Abstract
The effect of the antiviral agent ribavirin given alone or in combination with silymarin on the development of liver injury induced in rats with carbon tetrachloride (CCl4; 2.8 ml/kg followed by 1.4 ml/kg after one week) was studied. Ribavirin at three dose levels (30, 60 or 90 mg/kg), silymarin (25 mg/kg) or combination of ribavirin (60 mg/kg) and silymarin (25 mg/kg) was administered once daily orally for 14 days, starting at time of administration of CCl4. The administration of ribavirin decreased the elevations in serum alanine aminotransferase (ALT) by 78.5, 82.1, 75.1%, aspartate aminotransferase (AST) 47.5, 37.4, 38.8%, and alkaline phosphatase (ALP) by 23.4, 16, 21.6%, respectively and also pre-vented the development of hepatic necrosis caused by CCl4. In comparison, the elevated serum ALT, AST and ALP levels decreased to 43.3%, 46%, and 37.5% of controls, respectively by silymarin. When silymarin was combined with ribavirin, the serum activities of AST and ALP were further decreased, indicating a beneficial additive effect. Morphometric analysis indicated significant reduction in the area of necrosis and fibrosis on ribavirin treatment and this was further reduced after the addition of silymarin. Metabolic pertuberations caused by CCl4 as reflected in a decrease in intracellular protein content in hepatocytes were improved by ribavirin monotherapy and to higher extent by combined silymarin and ribavirin therapy. Proliferating cell nuclear antigen was reduced in nuclei of hepatocytes by ribavirin montherapy or the combination of ribavirin and silymarin compared with CCl4-control group. The study demonstrates that ribavirin treatment in the model of CCl4-induced liver injury results in less liver damage. Results also indicate that the combined application of ribavirin and sily-marin is likely to be a useful additive in reducing liver injury.
Keywords: Ribavirin, silymarin, carbon tetrachloride, liver injury, rat
Introduction
Ribavirin (1-β-d -ribofuranosyl-1,2,4, triazole-3 carboxamide) is an orally active synthetic guanosine analogue with antiviral and immunomodulatory actions. Ribavirin is a broad-spectrum antiviral drug, preventing the replication of a large number of RNA and DNA viruses by inhibiting the enzyme inosine monophosphate dehydrogenase, which is required for the synthesis of guanosine triphosphate. The final step in this chain of events is lethal mutagenesis of the RNA genome (Cameron and Castro, 2001). When used alone in the treatment of chronic hepatitis C virus infection, the drug normalizes serum aminotransferases, an effect that is not sustained and relapse was reported after discontinuing treatment. In patients with chronic hepatitis C, ribavirin is used more often in regimens employing interferon-alpha (INF-α) (Wartelle-Bladou et al. 2006).
The addition of ribavirin to interferon alpha is superior to interferon alpha in terms of virologic, biochemical, and histologic end points, resulting in improved end-of-treatment and sustained response rates, with an overall 41% sustained virological response rate in patients treated for 48 weeks (Pianko and McHutchison, 2000; Mukherjee and Lyden, 2006). This combined therapy has also resulted in an increased toxicity profile, which made therapy more difficult for both the patient and managing physician and prompted its discontinuation or a dosage reduction in a significant proportion of patients (Pianko and McHutchison, 2000; Chutaputti, 2000; Bonaccorsoa et al. 2000; Collier and Chapman, 2001; Fried et al. 2002; Burra et al. 2006). In addition, response is not obtained in up to 50% of cases and even in those where a response occurs, there is a 30% chance of relapse (Pianko and McHutchison, 2000; Hoofnagle et al. 2003).
In most studies, ribavirin monotherapy, improved liver enzyme levels, but without significant effects on HCV viraemia (Gane et al. 1995, 1996; Di_Bisceglie et al. 1995; Dusheiko et al. 1996; Cattral et al. 1999; Kamar, 2003; Hoofnagle et al. 2003). Nevertheless, histological improvement with reduction in hepatic necro-inflammation has been reported (Gane et al. 1995, 1998; Di Bisceglie et al. 1995; Hoofnagle et al. 2003) and ribavirin has been shown to possess anti-inflammatory properties and to decrease the synthesis of proinflammatory cyto-kines (e.g. IFN-gamma) (Meier et al. 2003; Barnes et al. 2004).
