Abstract
This review presents an overview about pharmaceutical and cosmetic topical products containing polymeric nanoparticles (nanospheres and nanocapsules), reporting the main preparation and characterization methods and the studies of penetration and transport of substances through the skin. The penetration and transport extent of those systems through the skin depends on the ingredients chemical composition, on the encapsulation mechanism influencing the drug release, on the size of nanoparticles and on the viscosity of the formulations. The polymeric nanoparticles are able to modify the activity of drugs, delay and control the drug release, and increase the drug adhesivity or its time of permanence in the skin. Briefly, the nanoparticles can be useful as reservoirs of lipophilic drugs to deliver them in the stratum corneum becoming an important strategy to control their permeation into the skin.
Keywords: polymeric nanoparticles, nanocapsules, semi-solid formulations, topical formulation, skin, sunscreen
Introduction
Topical administration of drugs has advantages such as minimal systemic effects and targeting only the areas of disease (Ting et al. 2004). Nevertheless, the stratum corneum, which is the non-viable uppermost layer of the epidermis, is an obstacle for the delivery of many molecules at therapeutic levels. In this way, the transport of drugs across the stratum corneum is complex (Kalia and Guy, 2001). Therefore, in order to enhance the transfer of a molecule across this layer, parameters such as partition, diffusion and solubility coefficients need to be manipulated and targeted. In addition, factors including the physico-chemical properties of the drug, its interaction with the membrane and its pharmacokinetic properties also influence the penetration-absorption of a molecule (Kalia and Guy, 2001).
In the last 30 years, different carrier systems have been extensively studied with the aim of controlling the drug release and improving the efficacy and selectivity of formulations (Barratt, 2000; Couvreur et al. 2002; Schaffazick et al. 2003). The controlled drug release systems are designed to provide appropriated response at the required site of action for prolonged time periods, improving the treatment (Vauthier et al. 2003). Those systems can be administered by different routes including intravenous, ocular, oral, intraperitoneal, intramuscular, subcutaneous and cutaneous (Barratt, 2000).
Drug targeting is defined as a selective drug release at specific physiological sites, organs, tissues or cells, in which the pharmacological effect is required (Yokoyama and Okano, 1996; Soppimath et al. 2001). Besides, in the case of a local treatment, the drug targeting systems can increase the therapeutic index due to the reduction of the systemic absorption and/or side effects of drugs, which occur when the drug acts at non-specific sites (Kreuter, 1994; Yokoyama and Okano, 1996).
The technological development of new dosage forms have been a promising approach to increase and control the drug skin penetration (Lboutounne et al. 2002; Müller et al. 2002; Alvarez-Román et al. 2004a; Alvarez-Román et al. 2004b). In the past years, different strategies have been proposed to increase drug skin permeation and to circumvent the inadequate physico-chemical characteristics of several substances (Bonina et al. 2001). Nanometric systems have a great surface area, which renders them highly satisfactory for the application of lipophilic substances promoting a homogeneous drug release (Bouchemal et al. 2004). Among the different approaches, nanostructured systems, that are colloidal aqueous suspensions, have been developed. The types of those systems are nanospheres (Shim et al. 2004), nanocapsules (Alvarez-Román et al. 2001; Milão et al. 2003; Miyazaki et al. 2003; Alvarez-Román et al. 2004a; Alvarez-Román et al. 2004b; Müller-Goymann, 2004; Shim et al. 2004), nanoemulsion (Calvo et al. 1996; Müller-Goymann, 2004; Sonneville-Aubrun et al. 2004; Yilmaz and Borchert, 2005), solid lipid nanoparticles (Jenning et al. 2000a; Jenning et al. 2000b; Lippacher et al. 2001; Mehnert and Mäder, 2001; Müller et al. 2002; Wissing and Müller, 2002a; Wissing and Müller, 2002b; Wissing and Müller, 2002c), microemulsions (Kreilgaard, 2002), liposomes (Barratt, 2000; Maghraby et al. 2000; Verma et al. 2003; Fang et al. 2006) and niosomes (Shahiwala and Misra, 2002). Moreover, such structures have been investigated as alternatives to the classical formulations based on chemical skin permeation enhancers (Asbill and Michniak, 2000; Foldvari, 2000; Santoyo and Ygartua, 2000). Applied epicutaneously, those systems modulate the transdermic diffusion, modifying the molecule activity and/or its partition and diffusivity. In consequence, their use can alter the drug pharmacokinetic and biodistribution through the skin (Cevc, 2004). Additionally, the nanostructure systems have small size which facilitates their formulation in dermatological products and enable confortable application to the skin (Perugini et al. 2002).
Liposomes were the first carriers introduced for topical delivery of drugs and, since then, they have been extensively studied (Barratt, 2000; Maghraby et al. 2000; Verma et al. 2003; Fang et al. 2006). They present advantages for example not being toxic or invasive, as well as they are able to deliver hydrophilic and/or lipophilic substances. Many drugs and cosmetic ingredients are already on the marked in liposomal formulations, presenting better dose/effect ratio and less adverse reactions compared to the free substances at the same concentration (Redziniak, 2003). In this way, some reviews have been consecrated to describe and discuss the preparation, physico-chemical characterization and cutaneous applications of liposomes (Redziniak, 2003; Choi and Maibach, 2005; Fang et al. 2006).
More recently, solid lipid nanoparticles (SLN) have been developed as novel topical carrier systems for cosmetic and pharmaceutical drugs (Mühlen et al. 1998; Müller-Goymann, 2004). SLN are formed by a matrix of lipids which are biodegradable raw materials that are physiologically well tolerated (Wissing and Müller, 2001). The main advantages of these systems include protection of labile substances from chemical degradation, control of the release of substances due to the solid state of the lipid matrix, and formation of films over the skin showing occlusive properties (Müller et al. 2000; Müller et al. 2002). Additional features are the avoidance of organic solvents during the preparation and no problem concerning large scale production and sterilization. Furthermore, the great ability of SLN to facilitate the contact of active substances with the stratum corneum, because of the small size of the particles and consequently the high surface area, leads to the high permeation of the carried substances through the viable skin (Jenning et al. 2000b; Maia et al. 2000). However, according to Mehnert and Mäder (2001) “SLN do not, as proposed, combine the advantages of other colloidal carriers and avoid the disadvantages of them”. Even though SLN are compounded by physiological ingredients and can be easily produced, they present some disadvantages such as low drug-loading capacities, presence of simultaneous alternative colloidal structures (micelles, liposomes, mixted micelles and drug nanocrystals), as well as physical instability during storage or administration due to the complexicity of the physical state of the lipid (Mehnert and Mäder, 2001). In the last decade, several articles and reviews have been published on this topic, a first review being published by Müller and co-workers (1995).
