Abstract
Sinomenine is one of the alkaloids extracted from Chinese medical plant, Sinomenium acutum Rehder et Wilson. Sinomenine has been used for Rheumatoid arthritis as an anti-inflammatory and immunomodulative drugs. We have so far been investigated the cardiovascular pharmacological actions of sinomenine. Sinomenine dilated NE (5 μM)-, KCl (60 mM)- and PDB (300 nM)-induced vasoconstrictions. The pretreatment with nicardipine (0.1 μM), staurosporine (30 nM), L-NMMA (100 μM), indomethacin (10 μM) or propranolol significantly attenuated the sinomenine-induced vasorelaxation. Therefore, these results indicate that sinomenine causes the vasorelaxation by the involvement with the inhibitions of Ca2+ current (ICa) and PK-C, β-adrenoceptor stimulation, and the activation of NO and PGI2 syntheses in endothelium. On the other hand, in the ventricular cardiomyocytes of guinea pig, sinomenine inhibits ICa and simultaneously decreases the delayed rectifier K+ current (IK), resulting in the prolongation of action potential duration. Sinomenine also suppresses the dysrhysmias induced by triggered activities under the Ca2+ overload condition. Therefore, sinomenine may be expected as one of effective therapeutic drugs for heart failure and dysrhythmias, and may maintain the cardiovascular functions due to modulation of cardiac ionic channels and blood vessels.
Keywords: Sinomenine, Vasodilation, Cardioprotective action, Ca2+ channel, Aorta, Cardiomyocytes
Introduction
Sinomenine (7, 8-didehydro-4-hydroxy-3, 7-dimethoxy-17-methyl-9α,13α,14α-morphinan-6-one) is one of the alkaloids extracted from Chinese herbs, Sinomenium acutum Rehder et Wilson (Li et al. 2004) or Sinomenium acutum var. cinereum (Zhao et al. 2005). In Chinese traditional medicine, Sinomenine acutum, a vine plant, has been used for rheumatic diseases for thousands years (Yamasaki, 1976; Liu et al. 1996). Its main constituent of Sinomenine acutum is sinomenine, which has also used for clinical treatment of Rheumatoid arthritis (RA), due to the anti-inflammatory and immunomodulative actions (Yamasaki, 1976; Liu et al. 1996).
We have already investigated the cardiovascular pharmacological actions of sinomenine. Mokuboito (Mu-Fang-Yi-Tang), a kind of Kampo formulation containing sinomenium acutum, has been used for heart failure (Inaki et al. 2005), which improves heart failure symptom and reduces New York Heart Association (NYHA) class and plasma brain natriuretic peptide (BNP) concentration (Yakubo et al. 2002). Mokuboito consists of Sinomeni Caulis et Rhizoma (rhizome of Sinomenium acutum Rehder et Wilson), Cinnamomi Cortex (bark of Cinnamomum cassia Blume), Ginseng Radix (roots of Panax ginseng C. A. Meyer) and Gypsum Fibrosum. In our recent reports (Satoh, 2005; Nishida and Satoh, 2006; 2007), Mokuboito, Sinomenium acutum and sinomenine might improve chronic heart failure, resulting from the modulation of cardiac and vascular systems. Mokuboito can exert the vasodilating and cardioprotective actions (Satoh, 2005), as discussed in the following parts.
In general, the basic treatments of heart failure consist of (1) reducing workload of heart, (2) protection of cardiomyocytes and (3) restriction and control of waters and sodium. In order to reduce both pre- and after-loads, the dilations of arterioles and veins are strongly required in the case of elevated filling pressures and reduced cardiac output. As a cardiovascular protective drug, sinomenine might contribute to clinical treatments via modulation of the ionic currents of cardiac cells, and control of the tension of blood vessels.
In this review, the cardiovascular pharmacological actions of sinomenine are mainly shown and discussed.