In the present study, it was aimed to examine whether ribavirin alone could exert protective effects in the CCl4 model of liver toxicity and if there is any benefit from combining ribavirin and silymarin. The latter, a standardized plant extract, derived from the milk thistle plant is widely used as a hepatoprotective agent, because of its anti-oxidant and membrane stabilizing properties (Flora et al. 1998; Muriel and Mourelle, 1990; Farghali et al. 2000; Wellington et al. 2001). The effect of ribavirin was evaluated on biochemical markers, histologically as well as by histochemical techniques. The area of damage or necrosis was calculated by image analysis system and immune staining by avidin biotin-peroxidae method for detection of proliferating cell nuclear antigen (PCNA), an endogenous cell replication marker (Shiina et al. 1996) was used.
Materials and Methods
Animals
Sprague-Dawley rats of both sex, weighing 150–160 g were used throughout the experiments and fed with standard laboratory chow and water ad libitum.
Drugs and chemicals
Carbon tertrachloride (BDH Chemicals, England), ribavirin (Virazole, October Pharma, Cairo) and silymarin (Sedico Pharmaceutical Co. Cairo) were used in the experiments.
The carbon tetrachloride induced hepatic damage
Hepatic injury was induced by treating rats by gavage with CCl4-olive oil (1:1, 2.8 ml/kg followed by 1.4 ml/kg after one week). Starting on the time of the first dose of CCl4 administration, rats also orally received either saline, silymarin (25 mg/kg), ribavirin (at three dose levels of 30, 60 and 90 mg/kg) alone or combined with silymarin 25 mg/kg. Control rats were treated with olive oil (2.8 ml/kg followed by 1.4 ml/kg after one week). The animals were killed on day 15 after the first dose of CCl4 or olive oil administration. Rats had free access to food and drinking water during the study.
Biochemical assessment
At the end of the experiments, blood samples were obtained from the retro-orbital vein plexuses, under ether anaesthesia. ALT and AST activities in serum were measured according to Reitman-Frankel colorimetric transaminase procedure (Crowley, 1967), whereas colorimetric determination of ALP activity was done according to the method of Belfield and Goldberg (1971), using commercially available kits (BioMérieux, France).Total protein in serum was measured spectrophotometrically (Bradford, 1976). Glucose concentrations in serum were measured enzymatically (Bauer, 1982).
Histopathological and histochemical studies
After the end of the treatment period, rats were killed, livers were excised and fixed in 10% formalin saline, Bouin’s and Carnoy’s fluids. Sections were prepared and stained with hema-toxylin and eosin (H & E) for the histological investigations. Bromophenol blue stain for intra-cellular proteins and avidin biotin-peroxidae method for detection of proliferating cell nuclear antigen (PCNA), an endogenous cell replication marker were used. Further histopathgological evaluation was done with morphometry. The percentage of liver tissue affected by necrosis and fibrosis (damaged area) was determined using a computer-assisted automated image analyzer. Qwin Leica image processing and analysis system (Cambridge, England) was used for interactive automatic measurement of the percentage of damaged areas on slides stained by H & E by analyzing 15 random fields per slide.
Statistical analysis
All results are expressed as means ± SE. Multiple group comparisons were performed by ANOVA followed by Duncan test. p < 0.05 was considered statistically significant.
Results
Biochemical changes
Results are presented in table 1. Serum alanine aminotransferase (ALT), aspartate aminotrans-ferase (AST), and alkaline phosphatase (ALP) levels were significantly higher in CCl4-treated rats compared with the vehicle-treated control group. Ribavirin administered to CCl4-treated rats at 30, 60 or 90 mg/kg resulted in a significant reduction in the levels of the serum enzymes. This effect of ribavirin was not dose-dependent. Thus, compared with CCl4 control group, serum ALT levels was reduced by the above doses of ribavirin by 78.5, 82.1 and 75.1%, respectively. When ribavirin and silymarin were given in combination, no further decrease in ALT values was noted (82.7, 80.2 and 82.1% vs CCl4 control group, respectively). Also a significant reduction in AST values by 47.5, 37.4 and 38.8% was noted after 30, 60 or 90 mg/kg of ribavirin, respectively. Ribavirin at these doses combined with silymarin caused 67.5, 66.2 and 68.6% decrease in serum AST levels, respectively. Serum ALP levels were reduced after ribavirin treatment by 23.4, 16 and 21.6%, respectively. However, 40 and 59.4% decrease in serum ALP was observed when ribavirin at 60 or 90 mg/kg was combined with silymarin. In comparison, silymarin given alone at 25 mg/kg to CCl4-treated rats decreased the elevated serum ALT, AST and ALP levels to 43.3%, 46%, and 37.5% of controls, respectively. Serum proteins increased by 19.2% after ribavirin monotherapy at 90 mg/kg, while the combined treatment with ribavirin (30, 60 or 90 mg/kg) plus silymarin resulted in 22.9, 20.5 and 35% increase in serum proteins compared with the CCl4 control group. Serum glucose level was reduced by 46.9% in CCl4-treated compared to vehicle-treated control. Significant increases in serum glucose by 56.9, 45.7, and 51.1% were observed after ribavirin monotherapy at 30, 60 or 90 mg/kg compared with the CCl4 control group. When ribavirin (30, 60 or 90 mg/kg) was combined with silymarin, 49.2, 93.5 and 120.2% increase in serum glucose was observed as compared to the CCl4 control group.