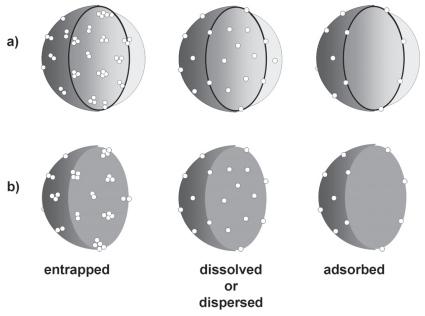
The use of polymeric materials for encapsulating drugs or other active substances is an important approach to mask the physico-chemical intrinsic properties of substances facilitating their skin penetration (Alvarez-Román et al. 2004a). Polymeric nanoparticles are carrier systems presenting diameters lower than 1 μm that can be named nanocapsules or nanospheres depending on their composition. The presence of oil in the nanocapsules leads to a vesicular structure while its absence in nanospheres provide a matricial organization of the polymeric chains (Soppimath et al. 2001; Couvreur et al. 2002; Schaffazick et al. 2003). Considering the encapsulation mechanisms (Lopes et al. 2001; Schaffazick et al. 2003, Cruz et al. 2006a; Cruz et al. 2006b), the drug can be entrapped, dispersed, dissolved within or adsorbed on the nanoparticles (Fig. 1).
Figure 1.
Encapsulation mechanism models: drug entrapped in, dissolved or dispersed within, and adsorbed on: a) nanocapsules and b) nanospheres.
Taking those considerations into account, the review focus is on the insights and data generated over the last few years based on the dermatological applications of pharmaceutical and cosmetic formulations containing polymeric nanoparticles reporting the main preparation and characterization methods and the studies of penetration and transport of substances through the skin. To our knowledge, this is the first review centered on the cutaneous applications of polymeric nanoparticles (nanospheres and nanocapsules).
The Skin as a Topical Route of Administration
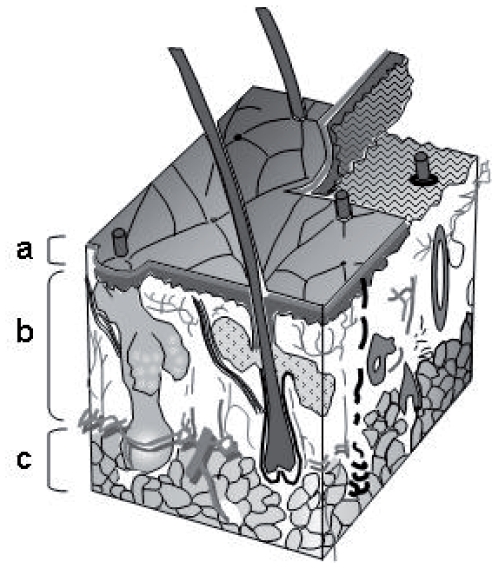
The skin is composed of the epidermis, the dermis and the hypodermis (Fig. 2) being a complex barrier as a consequence of its anatomical organization and special chemical composition. At the epidermis, the stratum corneum consists of 10 to 15 layers of corneocytes presenting a thickness of 10 to 20 μm (Foldvari, 2000). The intercellular junctions have corneocyte lipid envelopes and desmosomes (cell structures specialized for cell-to-cell adhesion). Quality and crystallinity as well as quantity of lipids in the stratum corneum determine the perfection of the skin barrier (Cevc, 2004). In this way, the stratum corneum and its compact structure are the main obstacle for the penetration of substances topically administrated on the skin (Suhonen et al. 1999; Hadgraft, 2001; Kalia and Guy, 2001; Morganti et al. 2001; Moser et al. 2001; Essa et al. 2002; Blanco et al. 2003; Ting et al. 2004).
Figure 2.
Scheme of the skin: a) epidermis, b) dermis and c) subcutaneous fat.
Many factors are able to govern the drug release into the skin after administration of topical formulations. Those factors include molecular weight and lipophilicity of the substance, type of formulation, presence of chemical penetration enhancers and physical state of the stratum corneum (Verma et al. 2003). Furthermore, the degree of hydration of the stratum corneum is important to determine the rate of drug percutaneous absorption. The hydration level is a function of the water concentration gradient between the dermis and the surface of the skin. Hence, an increase in skin water permeability corresponds to an augmentation in permeability to topical applied compounds (Morganti et al. 2001). The skin metabolic activity should also be regarded although its biotransformation capacity is considerably lower than the metabolic activity in the gut or in the liver (Tauber, 1989).
The partition coefficient between the vehicle and the stratum corneum is one of the factors which control the drug permeability, establishing a high initial drug concentration on the external layers of the skin (Morganti et al. 2001; Ting et al. 2004). The efficacy of a product for cutaneous application depends on the correlation between the permeability coefficients in the stratum corneum and the drug chemical characteristics. The absorption degree of a drug through the cutaneous structure is influenced by the physico-chemical characteristics of either the susbstance or the vehicle. Then, besides the partition coefficient between the vehicle and the stratum corneum lipids, the drug absorption is a function of the drug diffusion in the stratum corneum, the drug partition between the stratum corneum and the viable epidermis, the drug diffusion in the epidermis and dermis, as well as the drug ability to reach the systemic circulation through the cutaneous microvascularization (Morganti et al. 2001). Regarding the cosmetic and dermatological formulations, the systemic absorption must be avoided and only the skin permeation by the diffusion of the active substances in the epidermis is required.