Source of Sinomenine
Sinomenine is derived from natural plant such as Sinomenium acutum (Li et al. 2004) and Sinomenium acutum var. cinereum (Zhao et al. 2005). Sinomenium acutum and Sinomenium acutum var. cinereum are widely in a lot of regions of China. Caulis sinomeni is the dried plant stems of Sinomenium acutum and Sinomenium acutum var. cinereum. There is less or no difference in sinomenine content of Caulis sinomeni between the species and the varieties of growing regions. The variation is found among the samples collected from different parts of plant. The content of sinomenine is dependent on the size (diameter) of stem. The sinomenine content in Sinomenine acutum is 1.63 ± 0.64 (%, w/w) in large (>3 cm) stem, 0.96 ± 0.45 (%, w/w) in 1–3 cm stem, and 0.49 ± 0.16 (%, w/w) in <1 cm stem (Zhao et al. 2005).
Pharmacokinetics of Sinomenine
The pharmacokinetics and tissue distribution of sinomenine have been studied in rats (Liu et al. 2005). Sinomenine achieves high bioavilability (about 80%) by oral administration of 90 mg/kg. At 45min later, sinomenine is found widely in internal organs such as kidney, liver, lung, spleen, heart, brain and testis. Sinomenine is metabolized and eliminated by kidney and liver. Tmax is 39.5 ± 8.49 min, Cmax is 13.89 ± 4.29 μg/ml, T1/2A phase is 61.28 ± 53.62 min, AUC0-t is 2331.53 ± 1172.77 μg-min/ml, and CL is 42.95 ± 14.4 ml/min per Kg.
In clinical studies, oral administration of 80 mg sinomenine is performed for healthy volunteers (Yan et al. 1997). In the pharmacokinetic parameters, T1/2α is 1.04 ± 0.491h, T1/2β is 9.397 ± 2.425h, Tmax is 1.04 ± 0.274h, Cmax is 246.6 ± 71.165 ng/ml. Furthermore, AUC is 2651.158 ± 1039.050 ng.h/ml, and CL is 0.033 ± 0.010 ng/ml.
Vascular Pharmacology
Male Wistar rats (5–10 week-old) were anesthetized with ether, and euthanized by exsanguination. The thoracic aorta was quickly removed, and then the isolated aorta was cut into rings of 3-mm in length. All rings were stretched to generate a resting tension of 1.2 g in Krebs solution. After 40 min of resting, norepinephrine (NE) (5 μM) was added to the tissue bath. After the contractile response became steady, the drugs were cumulatively administrated into the bath solution. The similar methods are described in our previous reports (Nishida and Satoh, 2003, 2006).
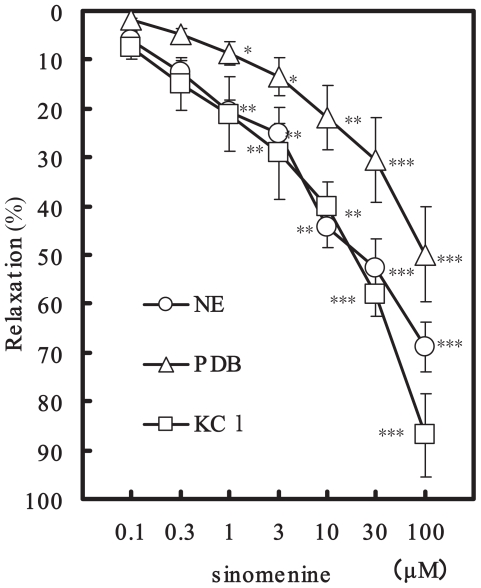
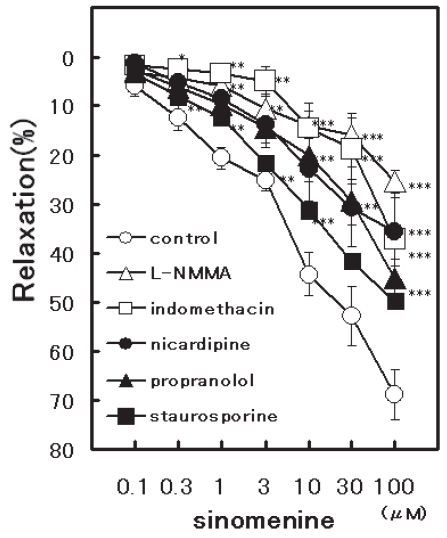
