Abstract
Camptothecin (CPT) derivatives are powerful anticancer drugs because of their ability to trap topoisomerase I (Top1)-DNA cleavage complexes. However, they exhibit clinical limitations due to the instability of their α-hydroxylactone six-membered E-ring structure. Additionally, they exhibit bone marrow and intestinal toxicity, especially in adults, and are drug efflux substrates. Here, we report a novel Top1 inhibitor, Genz-644282. We show that Genz-644282 and its metabolites induce Top1 cleavage at similar, as well as unique genomic positions, compared with CPT. The compound also induces protein-linked DNA breaks and Top1-DNA cleavage complexes that persist longer after compound removal than CPT. Concentration-dependent and persistent γH2AX formation was readily observed in cells treated with Genz-644282, and was present in greater than 50% of the cell population following 24 hours compound exposure. The compound shows partial cross-resistance in cell lines resistant to CPT. These cell lines include the human prostate DU145RC0.1 and the leukemic CEM/C2 cells. Limited cross-resistance to Genz-644282 was also found in the Top1-knockdown colon cancer (HCT116) and breast cancer (MCF7) cell lines and in human adenocarcinoma cells (KB31/KBV1) that overexpress (P-glycoprotein, ABCB1), a member of the ATP-binding cassette family of cell surface transport proteins known to confer multidrug resistance. Together, our results provide the first molecular and cellular characterization of Genz-644282 and its clinically relevant metabolites.
Keywords: Topoisomerase I, Genz-644282, camptothecin, DNA alkaline elution, DNA cleavage complexes
Introduction
The main activity of DNA topoisomerase I (Top1) is the relaxation of DNA supercoils generated during DNA replication, transcription, chromatin folding and possibly DNA repair. The enzyme nicks DNA by forming a reversible covalent tyrosyl-DNA bond, which allows the broken DNA strand to rotate around the complementary intact strand, followed by the religation of the breaks through reversal of the Top1-DNA covalent bond (1–3). Top1 is a well-established molecular target for anticancer drugs (4–6). Top1 inhibitors effectively trap the enzyme as it cleaves DNA by creating a Top1-DNA cleavage complex (Top1cc), which leads to cell killing after Top1ccs are converted into DNA damage by DNA replication and/or transcription (1). This DNA damage can be detected by the phosphorylation of the histone H2A variant H2AX (7, 8). Within minutes following the formation of a Top1-induced replication-dependent DNA double-strand break, the phosphorylated form of H2AX (γH2AX) accumulates and forms a nuclear focus around the break, which can be readily detected by immunofluorescence (7–9). The cytotoxicity of camptothecins is due to the trapping of Top1cc rather than by inhibiting Top1 catalytic activity. Since Top1 cleavage complexes need to be maintained for enough time before DNA damage can occur, the stability of these complexes is important.
Two camptothecin derivatives are currently the only clinically-approved Top1 inhibitors (6). In adults, topotecan is prescribed for ovarian and non-small cell lung cancer and irinotecan for colorectal cancers, and both drugs are also effective in pediatric tumors. The broad clinical activity of topotecan and irinotecan in different cancers validates the importance of Top1 as a therapeutic anticancer target. Camptothecin and its clinical derivatives inhibit Top1 with exquisite selectivity. A single drug molecule is sufficient to trap a Top1-DNA cleavage complex by binding at the interface of the DNA and the Top1 (10–12).
Camptothecins have several limitations. They rapidly diffuse from the Top1cc thereby allowing Top1cc reversal before the trapped Top1cc gives rise to a collision with a replication or transcription complex. In addition, the camptothecin α-hydroxylactone E-ring is rapidly hydrolyzed at physiological pH into an inactive carboxylate, thereby limiting the availability of active drug (1). Camptothecins also produce dose-limiting side effects (bone marrow suppression and diarrheas), especially in adults, which hinders the use of full therapeutic regimens. Lastly, camptothecins are pumped out of the cells by ABCG2, an ABC drug efflux transporter that confers a high degree of resistance to various anticancer drugs (13).
Two chemical approaches have thus far been taken to overcome the instability and inactivation of camptothecins. The first was to enlarge the E-ring by one carbon atom. This limits the opening of the E-ring; however it also prohibits its reclosure (6), which is potentially problematic, as irreversible E-ring opening inactivates the drug. Such synthetic analogs have been named the homocamptothecins with diflomotecan being the clinical derivative. The second approach has been to remove one carbon atom, which eliminates the α-hydroxylactone function and converts the E-ring from a 6- to a 5-membered highly stable ring while preserving potent inhibitory activity against Top1 (14). Those derivatives are referred to as the α-keto derivatives with S39625 selected for clinical trials (6).
Novel Top1 inhibitors have also been the focus of academic and industrial laboratories for several years (1, 4). Three classes of non-camptothecin Top1 inhibitors have reached clinical development: the indolocarbazoles, the indenoisoquinolines and the dibenzonaphthyridones (4, 15, 16). The indolocarbazoles were the first in clinical development. However, they affect other cellular targets besides Top1 and their current clinical development as anticancer drugs appears to have been put on hold. Two indenoisoquinolines have just begun clinical trials at the US National Cancer Institute with γH2AX as biomarker of genomic damage before and after treatment (17). Here we present molecular and cellular studies of Genz-644282 and its metabolites from the dibenzonaphthyridones class of non-camptothecin Top1 inhibitors (18, 19), which have also recently begun Phase 1 clinical trial. The basis for choosing compound Genz-644282 for clinical development is that the metabolite Genz-649975 is not an active antitumor agent, likely due to rapid metabolism. Genz-649974 is an active antitumor agent but is more toxic by body weight loss than Genz-644282.