Polymeric Nanoparticles for Cutaneous Administration
Preparation and characterization
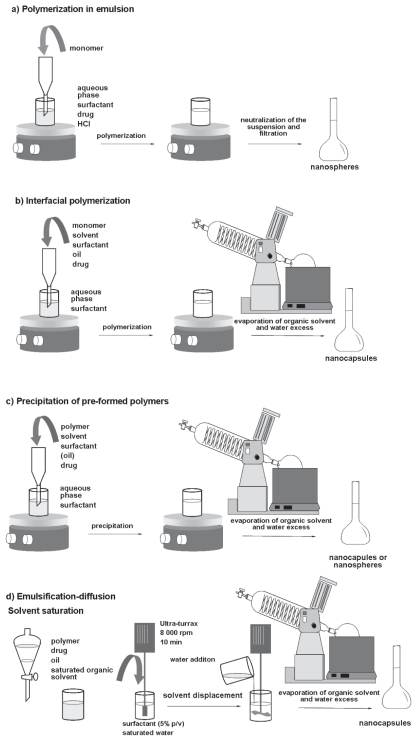
Different methods are described in the literature to obtain polymeric nanoparticles. From a general point of view, those methods are based on in situ polymerization or precipitation of preformed polymers (Fessi et al. 1989; Soppimath et al. 2001; Couvreur et al. 2002, Schaffazick et al. 2003; Sinha et al. 2004). The polymerization of alkyl cyanoacrylates in emulsion leads to matricial nanoparticles called nanospheres (Fig. 3a), while the addition of an organic solvent and oil in this medium gives vesicular nanostructures, the nanocapsules, by interfacial polymerization (Fig. 3b). Moreover, nanospheres and nanocapsules can be also prepared using preformed polymers by nanoprecipitation (omitting the oil in the formulation) or interfacial deposition of polymer (containing the oil), respectively (Fig. 3c). The method of emulsification-diffusion has been also introduced to obtain nanocapsules (Moinard-Checot et al. 2006) (Fig. 3d), being able to produce nanospheres by omitting the oil in the formulations. Nanoparticles can also be prepared using a solvent extraction method, in which the o/w emulsion is high-speed homogenized followed by addition of water and solvent evaporation (Luengo et al. 2006).
Figure 3.
Preparation of polymeric nanoparticles by a) polymerization in emulsion, b) interfacial polymerization, c) nanoprecipitation or interfacial deposition of preformed polymers, and d) emulsification-diffusion.
The techniques commonly used for the characterization of nanoparticles include size exclusion chromatography, liquid chromatography, ultrafiltration-centrifugation and ultracentrifugation, dynamic and static light scattering, electrophoretic mobility, potentiometry, small angle X-ray scattering, differential scanning calorimetry, atomic force microscopy, and scanning and transmission electron microscopies (Kumar et al. 2002; Schaffazick et al. 2003; Müller-Goymann, 2004; Astete and Sabliov, 2006; Pohlmann et al. 2007). The size exclusion chromatography provides polymer weight and weight distribution after the nanoparticle preparation furnishing information about the polymerization process or polymer degradation after storage. The liquid chromatography is employed to determine the drug loading (drug total content in the formulation), as well as it is used to quantify the drug in the supernatant or in the ultrafiltrate informing the non-encapsulated concentration of drug in the formulation (free drug concentration). The drug encapsulation rate is calculated by the ratio between the subtraction of the total content and the free drug concentration and the drug total content, multiplied by 100. The ultracentrifugation or the ultrafiltration-centrifugation of the native nanoparticle suspension is unable to distinguish either the mechanism of drug encapsulation (Fig. 1) or the simultaneous presence of nanocrystals and nanostructures in suspension. Dynamic light scattering measurements give the particle size and size distribution, and the static light scattering can provide the gyration radius of particles as well as the depolarization ratio, which is calculated by the correlation between the depolarized scattered light and the polarized scattered light, indicating the shape of the particles in suspension. Regarding the electrophoretic mobility, the attraction from the colloidal particles in suspensions causes some of the counter-ions to form a firmly attached layer around the surface of the nanoparticle. This layer of counter-ions is known as the Stern layer. The counter-ions have a high concentration near the surface which gradually decreases with the distance. The dynamic equilibrium of those counter-ions forms the diffuse layer, where they are repelled by the Stern layer. The electrical potential at the junction between the Stern layer and the diffuse layer is related to the mobility of the particles and is called zeta potential. The zeta potential is important to evaluate the physical stability of suspensions, to determine either the effectiveness of surface coating or the drug adsorption on the nanoparticles. Furthermore, the chemical stability of suspensions can be evaluated by monitoring the pH because its decrease can indicate the degradation of the polymer or other ingredient. DSC and SAXS analyses can provide information about the organization at a molecular level of the nanoparticle components. Additionally, microscopy techniques (SEM, TEM and AFM) characterize the surface morphology, shape and size of the particles as well as, in the case of TEM, the polymeric wall of nanocapsules.
Formulating polymeric nanoparticles for cutaneous applications
Up to now, the nanoprecipitation method is the most commonly used to formulate polymeric nanoparticles intended for cutaneous applications (Alverez-Román et al. 2001; Lboutounne et al. 2002; Milão et al. 2003; Alvarez-Román et al. 2004b; Jiménez et al. 2004a; Jiménez et al. 2004b; Lboutounne et al. 2004; Shim et al. 2004; Kim et al. 2006). The advantage of this method is the spontaneous, simple, efficient and reproducible formation of small particles exhibiting a high drug loading capacity (Jiménez et al. 2004a). Other methods of nanoparticle preparation include in situ polymerization (Miyazaki et al. 2003; Simeonova et al. 2003; Diaz-Torrez et al. 2005), solvent extraction (Luppi et al. 2004; Luengo et al. 2006), emulsification-diffusion (Olvera-Martinez et al. 2005) and salting out (Perugini et al. 2002).