The aorta ring strip of rat had a strong contraction after an initial application of 5 μM NE. Sinomenine (0.1 to 100 μM) applications potently relaxed the contraction induced by NE in a concentration-dependent manner (Nishida and Satoh, 2006). The relaxation was produced at the concentrations of over 0.3 μM sinomenine, and at 100 μM decreased the contractions by 68.8 ± 5.1% (n = 6, p < 0.001). PDB (300 nM) and KCl (60 mM) also caused strong contractions. Sinomenine dilated both PDB- and KCl-induced contractions in a concentration-dependent manner; at 100 μM by 49.9 ± 9.8% (n = 6, p < 0.001) and 86.9 ± 8.5% (n = 6, p < 0.001), respectively (Fig. 1). Further-more, the relaxation of sinomenine (0.1 to 100 μM) was attenuated significantly by the pretreatment with nicardipine (Fig. 2). At 100 μM the relaxation decreased from 68.8 ± 5.1% (n = 6) to 35.5 ± 6.9% (n = 5, p < 0.001). Since PK-C inhibition may be related to sinomenine-induced vasorelaxation, the pretreatment with staurosporine (30 nM) for 30 min was carried out (Satoh, 1996; Nishida and Satoh, 2004). Staurosporine attenuated the sinomenine-induced vasorelaxation; at 100 μM from 68.8 ± 5.1% (n = 6) to 49.5 ± 7.7% (n = 5, p < 0.001) (Fig. 2). In addition, propranolol (0.3 μM) also significantly attenuated the sinomenine-induced relaxation. The vasorelaxation at 100 μM sinomenine was attenuated to 45.2 ± 4.2% (n = 5, p < 0.01).
Figure 1.
Concentration-dependent relaxation of sinomenine on NE-, PDB- and KCl-induced vasorelaxations. Symbols used are sinomenine in pretreatments with NE (open circles, n = 6), PDB (triangles, n = 6) and KCl (squares, n = 6). Values (%) represent mean ± S.E.M. *: P < 0.05, **: P < 0.01, ***: P < 0.001, with respect to control value.
Figure 2.
Modulation by several inhibitors of the relaxation induced by sinomenine. Symbols used are in the absence (open circles, n = 6), in the presence of 100 μM L-NMMA (open triangles, n = 5), 10 μM indomethacin (open squares, n = 5), 0.1 μM nicardipine (closed circles, n = 5), 0.3 μM propranolol (closed triangles, n = 5), and 30 nM staurosporine (closed squares, n = 5). Values (%) represent mean ± S.E.M. *: P < 0.05, **: P < 0.01, ***: P < 0.001, with respect to control value.
Therefore, the modulation of both Ca2+ channel and PK-C is largely contributed to sinomenine-induced actions, and sinomenine also vasodilates mediated through β-adrenoceptor stimulation of aortic smooth muscle cells.
