Abstract
Several antiangiogenic drugs targeting VEGF/VEGFR approved by the FDA for many cancer types including colorectal and lung cancer can effectively reduce tumor growth. However, targeting the VEGF signaling pathway will likely influence the normal function of endothelial cells in maintaining homeostasis and cause unwanted adverse effects. Indeed, emerging experimental evidence suggests that VEGF-targeting therapy induced less tumor cell–specific cytotoxicity, allowing residual cells to become more resistant and eventually develop a more malignant phenotype. We report an antitumor therapeutic EndoCD fusion protein developed by linking endostatin (Endo) to cytosine deaminase and uracil phosphoribosyl transferase (CD). Specifically, Endo possesses tumor antiangiogenesis activity that targets tumor endothelial cells, followed by CD, which converts the nontoxic prodrug 5-fluorocytosine (5-FC) to the cytotoxic antitumor drug 5-fluorouracil (5-FU) in the local tumor area. Moreover, selectively targeting of tumor sites allows an increasing local intratumoral concentration of 5-FU, thus providing high levels of cytotoxic activity. We demonstrated that treatment with EndoCD plus 5-FC, compared with bevacizumab plus 5-FU treatment significantly increased the 5-FU concentration around tumor sites and suppressed tumor growth and metastasis in human breast and colorectal orthotropic animal models. In addition, in contrast to treatment with bevacizumab/5-FU, EndoCD/5-FC did not induce cardiotoxicity leading to heart failure in mice after long-term treatment. Our results showed that compared with currently used antiangiogenic drugs, EndoCD possesses potent anticancer activity with virtually no toxic effects and does not increase tumor invasion or metastasis. Together, these findings suggest that EndoCD/5-FC could become an alternative option for future antiangiogenesis therapy.
Keywords: antiangiogenesis, bevacizumab, chemotherapy, 5-fluorouracil, endothelial cell-targeting
Introduction
Several anticancer drugs that target angiogenesis by blocking vascular endothelial growth factor/receptor (VEGF/VEGFR) have shown promise and have been approved by the FDA for clinical treatment of various cancer types (1). These drugs include bevacizumab (Avastin), a humanized VEGF-neutralizing monoclonal VEFG antibody, and sorafenib and sunitinib, small molecular inhibitors that block VEGF receptor tyrosine kinase activity. Although these agents have successfully inhibited primary tumor growth and progression, there is no curative potential activity (2, 3). In addition to causing severe adverse effects such as cardiotoxicity, gastrointestinal perforation, poor wound healing, and hypertension, induced by antiangiogenic treatment (4, 5), recent reports also indicated that anti-VEGF therapy enhances tumor progression by increasing invasion and metastasis (6, 7).
Tumors require the VEGF pathway for progression; however, stimulation of other angiogenesis factors may occur after VEGF/VEGFR targeted therapy and induce tumor resistance to antiangiogenesis monotherapy (8). For example, in addition to VEGF-stimulated angiogenesis, six other proangiogenic growth factors contribute to various stages of breast cancer progression (9); in addition, several intracellular molecules have been identified that modulate tumor angiogenesis (10). Since it is not sufficient to inhibit tumor growth with use of antiangiogenesis monotherapy, a combination approach with chemotherapy is commonly used to improve the clinical benefits of antiangiogenic drug treatment (11). Although combination therapy may reduce tumor resistance and enhance therapeutic efficiency compared with use of a single antiangiogenic drug, increased toxicity results in shorter progression-free survival (12). Initially, anti-VEGF/VEGFR antiangiogenic drugs were not expected to cause toxic effects or drug resistance; however, increasing numbers of clinical reports have demonstrated that inhibition of the VEGF signaling pathway has led to off-target effects that influenced normal body homeostasis (13). Some adverse effects include potentially life-threatening complications such as gastrointestinal perforation with short-term treatment and a reduced left ventricular ejection fraction (LVEF) in the heart with long-term treatment.
Drugs that block angiogenesis can be classified as either direct or indirect inhibitors (2). Compared with direct angiogenic inhibitors, indirect anti-VEGF/VEGFR agents are more likely to induce drug resistance because they target genetically altered and unstable tumor cells rather than genetically stable endothelial cells (2, 14). Endostatin (Endo), an angiogenesis inhibitor that targets unique proliferating endothelial cells and inhibits proliferation, migration, and invasion (15) through interaction with the αvβ3 and α5β1 integrin receptors (16), was tested in multiple clinical trials (17, 18) without causing severe adverse effects or drug resistance. However, due to poor clinical response and its short protein half-life, Endo was not further pursued in clinical trials after phase II studies in the United States (19, 20).
