. Biol. 2007;19:634–645. doi: 10.1016/j.ceb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier JJ, Butler AE, Saisho Y, et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearl S, Kushner JA, Buchholz BA, et al. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 2010;95:E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997;29:301–307. doi: 10.1055/s-2007-979040. ▪ Excellent review describing the role of lactogens in pregnancy.
- 6.nBonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montana E, Bonner-Weir S, Weir GC. Transplanted β cell response to increased metabolic demand. Changes in β cell replication and mass. J. Clin. Invest. 1994;93:1577–1582. doi: 10.1172/JCI117137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of β cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- 10.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 11.Cebrian A, García-Ocaña A, Takane KK, Sipula D, Stewart AF, Vasavada RC. Overexpression of parathyroid hormone-related protein inhibits pancreatic β-cell death in vivo and in vitro. Diabetes. 2002;51:3003–3013. doi: 10.2337/diabetes.51.10.3003. [DOI] [PubMed] [Google Scholar]
- 12.Mathis D, Vence L, Benoist C. β-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 13.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in Type 1 diabetes. Nat. Rev. Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 14.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate post-transplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 15.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 16.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–2184. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes CJ. Type 2 diabetes. A matter of β cell life and death. Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 18.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with Type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Green IC, Howell SL, Montague W, Taylor KW. Regulation of insulin release from isolated islets of Langerhans of the rat in pregnancy. Biochem. J. 1973;134:481–487. doi: 10.1042/bj1340481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone AJ, Taylor KW. Metabolic adaptation to pregnancy shown by increased biosynthesis of insulin in islets of Langerhans isolated from pregnant rats. Nature. 1976;262:501–502. doi: 10.1038/262501a0. [DOI] [PubMed] [Google Scholar]
- 21.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 22.Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2 and glucose metabolism in islets during pregnancy and prolactin treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology. 1996;137:1640–1649. doi: 10.1210/endo.137.5.8612496. [DOI] [PubMed] [Google Scholar]
- 23.Green IC, Taylor KW. Effects of pregnancy in the rat on the size and insulin secretory response of islets of Langerhans. J. Endocrinol. 1972;54:317–325. doi: 10.1677/joe.0.0540317. [DOI] [PubMed] [Google Scholar]
- 24.Van Assche FA. Quantitative morphologic and histoenzymatic study of the endocrine pancreas in nonpregnant and pregnant rats. Am. J. Obstet. Gynecol. 1974;118:39–41. doi: 10.1016/s0002-9378(16)33642-0. [DOI] [PubMed] [Google Scholar]
- 25.Van Assche FA, Gepts W, Aerts L. Immunocytochemical study of the endocrine pancreas in the rat during normal pregnancy and during experimental diabetic pregnancy. Diabetologia. 1980;18:487–491. doi: 10.1007/BF00261705. [DOI] [PubMed] [Google Scholar]
- 26.Rieck S, Kaestner KH. Expansion of β cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Assche FA, Aerts L, Gepts W. Morphological changes in the endocrine pancreas in pregnant rats with experimental diabetes. J. Endocrinol. 1979;80:175–179. doi: 10.1677/joe.0.0800175. [DOI] [PubMed] [Google Scholar]
- 28. Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. ▪ Elegant study describing the role of the cell cycle regulator menin in maternal β-cell proliferation during gestation.
- 29. Gupta RK, Gao N, Gorski RK, et al. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. ▪ Elegant study describing the role of a transcription factor that is a MODY gene (HNF-4-α, MODY1) in maternal β-cell adaptation during pregnancy.
- 30. Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of β-cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. ▪ First study using a genetic mouse model to address the role of prolactin/PL in the β-cell adaptation in pregnancy.
- 31. Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired β-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59:143–152. doi: 10.2337/db09-0050. ▪ Important study describing the role of the transcription factor FoxM1 in the maternal β-cell adaptation during pregnancy.