Table 1.
Effect of ribavirin, silymarin or ribavirin combined with silymarin on serum alanine aminotransferase (ALT), aspartate minotransferase (AST), alkaline phosphatase (ALP), total proteins and glucose in CCl4-treated rats.
| ALT (U/l) | AST (U/l) | ALP (IU/l) | Total protein (g/dl) | Glucose (mg/dl) | Area of damage (%) | Glucose (mg/dl) | |
|---|---|---|---|---|---|---|---|
| Treatment | |||||||
| Saline control | 67.4 ± 1.7 | 77.8 ± 3.9 | 122.6 ± 11.1 | 9.1 ± 0.6 | 96.0 ± 7.6 | 0.2 ± 0.1 | 96.0 ± 7.6 |
| CCl4 control | 125.5 ± 6.3 | 156.2 ± 4.5 | 198.4 ± 5.8 | 8.3 ± 0.2 | 51.0 ± 4.5 | 21.6 ± 1.4 | 51.0 ± 4.5 |
| + Silymarin 25 mg/kg | 71.2 ± 6.6* | 84.8 ± 7.4* | 124.0 ± 11.6* | 8.7 ± 0.5 | 59.0 ± 7.8 | 9.0 ± 1.1* | 59.0 ± 7.8 |
| + Ribavirin 30 mg/kg | 27.0 ± 3.1* | 82.0 ± 5.7* | 152.0 ± 12.4* | 8.8 ± 0.4 | 80.0 ± 4.9* | 9.6 ± 0.8* | 80.0 ± 4.9 |
| + Ribavirin 60 mg/kg | 22.5 ± 2.8* | 97.8 ± 6.7 | 166.6 ± 14.6 | 8.9 ± 1.0 | 74.3 ± 4.1* | 6.2 ± 0.5* | 74.3 ± 4.1 |
| + Ribavirin 90 mg/kg | 31.2 ± 2.6* | 95.6 ± 8.4* | 155.6 ± 13.5 | 9.9 ± 0.6 | 77.1 ± 6.9* | 4.3 ± 0.5* | 77.1 ± 6.9 |
| + Ribavirin 30 mg/kg | 21.7 ± 3.4* | 50.7 ± 6.0*+ | 152.6 ± 7.8 | 10.2 ± 0.6 | 76.1 ± 6.1* | 8.9 ± 0.6* | 76.1 ± 6.1 |
| + silymarin 25 mg/kg | |||||||
| + Ribavirin 60 mg/kg | 24.9 ± 2.3* | 52.8 ± 5.7*+ | 119.0 ± 12.8*+ | 10.0 ± 0.7 | 98.7 ± 8.3*+ | 5.2 ± 0.4* | 98.7 ± 8.3 |
| + silymarin 25 mg/kg | |||||||
| + Ribavirin 90 mg/kg | 22.5 ± 1.8* | 49.0 ± 4.1*+ | 80.6 ± 7.0*+ | 11.2 ± 0.7* | 112.3 ± 9.6*+ | 4.8 ± 0.5* | 112.3 ± 9.6 |
| + silymarin 25 mg/kg | |||||||
Results are means ± S.E. Data were analyzed by one way ANOVA and means of different groups were compared by Duncan’s multiple range test. Two-tailed probabilities of less than 0.05 were considered significant.
p < 0.05 vs CCl4 control.
p < 0.05 vs corresponding ribavirin alone-treated group.