Materials and Methods
Drugs, Enzymes, Chemicals
Camptothecin (CPT) and topotecan (TPT) were obtained from the Drug Synthesis and Chemistry Branch, NCI (Bethesda, MD). MJ-III-65 (NSC 706744) was synthesized as described (20). Genz-644282 citrate salt, Genz-649974, Genz-649975, and Genz-649978 were provided from Genzyme Corporation, Framingham, MA. Doxorubicin and amsacrine (m-AMSA) were obtained from Sigma (St. Louis, MO). Drug stock solutions were made in 10 mM aliquots in DMSO for camptothecin and topotecan, 5 mM aliquots for MJ-III-65 and Genz-644282, and 1 mM aliquots for Genz-649975, Genz-649974, and Genz-649978. Aliquots were stored at −20ºC, and additional dilutions were made in DMSO immediately before use. For cytotoxicity assays, additional dilutions were done in cell culture medium. The final concentration of DMSO in the reaction mixtures did not exceed 10% (v/v).
Recombinant human recombinant Top1 was purified from baculovirus (17) and human recombinant Top2α was a generous gift from Dr. Neil Osheroff (Vanderbilt University).
Cell lines
Human colon HCT116 and breast MCF7 cancer cells were obtained from the NCI Developmental Therapeutics Program (DTP). The cells lines were characterized in our laboratory by DNA fingerprinting (21). The stably transfected HCT116 Top1-small interfering RNA (siRNA; HCT116-siTop1) and MCF-7 Top1- siRNA (MCF7-siTop1) cells were derived in our laboratory as described (22). HCT116 cells were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Gemini Bio-Products, Calabasas, CA) and MCF7 cells were maintained in DMEM supplemented with 10% FBS. Si-Top1 cells for both HCT116 and MCF7 were maintained with the addition of 100 μg/mL hygromycin. H460 human lung cancer stable transfectants expressing wild-type ABCG2 and KB human cervical carcinoma expressing MDR-1/P-glycoprotein were a kind gift from Dr. Matthew D. Hall and Dr. Michael M. Gottesman (Laboratory of Cell Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) and were maintained in RPMI supplemented with 10% FBS and either 20 ng/mL mitoxantrone or 1 μg/mL vinblastine, respectively. The DU145 cell line was obtained from the American Type Culture Collection (Rockville, MD). The RC0.1 cell subline was derived from DU145 cells as previously described (23, 24) and maintained in RPMI, 10% FBS. The CCRF-CEM cell line was obtained from the American Type Culture Collection and the CCRF-CEM C2 cell line was established as previously described (25, 26) and maintained in RPMI containing 10% FBS.
Top1/Top2-Mediated DNA Cleavage Assays
For Top1-mediated DNA cleavage reactions, a 117-bp DNA oligonucleotide (Integrated DNA Technologies, Coralville, IA) containing a single 5′-cytosine overhang was 3′-end labeled by fill-in reaction with [α-32P]-dGTP and DNA polymerase I. In addition, a shorter 23-b p 3′-end scissile strand–labeled duplex oligonucleotide (5′-AAAAAGACTT^GGAAAAATTTTTA-3′) with a single Top1 cleavage site (^ in Figure 2A) was also generated using [α-32P]-ddATP and terminal deoxyribonucleotidyl transferase. For Top2 reactions, the recessed strand from the 117-bp DNA oligonucleotide was 5′-labeled using T4 polynucleotide kinase and [γ-32P] ATP. The 5′-labeled single-stranded oligonucleotide was then annealed with its complementary strand by heating for 5 min at 95ºC and slowly cooling to room temperature. DNA cleavage reactions were prepared as previously reported (27). Briefly, approximately 2 nM of radiolabeled DNA substrate was incubated with or without recombinant Top1 or Top2 in 20 μL of reaction buffer (10 mM Tris-HCl, pH 7.5, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 15 μg/ml BSA) at 25ºC for 20 min in the presence of various concentrations of drugs. The reactions were terminated by adding SDS (0.5% final concentration) followed by the addition of two volumes of loading dye (80% formamide, 10 mM sodium hydroxide, 1 mM sodium EDTA, 0.1% xylene cyanol, and 0.1% bromophenol blue). The cleavage products were separated on a 16% sequencing PAGE gel. Imaging and quantification were performed using the Typhoon 8600 and ImageQuant software (Molecular Dynamics, Sunnyvale, CA), respectively.
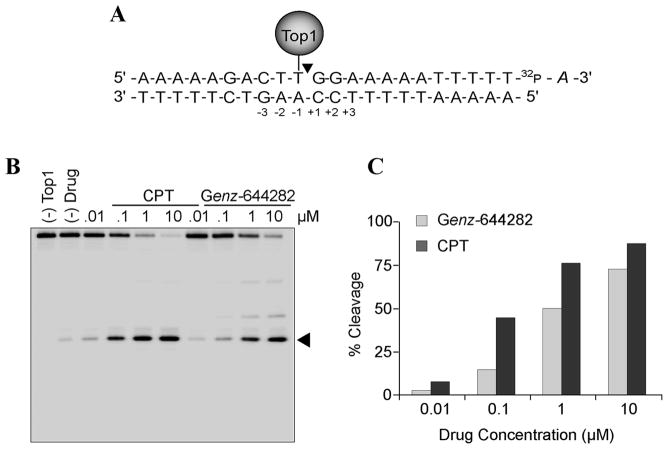
Figure 2. Top 1-cleavage complex induction by Genz-644282.
A) Schematic representation of the oligonucleotide substrate containing a single Top1-mediated cleavage site. The positions relative to the Top1 cleavage site [between positions (−3) and (+3)] are indicated. B) Comparisons of Top1-mediated DNA cleavage induced by Genz-644282 and CPT with the oligonucleotide substrate shown in panel A. C) Quantitative analysis of cleavage complexes induced by CPT or Genz-644282 are plotted as a function of compound concentrations.