Cytosine deaminase is an enzyme capable of converting 5-fluorocytosine (5-FC) prodrug into cytotoxic 5-fluorouracil (5-FU) (21). A fusion gene consisting of cytosine deaminase and uracil phosphoribosyl transferase (for simplicity, we will refer to this fusion gene as CD throughout this article) was reported to increase the 5-FC/5-FU conversion rate to 10,000 times the rate observed with use of a single enzyme alone (22) and is well known to produce bystander effect in cancer cell killing in a gene therapy setting (23, 24). However, since it is difficult to target chemotherapy specifically to the tumor site, systemic treatment will have off-target issues and cause unwanted adverse effects.
In this study, we report an engineered endostatin-CD (EndoCD) (25) fusion protein that provides a wider therapeutic window, higher therapeutic efficacy and safety, and lower drug resistance potential for cancer treatment. We compared the therapeutic efficacy of EndoCD plus 5-FC (EndoCD/5-FC) and bevacizumab plus 5-FU (bevacizumab/5-FU) and found that EndoCD/5-FC surpassed bevacizumab/5-FU in several aspects: 1) EndoCD/5-FC exhibited potent antitumor activity by increasing 5-FU concentration locally at the tumor site, resulting in higher therapeutic efficacy; 2) EndoCD/5-FC decreased metastasis; 3) EndoCD/5-FC did not induce cardiotoxicity or cardiac function failure as monitored by magnetic resonance imaging (MRI) over a prolonged period. Our findings demonstrate that EndoCD fusion protein is an excellent candidate as an antiangiogenic inhibitor to be tested in future clinical trials.
Materials and Methods
Detailed methodology is described in the Supplementary Methods.
Cell Lines
MDA-MB-231, and murine 4T1 breast adenocarcinoma cell lines were maintained in Dulbecco's modified Eagle's (DMEM)/F12 medium supplemented with 10% fetal bovine serum. Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell medium-2 (Cambrex, East Rutherford, NJ). The 620-L-1 colon cancer cell line was generated by our laboratory after several cycles of preselection from an orthotopic colon model that produced 100% liver metastasis and was maintained by G418 selection. All cell lines were validated by STR DNA fingerprinting with use of the AmpF&STR Identifiler kit according to manufacturer's instructions (Applied Biosystems cat. 4322288). The STR profiles were compared with known ATCC fingerprints (ATCC.org), to the Cell Line Integrated Molecular Authentication Database (CLIMA) version 0.1.200808 (http://bioinformatics.istge.it/clima/) (Nucleic Acids Research 37: D925-D932 PMCID: PMC2686526), and to the MD Anderson fingerprint database. The STR profiles matched known DNA fingerprints or were unique.
Recombinant Protein Purification
The coding sequence of the human endostatin (Endo) was amplified from pPICZaA/hE (EntreMed) by polymerase chain reaction and cloned into the pET28 bacterial expression vector (Novagen) to generate pET28Endo. The yeast cytosine deaminase and uracil phosphoribosyl transferase (CD) was sub-cloned from pORF5-Fcy∷Fur into pET28 (pET28CD). Recombinant proteins Endo, CD, and EndoCD were expressed from pET28Endo, pET28CD, and pET28EndoCD, respectively and purified from a liter of IPTG-induced bacterial culture based on procedures previously described (26). The molecular weights of Endo, CD, and EndoCD are 20 kDa, 40 kDa, and 60 kDa, respectively. Therefore, an equimolar ratio (1:2:3) of the proteins was used for all experiments.
Animal Models
All animals were maintained in the animal facility, and experiments were carried out under The University of Texas MD Anderson Cancer Center guidelines. For the syngeneic model, BALB/c mice were inoculated (mammary fat pad) with 1 × 105 4T1 murine breast adenocarcinoma cells. After the tumor volume reached 3–5 mm in diameter, equal molar amounts of proteins (Endo, CD, and EndoCD) were injected via tail vein. One hour after protein treatment, all groups received 5-FC (500 mg/kg) by intraperitoneal injection. For the orthotopic xenograft model, nude mice were inoculated with 3 × 106 MDA-MB-231 human breast cancer cells in the mammary fat pad or 3 × 106 620-L-1 human colon cancer cells in the cecal wall. Mice received 10 mg/kg of bevacizumab (once every 2 weeks, the clinical dose and schedule used in treating breast and colon cancer) or 60 mg/kg of EndoCD (twice per week; the protein dosage was based on the endostatin clinical dose of 20 mg/kg and the schedule was based on protein stability) via tail vein injection. For mice treated with EndoCD, 500 mg/kg of 5-FC was given 1 hour later by intraperitoneal injection. Mice treated with bevacizumab were given 15 mg/kg of 5-FU (clinical dose) intraperitoneally once per week. Tumor volume was monitored by measuring luciferase signals using IVIS (In Vivo Imaging System; Xenogen, Alameda, CA). In a reduced-treatment experiment, the number of treatments given was decreased from 10 to 5. All protein treatments were given intravenously, whereas chemical drugs were administered by intraperitoneal injection.