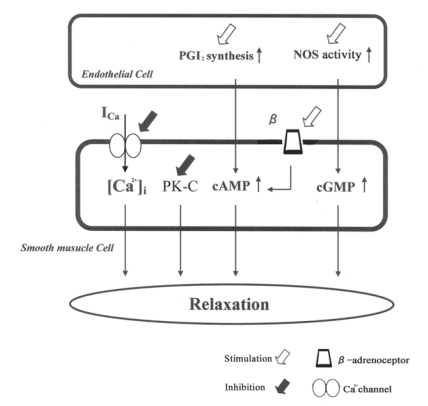
For involvement with endothelium-dependent relaxation via NO activation, a pretreatment with 100 μM L-NMMA (a non-selective NO synthesis inhibitor) was carried out (Nishida and Satoh, 2003, 2007). Under the conditions, the vasorelaxation induced by sinomenine was attenuated from 68.8 ± 5.1% (n = 6) to 25.3 ± 2.3% (n = 5, p < 0.01) (Fig. 2). The attenuation was supported by the results using the aorta with removal of endothelium; at 100 μM sinomenine by 53.7 ± 1.8% (n = 5, p < 0.01). Indomethacin (10 μM), as an inhibitor of prostanoid production, also strongly reduced the vasorelaxation induced by 100 μM sinomenine to 37.1 ± 9.3% (n = 5, p < 0.001). Thus, both L-NMMA and indomethacin affected the sinomenine-induced relaxation significantly. Therefore, sinomenine possesses the pharmacological characteristics for modulation of NO synthesis and PG production, as well as the inhibitions of Ca2+ channel and PK-C and the stimulation of β-adrenoceptor. The possible mechanisms for the vasodilating actions of sinomenine are summarized in Fig. 3.
Figure 3.
Summary of the multiple mechanisms induced by sinomenine. Sinomenine produces the vasorelaxation via NO and PGI2 releasings from endothelium. Also, sinomenine causes the vasorelaxation via modulation of Ca2+ channels and PK-C activity in smooth muscle cells. In addition, β-adrenoceptor stimulation is caused. NOS: nitric oxide synthetase, PK-C: protein kinase C, PGI2 : prostaglandin I2.
Cardiac Electropharmacology
Cardiac cells were taken from the ventricle muscles of guinea pig hearts, using methods similar to those described previously (Satoh, 2003, 2005). Current-clamp and whole-cell voltage-clamp were performed using an Axopatch patch-clamp amplifier (Axon Instruments, Burlingame, C.A, U.S.A.) and standard techniques.
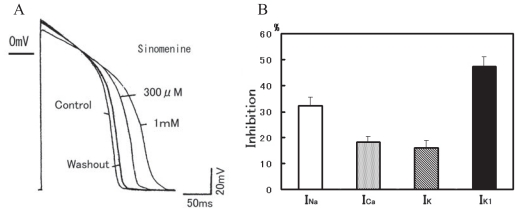
Current-clamp experiments were carried out to examine the modulation of the action potential configuration in guinea pig ventricular muscles (Fig. 4A) (Satoh, 2005). Sinomenine at 300 μM and 1 mM increased the 75% repolarization of action potential duration (APD75) by 24.9 ± 3.5% (n = 6, p < 0.05) and by 43.7 ± 3.3% (n = 6, p < 0.001), respectively. Sinomenine at 1 mM decreased the amplitude (APA), but not significantly (0.9 ± 2.1%, n = 5). The resting potential was not affected.
Figure 4.
Modulation by sinomenine on the action potentials in guinea pig ventricular cardiomyocytes. A: Concentration-dependent changes in the action potential configuration by sinomenine. Short lines at the left of action potential recordings represent zero mV level. B: The percentage inhibitions by sinomenine of cardiac ionic currents. Vertical bars represent mean ± SEM.
The modulation of L-type Ca2+ current (ICa) was investigated. Test pulses were applied to 0 mV from a holding potential of −30 mV. The average capacitance was 84.1 ± 2.4 pF (n = 23). At 1 mM sinomenine inhibited the ICa at 0 mV by 18.2 ± 2.1% (n = 6, p < 0.05). At 1 mM, the delayed rectifier K+ currents (IK) at 60 mV was inhibited by 16.2 ± 2.6% (n = 6, p < 0.05) (Fig. 4B), and the inwardly rectifying K+ current (IK1) at −120 mV by 47.2 ± 3.8% (n = 6, p < 0.01).
