Abstract
Sphingosine 1-phosphate (S1P), a pleiotropic lipid mediator, binds to five related G-protein-coupled receptors to exert its effects. As S1P1 receptor (S1P1R) activation blocks kidney inflammation in acute renal injury, we tested whether activation of S1P1Rs ameliorates renal injury in early-stage diabetic nephropathy (DN) in rats. Urinary albumin excretion increased in vehicle-treated diabetic rats (single injection of streptozotocin), compared with controls, and was associated with tubule injury and increased urinary tumor necrosis factor-α (TNF-α) at 9 weeks. These effects were significantly reduced by FTY720, a non-selective, or SEW2871, a selective S1P1R agonist. Interestingly, only FTY720 was associated with reduced total lymphocyte levels. Albuminuria was reduced by SEW2871 in both Rag-1 (T- and B-cell deficient) and wild-type diabetic mice after 6 weeks, suggesting that the effect was independent of lymphocytes. Another receptor, S1P3R, did not contribute to the FTY720-mediated protection, as albuminuria was also reduced in diabetic S1P3R knockout mice. Further, both agonists restored WT-1 staining along with podocin and nephrin mRNA expression, suggesting podocyte protection. This was corroborated in vitro, as SEW2871 reduced TNF-α and vascular endothelial growth factor mRNA expression in immortalized podocytes grown in media containing high glucose. Whether targeting kidney S1P1Rs will be a useful therapeutic measure in DN will need direct testing.
Keywords: diabetic nephropathy, inflammation, lymphocytes, podocyte
Diabetes mellitus and diabetic nephropathy (DN) are disorders that result, in part, from a dysregulation of the immune system. Proinflammatory markers such as tumor necrosis factor-α (TNF-α), interleukin-6, and C-reactive protein in patients with DN suggest that chronic low-grade inflammation is a characteristic of DN.1–3 Activated T-lymphocytes have been associated with DN and correlated with initiation of early-stage proteinuria in type 1 and type 2 diabetes.4,5 Infiltration of CD4+ and CD8+ T cells, as well as overexpression of major histocompatibility complex class I and II molecules, on lymphocytes may have an important role in the pathogenesis of both immune-mediated and nonimmune-mediated inflammatory kidney disease associated with DN.6 These findings point to the role of chronic mild inflammation and T cells in the pathogenesis of DN.
Sphingosine 1-phosphate (S1P), a pleiotropic lipid mediator, is produced by phosphorylation of sphingosine by sphingosine kinases (SphKs; SphK1, and SphK2) in response to a variety of stimuli. S1P binds to five related G-protein-coupled receptors, termed S1P1–5Rs.7 S1P receptors regulate a wide variety of important cellular functions, including cell survival, cytoskeletal rearrangements, and cell motility.7,8 We and others have shown that S1P1 receptors (S1P1Rs) are expressed in kidneys as well as on bone-marrow-derived cells, and on activation reduce inflammation.9,10 FTY720, a non-selective S1P1R agonist, and SEW2871, a selective S1P1R agonist, elicit lymphopenia resulting from reversible redistribution of lymphocytes from the circulation to secondary lymphatic tissue.11,12 FTY720 is a pro-drug and is phosphorylated by SphK1 and SphK2 into its active form.13 Notably, previous studies demonstrated a remarkable protective effect of FTY720 in models of multiple sclerosis,13,14 autoimmune myocarditis,15 uveoretinitis,16 systemic lupus erythematosus,17 and atherosclerosis.18 FTY720 prevents autoimmune diabetes in non-obese diabetic mice by preventing the development of hyperglycemia and insulinitis.19–21 However, these studies mainly focused on the effect of FTY720 on the pancreas. In humans, FTY720 has been shown to be efficacious in relapsing multiple sclerosis,22 and has been recently approved by the Food and Drug Administration for the treatment of multiple sclerosis.
We have demonstrated that activation of S1P1Rs protects the kidney by reducing inflammation, leukocyte infiltration, and vascular permeability associated with acute renal ischemia–reperfusion injury, primarily by peripheral lymphocyte depletion or direct effects on kidney cells expressing S1P1Rs.9,23 Whether FTY720 or other S1P1R agonists have an effect to reduce inflammation, proteinuria, and histological changes associated with DN is not known. Because lymphocytes may contribute to early-stage proteinuria,4,5 we hypothesized that activation of S1P1Rs would ameliorate renal tissue injury associated with early-stage DN. Using streptozotocin (STZ)-induced rat and mouse animal models, we show that chronic S1P1R activation by FTY720 or SEW2871 prevents functional and structural changes induced by diabetes by ameliorating urinary albumin excretion (UAE), urinary TNF-α, podocyte-specific proteins, podocyte TNF-α and vascular endothelial growth factor (VEGF) mRNA expression, and tubule vesicular injury and vacuolization. The mechanism(s) by which FTY720 and SEW2871 reduce the functional and morphological changes induced by diabetes appear(s) to be independent of lymphocytes, and is likely mediated primarily by S1P1Rs and not by S1P3Rs. To our knowledge, this study provides for the first time evidence of the protective role of S1P1R agonists in DN and demonstrates that targeting kidney S1P1Rs may be a novel therapeutic intervention in the treatment of DN.
RESULTS
Effects of FTY720 and SEW2871 on blood glucose, body weight, systolic blood pressure, and urinary volume in rats at 9 weeks were studied. Blood glucose levels increased significantly in the diabetes groups treated with vehicle, FTY720, or SEW2871 throughout the study period (Table 1). Control rats gained weight as expected. The increase in body weight in vehicle- and drug-treated diabetic groups was modest, and body weight in diabetic groups was markedly lower than control rats at week 9. There was a significant increase in urinary volume in diabetic groups. There were no significant changes in systolic blood pressure at 9 weeks after STZ induction of diabetes in any group.
Table 1.