Detection of cellular Top1-DNA complexes
Top1-DNA adducts were isolated using cesium chloride gradient centrifugation (17, 28, 29). Briefly, 106 HCT116 cells were treated with 1 μM of each compound for 1 hr or left untreated. The medium was removed and cells were scraped and collected in buffer containing 1% Sarkosyl, 8 mol/L guanidine hydrochloride, 30 mmol/L Tris pH 7.5 and 10 mmol/L EDTA. Samples were homogenized with a Dounce homogenizer, cell lysates were gently layered on cesium chloride step gradients, and centrifuged at 30700 rpm for 20 hr (g = 167,400) at 20ºC. DNA fractions of 0.5 mL were pooled and diluted with an equal volume of 25 mmol/L sodium phosphate buffer (8.8 mmol/L Na2HPO4, 16.2 mmol/L NaH2PO4, pH 6.5). Serial dilutions of each DNA fraction were made and blotted on Immobilon-P membranes (Millipore, Bedford, MA) using a slot-blot vacuum. Top1-DNA complexes were detected using the C21 Top1 monoclonal antibody (a kind gift from Dr. Yung-Chi Cheng, Yale University, New Haven, CT) and standard Western blotting procedures.
Alkaline elution assay for the detection of Top1-DNA crosslinks
The alkaline elution assay was done to detect DNA-protein cross-links (DPCs) as previously described (17, 30, 31). Human colon cancer HCT116 cells were pre-labeled with 0.2 μCi/mL of [14C]-thymidine for one to two doubling times at 37ºC and chased in non-radioactive medium overnight. Cells were treated for 1 hr with 1 μmol/L of Genz-644282 or CPT. After compound exposure, cells were scraped in Hanks’ balanced salt solution. For the reversal experiments, the cells were cultured in compound-free medium for the indicated times before scraping. DPCs were analyzed under non-deproteinizing, DNA-denaturing conditions using protein-adsorbing filters and the DPC frequencies were calculated as previously described (17, 30, 31).
Immunofluorescence microscopy detection of γH2AX
HCT116 and MCF7 cells were grown in culture medium on chamber slides. After 1 hr treatment with 1 μM drug, cells were fixed with 2% paraformaldehyde in phosphate buffered saline (PBS; Mediatech Inc., Manassas, VA), washed in PBS, and permeabilized in ice-cold 70% ethanol. Slides were blocked with PBS containing 8% bovine serum albumin (BSA; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hr at room temperature and incubated in 1% BSA-PBS solution with anti-γH2AX antibody (05–636 Millipore, Bedford, MA). Slides were then washed 3 times in PBS for 10 min, incubated for 90 min at room temperature with Alexa Fluor 488-conjugated goat-anti mouse IgG secondary antibody (Molecular Probes, Carlsbad, CA) in 1% BSA-PBS solution at a 500x dilution, and washed in PBS. Slides were stained and sealed with mounting medium containing DAPI (Vectashield with DAPI, Vector Laboratories, Inc., Burlingame, CA) and were viewed using a BD Pathway 800 Series microscope with a 60x objective.
Two-Dimensional flow cytometry analysis for DNA and γH2AX
HCT116 cells were treated with 1 μM CPT or Genz-644282 for 1, 3, 6 or 24 hr. After treatment or the appropriate reversal time in compound-free medium, cells were harvested, washed twice with ice-cold PBS, and fixed in 4% paraformaldehyde for 10 min at room temperature. Cell pellets were then washed with 1 ml of ice-cold PBS and permeabilized with 1 ml pre-chilled (−20ºC) 70% ethanol for 20 min at room temperature. Cells were again washed in PBS, and further permeabilized with ice-cold 0.25% Triton X-100 in PBS for 5 min on ice, washed in PBS, and incubated with anti-γH2AX antibody at a 250x dilution in PBS/1% BSA for 1 hr at room temperature. Cells were washed with PBS, and incubated with goat anti-mouse Alexa Fluor-488 antibody (Molecular Probes, Carlsbad, CA) at 250x dilution in PBS/1% BSA for 30 min at room temperature. Cells were washed with PBS and resuspended in 500 μl of PBS solution containing 50 μg/ml propidium iodide (PI) and 0.5 mg/ml RNase A. Analyses of FL2-A (PI) versus FL1-H (γ-H2AX) were done using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cell cycle distributions were calculated using ModFit LT software (Verity Software House, Inc., Topsham, ME).
DNA Unwinding Assays
DNA unwinding was performed as previously described (32). Briefly, reaction mixtures (10 μl final volume) contained 0.3 μg supercoiled SV40 DNA (New England Biolabs, Ipswich, MA) in reaction buffer (10 mm Tris·HCl, pH 7.5, 50 mm KCl, 5 mm MgCl2, 0.1 mm EDTA, and 15 μg/ml bovine serum albumin) and 10 units Top1. Reactions were performed at 37ºC for 30 min and terminated by the addition of 1% SDS. 1.1 μl of 10x concentrated compound solutions were added to relaxed DNA and incubated for 30 min. 1 mg/mL Proteinase K and 1x SDS were added to samples and incubated for an additional 30 min at 37ºC. Loading buffer (20% Ficoll 400; 0.1 M Na2EDTA, pH 8.0, 1.0% SDS, and 0.25% bromophenol blue) was added and reactions mixtures were loaded onto a 1% agarose gel. After electrophoresis, DNA bands were stained in 1 μg/ml of ethidium bromide and visualized by transillumination with ultraviolet light (300 nm).
Cytotoxicity Assays
Briefly, cells were seeded in 96 well plates 24 hr prior to drug treatment. Cytotoxicity of CPT, TPT, doxorubicin, and Genz-644282 in HCT116, MCF7, DU145, and their CPT-resistant subclones were assessed by the sulforhodamine B (Sigma Aldrich, St. Louis, MO) assay. In CEM and CEM/C2 cells, H460 and H460/MX20, and KB31 and KBV1 cells, cytotoxicity was measured by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega, San Luis Obispo, CA) colorimetric assay (17). Compound exposures were continuous for 72 hr for both assays. Percentage of growth was calculated relative to control (vehicle-treated cells) after 3 days of culture with control taken as 100.