Immunofluorescence Staining
Frozen sections (4 μm) were fixed in cold 100% acetone for 5 minutes and then air-dried. After immersion in 1× PBS for 15 minutes, the slides were incubated with rat monoclonal anti-CD31 antibody (BD Biosciences, San Jose, CA) at room temperature for 1 hour, rinsed with 1× PBS, and then incubated with goat anti-rat immunoglobulin G conjugated to Texas Red (1:200; Jackson ImmunoResearch Laboratory, West Grove, PA) in the dark at ambient temperature for 60 minutes. CD31-positive blood vessels were counted in 10–30 fields at 200× magnification in a blinded fashion.
Results
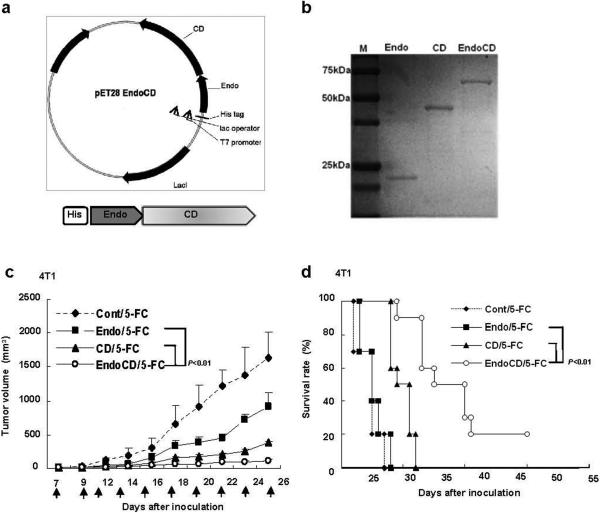
Previously, we developed a gene therapy-based EndoCD treatment that was both antiangiogenic and cytotoxic and had significant antitumor effect in vivo (25). However, since the FDA has not approved a single human gene therapy as a drug to date, a protein-based therapy of EndoCD would be more practical for obtaining FDA approval. To achieve this goal, we constructed a recombinant protein expression vector containing the human Endo and yeast CD fusion gene that was engineered to express EndoCD as a single polypeptide (Fig. 1a). As an endothelial cell-targeting agent, Endo would bring CD to the tumor site as Endo targets tumor vasculature (27), and once at the tumor site, CD could then convert 5-FC to cytotoxic 5-FU and induce apoptosis. Due to the differences in the molecular weight of Endo, CD, and EndoCD (20 kDa, 40 kDa, and 60 kDa, respectively), we used a ratio of 1:2:3 of the proteins for all the experiments in this study to ensure equal molarity (Fig. 1b).
Figure 1.
Construction of EndoCD protein expression vector, EndoCD protein purification, and its antitumor activity. (a) Vector map of the EndoCD fusion protein. EndoCD was cloned into the pET28 vector (Novagen) and expressed in Escherichia coli (BL21) with an N-terminal histidine tag. (b) SDS-PAGE analysis of the purified Endo, CD, and EndoCD protein. (c) EndoCD antitumor activity in breast cancer orthotopic animal model. 4T1 cells were injected into mammary fat pad, and 2.5 mg/kg of Endo, 5 mg/kg of CD, or 7.5 mg/kg of EndoCD was injected intravenously. All mice were given 500 mg/kg of 5-FC by intraperitoneal injection 1 hr after protein treatment. Arrows represent each protein treatment. EndoCD/5-FC had the best therapeutic efficacy in suppressing tumor growth and prolonged the overall mean survival of mice (d). ◆, Cont/5-FC; ■, Endo/5-FC; ▲, CD/5-FC; ο, EndoCD/5-FC.
To determine the stability of the purified protein in serum, 12.5 μM of each purified, his-tagged protein (Endo, CD, and EndoCD) was mixed immediately with mice serum, incubated at 37°C for the number of days indicated, and analyzed by immunoblotting with use of anti-his-tag antibody (Supplementary Fig. 1a). We found that although Endo had a short protein half-life of less than one day, which is consistent with previous clinical reports (28), EndoCD fusion protein had much longer protein stability with a half-life of about 3 days compared with Endo in the presence of mice serum (Supplementary Fig. 1b). Once we validated the protein stability of EndoCD in serum, we then tested the antiangiogenic activity and cytotoxicity in vitro. As shown in Supplementary Fig. 2, and consistent with the previously established EndoCD gene therapy, purified EndoCD protein significantly decreased tube formation and the number of migrated cells and suppressed cell growth. As expected, EndoCD/5-FC and CD/5-FC demonstrated similar cell killing effect in mouse 4T1 breast cancer cell line. However, we observed a slight difference cell killing activity between EndoCD/5-FC and CD/5-FC in human MDA-MB-231 cells for which the mechanism is unclear. Nonetheless, these results demonstrate that the purified EndoCD fusion protein maintained the function of Endo and CD in vitro.