In multicellular preparations, the modulation of action potential configuration by sinomenine was also examined (Satoh, 2005). The preparations were stimulated at 1 Hz. Sinomenine (100 μM to 1 mM) had inhibitory effects on the action potentials, and tended to increase the action potential durations (APD); at 1 mM, by 4.1± 1.6% (n = 6) in APD50 and by 4.5 ± 1.4% (n = 6) in APD90 (but not significantly). The maximum rate of depolarization (Vmax) was also inhibited by 20.0 ± 2.4% (n = 6, p < 0.05) at 300 μM and by 32.1 ± 3.3% (n = 6, p < 0.05) at 1 mM of sinomenine.
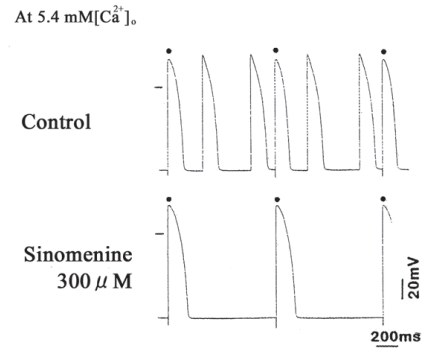
In high extracellular Ca2+ ([Ca2+]o) solution (5.4 mM) to cause cellular Ca2+ overload, abnormal action potentials occurred irregularly, in spite of constant stimulation (1 Hz) (Satoh, 2005). Application of 300 μM sinomenine suppressed and abolished the abnormal action potentials (dysrhythmias) (Fig. 5).
Figure 5.
Antiarrhythmic actions of sinomenine in guinea pig papillary muscles. Sinomenine abolishes the abnormal action potentials in high Ca2+ concentration (5.4 mM). Dots above the action potential recordings are represented the regular rhythms induced by 1 Hz stimulation. The horizontal line indicates zero mV.
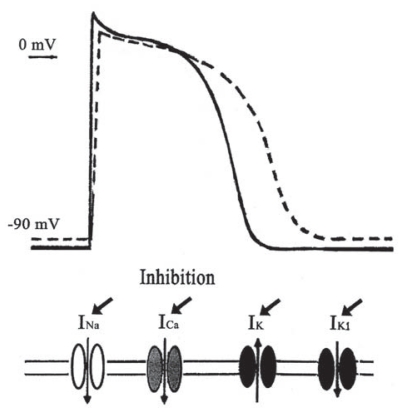
The changes in the action potential configurations and the modulation of the ionic channel currents on the membrane of cardiomyocyte by sinomenine are summarized in Fig. 6.
Figure 6.
Shame for the changes in cardiac ionic channels induced by sinomenine. Superimposed action potentials are in control (solid line) and in sinomenine (dash line). INa: the Na+ channel, ICa: the Ca2+ channel, IK: the delayed rectifier K+channel, IK1: the inwardly rectifying K+channel.
Immunomodulative Action
Sinomenium acutum has been used for treatment for various rheumatic diseases as a Chinese traditional medicine (Yamasaki, 1976; Liu et al. 1996). In basic pharmacological studies of Sinomenium acutum, the inhibitory actions on some enzymes relating to inflammation have been shown (Li et al. 2003).
One of most famous pharmacological actions of sinomenine is an immunomodulative effect. Sinomenine is also clinically used for RA treatment (Yamasaki, 1976; Liu et al. 1996). A lot of reports concerning about not only an anti-rheumatic effect, but also anti-inflammatory and immunomodulative effects have already been shown. As anti-rheumatic direct effects, it has been reported that sinomenine reduces inflammatory parameters and attenuates proliferation of synovial fibroblasts in rat adjuvant arthritis models (Liu et al. 1996). The anti-inflammatory and immunomodulative actions of sinomenine are responsible for various mechanisms via complex modulation of leukocytes and cytokine. Sinomenine reduces the production of prostagrandin (PG) E2 and NO from macrophage (Liu et al. 1994a). Also, sinomenine possesses anti-proliferative effects on lymphocytes (Liu et al. 1994b), contributing to anti-inflammatory and anti-rheumatic effects. In addition, sinomenine depressed mRNA expression of tumor necrosis factor (TNF)-α and interleukin (IL)-β of peritoneal macrophages (Wang et al. 2005). Therefore, sinomenine may act as an anti-rheumatic drug through the anti-inflammatory effects on lymphocytes and cytokine. Sinomenine inhibited bFGF-induced angiogenesis in vitro and in vivo (Kok et al. 2005). Sinomenine also attenuates transmigration of granulocyte. The inhibition of leukocytes migration across the vessel wall and anti-angiogeneic effect of sinomenine may also contribute to therapeutic effects for RA. Immunomodulative actions have been studied as the other aspect of sinomenine concerning about the cardiac transplantation model. It has been reported that acute and chronic cardiac allograft ejections are blocked by the immunomodulatory effects of sinomenine (Mark et al. 2003).