Histopathological changes
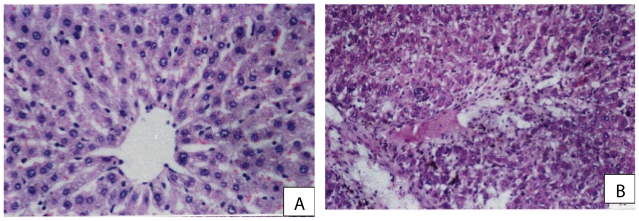
Examination of H & E stained sections of control liver showed the characteristic hepatic architecture (Fig. 1A). In rats treated with CCl4, patchy areas of necrosis, damaged bile ducts and focal inflam-matory cell infiltrate were observed (Fig. 1B). The hepatocytes from rats treated with silymarin (Fig. 2A), the high dose of ribavirin (Fig. 2C) or ribavirin (60 mg/kg) plus silymarin (Fig. 2B) showed more or less normal appearance.
Figure 1.
(A) A photomicrograph from a section of control rat liver showing normal hepatic architecture: central vein with radiating cords of liver cells, the hepatocytes had vesicular nuclei and granular cytopolasm and blood sinusoids were evident between the cords of hepatocytes (H× & E× 300). (B) A photomicrograph from a section of rat liver treated with CCl4 showing loss of normal architecture, patchy areas of necrosis, damaged bile ducts, vascular odema, inflammatory cellular infiltrate (H× & E × 150).
Figure 2.
(A) A photomicrograph from a section of rat liver treated CCl4 and silymarin showing normal hepatic architecture, while the sinusoids were dilated (H× & E × 500). (B) A photomicrograph from a section of control rat liver treated with CCl4 and a combination of ribavirin (60 mg/kg) and silymarin, showing that hepatocytes have almost regained their normal pattern (H× & E × 150). (C) A photomicrograph from a section of rat liver treated with CCl4 and ribavirin at 90 mg/kg, showing more or less normal hepatocytes and blood sinusoids (H× & E × 150).
Quantitative analysis of the area of damage
Significant increase in the percentage of damaged areas was observed in CCl4-treated rats when compared to normal animals; 21.6 ± 1.4% vs 0.2 ± 0.1%. Morphometric analysis of liver sections showed that ribavirin administration to CCl4-treated rats resulted in a significant and dose-dependent decrease in damaged areas; 9.6 ± 0.8, 6.2 ± 0.5, 4.3 ± 0.5% vs control value of 21.6 ± 1.4% and vs 9.0 ± 1.1% for silymarin at 25 mg/kg. When ribavirin and silymarin were given in combination, further reduction in the damaged area by 16.1% (p < 0.05) was noted in rats given 60 mg/kg ribavirin plus silymarin (5.2 ± 0.4 vs 6.2 ± 0.5) (Table 1).
Histochemical observations
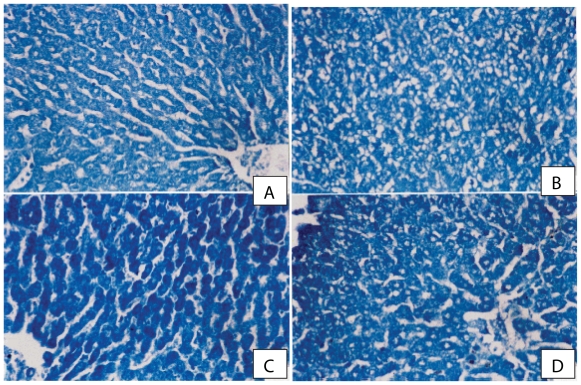
As regards bromophenol blue reactivity, the hepa-tocytes of control rats showed +ve reaction and moderate protein content (Fig. 3A). In CCl4-treated rats, the hepatocytes in pericentral and periportal regions showed faint reaction, resulting from a decrease in protein contents (Fig. 3B). After sily-marin and ribavirin (60 mg/kg) treatment, marked increase in protein content was observed compared to the CCl4 control group (Fig. 3C). Treatment with ribavirin at 90 mg/kg, resulted in moderate improvement in protein content in liver cells (Fig. 3D).
Figure 3.