To determine the antitumor activity of EndoCD in vivo, we first injected 4T1 mammary tumor cells into the mammary fat pad of BALB/c mice to establish a syngeneic mouse model. When the tumor size reached 3 to 5 mm in diameter, 2.5 mg/kg of Endo, 5 mg/kg of CD, or 7.5 mg/kg of EndoCD was administered by intravenous injection of mice for a total of 10 treatments (indicated by arrows), with intraperitoneal injection of 500 mg/kg 5-FC (22) given 1 hour after protein treatment. We chose 2.5 mg/kg for Endo as the starting treatment dose because it was previously shown to effectively inhibit tumor growth (15). Mice that received EndoCD/5-FC showed more significant tumor regression (Fig. 1c) and had a prolonged overall survival rate (Fig. 1d) compared with those that received Endo/5-FC or CD/5-FC. These results indicate that the EndoCD fusion protein did not alter the original biological function of either Endo or CD and inhibited tumor growth more effectively than the two proteins alone.
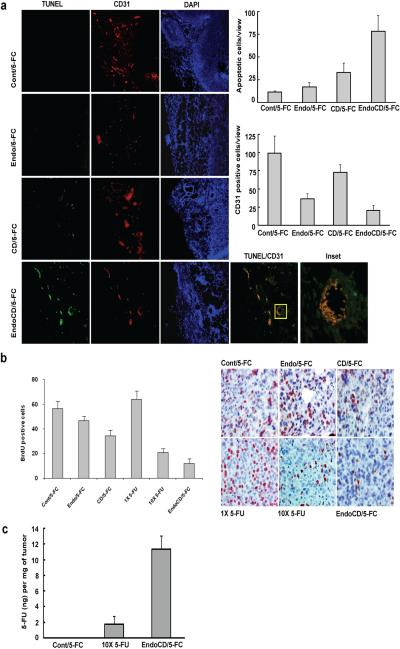
To characterize the biological effects of EndoCD-mediated antitumor and antiangiogenic activities in vivo, we harvested tumor samples from the treated mice described above to examine in vivo angiogenesis inhibition as well as induction of cell death induced by the purified fusion proteins. Immunofluorescence staining of tumor tissue with use of CD31 antibody (a marker for endothelial cells) as well as use of the TUNEL assay demonstrated that EndoCD/5-FC reduced tumor vascular density and induced endothelial and cancer cell apoptosis. Based on the merged image of CD31 and apoptosis double staining in EndoCD/5-FC–treated tumor samples, the majority of the apoptotic signal was found in and around endothelial cells (TUNEL/CD31 panel and inset, Fig. 2a). The signal (green) from the TUNEL assay overlapped with the CD31 signal (red) in the tumor of EndoCD/5-FC-treated mice, indicating that the endothelial cells underwent active apoptosis. Moreover, the presence of TUNEL signal (green) around the endothelial cells (inset, Fig. 2a) only in EndoCD/5-FC- but not Endo/5-FC- or CD/5-FC-treated cells suggests that it is most likely from apoptotic tumor cells. These observations suggest that the cytotoxic effect is a result of increased local concentration of 5-FU in the tumor microenvironment.
Figure 2.
Biological effect of 5-FC to 5-FU conversion by EndoCD in tumor microenvironment. (a) Representative example of immunostaining of tumor tissues from mice shown in Fig. 1c for vascular density by CD31 antibody staining (red) and apoptosis by TUNEL assay staining (green). EndoCD/5-FC reduced the vascular density and induced endothelial cell and tumor cell apoptosis. (b) Mice were treated with purified Endo (20 mg/kg), CD (40 mg/kg), EndoCD (60 mg/kg) proteins plus 500 mg/kg of 5-FC, a clinically sufficient dose of 5-FU (15 mg/kg; 1× 5-FU), or 10 times the clinically sufficient dose (150 mg/kg; 10× 5-FU). Tumor sections were labeled with BrdU (brown) antibody. BrdU was intraperitoneally injected at 1 mg/kg 18 hr before tumors were harvested. EndoCD/5-FC showed higher tumor cell proliferation inhibition than with 10× 5-FU. (c) LC/MS/MS analytical profile of 5-FU concentration in tumor from mice injected with 500 mg/kg of 5-FC, 10× 5-FU via intraperitoneal injection, or 60 mg/kg of EndoCD plus 500 mg/kg of 5-FC via intravenous injection.