Conclusion
Endothelium-dependent and -independent relaxations
We have been demonstrated that sinomenine possesses strong vasodilating actions by multiple mechanisms (Nishida and Satoh, 2006). The summarized mechanisms of sinomenine-induced vasorelaxation are shown in Fig. 3. Sinomenine possesses endothelium-dependent vasorelaxation via NO and PGI2 releasing from endothelium. NOS activation and PGI2 release are elicited by an increase in the intracellular Ca2+ concentration ([Ca2+]i) in endothelium cells (Busse et al. 1998; Quignard et al. 1999). The mechanisms for the endothelium-dependent relaxations are not yet unclear. However, sinomenine might increase [Ca2+]i in endothelium cells and then, activates NOS activity and PGI2 releasing, as reported previously (Nishida and Satoh, 2003).
Sinomenine causes the vasorelaxation via modulation of Ca2+ channels and PK-C activity in vascular smooth muscle cells. In vascular muscle cells, the contraction systems and the ion channels are regulated through the intracellular signal conditions (Satoh and Sperelakis, 1991, 1995; Satoh, 1996). Therefore, sinomenine might modulate the contraction systems, Ca2+ and Na+ channels, delayed rectifier K+ channels, and Ca2+-activated K+ (KCa) channel, accompanied with the activation of PK-C (Nishida and Satoh, 2007). Also, sinomenine possesses β-adrenoceptor stimulating action to inhibit the aortic constriction.
Clinically possibility of cardiovascular pharmacological effects
Sinomenine is included in Sinomenine acutum of Mokuboito. Mokuboito is traditionally used for dyspnea and edema (Shuji et al. 2002). Therefore, sinomenine may be expected as one of the therapeutic agents for heart failure. Most recently, Satoh (2005) has demonstrated that sinomenine effectively modulates cardiac ionic channels. Sinomenine inhibits ICa, and simultaneously produces the IK decerease in cardiomyocytes which results in the APD prolongation. Modulation of Ca2+ channel induced by sinomenine is similarly exerted in vascular smooth muscle cells. In addition, sinomenine possesses the regulatory actions for dysrhythmias under Ca2+ overload conditions. It has been well known that under the ischemia and heart failure, the cellular Ca2+ overload of heart muscles elicits some arrhythmias and dysfunctions (Satoh, 2001; 2003). The regulation of Ca2+ influx may modulate Ca2+ overload in cardiomyocytes and produces protective actions for Ca2+-overloaded myocardial cells (Satoh and Spererakis, 1998). Therefore, sinomenine might restrain the cell damages of heart muscles via modulation of [Ca2+]i, and as a result, exert a cardioprotective action.
Cardiopotective action of sinomenine on rat acute myocardial ischemia has also been demonstrated. Reperfusion injury is induced by ligating the rat left coronary artery for 15 min and reopening. Sinomenine can inhibit the incidence of arrhythmias and reduce intracellular Ca2+ concentration (Xie et al. 1993), well consistent with our results.