Changes in blood glucose, body weight, systolic blood pressure (BP), and urinary volume in control, vehicle-treated diabetic rats, and diabetic rats treated with FTY720 or SEW2871 for 9 weeks
| Control | Diabetes + vehicle | Diabetes + FTY720 | Diabetes + SEW2871 | |
|---|---|---|---|---|
| Blood glucose (mg/dl) | ||||
| Week 0 | 81 ± 4 | 78 ± 2 | 80 ± 4 | 80 ± 5 |
| Week 3 | 77 ± 4 | 326 ± 16* | 321 ± 28* | 333 ± 8* |
| Week 6 | 89 ± 5 | 334 ± 24* | 390 ± 12* | 418 ± 30*,† |
| Week 9 | 88 ± 7 | 465 ± 22* | 354 ± 18*,† | 414 ± 29* |
| Body weight (g) | 514 ± 15 | 264 ± 28* | 285 ± 13* | 268 ± 8* |
| Systolic BP (mm Hg) | 135 ± 3 | 136 ± 2 | 138 ± 3 | 138 ± 2 |
| Urinary volume (ml per 24 h) | 14 ± 1 | 41± 4* | 33 ± 4* | 39 ± 6* |
Data are mean ± s.e.m.
P<0.001 compared to control
P<0.05 compared to diabetes + vehicle.
Effects of FTY720 and SEW2871 on renal function in diabetic rats
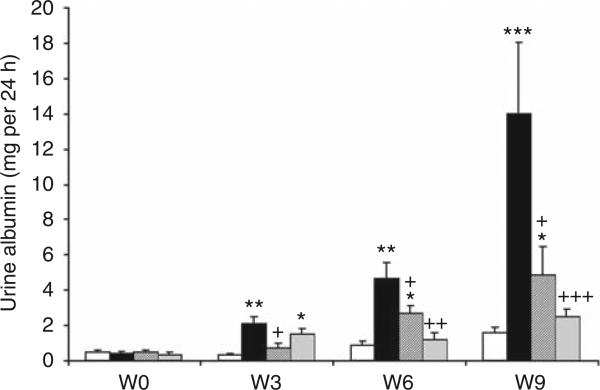
As expected, diabetic rats treated with vehicle showed a significant progressive increase in UAE that began as early as 3 weeks after STZ, continued at week 6, and peaked at week 9. Both FTY720 and SEW2871 significantly reduced UAE at week 6 and at week 9, compared with vehicle (Figure 1).
Figure 1. FTY720 and SEW2871 reduce urinary albumin excretion in diabetic rats.
To determine the effect of FTY720 and SEW2871 in diabetic rats, 24-h urine collections were obtained for measurement of urine albumin at baseline (W0) and at weeks (W) 3, 6, and 9 of the study. Open bars, control group; black bars, vehicle-treated diabetes group; dark gray bars, diabetes group treated with FTY720 (0.3 mg/kg, oral gavage); and light gray bars, diabetes group treated with SEW2871 (0.1 mg/kg, i.p.). Data are means ± s.e.m. *P < 0.05, **P < 0.005 and ***P < 0.0005 compared with control; +P < 0.05, ++P < 0.01 and +++P < 0.005 compared with vehicle-treated diabetes group.
Effects of FTY720 and SEW2871 on renal histology in DN
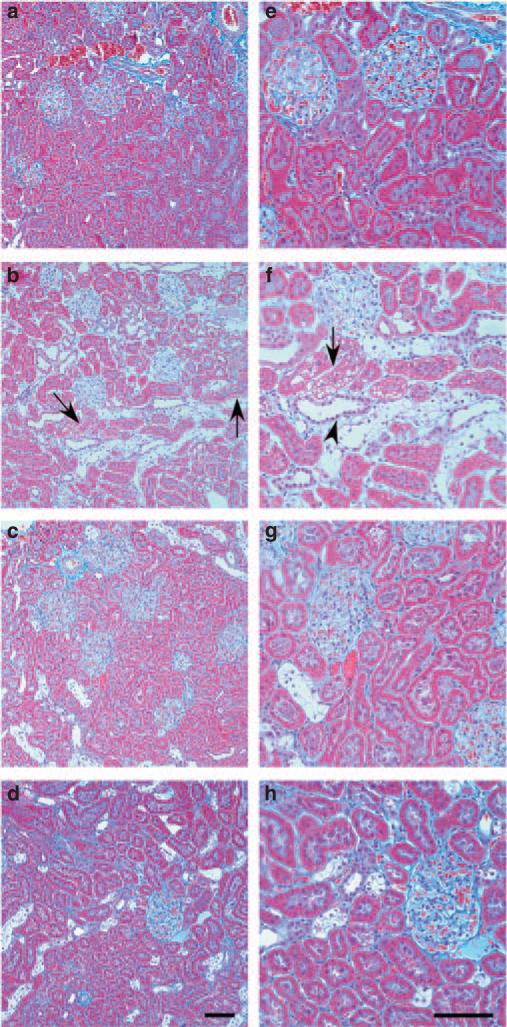
Masson's trichrome stain of rat kidneys from control rats (Figure 2a and e), diabetic rats treated with vehicle (Figure 2b and f), and diabetic rats treated with FTY720 (Figure 2c and g) or SEW2871 (Figure 2d and h) at week 9 of the study was used to evaluate renal histology. Diabetic rats treated with vehicle (Figure 2b and f) had severe tubular injury associated with vacuolization (tubular injury score, 3.4 ± 0.4; P < 0.0001, n = 5) compared with control (score, 0.2 ± 0.1, n = 7), with slight interstitial fibrosis. Both FTY720 and SEW2871 significantly reduced these histological effects (score 2.3 ± 0.5, n = 6 and 2.2 ± 0.3, n = 9, respectively; P < 0.05 compared with vehicle).
Figure 2. FTY720 and SEW2871 improve renal histology in diabetic nephropathy.
Masson's trichrome staining of kidney sections from normal control rat (a, e), diabetic rat treated with vehicle (b, f), and a diabetic rat treated with FTY720 (c, g) or SEW2871 (d, h) at 9 weeks of the study. Representative photographs show extensive tubular damage with vacuolization (arrows), interstitial fibrosis (blue staining of interstitial collagen), and increased intertubular space (arrowhead) in vehicle-treated diabetic rats and minimal changes in treated animals. Bar = 50 μm.