Results
Genz-644282, Genz-649974, Genz-649975, and Genz-649978 trap Top1cc
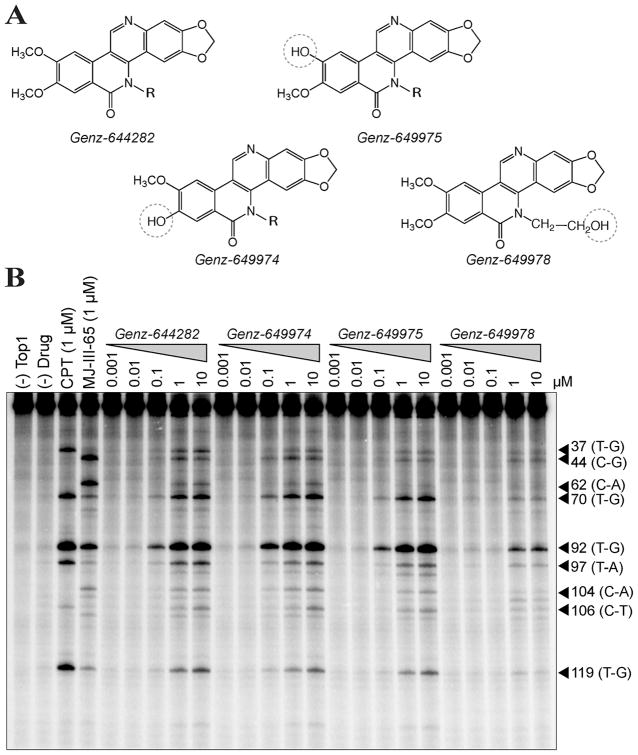
The primary difference between Genz-644282 and its metabolites, Genz-649975, Genz-649974, and Genz-649978, (shown in Fig. 1A) is the conversion of the methoxy to a hydroxyl group at the positions indicated by the dashed circles. To determine the effects of those compounds on Top1, the cleavage site distribution of Genz-644282 and its metabolites was tested using the 3′-end labeled 117-bp oligonucleotide in the presence of recombinant Top1 (17, 27). Figure 1B shows the induction of Top1cc by Genz-644282, Genz-649974, and Genz-649975 and to a lesser extent by Genz-649978. All four compounds exhibit cleavage at similar as well as unique sites from CPT and the indenoisoquinoline MJ-III-65 (17). Like CPT, Genz-644282 and all three metabolites showed a strong preference for site 92. However, this cleavage occurred with differences in relative intensity (see sites 37, 44, 62, 70, 97, 119). These results demonstrate the effectiveness of Genz-644282, Genz-649974, and Genz-649975 as Top1 inhibitors at potentially achievable concentrations. Both the similarities and differences of the DNA cleavage patterns from those of CPT and the indenoisoquinolines (MJ-III-65) suggest Genz-644282 and its metabolites have comparable binding modes to both CPT and MJ-III-65 in the Top1cc (33) but target both common and unique genomic sites.
Figure 1. Genz-644282, Genz-649974, Genz-649975, Genz-649978 trap Top 1-cleavage complexes.
A) Chemical structures of Genz-644282, Genz-649974, Genz-649975, Genz-649978. B) A 3′-end-labeled 117-bp oligonucleotide was reacted with Top1 in the presence or absence of compound at the indicated concentrations. Arrowheads and numbers indicate the positions of DNA fragments cleaved by Top1 in the oligonucleotide. The bases flanking each cleavage site are indicated in parentheses.
To further investigate the relative potency of Genz-644282 for Top1cc compared to CPT, a 22-bp double-stranded oligonucleotide containing a high-affinity Top1-DNA cleavage site was used (27). Cleavage intensity can be measured by the generation of a 13-mer cleavage product labeled at the 3′-end with [32P]cordycepin (Fig. 2A). The sequence of the 22-mer oligonucleotide is depicted with its Top1 cleavage site indicated by the caret between the T and G bases (Fig. 2A). Genz-644282 generates slightly less cleavage at the indicated site for each concentration of drug compared to CPT (Fig. 2B). Together the results obtained with the short oligonucleotide substrate (Fig. 2) and the long oligonucleotide substrate (Fig. 1B) demonstrate the effectiveness of Genz-644282 and its metabolites as Top1 inhibitors.
Induction of persistent Top1 cleavage complexes (Top1cc) and DNA-Protein Crosslinks (DPCs) by Genz-644282 in cancer cells
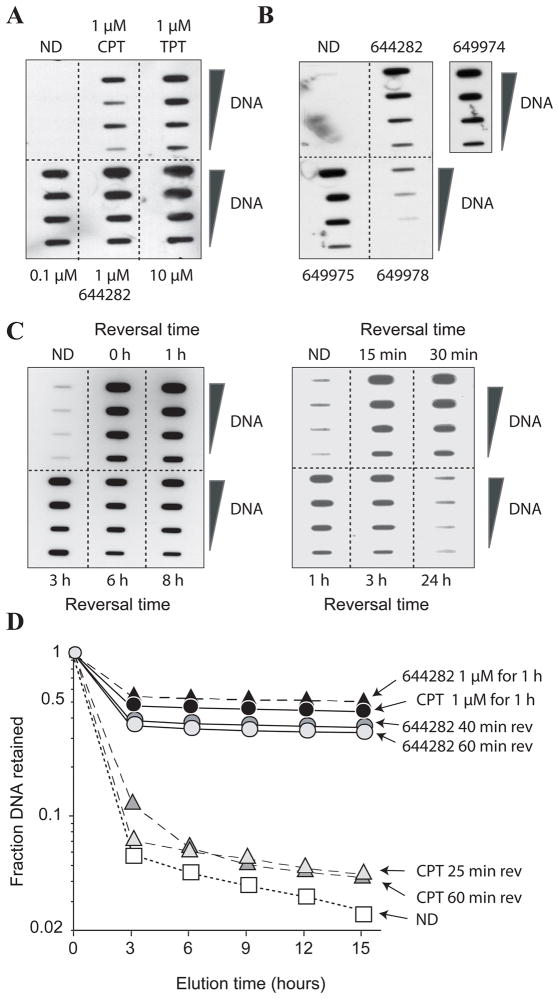
The induction and stability of Top1cc produced by Genz-644282 were evaluated in human colon cancer carcinoma HCT116 cells using the Immunocomplex of Enzyme (ICE) and alkaline elution assays (17). Figure 3A shows that Genz-644282 is more potent at trapping Top1-DNA covalent cleavage complexes than either CPT or TPT at the same concentration. Additionally, even metabolites of Genz-644282, Genz-649974, Genz-649975, and Genz-649978, are active and effective at trapping Top1 with Genz-649978 being less potent than the others (Fig. 3B).