- 32.Cheng YW, Caughey AB. Gestational diabetes: diagnosis and management. J. Perinatol. 2008;28:657–664. doi: 10.1038/jp.2008.62. [DOI] [PubMed] [Google Scholar]
- 33.Perkins JM, Dunn JP, Jagasia SM. Perspectives in gestational diabetes mellitus: a review of screening, diagnosis, and treatment. Clin. Diabetes. 2007;25:57–62. [Google Scholar]
- 34.Devlieger R, Casteels K, Van Assche FA. Reduced adaptation of the pancreatic β cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstet. Gynecol. Scand. 2008;87:1266–1270. doi: 10.1080/00016340802443863. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Department of Health and Human Services. GA, USA: Centers for Disease Control and Prevention; 2008. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. [Google Scholar]
- 36.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of Type 2 diabetes. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 39.Löbner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55:792–797. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]
- 40.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18:611–617. doi: 10.2337/diacare.18.5.611. [DOI] [PubMed] [Google Scholar]
- 41.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 42.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic β-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 43.Abouna S, Old RW, Pelengaris S, et al. Non-β-cell progenitors of β-cells in pregnant mice. Organogenesis. 2010;6:125–133. doi: 10.4161/org.6.2.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm. Res. 2006;65 Suppl. 3:41–49. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- 45.Cunha DA, Amaral ME, Carvalho CP, Collares-Buzato CB, Carneiro EM, Bischero AC. Increased expression of SNARE proteins and synaptotagmin IV in islets from pregnant rats and in vitro prolactin-treated neonatal islets. Biol. Res. 2006;39:555–566. doi: 10.4067/s0716-97602006000300016. [DOI] [PubMed] [Google Scholar]
- 46.Moldrup A, Petersen ED, Nielsen JH. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin producing cells. Endocrinology. 1993;133:1165–1172. doi: 10.1210/endo.133.3.8365359. [DOI] [PubMed] [Google Scholar]
- 47.Vasavada RC, Garcia-Ocana A, Zawalich WS, et al. Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 48.Brelje TC, Allaire P, Hegre O, Sorenson RL. Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology. 1989;125:2392–2399. doi: 10.1210/endo-125-5-2392. [DOI] [PubMed] [Google Scholar]
- 49.Sorenson RL, Johnson MG, Parsons JA, Sheridan JD. Decreased glucose stimulation threshold, enhanced insulin secretion, and increased β cell coupling in islets of prolactin-treated rats. Pancreas. 1987;2:283–288. doi: 10.1097/00006676-198705000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Brelje TC, Scharp DW, Lacy PE, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet β-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132:879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 51.Friedrichsen BN, Galsgaard ED, Nielsen JH, Moldrup A. Growth hormone- and prolactin-induced proliferation of insulinoma cells, INS-1, depends on activation of STAT5–signal transducer and activator of transcription 5. Mol. Endocrinol. 2001;15:136–148. doi: 10.1210/mend.15.1.0576. [DOI] [PubMed] [Google Scholar]
- 52.Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J. Endocrinol. 2007;193:367–381. doi: 10.1677/JOE-07-0043. [DOI] [PubMed] [Google Scholar]
- 53.Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote β cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J. Biol. Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- 54.Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 55.Rieck S, White P, Schug J, et al. The transcriptional response of the islet to pregnancy in mice. Mol. Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. ▪ Elegant study describing the first extracellular factor downstream of the lactogens and serotonin, that regulates maternal β-cell proliferation in pregnancy.
- 57.Layden BT, Durai V, Newman MV, et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J. Endocrinol. 2010;207(3):265–279. doi: 10.1677/JOE-10-0298. [DOI] [PubMed] [Google Scholar]
- 58.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocaña A. Growth factors and β cell replication. Int. J. Biochem. Cell. Biol. 2006;38:931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Horibe N, Okamoto T, Itakura A, et al. Levels of hepatocyte growth factor in maternal serum and amniotic fluid. Am. J. Obstet. Gynecol. 1995;173:937–942. doi: 10.1016/0002-9378(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 60.Otonkoski T, Beattie GM, Rubin JS, Lopez AD, Baird A, Hayek A. Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43:947–953. doi: 10.2337/diab.43.7.947. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases β cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Ocana A, Vasavada RC, Cebrian A, et al. Transgenic overexpression of hepatocyte growth factor in the β-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera J-C, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces b cell death. J. Biol. Chem. 2003;278:343–351. doi: 10.1074/jbc.M207848200. [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Talavera JC, Garcia-Ocana A, Takane KK, Cozar I, Stewart AF. Hepatocyte growth factor gene therapy, for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucorcorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145:467–474. doi: 10.1210/en.2003-1070. [DOI] [PubMed] [Google Scholar]