Effects of FTY720 and SEW2871 on urinary TNF-α production
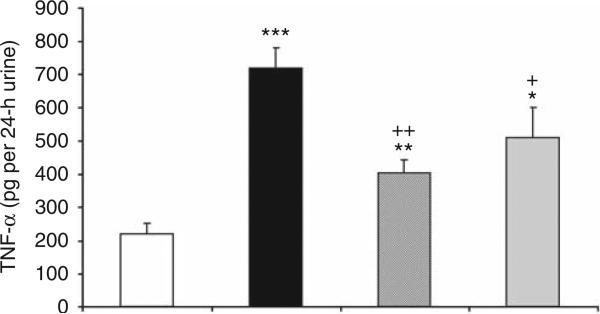
Inflammatory cytokines have an important role in DN. Therefore, we assessed the anti-inflammatory effect of FTY720 and SEW2871 treatment on urinary TNF-α level in diabetic rats. Urinary TNF-α excretion increased significantly in the vehicle-treated diabetic rats at week 9, and this increase was reduced by treatment with either FTY720 or SEW2871 administration (Figure 3).
Figure 3. FTY720 and SEW2871 reduce urinary tumor necrosis factor-α (TNF-α) level.
Urine collections (24-h) were obtained for measurement of TNF-α in rats after 9 weeks of streptozotocin (STZ)-induced diabetes. Open bar, control group; filled bar, vehicle-treated diabetes group; hatched bar, diabetes group treated with FTY720 (0.3 mg/kg, oral gavage); and gray bar, diabetes group treated with SEW2871 (0.1 mg/kg, i.p.). Values are means ± s.e.m. *P < 0.05, **P < 0.01, and ***P < 0.005 compared with control; +P < 0.05 and ++P < 0.01 compared with vehicle-treated diabetes group.
Effects of FTY720 and SEW2871 on kidney tissue content of T cells, B cells, and macrophages in diabetes
As S1P1R activation has been shown previously to reduce peripheral blood and tissue lymphocyte count,11,12,24 we investigated whether the protective effect of FTY720 and SEW2871 in diabetes is mediated by lymphopenia. As shown in Table 2, fluorescence-activated cell sorting analysis revealed no significant effects of FTY720 or SEW2871 treatment compared with vehicle on the kidney content of T cells, B cells, or macrophages in diabetic rats. In contrast, FTY720 but not SEW2871 treatment significantly reduced blood lymphocyte count in diabetic rats (Table 2).
Table 2.
Effects of FTY720 and SEW2871 on blood lymphocyte count and kidney tissue content of T cells, B cells, and macrophages in diabetes
| Method | Diabetes + vehicle | Diabetes + FTY720 | Diabetes + SEW2871 | |
|---|---|---|---|---|
| CD3 | FACSa | 1.1 ± 0.3 | 0.6 ± 0.3 | 2.0 ± 0.3 |
| CD8 | FACS | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.7 ± 0.2 |
| CD4 | FACS | 1.4 ± 0.3 | 0.7 ± 0.2 | 2.5 ± 0.7 |
| CD45R | FACS | 0.6 ± 0.3 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| CD11b | FACS | 0.7 ± 0.1 | 0.8 ± 0.04 | 2.9 ± 0.5 |
| Lymphocyte | HEMAVETb | 4.3 ± 0.7 | 2.5 ± 0.5* | 4.5 ± 0.7 |
Abbreviations: HEMAVET, veterinary hematology system; FACS, flow cytometry analysis.
Kidney tissue content of T cells (CD3+, CD8+, CD4+), B cells (CD45R+), and macrophages (CD11b+) was determined by FACS (no of cells × 104) after 9 weeks in diabetic rats.
Blood lymphocyte count (K/μl: 1000/μl) was determined by using HEMAVET.
P<0.05 compared with vehicle-treated diabetic rats.
Effects of SEW2871 on UAE in Rag-1 diabetic mice
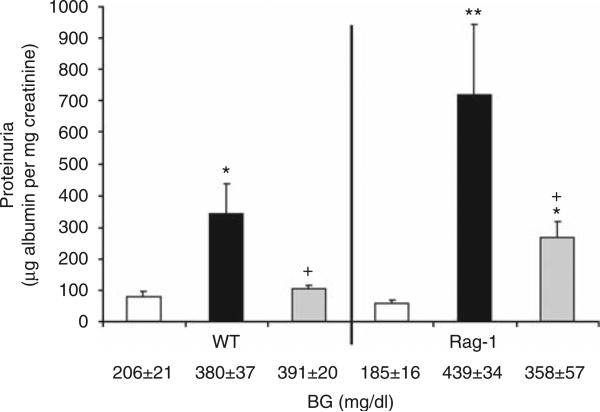
We further questioned whether the effect of S1P1R agonist was mediated, at least in part, by inducing lymphopenia in the STZ-induced diabetic model. Therefore, we used Rag-1 mice (which lack T- and B cells) and wild-type (WT) mice. As shown in Figure 4, UAE was increased significantly by STZ-induced diabetes in WT and Rag-1 mice. SEW2871 treatment significantly reduced UAE in diabetic WT group and was associated with reduced lymphocyte count (0.36 ± 0.1 K/μl; P < 0.01) from vehicle-treated diabetes group (1.27 ± 0.2 K/μl). SEW2871 still reduced UAE in diabetic Rag-1 mice.
Figure 4. SEW2871 reduces urinary albumin excretion (UAE) in Rag-1 diabetic mice.
Urine collections were obtained for measurement of UAE at week 6 in wild-type (WT) and Rag-1 mice after streptozotocin (STZ)-induced diabetes. Open bars, control group; black bars, vehicle-treated diabetes group; and gray bars, diabetes group treated with SEW2871 (1 mg/kg, i.p.). Data are means ± s.e.m. *P < 0.05 and **P < 0.01 compared with control; +P < 0.05 compared with vehicle-treated diabetes group. Blood glucose (BG) values are shown below the bars for each group.