Concerning the polymers, the poly(ɛ-caprolactone) (Alvarez-Roman et al. 2001; Alvarez-Roman et al. 2004b; Jiménez et al. 2004a) is the most employed due to its biocompatibility, biodegradability and mechanical properties (Kim and Rhee 2003). Because poly(ɛ-caprolactone) is a semi-cristalline polymer its degradation is delayed compared to amorphous polyesters, like poly(lactide) and its copolymers with glycolide. Other polymers have also been used to prepare nanoparticulated systems, such as poly (lactide-co-glycolide) (Perugini et al. 2002), poly(ɛ-caprolactone)-block-poly(ethylene glycol) (Shim et al. 2004), poly(butyl cyanoacrylate) (Simeonova et al. 2003), poly(ethyl cyanoacrylate) (Diaz-Torres et al. 2005), ethyl cellulose (Perugini et al. 2002), cellulose acetate phtalate (Calderilla-Fajardo et al. 2006) and a fatty acid-conjugated poly(vinyl alcohol) (Luppi et al. 2004). Regarding the reported works, the nanoparticles presented diameters varying between 100 and 615 nm, excepting the particles prepared with poly(lactide-co-glycolide) by salting out, which sizes ranged from 0.68 to 5.71 μm. In this work (Perugini et al. 2002) the presence of chloroform in the organic phase probably influenced the size of those particles because organic solvents of low water solubility in this phase result in slow precipitation, and microparticles are formed (Choi et al. 2002).
Semi-solid formulations containing polymeric nanoparticles
Skin care formulations are often based on emulsions and gels because of the technological ability of controlling their viscosity, which can provide appropriated characteristics for cutaneous application by the patient/costumers. The choice of their ingredients defines the rheology of formulations that is reflected by spreading properties on the skin. Besides, rheological properties affect all stages of manufacture such as mixing, pumping and filling; in addition they are valuable tools in quality control (Lippacher et al. 2001). Nevertheless, only few studies have been carried out on semi-solid formulations containing polymeric nanoparticles (Alvarez-Román et al. 2001; Milão et al. 2003; Miyazaki et al. 2003; Jiménez et al. 2004a; Alves et al. 2005; Luengo et al. 2006).
The thickening agents usually used to prepare gel formulations containing polymeric nanoparticles are: 1) Pluronic F127 [poly(oxyethylene)-b-poly(oxypropylene)] (Miyazaki et al. 2003), 2) Satiaxane CX 91 (purified xanthan gum) (Alvarez-Román et al. 2001), 3) Natrosol® 250 M (hydroxyethyl cellulose) (Luengo et al. 2006) and 4) Carbopol® 940 [cross-linked poly(acrylic acid)] (Milão et al. 2003; Alves et al. 2005). Two emulsions containing nanoparticles, one oil-in-water (O/W) and another water-in-oil (W/O), have also been studied (Jiménez et al. 2004a).
Our research group reported the only two works, as far as we know, concerning the rheological characterization of semi-solid formulations containing polymeric nanoparticles (Milão et al. 2003; Alves et al. 2005). The non-Newtonian behavior and the pseudo-plastic character of the hydrogels have not been affected by the incorporation of nanocapsules or nanospheres.
Cutaneous applications
In 1995, the polymeric nanocapsules were introduced in the cosmetic market by L’Oreal. Subsequently, scientific articles based on skin penetration and distribution of drugs or cosmetic ingredients, encapsulated in polymeric nanoparticles, were published. Those works are commented in the following sections according to the type of encapsulating active ingredient: sunscreens or other drugs.
Sunscreen formulations
Formulations containing sunscreens are usually applied superficially to large skin areas. Consequently, their effectiveness indicates that sunscreen molecules adhere to the skin like a protecting film. The UV filters are designed to remain on the uppermost layers of the skin (Jiang et al. 1997). Ideally sunscreen molecules should be bound to the outer section of the stratum corneum being immobilized near to the skin sufarce. Definitely, penetration to the viable tissues and beyond characterizes a loss from the desired deposition sites being counterproductive (Gupta et al. 1999).
The main application of polymeric nanoparticles is focused on the new formulations of sunscreens as a result of the ability of those nanoparticles in carrying highly lipophilic substances and their potentialities in modifying and/or masking the physico-chemical properties of loaded drugs; in addition to the need of developing new sunscreen formulations showing an important remanence and a limited penetration in the skin. The works reported in the literature concerning the use of polymeric structures to the nanoencapsulation of sunscreens have been published in the last 5 years. Table 1 summarizes the type of nanostructure, the preparation method, the particle size, the active ingredient, the vehicle used and the type of skin evaluation.
Table 1.
Characteristics of formulations containing sunscreen-loaded nanstructures and types of cutaneous evaluation.
| Type | Polymer | Method | Size (nm) | Sunscreen | Vehicle | Cutaneous evaluation | Reference |
|---|---|---|---|---|---|---|---|
| NC | PCL | nanoprecipitation | 255 ± 3 to 427 ± 4 | OMC | Gels | Irradiation of Guinea pig skin with UV 365/312 nm | Alvarez-Roman et al. 2001 |
| NP | PCL | nanoprecipitation | 250 | OMC | Suspension | Diffusion cells and tape stripping using Porcine ear skin | Alvarez-Roman et al. 2004b |
| NC | PCL | nanoprecipitation | 374 | OMC | O/W and W/O emulsions | Static diffusion cells in a modified Franz cells and tape stripping using flank of female pigs | Jiménez et al. 2004a |
| NC and NE | CAP – |
emulsification-diffusion for both | 396 ± 41 to 615 ± 27 162 ± 19 |
OMC OMC |
Suspension Suspension |
Stratum corneum penetration after tape stripping in healthy volunteers | Olvera-Martinez et al. 2005 |
| NC and NE | CAP – |
emulsification-diffusion for both | 363 ± 40 to 458 ± 26 124 ± 15 to 162 ± 24 |
OMC | Suspension Suspension |
Stratum corneum penetration after tape stripping in healthy volunteers | Calderilla-Fajardo et al. 2006 |
| NP | PVA-FA | solvent extraction | 312 ± 10 to 440 ± 25 | BZP | Suspension | Static diffusion cell based on the Franz design and tape stripping using pig ear skin | Luppi et al. 2004 |
NC, nanocapsules; NP, nanoparticles, NE, nanoemulsion; PCL, poly(ɛ-caprolactone); CAP, cellulose acetate phthalate; PVA-FA, fatty acid conjugated poly(vinyl alcohol); OMC, octyl methoxycinnamate; BZP, benzophenone-3; O/W, oil-in-water; W/O, water-in-oil.