Figure 3. Induction of persistent Top1 cleavage complexes (Top1cc) and DNA-Protein Crosslinks (DPCs) by Genz-644282 in HCT116 cells.
A) Top1cc induced by Genz-644282, CPT, and TPT (1 h) measured by Immunocomplex of Enzyme (ICE) assay. DNA fractions were pooled and serial dilutions were blotted. Top1cc were detected using Top1 C21 monoclonal antibody. B) Top1cc induced by Genz-644282 or its metabolites Genz-649974, Genz-649975, and Genz-649978 (1 μM for 1 h). C) Persistent Genz-644282-induced Top1cc following compound removal. After 1 hour of treatment, cells were cultured in compound-free medium and assayed for Top1cc at the indicated time points. D) DNA-Protein Crosslinks (DPCs) induced by treatment with CPT and Genz-644282. Persistence of DPCs at various time points following drug removal is shown. Cells were pre-labeled with 14C-thymidine and treated with either drug. Fraction of DNA remaining on the filter is plotted against time (hours).
Reversal experiments were performed to determine the stability of the Top1 complexes trapped by Genz-644282. Figure 3C shows the persistence of the Top1 complexes for up to 8 hours following compound removal with complete reversal of complexes at 24 hours (Fig. 3C). This cleavage complex stability is greater than that induced by CPT, which has been shown to reverse within 1 hour (17, 34). Alkaline elution, another method to measure topoisomerase cleavage complexes (30, 31, 35), was used to quantify Top1cc as DNA-protein crosslinks (DPC). Genz-644282 induced slightly more DPC than CPT. Additionally, the DPC induced by Genz-644282 were significantly less reversible following compound removal than those induced by CPT (Fig. 3D). Together these results indicate greater stability of Top1cc induced by Genz-644282 than CPT.
Generation and persistence of γH2AX foci in MCF7 and HCT116 cells in response to Genz-644282
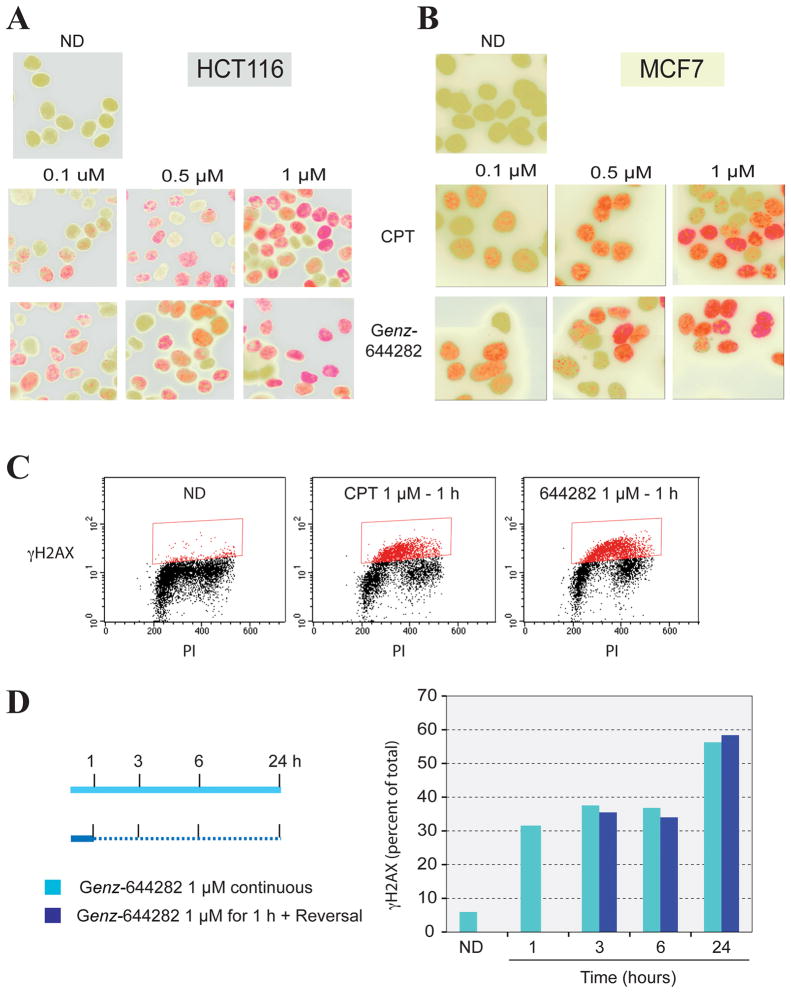
To demonstrate the DNA damage produced by Genz-644282, γH2AX induction was assessed in cells (8, 17). Both human colon cancer HCT116 cells and breast cancer MCF7 cells were treated with 0.1, 0.5, or 1 μM of Genz-644282 or CPT and studied by immunofluorescence after staining with γH2AX antibodies (Fig. 4A and B). γH2AX foci were induced by Genz-644282 at concentrations as low as 0.1 μM, and the γH2AX staining increased with compound concentration. γH2AX induction was also evaluated by flow cytometry to determine the cell cycle-dependence and percent of γH2AX-positive cells (17) following 1 hour of Genz-644282 treatment (Fig. 4C). γH2AX positive cells were mostly in S-phase as previously demonstrated for CPT (7, 36) and the indenoisoquinolines (17). Because the Top1cc induced by Genz-644282 were reversed by 24 hours (Fig. 3C), γH2AX flow cytometry was also used to study whether the DNA damage induced by these complexes was persistent. The percent of γH2AX-positive cells induced by Genz-644282 was compared between 24 hours of continuous exposure and 1 hour of exposure followed by 23 hours of compound free medium. There was no substantial difference between cells that underwent continuous compound treatment and those treated for 1 hour, indicating that the DNA damage response induced by 1 hour of exposure to Genz-644282 was persistent after drug removal (Fig. 4D).