Sinomenine has multiple vasodilating mechanisms. The vasodilating agent is one of the great useful tools for heart failure and regulates pre- and after-loads of cardiovascular systems. Therefore, sinomenine-induced vasodilating actions may improve cardiac functions via the regulation of both pre- and after-loads under heart failure. In summary, sinomenine caused a concentration-dependent vasorelaxation on NE-, KCl- and PDB-induced contractions, and sinomenine-induced vasorelaxation is attenuated by the pretreatments with L-NMMA, indomethacin, staurosporine, nicardipine and propranolol. In electropharmacological mechanisms, sinomenine inhibits the ICa and the IK in cardiomyocytes which results in the APD prolongation. In addition, sinomenine depressed the dysrhysmias induced by triggered activities under the Ca2+ overload. Finally, sinomenine also possesses the anti-inflammatory and immunomodulative actions.
In future, therefore, sinomenine as a cardioprotective drug may be expected to the respectable effectiveness for heart failure, mediated through the modulation of cardiac ion channels (including the regulation for dysrhythmias) and blood vessels. Further experiments need to elucidate more in detail mechanisms of sinomenine.
Acknowledgements
The authors wish to express thanks for the supply of Mokuboito and Sinomenium acutum extracts (Tsumura Co.). This work was in part supported by the research fund of Japan Kampo Medicine Manufactures Association.
References
- Busse R, Fichter H, Luckhoff A, et al. Hyperpolarizaion and increased free calcium in acethylcholine-stimulated endothelium cells. Am J physiol. 1998;255:H965–9. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Inaki K. Diseases and Kampo. In: Sato Y, Hanawa T, Arai M, Cyong J, Fukuzawa M, Mitani K, Ogihara Y, Sakiyama T, Shimada Y, Toriizuka K, Ymamada T, editors. Introduction to Kampo. Elsevier; Japan: 2005. p. 125. [Google Scholar]
- Kok TW, Yue PY, MaK NK, et al. The anti-angiogenic effect of sinomenine. Angiogenesis. 2005;8(1):3–12. doi: 10.1007/s10456-005-2892-z. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Tanaka T, Umegaki K. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–36. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- Li RW, David, Lin G, Myers SP, et al. Anti-inflammatory activity of Chinese medical vine plants. J. Ethnopharmacol. 2003;85(1):61–7. doi: 10.1016/s0378-8741(02)00339-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Cui S, Cheng Y, et al. Application of nonaqueous capillary electrophoresis for quantitative analysis of quinolizidine alkaloids in Chinese herbs. Anal Chim Acta. 2004;508:17–22. [Google Scholar]
- Liu L, Riese J, Resch K, et al. Impairment of macropharge eicosanoids and nitric oxide production by alkaloid from Sinomenium acutum. Arzneimittelforschung. 1994a;44:1223–26. [PubMed] [Google Scholar]
- Liu L, Resch K, Kaever V. Inhibition of lymphocyte proliferation by the anti-arthritc drug of sinomenine. Int. J. Immunopharmacol. 1994b;16(8):685–91. doi: 10.1016/0192-0561(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Buchner E, Beitze D, et al. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int. J. Immunopharmacol. 1996;18(10):529–43. doi: 10.1016/s0192-0561(96)00025-2. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Chan K, Zhou H, et al. The pharmacokinetics and tissues distribution of sinomenine in rats and its protein binding ability in vitro. Life Sci. 2005;77(25):3197–209. doi: 10.1016/j.lfs.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Lopez-Jaramillo P, Gonzalez MC, Palmer RM, et al. The crucial role of physiological Ca2+ concentrations in the production of endothelial nitric oxide and the control of vascular tone. Br. J. Pharmacol. 