Alvarez-Román and co-workers (2001) have conducted the first study concerning the nanoencapsulation of a sunscreen, the octyl methoxycinnamate (OMC). OMC-loaded nanocapsules have been evaluated regarding the in vitro OMC release, during the time of contact with the skin, and the in vivo ability to protect the skin against UVB radiation. OMC-loaded nanocapsules have been prepared as aqueous suspension and incorporated in gel formulation. For these suspension and gel, the OMC release profiles showed similar shape, but the release rate was faster from the nanocapsule suspension than from the gel. The higher viscosity of the gel compared to the nanocapsule suspension could explain the findings. In addition, the gel containing OMC nanocapsules significantly reduced UV-induced erythema, compared to the corresponding OMC-free gel. The authors attributed those results to the nanocapsule film formation on the skin surface.
Other subsequent studies have been performed to further define the passive skin penetration, permeation and distribution of OMC encapsulated within nanoparticles (Alvarez-Román et al. 2004b). For comparison, OMC-loaded formulations have been prepared by interfacial deposition (nanocapsules) and by spontaneous emulsification (emulsion). The penetration of OMC into the stratum corneum from the nanocapsules was 3.4-fold higher than that from the emulsion. Furthermore, after 6 hours of experiment the sunscreen was not detected in the receptor compartment. The authors suggested that the thermodynamic activity of the nanoencapsulated sunscreen molecules, compared to solution formulations, could be higher facilitating their partitioning into the membrane. In addition, the high surface area of nanoparticulated systems may also play an important role in dermal penetration, and facilitates the contact of the encapsulated molecules with the stratum corneum. Confocal laser scanning microscopy has been used to visualize the skin penetration of Nile red, a fluorescent probe, from nanoparticles. The confocal images clearly demonstrated the enhanced distribution of Nile red when delivered from nanoparticles. However, the study did not allow determining unequivocally whether the fluorescence observed in the images have been originated from Nile red associated with the nanoparticles or from free Nile red. On the other hand, confocal microscopy studies performed using fluorescein-conjugated polystyrene nanoparticles showed that they preferentially accumulate in the follicular openings in a time dependent-manner (Alvarez-Román et al. 2004a). Moreover, the influence of the particle size (20 or 200 nm) on the skin deposition and/or permeation was also examined. The accumulation was higher as the smaller the particles were. The nanoparticles have been also detected in the furrows on the skin.
The influence of OMC nanoencapsulation on the in vitro transdermal permeation and skin accumulation has been evaluated applying four different formulations: oil-in-water (O/W) and water-in-oil (W/O) emulsions containing the free sunscreen, and similar emulsions containing OMC-loaded nanocapsules (Jiménez et al. 2004a). Results have shown that the incorporation of OMC in nanocapsules decreased the release compared to the free OMC emulsions. The encapsulation of OMC in nanocapsules decreased the penetration of the sunscreen in the skin compared to the free OMC emulsions, as well as OMC has shown a slower diffusion rate when nanoencapsulated. In consequence, the sunscreen remained longer on the surface of the skin where it was designed to act.
Stratum corneum penetration degree of OMC formulated in nanocapsules has been compared to those obtained for OMC-loaded nanoemulsion and for a conventional O/W emulsion containing OMC (Olvera-Martínez et al. 2005). Incorporation of OMC in nanoemulsion increased the penetration rate compared to its incorporation in nanocapsules or in the conventional emulsion. The aptitude of nanoemulsion to enhance the penetration of OMC have been attributed to the size and flexibility of the droplets compared to those of nanocapsules, which are larger and have a rigid structure, or those of conventional emulsion showing the largest droplet size. These authors have also studied the effect of sucrose laurate and sucrose oleate on the in vivo percutaneous penetration of OMC from nanocapsules and nanoemulsion (Calderilla-Fajardo et al. 2006). The inclusion of sucrose laurate in the nanoemulsion increased the transport of OMC into the stratum corneum. This enhancement was not the result of only one factor such as the size, the nature of the systems or the type of enhancer, but a combination of them. Indeed the enhancement was a result of a synergic effect of the size, the interaction of sucrose laurate with intercellular lipids, and the deformability of the globules. In the case of nanocapsules, the interaction of sucrose esters with the lipid domain of stratum corneum did not enhance the penetration, probably because the rigidity of the particles due to the polymeric matrix of nanocapsule wall.
The degree of sunscreen penetration through the skin depends mainly on the physico-chemical properties and nature of the carrier. Varying the chemical nature of polymers used to formulate polymeric nanoparticles, different physico-chemical and functional properties can be obtained. In this way, the effects of various fatty acid-conjugated PVA (PVA-FA) on the skin permeation of benzophenone-3 have been evaluated with the purpose of developing a new formulation that can limit the benzophenone-3 penetration into the skin and into the systemic circulation (Luppi et al. 2004). Nanoparticles, prepared using PVA-FA at two different degrees of substitution (40% and 80%), have shown the prevention of the movement of benzophenone-3 towards the skin, as a result of the limitation of its percutaneous absorption. Precisely, nanoparticles prepared with a low degree of substitution have been the best formulations for enhancing sunscreen location in the epidermis, while nanoparticles prepared with a high degree of substitution have prevented benzophenone-3 percutaneous absorption.
Other drugs
The topical antimicrobial efficacy of chlorhexidine-loaded nanocapsules has been compared to the efficacy of a disinfectant-detergent solution of chlorhexidine digluconate (Lboutounne et al. 2002). A sustained release of chlorhexidine from those nanocapsules enhanced the drug delivery by mediating a more direct and prolonged contact between the carrier and bacteria, skin surface and skin follicles. Furthermore, the formulation presented a prolonged ex vivo topical antimicrobial activity against Staphylococcus epidermidis. More recently, the transport of chlorexidine-loaded poly(ɛ-caprolactone) nanocapsules through full-thickness and the stripped hairless rat skin has been investigated in static diffusion cells (Lboutounne et al. 2004). After modeling the permeation profiles using the Fickian diffusion equation, data showed that the drug encapsulation decreased the percutaneous absorption through stripped skin. Confocal laser microscopy confirmed that nanocapsules transport has taken place by the skin conducts. The small wetting of nanocapsules on the stratum corneum surface, estimated by the contact angle and the surface tension, has maintained the mechanical integrity of the nanocapsules. That is, the flexibility of the nanocapsules has guaranteed a bioadhesion to the skin while the rigidity of the nanoparticle restricted the molecular leakage into the skin, controlling the chlorexidine delivery.