Figure 4. Generation and persistence of γH2AX foci in MCF7 and HCT116 cells in response to Genz-644282 treatments.
A and B) Immunofluorescence microscopy of γ-H2AX foci formation in HCT116 and MCF7 cells. Cells were treated with Genz-644282 or CPT for 1 hour. Accumulation of γ-H2AX is shown in red, nuclei are shown in green. C) Comparison of γ-H2AX-positive cells induced by CPT or Genz-644282 as analyzed by flow cytometry (PI: propidium iodide). D) Percentage of γ-H2AX-positive cells induced by continuous treatment with 1 μM Genz-644282 or 1 hour of treatment followed by incubation in compound-free medium for the indicated times. HCT116 cells were treated as shown in the left panel. Cells were fixed and incubated with γ-H2AX antibody and PI. The right panel depicts a quantitative comparison of γ-H2AX-positive cells induced by continuous compound exposure versus a 1-hour treatment followed by incubation in compound-free medium.
Activity of Genz-644282 in camptothecin-resistant cell lines
The development of drug resistance often occurs during treatment and presents a major obstacle to curing potentially sensitive cancers (37). Table 1 shows the testing of a panel of camptothecin-resistant cells. It shows that Genz-644282 is able to partially and fully overcome resistance in two cell lines resistant to CPT that have mutations in Top1. In the prostate cancer RC0.1 cells, the Top1 is catalytically active but is highly resistant to inhibition by CPT and its derivatives because of an R364H mutation (24). Genz-644282 displays cross-resistance in the RC0.1 cell line (Table 1). The human leukemia cell line CCRF-CEM is 974-times more resistant to CPT than the parental CEM cell line. This is due to the mutation N722S (25), as the asparagine immediately flanking the catalytic tyrosine is important for the binding of CPT and TPT to Top1 in the Top1cc (10, 11, 33, 38). Genz-644282 displays almost no resistance in the resistant CCRF-CEM cell line compared to the parental (Table 1).
Table 1.
Cytotoxicity of Genz-644282 in CPT-resistant human cancer cell lines.
| Compound | Parental Cell Line, IC50 nM | Resistant Subline, IC50 nM | Resistance Ratio | Mechanism of Resistance (ref) |
|---|---|---|---|---|
| DU145 Prostate Cancer | RC0.1 | Mutant Top1 (23, 24) | ||
| CPT | 11.5 +/− 6.4 | >1000 | >87 | |
| Genz-644282 | 4.8 +/− 0.3 | 606.3 +/− 456.7 | 126 | |
| CCRF-CEM Leukemia | CCRF-CEM C2 | Mutant Top1 (25, 26) | ||
| CPT | 5.5 | >1000 | >182 | |
| Genz-644282 | 5.4 +/− 1.8 | 9.3 | 1.7 | |
| MCF7 Breast Cancer | MCF7-siTop1 | siRNA Top1 (22) | ||
| CPT | 13.3 +/− 2.1 | 39.8 | 3 | |
| Genz-644282 | 2.8 +/− 0.1 | 5.9 +/− 0.1 | 2.1 | |
| HCT116 Colon Cancer | HCT116-siTop1 | siRNA Top1 (22) | ||
| CPT | 14.3 +/− 5.0 | 36.8 +/− 0.6 | 2.6 | |
| Genz-644282 | 9 +/− 0.1 | 11.9 +/− 2.7 | 1.3 | |
| H460 NSCLC | H460/Mito | Mitoxantrone-treated (ABCG2 over-expresser) | ||
| Topotecan | 175.5 +/− 139.2 | >767 +/− 403 | >4.4 | |
| Genz-644282 | 15.8+/− 14.7 | >678 +/− 558 | >43 | |
| KB3.1 Cervical Cancer | KB3.1/Vinbl | Vinblastine-treated (ABCB1 over-expresser) | ||
| Doxorubicin | 86.7 +/− 15.7 | >1000 | >11.5 | |
| Genz-644282 | 67.6 +/− 15.1 | 314 +/− 129.6 | 4.6 |
IC501 (concentration of drug required for 50% cell growth inhibition) and relative resistances (RR)2 to Genz-644282 and the appropriate positive control [CPT, TPT, or doxorubicin (Dox)] in 6 different pairs of matched cell lines. Individual values correspond to independent experiments.
RR (relative resistance) was calculated by dividing the IC50 of the mutant cell line by the IC50 of the corresponding parental cell line.
The MCF7-siTop1 and HCT116-siTop1 sublines developed by transfection of MCF7 breast cancer cells and HCT116 colon cancer cells with shRNA vectors expressing siRNA for Top1 are about 3-fold resistant to CPT and less resistant to Genz-644282 (Table 1).
The drug efflux ABC transporters, ABCG2 (mitoxantrone resistant/breast cancer resistance proteins) and ABCB1 (MDR-1) also confer a high degree of resistance to various anticancer drugs (37). TPT and irinotecan are substrates for both. We find that Genz-644282 appears to be a substrate for the ABCG2 mitoxantrone resistant transporter (Table 1). However, it appears to be less of a substrate for the ABCB1 pump when compared to the reference substrate doxorubicin.