S1P3Rs do not contribute to the FTY720 protective effect in DN
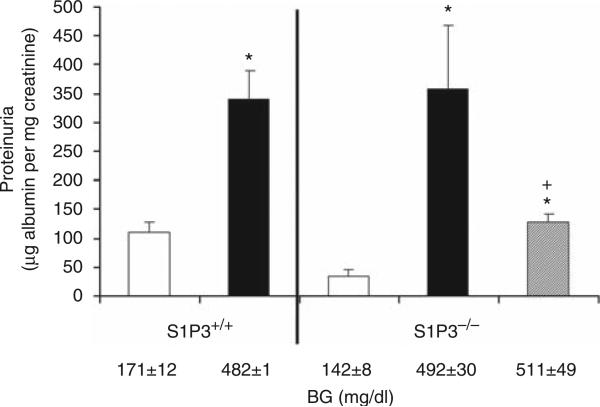
FTY720 is a non-selective S1P1R agonist, and it may activate other S1P receptors such as S1P3R, S1P4R, and S1P5R. We have shown previously that, of the five subtypes, S1P1R and S1P3R have the highest expression in the kidney.9 To examine the relative contribution of these two receptor subtypes to FTY720 effect in DN, we induced diabetes in mice deficient in S1P3Rs (S1P3–/–) and their corresponding WT littermate S1P3+/+ controls. FTY720 significantly reduced UAE in STZ-treated S1P3–/– mice (Figure 5).
Figure 5. FTY720 reduces albuminuria independent of sphingosine 1-phosphate 3 receptor (S1P3R) effects.
Urine collections were obtained for measurement of urinary albumin excretion (UAE) at week 4 in S1P3+/+ (wild type, WT) and S1P3–/– mice after streptozotocin (STZ)-induced diabetes. Open bars, control group; black bars, vehicle-treated diabetes group; and gray bar, diabetes group treated with FTY720 (0.3 mg/kg, oral gavage). Data are means ± s.e.m. *P < 0.05 compared with control; +P < 0.05 compared with vehicle-treated diabetes group. Blood glucose (BG) values are shown below the bars for each group.
S1P1R agonists regulate podocyte-specific proteins
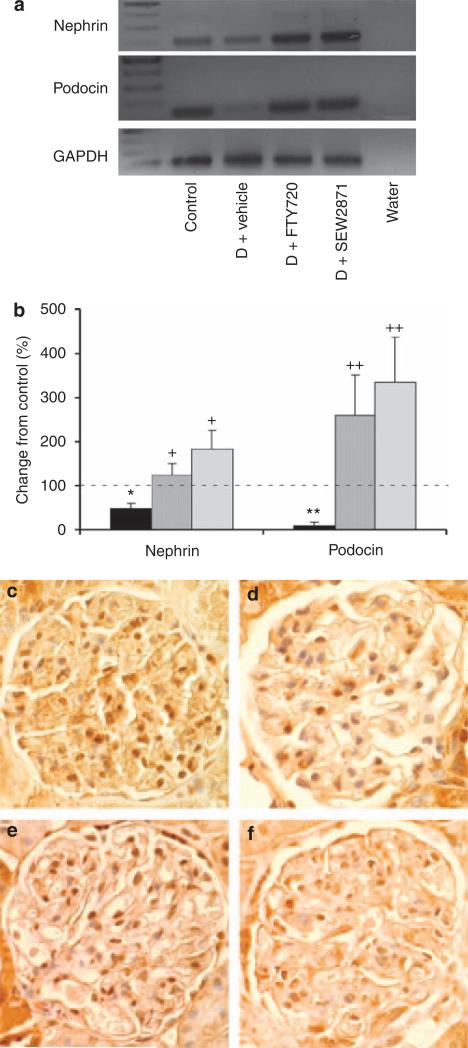
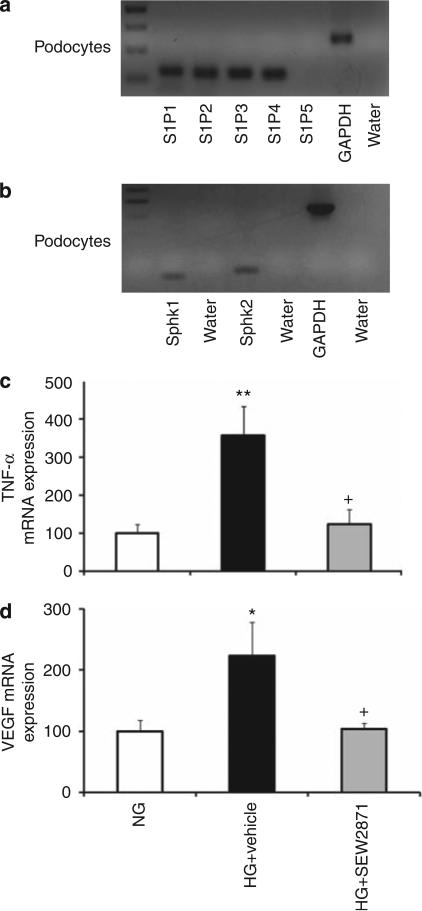
To determine the possible mechanisms by which FTY720 and SEW2871 reduce UAE and mediate renal tissue protection, we examined their effects on podocytes. At 9 weeks of diabetes, both nephrin and podocin (podocyte-specific proteins) mRNA were significantly reduced and were associated with reduced number of WT-1-positive staining (16.2 ± 1.2 cells; P < 0.0001) in kidneys from vehicle-treated diabetic rats compared with control (WT-1-positive staining: 30 ± 1 cells). FTY720 and SEW2871 restored and markedly increased the expression of nephrin and podocin in diabetic rats and increased the number of WT-1-positive staining (28 ± 1 cells, P < 0.0001; 22 ± 2 cells, P < 0.05), respectively, compared with vehicle (Figure 6). These data suggest that FTY720 and SEW2871 could have direct effects on podocytes. To explore this option, first, we examined the expression of S1P receptors and S1P synthetic enzymes in podocytes. We have shown previously that S1P1-4Rs but not S1P5Rs are expressed in kidney, with S1P1Rs having the highest receptor expression.9 We also showed that both SphK1 and SphK2 are expressed in the kidney.25 We now extend these findings and show mRNA expression of S1P1–4Rs, but not of S1P5R (Figure 7a), and of SphK1 and SphK2 (Figure 7b) in an immortalized mouse podocyte cell line, indicating a possible role of endogenous S1P in podocytes under physiological or pathophysiological conditions. To explore this possibility, we cultured immortalized podocytes with normal glucose or high glucose (HG) media for 14 days. Our data show enhanced TNF-α and VEGF mRNA expression after HG media, an effect significantly reduced using SEW2871 treatment (Figure 7c and d). In contrast, there were no effects on podocyte apoptosis (data not shown) in all groups.