1990;101(2):489–93. doi: 10.1111/j.1476-5381.1990.tb12735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark W, Schneeberger S, Seiler R, et al. Sinomenine blocks tissue remodeling in a rat model of chronic cardiac allograft rejection. Transplantation. 2003;15:940–5. doi: 10.1097/01.TP.0000056610.22062.03. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 2003;72:2659–67. doi: 10.1016/s0024-3205(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clinica Chimica Acta. 2004;339:129–33. doi: 10.1016/j.cccn.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. In vitro pharmacological actions of sinomenine on the smooth muscle and endothelial cell activity in rat aorta. Life Sci. 2006;79:1203–06. doi: 10.1016/j.lfs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. Vascular pharmacology of Mokuboito (Mu-Fang-Yi-Tang) and its constituents on the smooth muscle and the endothelium in rat aorta. eCAM. 2007 doi: 10.1093/ecam/nel097. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignard JF, Feleton M, Thollon C, et al. Pottasium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br J Pharmacol. 1999;127:27–37. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H. Modulation of Ca2+-activated K+ current by isoprenaline, carbachol, and phorbol ester in cultured (and fresh) rat aortic vascular smooth muscle cells. Gen Pharmacol. 1996;27:319–24. doi: 10.1016/0306-3623(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Satoh H. [Ca2+]i- dependent actions of taurine in spontaneously beating rabbit sino-atrial nodal cells. Eur J Pharmacol. 2001;424:19–25. doi: 10.1016/s0014-2999(01)01128-1. [DOI] [PubMed] [Google Scholar]
- Satoh H. Effects of Ginkgo biloba extract and bilobalide, a main constituent, on the ionic currents in guinea pig ventricular cardio-myocytes. Arzneimittelforschung. 2003;53:407–13. doi: 10.1055/s-0031-1297128. [DOI] [PubMed] [Google Scholar]
- Satoh H. Electropharmacology of sinomeni caulis et rhizome and its constituents in cardiomyocytes. Am J Chin Med. 2005;33:967–79. doi: 10.1142/S0192415X05003569. [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Calcium and potassium currents in cultured rat aortic vascular smooth muscle cell lines. In: Sperelakis N, editor. Ion Channels of Vascular Smooth Muscle Cells and Endothelial Cells. Academic Press; New York: 1991. pp. 55–63. [Google Scholar]
- Satoh H, Sperelakis N. Modulation of L-type Ca2+ current by isoprenaline, carbachol and phorbol ester in cultured rat aortic vascular smooth muscle (A7r5) cells. Gen Pharmacol. 1995;26:369–79. doi: 10.1016/0306-3623(94)00193-q. [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Review of some actions of taurine on ion channels of cardiac muscle cells and others. Gen Pharmacol. 1998;30:451–63. doi: 10.1016/s0306-3623(97)00309-1. [DOI] [PubMed] [Google Scholar]
- Shuji Y, Kinoshita Y, Arakawa Y, et al. Clinical evaluation of Moku-boi-to(Mu-Fang-Yi-Tang): a Japanese and Chinese traditional medicine for heart failure. J Trad Med. 2002;19:159–63. [Google Scholar]
- Wang Y, Fang Y, Huang W, et al. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J Ethopharmacol. 2005;98:37–43. doi: 10.1016/j.jep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Pharmacology of sinomenine, an anti-rheumatic alkaloids from sinomenine acutum. Acta Med Okayama. 1976;30:1–20. [PubMed] [Google Scholar]
- Xie SX, Jin QQ. Prevention of sinomenine on isolated rat myocardial reperfusion injury. Zhongguo Yao Li Xue Bao. 1993;(Suppl):S12–5. [PubMed] [Google Scholar]
- Yan XH, Li HD, Peng WX, et al. Determination of sinomenine HCl in serum and urine by HPLC and its pharmacokinetics in normal volunteers. Yao Xue Xue Bao. 1997;32(8):620–4. [PubMed] [Google Scholar]
- Zhao ZZ, Liang ZT, Zhou H, et al. Quantification of sinomenine in caulis sinomenii collected from different growing regions and wholesale herbal markets by a modified HPLC method. Biol. Pharm. Bull. 2005;28(1):105–9. doi: 10.1248/bpb.28.105. [DOI] [PubMed] [Google Scholar]