Discussion
Here we show that Genz-644282, which is undergoing Phase 1 clinical trial, and its metabolites are potent Top1 inhibitors. Genz-644282 appears to overcome some of the limitations of topotecan and irinotecan, the only clinically approved Top1 inhibitors. One of the primary therapeutic limitations of CPT derivatives is the conversion of their α-hydroxyl lactone into an inactive carboxylate form at physiological pH. This happens rapidly within minutes and limits the availability of active drug. Unlike CPT, the metabolites of Genz-644282 trap Top1cc both in vitro and in cells (Fig.1B, 3B). The metabolites of Genz-644282 that are most active against Top1 (Genz-649975 and Genz-649974) differ from the parent compound by the demethylation of Genz-644282 (see Fig. 1A). In the least active metabolite, Genz-649978, the side chain of the compound (designated by R) is hydroxylated. The side chain in the parental compound, Genz-644282, and its two active metabolites, Genz-649975 and Genz-649974, is critical to achieving the desired properties of the compound as a Top1 inhibitor and increases the affinity of the compounds for DNA.
The similarities of the DNA cleavage patterns compared to those of CPT and the indenoisoquinolines and the chemical similarity between Genz-644282 and the indenoisoquinoline derivatives suggest that Genz-644282 and its metabolites have comparable binding modes to both CPT and MJ-III-65 in the Top1cc but may have potential uniqueness in the way they target genomic sites (33). The ternary complex structures of camptothecin, indenoisoquinolines, and indolocarbazoles demonstrate flat planar ring structures that intercalate between the −1 and +1 base pairs at the site of DNA-enzyme cleavage (6, 10, 11, 33, 38). In addition, each possesses a major groove substituent and a hydrogen bond acceptor that faces the minor groove. Genz-644282 also has a relatively flat planar ring structure, and it is possible that the substituents on the nitrogen in the lactam ring in Genz-644282 may be directed into the major groove.
Another limitation of the camptothecins is their rapid dissociation and diffusion from the Top1cc due to their noncovalent binding. This creates a requirement for extended clinical infusions to maintain the persistent cleavage complexes necessary for killing cancer cells (1, 6). Genz-644282 appears to overcome this limitation by creating persistent Top1-DNA cleavage complexes for up to 8 hours following drug removal (Fig. 3C). The stereochemistry produced by the methoxy-substituents at the 8 and 9 position tends to limit intercalation. One indication of intercalation is DNA unwinding (32), which we found at a concentration of Genz-644282 of 100 μM (Supplemental Fig. 1A). This dual effect is reminiscent of intercalating topoisomerase II (Top2) inhibitors such as acridines (amsacrine) and anthracyclines (doxorubicin) (39, 40). Thus, it is plausible that Genz-644282 primarily targets Top1 cleavage sites in DNA at low concentrations that may be clinically relevant. At higher concentrations the compound may intercalate into DNA at other locations thereby preventing proteins, such as Top2, from binding (Supplemental Fig. 1B). This additional effect might account at least in part for the low cross-resistance of the camptothecin-resistant cells to Genz-644282.
Histone γH2AX has been found to form rapidly in response to Top1 inhibitors (7) and is considered to be a sensitive and selective marker for DNA double-strand breaks (8). The present data show that Genz-644282 induces significant replication-dependent γH2AX at nanomolar concentrations within 1 hour of treatment. Foci could be detected at concentrations as low as 0.1 μM. Additionally, the induction of γH2AX by Genz-644282 is persistent and actually increases following drug removal indicating continual DNA damage. This increase in signal could be due to irreversible DNA damage or secondary to apoptosis, which has recently been shown to induce a typical “γH2AX ring pattern” by confocal microscopy at the periphery of the nucleus as cells initiate their apoptotic response (41, 42). Thus, our data suggest that γH2AX may be used as a pharmacodynamic biomarker to clinically evaluate and monitor the efficacy of Genz-644282 several hours following infusion.
Supplementary Material
Acknowledgments
This work was supported by the NCI intramural program, Center for Cancer Research and by Genzyme Corporation.
References
- 1.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 2.Champoux JJ. DNA TOPOISOMERASES: Structure, Function, and Mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 3.Wang JC. A Journey in the World of DNA Rings and Beyond. Annual Review of Biochemistry. 2009;78:31–54. doi: 10.1146/annurev.biochem.78.030107.090101. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA. Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol. 2008;75:1262–71. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: current perspectives. Bioorg Med Chem. 2004;12:1585–604. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109:2894–902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA-double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–12. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 8.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. gammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura AJ, Rao VA, Pommier Y, Bonner WM. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell Cycle. 2010:9. doi: 10.4161/cc.9.2.10475. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002;99:15387–92. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioanoviciu A, Antony S, Pommier Y, Staker BL, Stewart L, Cushman M. Synthesis and Mechanism of Action Studies of a Series of Norindenoisoquinoline Topoisomerase I Poisons Reveal an Inhibitor with a Flipped Orientation in the Ternary DNA-Enzyme-Inhibitor Complex As Determined by X-ray Crystallographic Analysis. J Med Chem. 2005;48:4803–14. doi: 10.1021/jm050076b. [DOI] [PubMed] [Google Scholar]
- 12.Pommier Y, Cherfils J. Interfacial protein inhibition: a nature’s paradigm for drug discovery. Trends Pharmacol Sci. 2005;28:136–45. doi: 10.1016/j.tips.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, et al. Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999;59:5938–46. [PubMed] [Google Scholar]