Figure 6. Sphingosine 1-phosphate 1 receptor (S1P1R) agonists regulate podocyte-specific proteins.
(a, b) Reverse transcriptase-PCR (RT-PCR) was performed on whole rat kidney total RNA isolated after 9 weeks of diabetes from control, vehicle-treated diabetic (D) rats, and diabetic rats treated with FTY720 or SEW2871. (a) Gel analysis of PCR products. (b) Expression of nephrin and podocin mRNA was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and data were calculated as expression relative to control. Black bars, vehicle-treated diabetic rats; dark gray bars, diabetic rats treated with FTY720 (0.3 mg/kg, oral gavage); and light gray bars, diabetic rats treated with SEW2871 (0.1 mg/kg, i.p.). Data are means ± s.e.m. *P < 0.05, **P < 0.005 compared with control (dashed line); +P < 0.05, ++P < 0.01 compared with vehicle-treated diabetes group. (c–f) WT-1 staining of rat kidney after 9 weeks of diabetes from control (c), vehicle-treated diabetic (d) rats, and diabetic rats treated with FTY720 (e) or SEW2871 (f) (magnification × 400).
Figure 7. Role of sphingosine 1-phosphate 1 receptor (S1P1R) agonists in immortalized podocytes in vitro.
(a, b) Total RNA from immortalized podocytes was extracted and subjected to analysis by reverse transcriptase-PCR (RT-PCR) for S1P receptor mRNA expression (a) and sphingosine kinase 1 and 2 (SphK1 and SphK2) mRNA expression (b). (c, d) Total RNA from immortalized podocytes subjected to normal glucose (NG) or high glucose (HG) media for 14 days with or without SEW2871 (n = 5–6 each group) was extracted and subjected to analysis by RT-PCR for tumor necrosis factor-α (TNF-α) mRNA expression (c) and vascular endothelial growth factor (VEGF) mRNA expression (d). Expression of TNF-α and VEGF mRNA was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Open bars, NG; black bars, HG treated with vehicle; and gray bars, HG treated with SEW2871 (1 μmol/l). Data are means ± s.e.m. *P < 0.05, **P < 0.01 compared with NG; +P < 0.05 compared with HG + vehicle.
DISCUSSION
This study demonstrates a finding previously unrecognized that FTY720 and SEW2871 have protective effects on renal function and histology in an STZ-induced diabetic rat model. The effect of S1P1R activation in diabetes is probably not due to lymphocyte depletion, as SEW2871 continues to induce renal tissue protection in Rag-1 mice that lack both T and B cells. Furthermore, FTY720 effects are mediated mainly through S1P1R activation, as FTY720 was protective in S1P3–/– mice. FTY720 or SEW2871, either directly by maintaining podocyte-specific proteins and expression of TNF-α and VEGF or indirectly, control glomerular permeability and podocyte function. Additionally, we observed marked reduction of tubule injury and vacuolization, suggesting effects of these compounds on proximal tubule cells. Because progressive kidney disease due to DN results from abnormalities of the glomerulus and the tubulointerstitium, the broad ranging effects of S1P1R agonists might be more effective than current therapies for DN.
The biological functions of S1P have been explored in diverse cell types and tissues. However, the exact role of S1P in kidney physiology and pathophysiology remains unclear. S1P regulates a wide variety of important cellular functions.7,8 S1P1Rs are expressed in the kidney,9,26 and have an important role in maintaining endothelial cell integrity27,28 and in trafficking of lymphocytes.29
Recently, we demonstrated that both FTY720 and SEW2871 prevent renal ischemia–reperfusion injury by activating S1P1Rs.9,23 Our current study extends the role of S1P1Rs from an acute to a chronic model of DN. In this study, we demonstrated a significant increase in UAE after 3 weeks of diabetes in conscious rats, which persisted throughout the study period. Administration of FTY720 or SEW2871 significantly reduced albuminuria associated with diabetes. The effect of S1P1R activation was associated with marked reduction of tubular injury and vacuolization in DN. It is now clear that DN is a tubule–glomerular disorder. Whether S1P1R activation has a direct effect on tubular cells in DN is not clear in this study. However, data from our laboratory show that S1P1R activation mediates renal tissue protection through a direct effect on proximal tubule S1P1Rs in renal ischemia–reperfusion injury.23 It is possible that tubular cell injury associated with DN contributes, in part, to increased UAE in diabetes and that S1P1R activation reduced albuminuria by protecting tubular injury. Additional study is needed to explore the direct effect of S1P1R activation on tubular cells in DN. Furthermore, the reduction of podocyte mRNA expression of VEGF after SEW2871 treatment of immortalized podocytes following HG suggests that the effects of S1P1R agonists on reducing proteinuria early in the course of diabetes may be mediated by a direct effect on podocytes.
We have shown previously that levels of urinary TNF-α were correlated with increases in UAE in diabetes.30 TNF-α is produced mainly by monocytes/macrophages, T and B lymphocytes, and glomerular mesangial cells.31,32 Both TNF-α and interleukin-1β have been associated with increased vascular endothelial permeability33 and have been detected in isolated glomerular basement membrane in diabetes.34 It is possible that S1P1R activation by FTY720 or SEW2871 protects kidneys in DN by reducing kidney inflammatory cytokines such as TNF-α. The fact that TNF-α was undetectable in the blood30 indicates that the source of urinary TNF-α was mainly from the kidney. Interestingly, neither FTY720 nor SEW2871 affected the number of kidney macrophages, as shown by fluorescence-activated cell sorting, suggesting that their effects are mainly mediated through kidney-derived cells producing TNF-α. Our data in podocytes support this hypothesis and show that SEW2871 reduced podocyte mRNA expression of TNF-α after HG. Another observation from our study indicates that SEW2871 treatment elicits more kidney macrophage recruitment than the vehicle group. These data are consistent with a recent report indicating that an increase in the percentage and absolute number of circulatory monocytes in response to SEW2871 treatment occurs by enhanced monocyte emigration from the bone marrow.35
The mechanism by which S1P1R agonists mediate protection in STZ-induced DN is not completely clear. Previous reports showed that S1P1R activation induces lymphopenia resulting from a reversible redistribution of lymphocytes from the circulation to secondary lymphatic tissue.11,12,36 Therefore, we questioned whether FTY720 and SEW2871 have a similar effect in diabetes. Our data show that FTY720, but not SEW2871, reduced elevated blood lymphocyte counts that occurred in diabetic rats. These studies suggested that S1P1R agonists mediate their effect independent of lymphocytes. To test this hypothesis, we examined the effect of SEW2871 at doses known to induce lymphopenia in mice that lack T- and B lymphocytes (Rag-1) and found that even in the absence of lymphocytes, SEW2871 reduced albuminuria. These results raise the possibility that S1P1R agonists mediate their protective effect independent of their canonical effect on lymphocytes and may induce direct effects to alter glomerular permeability. Our data demonstrating direct effects of SEW2871 on cultured podocytes are consistent with this hypothesis.