(A) A photomicrograph from a section of control rat liver showing normal protein distribution in hepatocytes cytoplasm (Bromo-phenol blue reaction × 300). (B) A photomicrograph from a section of rat liver given CCl4 showing marked reduction in protein content especially in damaged areas (Bromophenol blue reaction × 300). (C) A photomicrograph from a section of rat liver given CCl4 and a combination of ribavirin (60 mg/kg) and silymarin. Marked improvement of protein content in cytoplasm of hepatic cells is seen. (Bromophenol blue reaction × 300). (D) A photomicrograph from a section of rat liver given CCl4 with ribavirin at 90 mg/kg, showing moderate improvement in protein content in liver cells (Bromophenol blue reaction × 300).
Immunohistochemistry for PCNA
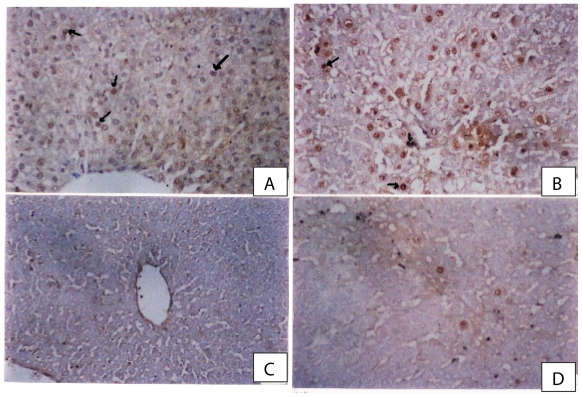
Proliferating cell nuclear antigen (PCNA) known as cyclin, is a non-histone nuclear protein whose level of synthesis correlates directly with rates of cellular proliferation and DNA synthesis (Shiina et al. 1996). Cells were considered PCNA positive if there was brown nuclear staining of the cells and negative nuclei not stained and appear blue. In the control group there were few nuclei that showed positive reaction (brown nuclei) (Fig. 4A). Increase in the number of PCNA staining of hepatocyte nuclei was evident in CCl4-treated rats (Fig. 4B). In rat liver given CCl4 and a combination of riba-virin (60 mg/kg) and silymarin there was a marked reduction in the number of PCNA positive nuclei especially in peripheral zones compared with sections from rats treated with CCl4-olive oil (Fig. 4C). The hepatocyte nuclei of rats treated with CCl4 + ribavirin showed a reduction in PCNA +ve reaction compared to CCl4 control group (Fig. 4D).
Figure 4.
(A) A photomicrograph from a section of control rat liver showing normal hepatocytes with few PCNA positive nuclei (arrows) (PCNA immunostaining × 300). (B) A photomicrograph from a section of rat liver given CCl4 showing prominent increase in the number of PCNA positive nuclei (proliferating cells) in regenerated cells in necrotic areas around central veins (PCNA immunoperoxidae × 300). (C) A photomicrograph from a section of rat liver given CCl4 and a combination of ribavirin (60 mg/kg)and silymarin showing marked reduction in the number of PCNA positive nuclei especially in peripheral zones compared with sections from rats treated with CCl4-olive oil (PCNA immunostaining × 300). (D) A photomicrograph from a section of rat liver given CCl4 and ribavirin at 90 mg/kg showing few number of PCNA positive nuclei in hepatocytes adjacent to the central vein (PCNA immunostaining × 300).
Discussion
The present study provides evidence that in the CCl4 model of hepatic toxicity, ribavirin, an antiviral drug, exerts hepatic protective effects. Leakage of hepatocellular enzymes ALT and AST into plasma was significantly reduced and the histological degree of hepatocyte necrosis was attenuated. Improved liver function tests are likely a consequence of the lower degree of organ damage and fibrosis. The hepatocytes from rats treated with the high dose of ribavirin showed more or less normal appearance. Morphometric analysis of liver sections showed that ribavirin administration to CCl4-treated rats resulted in a significant and dose-dependent decrease in damaged areas. Metabolic pertuberations caused by the hepatotoxin CCl4 as reflected in a decrease in intracellular protein content in hepatocytes were improved by ribavirin monotherapy and also by combined silymarin and ribavirin therapy. These results point to a hepatic protective effect of ribavirin distinct from its antiviral activity.