- 14.Takagi K, Dexheimer TS, Redon C, Sordet O, Agama K, Lavielle G, et al. Novel E-ring camptothecin keto analogues (S38809 and S39625) are stable, potent, and selective topoisomerase I inhibitors without being substrates of drug efflux transporters. Mol Cancer Ther. 2007;6:3229–38. doi: 10.1158/1535-7163.MCT-07-0441. [DOI] [PubMed] [Google Scholar]
- 15.Long BH, Rose WC, Vyas DM, Matson JA, Forenza S. Discovery of antitumor indolocarbazoles: rebeccamycin, NSC 655649, and fluoroindolocarbazoles. Curr Med Chem Anticancer Agents. 2002;2:255–66. doi: 10.2174/1568011023354218. [DOI] [PubMed] [Google Scholar]
- 16.Pommier Y, Cushman M. The indenoisoquinoline noncamptothecin topoisomerase I inhibitors: update and perspectives. Mol Cancer Ther. 2009;8:1008–14. doi: 10.1158/1535-7163.MCT-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antony S, Agama KK, Miao ZH, Takagi K, Wright MH, Robles AI, et al. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 2007;67:10397–405. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 18.Li TK, Houghton PJ, Desai SD, Daroui P, Liu AA, Hars ES, et al. Characterization of ARC-111 as a novel topoisomerase I-targeting anticancer drug. Cancer Res. 2003;63:8400–7. [PubMed] [Google Scholar]
- 19.Kurtzberg LS, Battle T, Rouleau C, Bagley RG, Agata N, Yao M, et al. Bone marrow and tumor cell colony-forming units and human tumor xenograft efficacy of noncamptothecin and camptothecin topoisomerase I inhibitors. Mol Cancer Ther. 2008;7:3212–22. doi: 10.1158/1535-7163.MCT-08-0568. [DOI] [PubMed] [Google Scholar]
- 20.Nagarajan M, Morrell A, Ioanoviciu A, Antony S, Kohlhagen G, Agama K, et al. Synthesis and evaluation of indenoisoquinoline topoisomerase I inhibitors substituted with nitrogen heterocycles. J Med Chem. 2006;49:6283–9. doi: 10.1021/jm060564z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, et al. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther. 2009;8:713–24. doi: 10.1158/1535-7163.MCT-08-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao ZH, Player A, Shankavaram U, Wang YH, Zimonjic DB, Lorenzi PL, et al. Nonclassic Functions of Human Topoisomerase I: Genome-Wide and Pharmacologic Analyses. Cancer Res. 2007;67:8752–61. doi: 10.1158/0008-5472.CAN-06-4554. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee D, Wyche JH, Pantazis P. Induction of apoptosis in malignant and camptothecin-resistant human cells. Ann N Y Acad Sci. 1996;803:143–56. doi: 10.1111/j.1749-6632.1996.tb26383.x. [DOI] [PubMed] [Google Scholar]
- 24.Urasaki Y, Laco G, Pourquier P, Takebayashi Y, Kohlhagen G, Gioffre C, et al. Characterization of a novel topoisomerase I mutation from a camptothecin-resistant human prostate cancer cell line. Cancer Res. 2001;61:1964–9. [PubMed] [Google Scholar]
- 25.Fujimori A, Harker WG, Kohlhagen G, Hoki Y, Pommier Y. Mutation at the catalytic site of topoisomerase I in CEM/C2, a human leukemia cell resistant to camptothecin. Cancer Res. 1995;55:1339–46. [PubMed] [Google Scholar]
- 26.Fujimori A, Hoki Y, Popescu NC, Pommier Y. Silencing and selective methylation of the normal topoisomerase I gene in camptothecin-resistant CEM/C2 human leukemia cells. Oncol Res. 1996;8:295–301. [PubMed] [Google Scholar]
- 27.Dexheimer TS, Pommier Y. DNA cleavage assay for the identification of topoisomerase I inhibitors. Nat Protoc. 2008;3:1736–50. doi: 10.1038/nprot.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw JL, Blanco J, Mueller GC. A simple procedure for isolation of DNA, RNA and protein fractions from cultured animal cells. Anal Biochem. 1975;65:125–31. doi: 10.1016/0003-2697(75)90498-4. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian D, Kraut E, Staubus A, Young DC, Muller MT. Analysis of topoisomerase I/DNA complexes in patients administered topotecan. Cancer Res. 1995;55:2097–103. [PubMed] [Google Scholar]
- 30.Covey JM, Jaxel C, Kohn KW, Pommier Y. Protein-linked DNA strand breaks induced in Mammalian cells by camptothecin, an inhibitor of topoisomerase I. Cancer Res. 1989;49:5016–22. [PubMed] [Google Scholar]
- 31.Kohn KW. DNA filter elution: a window on DNA damage in mammalian cells. Bioessays. 1996;18:505–13. doi: 10.1002/bies.950180613. [DOI] [PubMed] [Google Scholar]
- 32.Pommier Y, Covey JM, Kerrigan D, Markovits J, Pham R. DNA unwinding and inhibition of mouse leukemia L1210 DNA topoisomerase I by intercalators. Nucleic Acids Res. 1987;15:6713–31. doi: 10.1093/nar/15.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchand C, Antony S, Kohn KW, Cushman M, Ioanoviciu A, Staker BL, et al. A novel norindenoisoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of topoisomerase I-DNA covalent complexes. Mol Cancer Ther. 2006;5:287–95. doi: 10.1158/1535-7163.MCT-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86:836–42. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- 35.Ross WE, Glaubiger DL, Kohn KW. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978;519:23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- 36.Seiler JA, Conti C, Syed A, Aladjem MI, Pommier Y. The Intra-S-Phase Checkpoint Affects both DNA Replication Initiation and Elongation: Single-Cell and -DNA Fiber Analyses. Mol Cell Biol. 2007;27:5806–18. doi: 10.1128/MCB.02278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 38.Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, et al. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J Med Chem. 2005;48:2336–45. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 39.Tewey KM, Chen GL, Nelson EM, Liu LF. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984;259:9182–7. [PubMed] [Google Scholar]
- 40.Pommier Y, Schwartz RE, Kohn KW, Zwelling LA. Formation and rejoining of deoxyribonucleic acid double-strand breaks induced in isolated cell nuclei by antineoplastic intercalating agents. Biochemistry. 1984;23:3194–201. doi: 10.1021/bi00309a013. [DOI] [PubMed] [Google Scholar]
- 41.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–5. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 42.Solier S, Pommier Y. The apoptotic ring: a novel entity with phosphorylated histones H2AX and H2B and activated DNA damage response kinases. Cell Cycle. 2009;8:1853–9. doi: 10.4161/cc.8.12.8865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.