FTY720 is rapidly phosphorylated in vivo by SphKs to form FTY720 phosphate, which is an agonist for S1P1R and S1P3–5Rs, but not for S1P2R,11,13 and mediates the immunosuppressive effect of FTY720.11,13 Therefore, the relative contributions of different actions of FTY720 may depend on tissue expression of receptor subtypes. Both S1P1R and S1P3R are highly expressed in the kidney.9 S1P1R activation mediates cell survival and proliferation of vascular endothelial cells, as well as stimulation of endothelial cell nitric oxide synthase.27,37 These effects on endothelial cells may lead to maintenance of endothelial barrier function. We have shown previously that FTY720 is necessary to maintain vascular integrity after ischemia–reperfusion injury.9 Whether the protective effect of FTY720 and SEW2871 is due to action on kidney endothelial cells cannot be answered by our study, but will require additional investigation. In contrast to S1P1R, S1P3R activation is thought to lead to a loss of epithelial integrity in the lung38 and may have a similar function in the kidney. Therefore, we questioned whether S1P3Rs are involved in FTY720 protective effects in DN. Our data clearly show that FTY720 still reduced UAE in diabetic S1P3–/– mice. This excludes any possible role of S1P3R to mediate FTY720 effects in DN and provides additional evidence that the effect of FTY720 is mainly mediated through S1P1R activation.
We further investigated a possible mechanism of S1P1R activation in DN. As podocytes have a pivotal role in maintaining the glomerular filtration barrier, we questioned whether podocytes either directly or indirectly mediate S1P1R agonist effect. Our data demonstrate that FTY720 and SEW2871 restored both nephrin and podocin mRNA expression and ameliorated the reduction in WT-1-positive staining after 9 weeks of diabetes, indicating a potential direct or indirect role of S1P1R activation on kidney cells such as podocytes. Both podocin and nephrin are crucial complex proteins in the assembly and reinforcement of the slit diaphragm by binding to the actin cytoskeleton.39,40 Dysregulation of these podocyte structural proteins has been associated with glomerular injury in diabetes.30,41,42
Recently, we demonstrated a rank order of expression of S1P1R > S1P3R > S1P2R > S1P4R in mouse kidneys, whereas S1P5R mRNA was not found.9 In addition, both SphK1 and SphK2 are expressed in the kidney.25 In this study, we further confirmed the expression of S1P1R and the S1P biosynthetic enzymes, SphK1 and SphK2, on podocytes, indicating a possible direct site of action for FTY720 and SEW2871 on podocytes in diabetes and that S1P may have a role, possibly autocrine, in podocytes under physiological or pathophysiological conditions. Treatment with FTY720 or SEW2871 did not change S1P1R expression in diabetes (data not shown); therefore, S1P1R agonists likely act by increasing S1P1R signaling. In addition, these data provide evidence that chronic administration of FTY720 or SEW2871 does not downregulate S1P1Rs. To investigate the direct role of S1P1R agonist in podocytes, we carried out in vitro experiments in immortalized podocytes grown in culture and treated with HG. Our data show that SEW2871 reduced the induction of podocyte mRNA expression of TNF-α and VEGF after HG, indicating a direct effect of S1P1R agonist in podocytes.
In summary, this study extends our previous observations of the protective effect of S1P from an acute to a chronic diabetic model and demonstrates that chronic administration of S1P1R agonists attenuates renal dysfunction in early-stage DN. We believe that the renal tissue protective effect of S1P1R agonist is not mediated by inducing lymphopenia, but may have a direct effect on kidney-derived cells such as podocytes. We conclude that S1P1R activation may represent a novel therapeutic option for the treatment of early-stage diabetic kidney disease.
MATERIALS AND METHODS
Induction of diabetes
Experiments were conducted in 14-week-old conscious Sprague—Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 240–260 g. Additional experiments were conducted in 7- to 8-week-old C57BL/6J and Rag-1 mice (B6 background; Jackson Laboratories, Bar Harbor, ME) and in mice that lack S1P3R; S1P3–/– mice (congenic on C57BL/6) or S1P3+/+ mice (littermate controls) were provided by Dr Richard L Proia (National Institutes of Health). All experiments were performed in accordance with the NIH and the Institutional Animal Care and Use Guidelines, and all protocols and procedures were approved by the University of Virginia Animal Care and Use Committee. After an overnight fast, animals were given a single intravenous injection via tail vein of vehicle or STZ (Sigma, St Louis, MO; rats 50 mg/kg body weight and mice 80 mg/kg body weight; dissolved in lactated Ringer solution). Establishment of diabetes was confirmed 48 h after STZ induction by measuring random blood glucose levels (Accu-Chek glucometer, Boehringer Mannheim, Indianapolis, IN).