One of the principal functions of the liver is the regulation of carbohydrate metabolism and blood glucose homeostasis. In the present study, serum glucose was reduced by 50% in CCl4-treated rats which was prevented by ribavirin montherapy and also by ribavirin plus silymarin. Studies have demonstrated a decreased hepatic glycogen content after treatment with CCl4, reflecting decreased gluconeogenesis by the liver (Muriel et al. 2001). Without sufficient glycogen levels to provide glucose to drive glycolysis, cellular ATP levels may drop below critical levels when secondary stress such as hypoxia is present, precipitating cell death (Ulrich et al. 2001). Glucose also has a role in protecting cells from oxidative injury (Tian et al. 1999). Low O2 tensions which are found in the centrilobular areas of the liver favor conversion of CCl4 to free radical products which cannot be detoxified by the glutathione-dependent mechanism (Burk et al. 1984). Abnormalities in glucose metabolism are also present in patients with liver disease and type 2 diabetes mellitus seems to be more common in patients with chronic hepatitis C infection (Allison et al. 1994; Caronia et al. 1999). Insulin resistance, hepatocyte dysfunction, or an HCV-related autoimmune process might be implicated (Alexander, 2000). Insulin resistance in chronic hepatitis C is relevant because it promotes steatosis and fibrosis. Insulin resistance together with fibrosis and genotype has been found to be independently associated with impaired response rate to peginterferon plus ribavirin (Romero-Gomez, 2006). Alternatively, diabetic status is one of the more important variables determining the severity of HCV recurrence after liver transplantation (Foxton et al. 2006). In decompensated cirrhosis, there may be, however, acute post absorptive hypoglycaemia, primarily due to a reduction in hepatic glycogen capacity (McCullough and Tavill, 1991; Krahenbuhl et al. 1991) and there is evidence for altered hepatic gluconeogenesis (Changani et al. 2001).
The present study also provides evidence of additional beneficial effect of combining silymarin with ribavirin. Silymarin (Milk Thistle, Silybum marianum) is a commonly used herbal therapy, particularly by patients who have liver disease including those with chronic hepatitis C infection. There are clinical data to support its use in chronic alcoholic liver disease (Feher et al. 1989; Pares et al. 1998) where it resulted in reducing serum bilirubin, and transaminases. Others, however, failed to demonstrate such benefit from silymarin (Trinchet et al. 1989; Buzzelli et al. 1993). In patients with chronic hepatitis C, silymarin has been shown not to affect serum HCV RNA, ALT levels, quality of life or psychological well-being in subjects with this condition (Gordon et al. 2006). In animal models of hepatotoxicity, silymarin showed marked protective properties. This hepa-toprotective effect of silymarin is due to membrane-stabilizing action, free radicals scavenging properties, inhibition of lipid peroxidation and modulation of hepatocyte Ca++ (Muriel and Mourelle, 1990; Flora et al. 1998; Farghali et al. 2000). In rats with secondary biliary cirrhosis, silymarin reduced hepatic collagen accumulation by 35% (Boigk et al. 1997). Silymarin thus might be of benefit in reducing liver injury in patients on ribavirin therapy.
Cell proliferation is of interest since abnormal cell proliferation is a precursor of tumorigensis. Identification of proliferating cells was studied by the avidin-biotin-peroxidase technique. Proliferating cell nuclear antigen (PCNA) known as cyclin, is a non-histone nuclear protein whose level of synthesis correlates directly with rates of cellular proliferation and DNA synthesis (Shiina et al. 1996). The elevated levels of PCNA expression appear in the nucleus during the late G1 phase with maximum expression during S-phase and decline during G2 and M phase. Therefore, the accumulation of PCNA gene products in cycling cells can be an index of the degree of cellular proliferation and DNA synthesis (Bravo et al. 1987). Carbon tetrachloride administration in rats concomitantly induces both processes in acute injury and liver regeneration. Hepatocyte growth factor and PCNA were induced in the early stage (6 h) and 36 h, respectively (Taniguchi et al. 2004). Liver cirrhosis induced by CCl4 is associated with alterations in cell cycle-related proteins, and that the expression of these proteins is responsible for hepatocyte regeneration in the damaged liver and may be involved in liver carcinogenesis (Jeong et al. 2001). In the present study hepatocyte proliferation in CCl4-treated rats, as evidenced by the increase in the number of PCNA staining of hepatocyte nuclei was reduced by ribavirin administration.