To determine the effect of protein treatments in cell proliferation, MDA-MB-231 breast cancer cells were first injected into the mammary fat pad of nude mice, and when tumors reached 10 mm in diameter, mice were treated once only with purified Endo (20 mg/kg), CD (40 mg/kg), or EndoCD (60 mg/kg) proteins plus 500 mg/kg 5-FC, a clinically sufficient dose of 5-FU (15 mg/kg; 1× 5-FU), or 10 times the clinically sufficient dose (150 mg/kg; 10× 5-FU). The choice of 20 mg/kg Endo was based on a previous preclinical study (15) and is also within the dose tested in the phase I clinical trial (18) (15–600 mg/m2 in human is equivalent to 4.8–194.4 mg/kg in mouse (29)). Tumors were harvested from mice 48 hours after treatment and labeled with BrdU antibody for in vivo BrdU incorporation analysis. The results show that EndoCD/5-FC most significantly reduced cancer cell proliferation (Fig. 2b) compared with all other treatment groups.
The potent inhibitory activity of EndoCD/5-FC treatment on cancer cell proliferation in vivo, which is even stronger than that of 10× 5-FU treatment (Fig. 2b), prompted us to measure the local concentration of 5-FU in the tumor sites. To this end, we used liquid chromatography-mass spectrometry (LC/MS/MS) to measure a 5-FU concentration in the tumor microenvironment. First, tumor-bearing mice were given 500 mg/kg 5-FC, 10× 5-FU, or 60 mg/kg EndoCD plus 500 mg/kg 5-FC. Then, tumors were removed 2 hours after drug treatment, and 5-FU was directly extracted from tumor samples for detection by LC/MS/MS. The 5-FU concentration in tumors from the EndoCD/5-FC–treated group was about 7-fold higher than that from the 10× 5-FU treatment group (Fig. 2c). Together, the results show that EndoCD/5-FC can reduce tumor site vascular density and increase 5-FU concentration around tumor sites to enhance apoptosis in both tumor and tumor endothelial cells.
To investigate the acute toxicity of EndoCD, mice were administered 60 mg/kg EndoCD with 500 mg/kg 5-FC given 1 hour after the protein treatment. Blood samples were collected every other day for 7 days from all mice treated with Endo, CD, or EndoCD fusion protein and analyzed for changes in the serum level of liver aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine. Clearly, AST, ALT, BUN, and creatinine levels in EndoCD-treated mice were all within the normal range (30) (Supplementary Fig. 3). In addition, no mice in the EndoCD/5-FC treatment group displayed any obvious symptoms such as loss of appetite or inactivity or died more than 2 months after initial treatment, when the experiment was terminated (data not shown). Thus, these results suggest that treatment with EndoCD/5-FC produced virtually no toxicity or nondetectable toxicity in mice.
To further determine the antitumor activity of EndoCD/5-FC, we compared the therapeutic efficacy of EndoCD/5-FC and bevacizumab/5-FU in orthotopic mouse models of human colorectal cancer with liver metastasis (620-L-1) and human breast cancer (MDA-MB-231). Seven days after injection, when tumors were established, EndoCD (60 mg/kg, twice per week) or bevacizumab (10 mg/kg, once every 2 weeks) (31) was intravenously injected, and 5-FU (15 mg/kg, once per week) (31) or 5-FC (500 mg/kg; given 1 hour after EndoCD treatment) was administered by intraperitoneal injection. For practical clinical reasons, the treatment protocols for bevacizumab and 5-FU were essentially derived from previously established clinical doses and schedules (31).
We observed significantly better tumor suppression in mice treated with EndoCD/5-FC than in those treated with bevacizumab or 5-FU alone (Fig. 3a, 3b). Although we did not observe a significant difference in tumor reduction between EndoCD/5-FC and bevacizumab/5-FU under this condition, EndoCD/5-FC–treated mice had a better overall mean survival rate than did those treated with bevacizumab/5-FU (p = 0.004) in the 620-L-1 model (Fig. 3c).
Figure 3.
Therapeutic efficacy of EndoCD/5-FC and bevacizumab/5-FU in an orthotopic animal model. In a metastatic colorectal cancer model (a) and a human breast cancer model (b), 60 mg/kg of EndoCD proteins were injected intravenously twice per week. An hour after protein treatment, mice were given 500 mg/kg of 5-FC by intraperitoneal injection. For bevacizumab treatment, 10 mg/kg of the drug was injected intravenously once every 2 weeks, and mice were given with 15 mg/kg of 5-FU by intraperitoneal injection once per week. (c) EndoCD/5-FC–treated mice from (a) exhibited prolonged overall survival. EndoCD/5-FC showed better antitumor activity than bevacizumab/5-FU in mice treated only for 3 weeks for a total of 5 treatments in a metastatic colorectal cancer model (d) and breast cancer model (e). (f) EndoCD/5-FC reduced liver metastasis in a metastatic colorectal cancer model. Bec, bevacizumab. Arrows represent each protein treatment. ◆, Cont/5-FC; ■, 5-FU; ▲, Bec; □, Bec/5-FU; ο, EndoCD/5-FC.