The penetration mechanism of minoxidil encapsulated in polymeric nanoparticles prepared with a diblock, the poly(ɛ-caprolactone)-b-poly(ethylene glycol), has been studied (Shim et al. 2004). In addition, the effect of the nanoparticle diameters on the permeation on both hairy and hairless Guinea pig skin using Franz diffusion cells has been evaluated. In the hairy Guinea pig skin, minoxidil permeated 1.5-fold higher in the epidermal layer and 1.7-fold higher in the receptor solution, when encapsulated in the smaller nanocapsules (40 nm) compared to the larger ones (130 nm). On the other hand, regarding the hairless Guinea pig, the permeation of minoxidil has not been dependent upon the nanoparticle sizes. In this way, nanoparticles released minoxidil in the skin mainly by the hair follicles.
Distribution of poly(lactide-co-glycolide) (4.6 ± 0.8 μm) in porcine skin after its topical administration has been studied in vitro using rhodamine as a fluorescent probe (Jalón et al. 2001a). Fluorescence photomicrographs revealed that microparticles penetrated through the stratum corneum and reached the epidermis. A subsequent study (Jalón et al. 2001b) showed that acyclovir-loaded microparticles can increase drug retention in the porcine basal epidermis. At 6 and 24 h, the quantity of drug was similar to that obtained with the control suspension, while after 88 h the acylovir reservoir in the basal epidermis was higher with the microparticles in comparison with the control suspension.
The influence of nanoencapsulation of flufenamic acid, used as lipophilic model of drug, on its transport into excised human skin has been investigated (Luengo et al. 2006). In order to estimate drug penetration, the Saarbrücken model has been employed. In this model the skin itself acts as a receptor compartment. A tape stripping technique of the deeper skin layers allowed quantifying penetrated drug concentration. Additionally, drug release and permeation through the epidermis have been measured using static Franz diffusion cells. In the case of the stratum corneum no differences have been found between the nanoencapsulated and the free drug. On the contrary, the drug accumulation in deeper layers of the skin has been slightly delayed for the nanoencapsulated drug compared to the free drug after shorter incubation times (t <12 h). After longer incubation times (t >12 h), the drug transport has been enhanced for the nanoencapsulated drug compared to the free drug.
Despite all the works mentioned in this section (Cutaneous applications) have reported the use of polymeric nanoparticles as local delivery systems, Miyazaki and co-workers (2003) showed the ability of poly(n-butyl cyanoacrylate) nanocapsules containing indomethacin to deliver the drug systemically after topical application. Confocal laser microscopy (rhodamin 6G-loaded nanoparticles), in vitro release, in vitro permeation and in vivo percutaneous absorption have been used to compare the behavior of the indomethacin-loaded nanocapsule suspension with a gel containing the drug loaded-nanocapsules and a conventional gel containing free indomethacin. Indomethacin-loaded nanocapsules improved the transdermal delivery of indomethacin compared to the conventional gel. Furthermore, the authors suggested that the nanocapsules have been penetrated intact through the rat skin.
Concluding Remarks
Polymeric nanoparticles intended for cutaneous delivery are prepared with biocompatible polymers generally presenting particle diameters arround 200 to 300 nm. The penetration and transport extent of those systems through the skin seem to be mainly dependent on the chemical composition of ingredients, on the encapsulation mechanism, which, by consequence, influences the drug release mechanism, on the size of nanoparticles and, as much as, on the viscosity of formulations. Despite some of the reports have compared formulations in a qualitative way, the influence of the rheological properties of formulations have not been considered under a quantitative point of view in the case of the studies carried out in vivo. This is an important feature because the topical application of polymeric nanoparticles implies the use of semi-solid formulations due to the low viscosity of the nanoparticle suspensions.
Taking all findings toghether, it was clearly demonstrated that polymeric nanoparticles are able to modify the activity of drugs by altering the physico-chemical properties of formulations, to delay and control the drug release and increase the drug adhesivity or its time of permanence in the skin. Briefly, the polymeric nanoparticles can be useful as reservoirs of lipophilic drugs to deliver them in the stratum corneum being an important strategy to control their permeation into the skin.
Acknowledgments
The authors thank CNPq/Brasil, Rede Nanocosméticos CNPq/MCT, CAPES/COFECUB, Rede Brasil/França CNPq/MCT and FAPERGS.