Ribavirin monotherapy has been employed in hepatitis C virus infection such as in patients with hepatitis C recurring after liver transplant (Quadri et al. 2002) and renal transplant patients (Kamar et al. 2003), often with discrepancy in results. In most studies, ribavirin monotherapy, improved liver enzyme levels, but without significant effects on HCV viraemia (Gane et al. 1995, 1998; Di_Bisceglie et al. 1995; Dusheiko et al. 1996; Cattral et al. 1999; Stanimirovic et al. 2002; Hoofnagle et al. 2003; Kamar et al. 2003). Others noted slight, albeit not significant, decrease of serum HCV RNA level and intrahepatic HCV antigen staining score (Quadri et al. 2002). Significant histological improvement with reduction in hepatic inflammation and necrosis (Gane et al. 1995, 1996; Di_Bisceglie et al. 1995; Stanimirovic et al. 2002; Hoofnagle et al. 2003), no change in necroinflammation (Cattral et al. 1999; Izopet and Rostaing, 2003) or even worsening of fibrosis (Quadri et al. 2002; Kamar et al. 2003) has been reported. The exact mechanism of action of ribavirin is unknown and direct antiviral properties are unclear (Lee et al. 1998; Querenghi et al. 2001). It was suggested that the beneficial effects of riba-virin are mediated by inhibition of induction of macrophage proinflammatory cytokines and Th2 cytokines while preserving Th1 cytokines (Ning et al. 1998). Ribavirin could decrease the synthesis of proinflammatory cytokines (e.g. IFN-gamma) by an inhibition of total DNA-, RNA-, and protein-synthesis and by induction of apoptosis in the cells of the inflammatory infiltrate (Meier et al. 2003). Ribavirin at physiological doses markedly suppressed the production of TNF-alpha, IL-10, and IL-12 (p70) which may explain the reduction in hepatic inflammation observed during ribavirin monotherapy (Barnes et al. 2004). In liver injury induced by CCl4, secondary hepatic injury occurs from inflammatory processes originating from products released by activated Kupffer cells, which play a central role in hepatic inflammation. Kupffer cells isolated from rats with CCl4-induced steatonecrosis produced more reactive oxygen intermediates than cells isolated from normal rats. These oxidants could activate NF-kappa B and lead to an overexpression of TNF-alpha, observed in liver tissue sections. This cytokine expressed in the CCl4-induced inflammatory process is associated with the development of fibrosis and may contribute to disease severity (Orfila et al. 1999, 2000). In CCl4-induced liver injury IL-6, IL-1beta, TNF-alpha and IFN-gamma upregulation was found at the maximum 12 hours after administration of the toxin (Sheikh et al. 2006). Studies also linked protection from CCl4-induced hepatic injury with suppression of TNF-alpha level (Yang et al. 2005, 2006). Based on the above data, it is suggested that the inhibition of proinflammatory cytokines by ribavirin might be involved in the protection observed in the present study.
In conclusion, protective effects for the antiviral agent ribavirin were observed against liver damage induced by CCl4 treatment. Additional benefit from combining ribavirin and silymarin was observed. These results suggest that ribavirin lessens hepatic necro-inflammation through mechanisms distinct from antiviral activity. Furthermore, these effects of ribavirin were associated with a reduction in PCNA staining of hepatocyte nuclei, a finding that could have relevance for humans in view of the risk of developing hepatocellular carcinoma in patients with chronic hepatitis C infection. The results suggest that a therapeutic approach using both ribavirin and silymarin could be effective in chronic liver disease. This regimen is likely to benefit those patients who can not tolerate, failed to respond to, or relapsed after combined interferon and ribavirin therapy.