To further investigate whether the treatment schedule might affect antitumor activity, we decreased the number of drug treatments to only 5 and showed that EndoCD/5-FC had much better antitumor activity and metastasis suppression activity than did bevacizumab/5-FU in the metastatic colon cancer model on day 35 after tumor inoculation (Fig. 3d). Similar results were obtained in the MDA-MB-231 orthotopic tumor mouse model, although the efficiency in the breast cancer model (Fig. 3e) was not as dramatic as that in the colon cancer model (Fig. 3d). These results suggested that EndoCD/5-FC treatment resulted in stronger antitumor activity than did bevacizumab/5-FU treatment.
As mentioned above, VEGF/R inhibitors reduce primary tumor growth; however, due to the cytostatic nature of these drugs, tumor cells that might have survived could become more malignant, as suggested in recent studies that presented evidence that these drugs may increase tumor invasiveness and metastasis (32). To address whether EndoCD/5-FC treatment also promotes tumor invasiveness and metastasis, we used the stable 620-L-1 cell line expressing luciferase to facilitate detection of colon to liver metastasis in real time by IVIS imaging. After 35 days of tumor cell inoculation, we found that mice that were treated with EndoCD did not have increased liver metastasis compared with those that received bevacizumab/5-FU (Fig. 3f). Taken together (Figs. 2 and 3), our results suggested that EndoCD/5-FC induces not only potent cytostatic effects but also cytotoxic activity to reduce the number of surviving tumor cells and further inhibit colorectal liver metastasis.
Bevacizumab treatments have resulted in a 1.7 to 3% incidence of left ventricular dysfunction, and 5-FU is known to have caused ischemic complications in treated patients (33). To examine the contribution of these drug treatments to cardiotoxicity, we collected serum from mice (Fig. 3b) treated with 15 mg/kg of 5-FU, 60 mg/kg of EndoCD plus 500 mg/kg of 5-FC, 10 mg/kg of bevacizumab, or 10 mg/kg of bevacizumab plus 15 mg/kg of 5-FU and compared levels of troponin I, a marker of cardiomyocyte damage, from these groups with levels from the untreated control group. We found that bevacizumab- and bevacizumab/5-FU–treated mice had a substantially higher troponin I serum concentration than the other groups did, which showed essentially no troponin I (Supplementary Fig. 4).
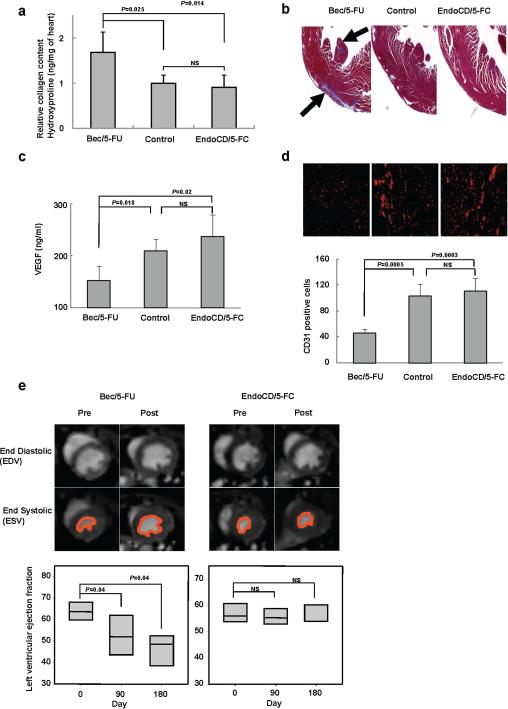
To further investigate the impact of EndoCD/5-FC and bevacizumab/5-FU on the heart, we determined the occurrence of cardiac fibrosis by measuring hydroxyproline for collagen accumulation and using trichrome staining to directly evaluate the amount of collagen in the heart tissue. Mice were treated with 60 mg/kg of EndoCD plus 500 mg/kg of 5-FC (twice per week) and 10 mg/kg of bevacizumab (once every 2 weeks) plus 15 mg/kg of 5-FU (once per week); after 6 months of treatment, the hearts were harvested from mice for proline hydroxylation measurement. Mice that were given bevacizumab/5-FU had higher levels of proline hydroxylation (Fig. 4a) and increased cardiac fibrosis (blue color indicated by arrows; Fig. 4b) than did mice of the same age in the control and EndoCD/5-FC treatment groups. Previously, it was shown that VEGF plays an important role in myocardial angiogenesis and that the loss of VEGF-impaired cardiac function leads to ischemic cardiomyopathy in mice (34). As serum VEGF concentration significantly decreased under anti-VEGF treatment (Fig. 4c), a concurrent reduction in vascular density of heart tissue was observed, indicated by the loss of CD31 signal under anti-VEGF treatment (Fig. 4d), which is consistent with the expected effect of anti-VEGF treatment. These findings therefore suggest that more severe cardiomyopathy in patients could be caused by bevacizumab/5-FU treatment than by EndoCD/5-FC treatment.