- 65.Fiaschi-Taesch NM, Berman DM, Sicari BM, et al. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57:2745–2754. doi: 10.2337/db08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gahr S, Merger M, Bollheimer LC, Hammerschmied CG, Schölmerich J, Hügl SR. Hepatocyte growth factor stimulates proliferation of pancreatic β-cells particularly in the presence of subphysiological glucose concentrations. J. Mol. Endocrinol. 2002;28:99–110. doi: 10.1677/jme.0.0280099. [DOI] [PubMed] [Google Scholar]
- 67.Vasavada RC, Wang L, Fujinaka Y, et al. Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes. 2007;56:2732–2743. doi: 10.2337/db07-0461. [DOI] [PubMed] [Google Scholar]
- 68.Mellado-Gil JM, Rosa TC, Demirci C, et al. Disruption of HGF/c-met signaling enhances pancreatic β cell death and accelerates the onset of diabetes. Diabetes. 2011;60(2):525–536. doi: 10.2337/db09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J. Physiol. 2009;587:5031–5037. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green IC, El Seifi S, Perrin D, Howell SL. Cell replication in the islets of Langerhans of adult rats: effects of pregnancy, ovariectomy and treatment with steroid hormones. J. Endocrinol. 1981;88:219–224. doi: 10.1677/joe.0.0880219. [DOI] [PubMed] [Google Scholar]
- 71.Costrini NV, Kalkhoff RK. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet content. J. Clin. Invest. 1971;50:992–999. doi: 10.1172/JCI106593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology. 1993;133:2227–2234. doi: 10.1210/endo.133.5.8404674. [DOI] [PubMed] [Google Scholar]
- 73.Holness MJ, Sugden MC. Dexamethasone during late gestation exacerbates peripheral insulin resistance and selectively targets glucose-sensitive functions in β cell and liver. Endocrinology. 2001;142:3742–3748. doi: 10.1210/endo.142.9.8379. [DOI] [PubMed] [Google Scholar]
- 74.Nieuwenhuizen AG, Schuiling GA, Hilbrands LG, Bisschop EM, Koiter TR. Proliferation of pancreatic islet-cells in cyclic and pregnant rats after treatment with progesterone. Horm. Metab. Res. 1998;30:649–655. doi: 10.1055/s-2007-978952. [DOI] [PubMed] [Google Scholar]
- 75.Nieuwenhuizen AG, Schuiling GA, Liem SM, Moes H, Koiter TR, Uilenbroek JTJ. Progesterone stimulates pancreatic cell proliferation in vivo. Eur. J. Endocrinol. 1999;140:256–263. doi: 10.1530/eje.0.1400256. [DOI] [PubMed] [Google Scholar]
- 76.Picard F, Watanabe M, Schoonjans K, Lydon J, O’Malley BW, Auwers J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to β-cell proliferation. Proc. Natl Acad. Sci. USA. 2002;99:15644–15648. doi: 10.1073/pnas.202612199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amaral ME, Cunha DA, Anhê GF, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J. Endocrinol. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- 78.Rachdi L, Balcazar N, Osorio-Duque F, et al. Disruption of Tsc2 in pancreatic β cells induces β cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc. Natl Acad. Sci. USA. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balcazar N, Sathyamurthy A, Elghazi L, et al. mTORC1 activation regulates β-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J. Biol. Chem. 2009;284:7832–7842. doi: 10.1074/jbc.M807458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahr E, Molano RD, Pileggi A, et al. Rapamycin impairs in vivo proliferation of islet β-cells. Transplantation. 2007;84:1576–1583. doi: 10.1097/01.tp.0000296035.48728.28. [DOI] [PubMed] [Google Scholar]
- 81.Shih DQ, Stoffel M. Molecular etiologies of MODY and other early-onset forms of diabetes. Curr. Diab. Rep. 2002;2:125–134. doi: 10.1007/s11892-002-0071-9. [DOI] [PubMed] [Google Scholar]
- 82.Colom C, Corcoy R. Maturity onset diabetes of the young and pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:605–615. doi: 10.1016/j.beem.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 83.Ye H, Kelly TF, Samadani U, et al. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol. Cell. Biol. 1997;17:1626–1641. doi: 10.1128/mcb.17.3.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H, Ackermann AM, Gusarova GA, et al. The FoxM1 transcription factor is required to maintain pancreatic β-cell mass. Mol. Endocrinol. 2006;20:1853–1866. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
- 85.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology. 2003;38:1552–1562. doi: 10.1016/j.hep.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 86.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Evidence of increased islet cell proliferation in patients with recent-onset Type 1 diabetes. Diabetologia. 2010;53:2020–2028. doi: 10.1007/s00125-010-1817-6. [DOI] [PubMed] [Google Scholar]
- 87.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, et al. Molecular control of cell cycle progression in the pancreatic β-cell. Endocr. Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 88.Kirwan JP, Hauguel-de Mouzon S, Lepercq J, et al. TNF is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 89. Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br. J. Obstet. Gynaecol. 1978;85:818–820. doi: 10.1111/j.1471-0528.1978.tb15835.x. ▪ Pioneer study describing the maternal β-cell expansion during gestation in humans.
- 90. Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia. 2010;53:2167–2176. doi: 10.1007/s00125-010-1809-6. ▪ Most recent study addressing the effect of pregnancy in β-cell mass and β-cell biological processes in a large number of human samples.