Drug delivery
A 1 mmol/l stock solution of FTY7209,13 (Novartis, Basel, Switzerland) or SEW28719 was prepared in a 3% fatty acid-free bovine serum albumin (Sigma)/phosphate-buffered saline solution. For rat experiments, FTY720 was given at a dose of 0.3 mg/kg by oral gavage and SEW2871 at a dose of 0.1 mg/kg (i.p.) daily, Monday through Friday (n = 6–8 each group), for 9 weeks. For Rag-1 mouse experiments, SEW2871 was given at a dose of 1 mg/kg (i.p.) daily, Monday through Friday (n = 4 each group), for 6 weeks. For S1P3R experiments, FTY720 was given at a dose of 0.3 mg/kg by oral gavage daily, Monday through Friday (n = 4 each group), for 4 weeks. Administration of S1P agonists or vehicle (3% fatty acid-free bovine serum albumin/phosphate-buffered saline solution) started on the same day as STZ administration.
Podocyte cell culture
All experiments were carried out on differentiated immortalized mouse podocyte cell line (1 × 105 cells/well) kindly provided by Dr John Sedor (Case Western Reserve University, Cleveland, OH) in six-well collagen-coated culture dishes for 14 days, as previously described.43 Differentiated podocytes were then exposed to RPMI media containing either 11 mmol/l d-glucose (normal glucose) or 33 mmol/l d-glucose (HG), with or without vehicle or SEW2871 (1 μmol/l), for 14 days after differentiation. Medium was replaced three times per week.
Renal histology
Kidneys from rats were fixed in 4% paraformaldehyde and embedded in paraffin, and 4-μm sections were cut. Sections were stained with Masson's trichrome, hematoxylin and eosin, and Periodic acid-Schiff stains. Sections were examined (WKB) in a masked manner under × 400 magnification by light microscopy (Zeiss AxioSkop, Thornwood, NY) for mesangial expansion, interstitial fibrosis, and tubular injury. Semiquantitative scores (0 to 4+) were assigned based on the masked reading, as previously described.30 For WT-1 staining, sections were stained with rabbit polyclonal IgG (dilution 1:300; Santa Cruz Biotechnology, Santa Cruz, CA) as we described previously.43 A total of 20 glomeruli/kidney were selected randomly and the number of WT-1-positive cells of the entire glomerulus was counted in a masked manner.
Quantitative real-time PCR
Total RNA was extracted from rat kidneys and from podocytes using RNeasy Mini Kit (Qiagen, Gmbh, Hilden, Germany). Single-strand cDNA was synthesized using iScript cDNA Synthesis Kits (Bio-Rad, Hercules, CA) for two-step real-time RT-PCR. Gene-specific primers for nephrin, podocin, S1P1-5R, SphK1, SphK2, TNF-α, and VEGF were designed using Beacon Designer Probe/Primer Design Software (Premier Biosoft International, Palo Alto, CA; Table 3). Primers were obtained from Integrated DNA Technologies (Coralville, IA). Specificity of the PCR products was verified by melting curve analysis followed by gel electrophoresis. Quantitative real-time PCR was performed using a MyIQ Single-Color Real-Time PCR Detection System iCycler (Bio-Rad, Hercules, CA). Reactions were performed in duplicate, and threshold cycle numbers were averaged. Samples were calculated with normalization to glyceraldehyde 3-phosphate dehydrogenase, as described previously.30
Table 3.
Primer pairs for PCR
| Gene | Sense (5′-3′) | Antisense (5′-3′) | Length, bpa |
|---|---|---|---|
| Nephrin | GGCTAAGGATGG | GAGGTCACAGGCTTCAATAAG | 124 |
| Podocin | GCCTTGGACTCAGTGACC | GCAATCACCCGCACTTTG | 140 |
| S1P1R | TTCTCATCTGCTGCTTCATCATCC | GGTCCGAGAGGGCTAGGTTG | 117 |
| S1P2R | TTACTGGCTATCGTGGCTCTG | ATGGTGACCGTCTTGAGCAG | 107 |
| S1P3R | GCGTGTTCCTTCTGATTGG | GCAAGATGGTAGAGCAGTC | 111 |
| S1P4R | CTGTCAGGGACTCGTACC | CGTGAAGAGCAGACTGAAG | 105 |
| S1P5R | GAGGTTATTGTCCTTCACTAC | AAGAGCACAGCCAAGTTC | 140 |
| SphK1 | GGAACTTGACTGTCCATACC | TACCATCAGCTCTCCATCC | 103 |
| SphK2 | GCACGGCGAGTTTGGTTC | GAGACCTCATCCAGAGAGACTAG | 141 |
| TNF-α | CCTCCCTCTCATCAGTTCTATGG | CGTGGGCTACAGGCTTGTC | 83 |
| VEGF | AGGCTGCTGTAACGATGAAG | GTGCTGGCTTTGGTGAGG | 97 |
Abbreviations: S1P1R, sphingosine 1-phosphate 1 receptor; SphK, sphingosine kinase; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Length of PCR product.
Fluorescence-activated cell sorting analysis
We used flow cytometry to analyze kidney leukocyte content for T cells (CD4, CD3, and CD8), B cells (CD45R), and macrophages (CD11b) in vehicle-treated, FTY720-treated, or SEW287-treated diabetes groups at the end of the study as described previously.9 In brief, kidneys were extracted, minced, digested, and then passed through a filter and a cotton wool column. Fresh kidney suspensions were incubated with anti-rat CD45-fluorescein isothiocyanate (30-F11) for 30 min on ice. Anti-rat CD45 was used to determine the total leukocyte cell number. The remaining sample was stained for T cells, B cells, and macrophages, as previously described. 7-aminoactinomycin D was added 15 min before running the sample to exclude dead cells. Subsequent flow cytometry data acquisition was performed on FASCalibur (Becton Dickinson, San Jose, CA). Data were analyzed by FlowJo software 6.4 (Tree Star, Ashland, OR). All the antibodies were purchased from eBioscience (San Diego, CA). For measurement of apoptosis, cultured podocytes were washed with cold phosphate-buffered saline and resuspended in 1 × binding buffer (BD Pharmingen, San Diego, CA); 100 μl of cells were incubated with 5 μl of fluorescein isothiocyanate-conjugated Annexin V and 3 μl of 7-aminoactinomycin D for 15 min at room temperature in the dark. The reaction was stopped by addition of 400 μl of 1 × binding buffer, and cells were analyzed by fluorescence-activated cell sorting.