Figure 4.
In vivo cardiac function detection. (a) Hydroxyproline content was measured and normalized to heart tissue weight. The hydroxyproline content in the bevacizumab/5-FU treatment group was significantly higher than that in control mice of the same age, and there was no significant difference between levels in the EndoCD/5-FC group and control mice. (b) The presence of fibrosis is shown in blue by trichrome staining of heart histological section. (c) Mice serum VEGF level decreased in bevacizumab/5-FU–treated mice but not in EndoCD/5-FC–treated mice. (d) Representative example of vascular density immunostaining by CD31 antibody (red) of heart tissue. Bevacizumab/5-FU–treated mice showed significantly reduced vascular density in the heart tissue. (e) After drug treatment, left ventricular ejection fraction (LVEF) was significantly decreased in the bevacizumab/5-FU group, whereas there was no significant difference of LVEF in the EndoCD/5-FC group. The pretreatment percentage was used as the basal level of LVEF. All mice described in panels (a) through (e) were treated with 10 mg/kg of bevacizumab plus 15 mg/kg of 5-FU or 60 mg/kg of EndoCD plus 500 mg/kg of 5-FC. NS, no significance.
Finally, to determine whether these drug treatments would affect cardiac function, we used small animal MRI to determine the end-diastolic volume (EDV) and endsystolic volume (ESV) and calculated the left ventricular ejection fraction (LVEF) (35) of mice before (pretreatment basal level) and after treatment with 60 mg/kg of EndoCD plus 500 mg/kg of 5-FC or 10 mg/kg of bevacizumab plus 15 mg/kg of 5-FU. A decrease in LVEF is a major marker of left ventricular dysfunction as a result of a decrease in angiogenesis and increase in cardiac fibrosis (36). As shown in Fig. 4e, although LEVF is significantly decreased in mice that received bevacizumab/5-FU treatment for 3 months, no change in LEVF was observed in EndoCD/5-FC-treated mice, even after 6 months of treatment. Thus, EndoCD/5-FC has minimal cardiac toxicity, which would give it a relative advantage in the clinic.
Discussion
VEGF/VEGFR serves an important role in regulating tumor angiogenesis initiation and in controlling human homeostasis. In addition to its role in angiogenesis, VEGF also maintains normal biological functions including blood pressure, kidney function, and blood coagulation (13). Thus, starvation of tumor growth by systemically blocking VEGF/VEGFR would also influence human homeostasis and induce off-target adverse effects. While inhibition of a tumor angiogenesis pathway by a single drug alone is not sufficient to block redundancies in tumor angiogenesis regulators, leading to potential increases in tumor relapse, invasion, metastasis (37), a combination of antiangiogenic drugs and chemotherapy have been shown to causes more life-threatening adverse effects (12). Therefore, by targeting unique endothelial cells through the use of an endogenous antiangiogenic molecule, the off-target adverse effects and drug-induced resistance may be reduced. With one exception, most endogenous antiangiogenesis proteins are low in protein stability as well as cytotoxic antitumor activity (38).
Here, we show that in the presence of 5-FC, EndoCD endothelial cell-targeting fusion protein not only blocks angiogenesis but also provides a relatively high dose and site-specific chemotherapeutic effect, thereby offering potentially curative therapeutic benefits. Since the VEGF pathway is also essential for normal biological functions, inhibitors that interrupt it would no doubt produce off-target side effects in normal organs. Instead, the use of an approach that targets proliferating endothelial cells would most likely decrease the adverse effects (14). Therefore, by bringing chemotherapeutic drugs to the tumor site, treatment with drugs such as EndoCD further increases the local cytotoxic effect.
Recently, some studies have reported that cancer stem cells transdifferentiate into endothelial cell phenotypes to form tumor vasculature, explaining the failure of antiangiogenic drugs in the clinic and of proposed strategies to specifically target tumor endothelial cells to block angiogenesis in cancer treatment (39–41). However, it is not yet clear whether transdifferentiation of stem-like cancer cells into endothelial cells also takes place in solid tumors such as breast or colon cancer. This is an interesting issue to be address in a systematic approach in the future. In this study, we provided evidence that EndoCD/5-FC not only targets tumor angiogenesis specifically but also reduces surviving tumor cells, thus decreasing the incidence of tumor invasiveness and metastasis. Taken together, the dual-targeting antiangiogenic and chemotherapeutic strategy we have developed may provide a new therapy to prevent tumor recurrence, decrease tumor metastasis, and eliminate unwanted cytotoxic adverse effects.