References
- Alvarez-Román R, Barré G, Guy RH, Fessi H. Eur J Pharm Biopharm. 2001;52:191–5. doi: 10.1016/s0939-6411(01)00188-6. [DOI] [PubMed] [Google Scholar]
- Alvarez-Román R, Naik A, Kalia YN, Guy RH, Fessi H. J Control Release. 2004a;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Alvarez-Román R, Naik A, Kalia YN, Guy RH, Fessi H. Pharm Res. 2004b;21:1818–24. doi: 10.1023/b:pham.0000045235.86197.ef. [DOI] [PubMed] [Google Scholar]
- Alves MP, Pohlmann AR, Guterres SS. Pharmazie. 2005;60:900–4. [PubMed] [Google Scholar]
- Asbill CS, Michniak BB. Pharm Sci & Tech Today. 2000;3:36–41. doi: 10.1016/s1461-5347(99)00225-4. [DOI] [PubMed] [Google Scholar]
- Astete CE, Sabliov CM. J Biomaterials Sci Polymer Ed. 2006;17:247–89. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- Barratt GM. PSTT. 2000;3:163–71. doi: 10.1016/s1461-5347(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Blanco MD, Bernardo MV, Teijón C, Sastre RL, Teijón MJ. Int J Pharm. 2003;255:99–107. doi: 10.1016/s0378-5173(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Bonina FP, Puglia C, Barbuzzi T, Caprariis P, Palagiano F, Rimoli MG, Saija A. Eur J Pharm Sci. 2001;14:123–34. doi: 10.1016/s0928-0987(01)00163-4. [DOI] [PubMed] [Google Scholar]
- Bouchemal K, Briançon S, Perrier E, Fessi H, Bonnet I, Zydowicz N. Int J Pharm. 2004;269:89–100. doi: 10.1016/j.ijpharm.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Calderilla-Fajardo SB, Cazares-Delgadillo J, Villalobos-García R, Quintanar-Guerrero D, Ganem-Quintanar A, Robles R. Drug Dev Ind Pharm. 2006;32:107–13. doi: 10.1080/03639040500388540. [DOI] [PubMed] [Google Scholar]
- Calvo P, Alonso MJ, Vila-Jato JL, Robinson JR. J Pharm Pharmacol. 1996;48:1147–52. doi: 10.1111/j.2042-7158.1996.tb03911.x. [DOI] [PubMed] [Google Scholar]
- Cevc G. Adv Drug Delivery Rev. 2004;56:671–5. doi: 10.1016/j.addr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Choi MJ, Maibach HI. Skin Pharmacol Physiol. 2005;18:209–19. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kwon HI, Kim WS, Kim JH. Coll Surfaces A. 2002;201:283–9. [Google Scholar]
- Couvreur P, Barrat G, Fattal E, Legrand P, Vauthier C. Crit Rev Ther Drug Carrier Syst. 2002;19:99–134. doi: 10.1615/critrevtherdrugcarriersyst.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- Cruz L, Schaffazick SR, Dalla Costa T, Soares LU, Mezzalira G, da Silveira NP, Shapoval ES, Pohlmann AR, Guterres SS. J. Nanosci. Nanotechnol. 2006a;6:3154–62. doi: 10.1166/jnn.2006.417. [DOI] [PubMed] [Google Scholar]
- Cruz L, Soares LU, Dalla-Costa T, Mezzalira G, da Silveira NP, Guterres SS, Pohlmann AR. Int J Pharm. 2006b;313:198–205. doi: 10.1016/j.ijpharm.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Diaz-Torres R, Castano VM, Ganem-Quintanar A, Quintanar-Guerrero D, Rodriguez-Romo S. Nanotechnology. 2005;16:2612–8. [Google Scholar]
- Essa EA, Boner MC, Barry BW. J Pharm Pharmacol. 2002;54:1481–90. doi: 10.1211/002235702135. [DOI] [PubMed] [Google Scholar]
- Fang JY, Hwang TL, Huang YL. Curr Nanoscience. 2006;2:55–70. [Google Scholar]
- Fessi H, Puisieux F, Devissaguet JP, Amoury N, Benita S. Int. J. Pharm. 1989;113:r1–r4. [Google Scholar]
- Foldvari M. Pharm Sci & Tech Today. 2000;3:417–25. doi: 10.1016/s1461-5347(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Zatz JL, Rerek M. Pharm Res. 1999;16:1602–7. doi: 10.1023/a:1018916907263. [DOI] [PubMed] [Google Scholar]
- Hadgraft J. Int J Pharm. 2001;224:1–18. doi: 10.1016/s0378-5173(01)00731-1. [DOI] [PubMed] [Google Scholar]
- Jalón EG, Blanco-Príeto MJ, Ygartua P, Santoyo S. Int J Pharm. 2001a;226:181–4. doi: 10.1016/s0378-5173(01)00811-0. [DOI] [PubMed] [Google Scholar]
- Jalón EG, Blanco-Príeto MJ, Ygartua P, Santoyo S. J Control Release. 2001b;75:191–7. doi: 10.1016/s0168-3659(01)00395-9. [DOI] [PubMed] [Google Scholar]
- Jenning V, Gysler A, Schäfer-Korting M, Gohla S. Eur J Pharm Sci. 2000a;49:211–18. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Jenning V, Schäfer-Korting M, Gohla S. J Control Release. 2000b;66:115–26. doi: 10.1016/s0168-3659(99)00223-0. [DOI] [PubMed] [Google Scholar]
- Jiang R, Roberts MS, Prankerd RJ, Benson HA. J Pharm Sci. 1997;86:791–6. doi: 10.1021/js960523y. [DOI] [PubMed] [Google Scholar]
- Jiménez MM, Pelletier J, Bobin MF, Martini MC. Int J Pharm. 2004a;272:45–55. doi: 10.1016/j.ijpharm.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Jiménez MM, Pelletier J, Bobin MF, Martini MC, Fessi H. Pharm Dev Tech. 2004b;9:329–39. doi: 10.1081/pdt-200031456. [DOI] [PubMed] [Google Scholar]
- Kalia NY, Guy RH. Adv Drug Delivery Rev. 2001;48:159–72. doi: 10.1016/s0169-409x(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Kim DG, Jeong YI, Choi C, Roh SH, Kang SK, Jang MK, Nah JW. Int J Pharm. 2006;319:330–8. doi: 10.1016/j.ijpharm.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Kim DY, Rhee YH. Appl Microbiol Biotechnol. 2003;61:300–8. doi: 10.1007/s00253-002-1205-3. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M. Adv Drug Delivery Rev. 2002;54:S77–98. doi: 10.1016/s0169-409x(02)00116-3. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticles. In: Kreuter J, editor. Colloidal drug delivery systems. New York: Marcel Dekker; 1994. pp. 219–342. [Google Scholar]
- Kumar MNVR, Kumar N, Domb AJ, Arora M. Filled Elastomers Drug Del Systems Adv Polymer Sci. 160:45–117. [Google Scholar]