Analytical methods
Urinary albumin was measured by ELISA using a Nephrat kit for rats or Albuwell M kit for mice (Exocell, Philadelphia, PA), as described previously.30 Anticoagulated blood was analyzed for leukocyte counts (HEMAVET 850, CDC Technologies, Oxford, CT).9 Urine TNF-α was measured by ELISA (BD Pharmingen).30 Systolic blood pressure was measured via the tail-cuff method (IITC model 179, IITC/Life Science Instruments, Woodland Hills, CA), as described previously.30
Statistical analysis
For the comparisons among groups, we used analysis of variance, with adjusting for multiple comparisons by applying the Tukey's method or the Wilcoxon's test, depending on the distribution of data. To take into account repeated measurement, PROC MIXED procedure in SAS was invoked to compare urine albumin for diabetic group over the weeks. All analyses were performed in SAS 9.2 statistical software (SAS Institute, Arlington, VA), and the two-sided P-values < 0.05 were considered significant.
ACKNOWLEDGMENTS
This study was supported by NIH Grants, DK56223, DK58413, DK62324, HL37942, 1 PO1 HL073361, DK077444, GM067958, and DK076095. We gratefully acknowledge Dr Kwangmi Ahn (Penn State Hershey Medical Center) for help with statistics, Dr Amandeep Bajwa and members of the Okusa lab for helpful discussions and Ting Gao (Penn State Hershey Medical Center) for technical help. FTY720 was the generous gift of Dr Volker Brinkmann (Novartis, Basel, Switzerland).
Footnotes
DISCLOSURE
All the authors declare no competing interests.
REFERENCES
- 1.Parving HH, Osterby R, Ritz E. Diabetic nephropathy. In: Brenner BM, editor. The Kidney. WB Saunders Company; Philadelphia: 2000. pp. 1731–1773. [Google Scholar]
- 2.Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213–219. doi: 10.1016/S0140-6736(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 3.Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 5):3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- 4.Bending JJ, Lobo-Yeo A, Vergani D, et al. Proteinuria and activated T-lymphocytes in diabetic nephropathy. Diabetes. 1988;37:507–511. doi: 10.2337/diab.37.5.507. [DOI] [PubMed] [Google Scholar]
- 5.Moriya R, Manivel JC, Mauer M. Juxtaglomerular apparatus T-cell infiltration affects glomerular structure in Type 1 diabetic patients. Diabetologia. 2004;47:82–88. doi: 10.1007/s00125-003-1253-y. [DOI] [PubMed] [Google Scholar]
- 6.Mensah-Brown EP, Obineche EN, Galadari S, et al. Streptozotocin-induced diabetic nephropathy in rats: the role of inflammatory cytokines. Cytokine. 2005;31:180–190. doi: 10.1016/j.cyto.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Hla T, Lee MJ, Ancellin N, et al. Lysophospholipids—receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 9.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lien YH, Yong KC, Cho C, et al. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 11.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 12.Pinschewer DD, Ochsenbein AF, Odermatt B, et al. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 14.Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 15.Kitabayashi H, Isobe M, Watanabe N, et al. FTY720 prevents development of experimental autoimmune myocarditis through reduction of circulating lymphocytes. J Cardiovasc Pharmacol. 2000;35:410–416. doi: 10.1097/00005344-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Kurose S, Ikeda E, Tokiwa M, et al. Effects of FTY720, a novel immunosuppressant, on experimental autoimmune uveoretinitis in rats. Exp Eye Res. 2000;70:7–15. doi: 10.1006/exer.1999.0777. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki H, Hirata D, Kamimura T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol. 2002;29:707–716. [PubMed] [Google Scholar]
- 18.Keul P, Tolle M, Lucke S, et al. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 19.Maki T, Gottschalk R, Monaco AP. Prevention of autoimmune diabetes by FTY720 in nonobese diabetic mice. Transplantation. 2002;74:1684–1686. doi: 10.1097/00007890-200212270-00006. [DOI] [PubMed] [Google Scholar]
- 20.Maki T, Gottschalk R, Ogawa N, et al. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation. 2005;79:1051–1055. doi: 10.1097/01.tp.0000161220.87548.ee. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Chen M, Fialkow LB, et al. The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice small star, filled. Clin Immunol. 2003;107:30–35. doi: 10.1016/s1521-6616(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 22.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 23.Bajwa A, Jo SK, Ye H, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba K, Yanagawa Y, Masubuchi Y, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 25.Jo SK, Bajwa A, Ye H, et al. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2009;75:167–175. doi: 10.1038/ki.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 28.Krump-Konvalinkova V, Yasuda S, Rubic T, et al. Stable knock-down of the sphingosine 1-phosphate receptor S1P1 influences multiple functions of human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:546–552. doi: 10.1161/01.ATV.0000154360.36106.d9. [DOI] [PubMed] [Google Scholar]
- 29.Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114:1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awad AS, Huang L, Ye H, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 31.Baud L, Oudinet JP, Bens M, et al. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–1118. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- 32.Hruby ZW, Lowry RP. Spontaneous release of tumor necrosis factor alpha by isolated renal glomeruli and cultured glomerular mesangial cells. Clin Immunol Immunopathol. 1991;59:156–164. doi: 10.1016/0090-1229(91)90089-s. [DOI] [PubMed] [Google Scholar]
- 33.Royall JA, Berkow RL, Beckman JS, et al. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am J Physiol. 1989;257:L399–L410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Fukui M, Ebihara I, et al. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450–456. doi: 10.2337/diab.42.3.450. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, Egen JG, Klauschen F, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanagawa Y, Sugahara K, Kataoka H, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J Immunol. 1998;160:5493–5499. [PubMed] [Google Scholar]
- 37.Morales-Ruiz M, Lee MJ, Zollner S, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a G protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 38.Gon Y, Wood MR, Kiosses WB, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Dedhar S, Jewell K, Rojiani M, et al. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J Biol Chem. 1992;267:18908–18914. [PubMed] [Google Scholar]
- 40.Schwarz K, Simons M, Reiser J, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 42.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awad AS, Rouse M, Liu L, et al. Activation of adenosine 2A receptors preserves structure and function of podocytes. J Am Soc Nephrol. 2008;19:59–68. doi: 10.1681/ASN.2007030276. [DOI] [PMC free article] [PubMed] [Google Scholar]