Supplementary Material
Acknowledgments
We are grateful to Drs. Long-Sheng Lu and Sui Zhang for their advice on small animal MRI and to Dr. Qingqing Ding for providing the 620-L-1 liver metastasis colorectal cell line. We thank the Translational Chemistry Core Facility and the Pharmacology and Analytical Facility for tumor 5-FU analysis. We thank Dr. Tamara K. Locke for scientific editing.
Grant Support This work was supported by the Breast Cancer Research Foundation, the Breast Cancer SPORE grant, The National Breast Cancer Foundation, Patel Memorial Breast Cancer Research Fund, Sister Institution Fund of China Medical University and Hospital and MD Anderson Cancer Center, Cancer Research Center of Excellence (D0H99-TDC-111-005, Taiwan), NSC-3111-B-039 (Taiwan), and Cancer Center Support Grant (CA16672). In memoriam, we recognize Mrs. Serena Lin-Guo for her courageous fight against breast cancer.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 4.Kramer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32:1–14. doi: 10.1111/j.1365-2710.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 5.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 6.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–9. [PubMed] [Google Scholar]
- 10.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–79. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 11.Kerbel RS. Issues regarding improving the impact of antiangiogenic drugs for the treatment of breast cancer. Breast. 2009;18(Suppl 3):S41–7. doi: 10.1016/S0960-9776(09)70271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 13.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–85. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 14.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13:31–6. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 17.Yang L, Wang JW, Sun Y, Zhu YZ, Liu XQ, Li WL, et al. [Randomized phase II trial on escalated doses of Rh-endostatin (YH-16) for advanced non-small cell lung cancer] Zhonghua Zhong Liu Za Zhi. 2006;28:138–41. [PubMed] [Google Scholar]
- 18.Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792–803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Kulke MH, Bergsland EK, Ryan DP, Enzinger PC, Lynch TJ, Zhu AX, et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24:3555–61. doi: 10.1200/JCO.2006.05.6762. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Tang H, Huang Y, Song N, Luo Y. Unraveling the mysteries of endostatin. IUBMB Life. 2009;61:613–26. doi: 10.1002/iub.215. [DOI] [PubMed] [Google Scholar]
- 21.Pandha HS, Martin LA, Rigg A, Hurst HC, Stamp GW, Sikora K, et al. Genetic prodrug activation therapy for breast cancer: A phase I clinical trial of erbB-2-directed suicide gene expression. J Clin Oncol. 1999;17:2180–9. doi: 10.1200/JCO.1999.17.7.2180. [DOI] [PubMed] [Google Scholar]
- 22.Chung-Faye GA, Chen MJ, Green NK, Burton A, Anderson D, Mautner V, et al. In vivo gene therapy for colon cancer using adenovirus-mediated, transfer of the fusion gene cytosine deaminase and uracil phosphoribosyltransferase. Gene Ther. 2001;8:1547–54. doi: 10.1038/sj.gt.3301557. [DOI] [PubMed] [Google Scholar]
- 23.Ramnaraine M, Pan W, Goblirsch M, Lynch C, Lewis V, Orchard P, et al. Direct and bystander killing of sarcomas by novel cytosine deaminase fusion gene. Cancer Res. 2003;63:6847–54. [PubMed] [Google Scholar]
- 24.Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–22. [PubMed] [Google Scholar]
- 25.Ou-Yang F, Lan KL, Chen CT, Liu JC, Weng CL, Chou CK, et al. Endostatincytosine deaminase fusion protein suppresses tumor growth by targeting neovascular endothelial cells. Cancer Res. 2006;66:378–84. doi: 10.1158/0008-5472.CAN-05-1578. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Wong MK, Zhao Q, Zhu Z, Wang KZ, Huang N, et al. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res. 2001;61:478–81. [PubMed] [Google Scholar]
- 27.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eder JP, Jr., Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, et al. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20:3772–84. doi: 10.1200/JCO.2002.02.082. [DOI] [PubMed] [Google Scholar]
- 29.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- 30.Khatri A, Zhang B, Doherty E, Chapman J, Ow K, Pwint H, et al. Combination of cytosine deaminase with uracil phosphoribosyl transferase leads to local and distant bystander effects against RM1 prostate cancer in mice. J Gene Med. 2006;8:1086–96. doi: 10.1002/jgm.944. [DOI] [PubMed] [Google Scholar]
- 31.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 32.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–70. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–11. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zannad F, Rossignol P, Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. 15:319–29. doi: 10.1007/s10741-009-9143-0. [DOI] [PubMed] [Google Scholar]
- 37.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001;33:357–69. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 39.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 41.Bautch VL. Cancer: Tumour stem cells switch sides. Nature. 2010;468:770–1. doi: 10.1038/468770a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.