- Lboutounne H, Chaulet J, Ploton C, Falson F, Pirot F. J Control Release. 2002;82:319–34. doi: 10.1016/s0168-3659(02)00142-6. [DOI] [PubMed] [Google Scholar]
- Lboutounne H, Faivre V, Falson F, Pirot F. Skin Pharmacol Physiol. 2004;17:176–82. doi: 10.1159/000078820. [DOI] [PubMed] [Google Scholar]
- Lippacher A, Müller RH, Mäder K. Int J Pharm. 2001;214:9–12. doi: 10.1016/s0378-5173(00)00623-2. [DOI] [PubMed] [Google Scholar]
- Lopes E, Pohlmann AR, Bassani V, Guterres SS. Pharmazie. 2000;55:527–530. [PubMed] [Google Scholar]
- Luengo J, Weiss B, Schneider M, Ehlers A, Stracke F, König K, Kostka KH, Lehr CM, Schaefer UF. Skin Pharmacol Physiol. 2006;19:190–7. doi: 10.1159/000093114. [DOI] [PubMed] [Google Scholar]
- Luppi B, Cerchiara T, Bigucci F, Basile R, Zecchi V. J Pharm Pharmacol. 2004;56:407–11. doi: 10.1211/0022357022926. [DOI] [PubMed] [Google Scholar]
- Maghraby GMM, Williams AC, Barry BW. Int J Pharm. 2000;196:63–74. doi: 10.1016/s0378-5173(99)00441-x. [DOI] [PubMed] [Google Scholar]
- Maia CS, Mehnert W, Schäfer-Korting M. Int J Pharm. 2000;196:165–67. doi: 10.1016/s0378-5173(99)00413-5. [DOI] [PubMed] [Google Scholar]
- Mehnert W, Mäder K. Adv Drug Delivery Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Milão D, Knorst MT, Guterres SS. Pharmazie. 2003;58:325–9. [PubMed] [Google Scholar]
- Miyazaki S, Takahashi A, Kubo W, Bachynsky J, Löbenberg R. J Pharm Pharmaceutical Sci. 2003;6:238–45. [PubMed] [Google Scholar]
- Moinard-Checot D, Chevalier Y, Briancon S, Fessi H, Guinebretiere S. J Nanosci Nanotechnol. 2006;6:2664–81. doi: 10.1166/jnn.2006.479. [DOI] [PubMed] [Google Scholar]
- Morganti P, Ruocco E, Wolf R, Ruocco V. Clin. Dermatol. 2001;19:489–501. doi: 10.1016/s0738-081x(01)00183-3. [DOI] [PubMed] [Google Scholar]
- Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Eur J Pharm Biopharm. 2001;52:103–12. doi: 10.1016/s0939-6411(01)00166-7. [DOI] [PubMed] [Google Scholar]
- Mühlen A, Schwarz C, Mehnert W. Eur J Pharm Biopharm. 1998;45:149–55. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- Müller RH, Mäder K, Gohla S. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Müller RH, Mehnert W, Lucks JS, Schwarz C, Zurmuhlen A, Weyhers H, Freitas C, Ruhl D. Eur. J. Pharm. Biopharm. 1995;41:62–9. [Google Scholar]
- Müller RH, Radtke M, Wissing SA. Adv Drug Delivery Rev. 2002;54:S131–155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Müller-Goymann CC. Eur J Pharm Biopharm. 2004;58:343–56. doi: 10.1016/j.ejpb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Olvera-Martínez BI, Cázeres-Delgadillo J, Calderilla-Fajardo SB, Villalobos-García R, Ganem-Quintanar A, Quintanar-Guerrero D. J Pharm Sci. 2005;94:1552–9. doi: 10.1002/jps.20352. [DOI] [PubMed] [Google Scholar]
- Perugini P, Simeoni S, Scalia S, Genta I, Modena T, Conti B, Pavanetto F. Int J Pharm. 2002;246:37–45. doi: 10.1016/s0378-5173(02)00356-3. [DOI] [PubMed] [Google Scholar]
- Pohlmann AR, Cruz L, Mezzalira G, Soares LU, da Silveira NP, Guterres SS. Int. J. Nanotechnol. 2007;4 in press. [Google Scholar]
- Redziniak G. Phathologie Bio. 2003;51:279–81. doi: 10.1016/s0369-8114(03)00079-8. [DOI] [PubMed] [Google Scholar]
- Santoyo S, Ygartua P. Eur J Pharm Biopharm. 2000;50:245–50. doi: 10.1016/s0939-6411(00)00097-7. [DOI] [PubMed] [Google Scholar]
- Schaffazick SR, Guterres SS, Freitas LL, Pohlmann AR. Química Nova. 2003;26:726–37. [Google Scholar]
- Shahiwala A, Misra A. J Pharm Pharma Sci. 2002;5:220–25. [PubMed] [Google Scholar]
- Shim J, Kang HS, Park W, Han S, Kim J, Chang I. J Control Release. 2004;97:477–84. doi: 10.1016/j.jconrel.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Simeonova M, Velichkova R, Ivanova G, Enchev V, Abrahams I. Int J Pharm. 2003;263:133–40. doi: 10.1016/s0378-5173(03)00373-9. [DOI] [PubMed] [Google Scholar]
- Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Int J Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Adv Coll Interf Sci. 2004;108–109:145–9. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Suhonen MT, Bouwstra JA, Urtti A. J Control Release. 1999;59:149–61. doi: 10.1016/s0168-3659(98)00187-4. [DOI] [PubMed] [Google Scholar]
- Tauber U. Drug metabolism in the skin: advantages and disadvantages. In: Hadgraft JRH, editor. Transdermal Drug Delivery: Developmental Issues and Research Initiatives. NewYork: Marcel Dekker; 1989. pp. 99–112. [Google Scholar]
- Ting WW, Vest CD, Sontheimer RD. Int J Dermatol. 2004;43:538–47. doi: 10.1111/j.1365-4632.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Adv Drug Delivery Rev. 2003;55:519–48. doi: 10.1016/s0169-409x(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Verma DD, Verma S, Blume G, Fahr A. Int J Pharm. 2003;258:141–51. doi: 10.1016/s0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. Int J Pharm. 2001;23:233–43. doi: 10.1046/j.1467-2494.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. Int J Pharm. 2002a;242:377–9. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. J Control Release. 2002b;81:225–33. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Müller RH. Int J Pharm. 2002c;242:373–5. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Yilmaz E, Borchert HH. Eur J Pharm Biopharm. 2005;60:91–8. doi: 10.1016/j.ejpb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Okano T. Adv Drug Delivery Rev. 1996;21:77–80. [Google Scholar]