References
- Alexander GJM. An Association between Hepatitis C Virus Infection and Type 2 Diabetes Mellitus: What Is the Connection? 2000. Ann Intern Med. 2000;33:650–652. doi: 10.7326/0003-4819-133-8-200010170-00018. [DOI] [PubMed] [Google Scholar]
- Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–9. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- Bauer JD. Clinical Laboratory Methods. 9th ed. C.V. Mosby Company; St.Louis, Toronto, London: 1982. [Google Scholar]
- Belfield A, Goldberg DM. Revised assay for serum phenyl phos-phatase activity using 4-amino-antipyrine. Enzyme. 1971;12:561–73. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schup-pan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–9. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- Bonaccorsoa S, Meltzerc H, Maes M. Psychological and behavioural effects of interferons. Curr Opin Psychiat. 2000;13:673–677. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1997;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Burk RF, Lane JM, Patel K. Relationship of oxygen and glutathione in protection against carbon tetrachloride-induced hepatic microsomal lipid peroxidation and covalent binding in the rat. Rationale for the use of hyperbaric oxygen to treat carbon tetrachloride ingestion. J Clin Invest, 1984;74:1996–2001. doi: 10.1172/JCI111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra P, Targhetta S, Pevere S, Boninsegna S, Guido M, Canova D, Brolese A, Masier A, D’Aloiso C, Germani G, Tomat S, Fagi-uoli S. Antiviral therapy for hepatitis C virus recurrence following liver transplantation: long-term results from a single center experience. Transplant Proc. 2006;38:1127–1130. doi: 10.1016/j.transproceed.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lamper-tico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456–60. [PubMed] [Google Scholar]
- Cameron CE, Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis. 2001;14:757–764. doi: 10.1097/00001432-200112000-00015. [DOI] [PubMed] [Google Scholar]
- Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–63. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- Cattral MS, Hemming AW, Wanless IR, Al Ashgar H, Krajden M, Lilly L, Greig PD, Levy GA. Outcome of long-term riba-virin therapy for recurrent hepatitis C after liver transplantation. Transplantation. 1999;67:1277–1280. doi: 10.1097/00007890-199905150-00014. [DOI] [PubMed] [Google Scholar]
- Changani KK, Jalan R, Cox IJ, Ala-Korpela M, Bhakoo K, Taylor-Robinson SD, Bell JD. Evidence for altered hepatic gluconeogenesis in patients with cirrhosis using in vivo 31-phosphorus magnetic resonance spectroscopy. Gut. 2001;49:557–564. doi: 10.1136/gut.49.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutaputti A. Management of hepatitis C. adverse effects and other safety aspects of the hepatitis C antivirals. J. Gastroenterol. Hepatol. 2000;15(Suppl):E156–163. doi: 10.1046/j.1440-1746.2000.02114.x. [DOI] [PubMed] [Google Scholar]
- Collier J, Chapman R. Combination therapy with interferon-alpha and ribavirin for hepatitis C: practical treatment issues. BioDrugs. 2001;15:225–238. doi: 10.2165/00063030-200115040-00003. [DOI] [PubMed] [Google Scholar]
- Crowley LV. The Reitman-Frankel colorimetric transaminase procedure in suspected myocardial infarction. Clin Chem. 1967;13:482–487. [PubMed] [Google Scholar]
- Di-Bisceglie AM, Conjeevaram HS, Fried MW, Sallie RF, Park Y, Yurdaydin C, Swain M, Kleiner DE, Mahaney K, Hoofnagle JH. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dhillon A, Rassam S, Fryden A, Reesink H, Bassendine M, Norkrans G, Cuypers T, Lelie N, Telfer P, Watson J, Weegink C, Sillikens P, Weiland O. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Tibbs CJ, Ramage JK, Portmann BC, Williams R. Ribavirin therapy for hepatitis C infection following liver transplantation. Transpl Int. 1995;8:61–64. doi: 10.1007/BF00366714. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Lo SK, Riordan SM, Portmann BC, Lau JY, Naoumov NV, Williams R. A randomized study comparing ribavirin and interferon alfa monotherapy for hepatitis C recurrence after liver transplantation. Hepatology. 1998;27:1403–1407. doi: 10.1002/hep.510270530. [DOI] [PubMed] [Google Scholar]
- Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJP, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- Farghali H, Kamenikova L, Hynie S, Kmonickova E. Silymarin effects on intracellular calcuim and cytotoxicity: a study in perfused rat hepatocytes after oxidative stress injury. Pharmacol Res. 2000;41:231–237. doi: 10.1006/phrs.1999.0575. [DOI] [PubMed] [Google Scholar]
- Feher J, Deak G, Muzes G, Lang I, Niederland V, Nekam K, Karteszi M. Liver protective action of silymarin therapy in chronic alcoholic liver diseases. Orv Hetil. 1989;130:2723–7. [PubMed] [Google Scholar]
- Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- Foxton MR, Quaglia A, Muiesan P, Heneghan MA, Portmann B, Norris S, Heaton ND, O’Grady JG. The impact of diabetes mel-litus on fibrosis progression in patients transplanted for hepatitis C. Am. J. Transplant. 2006;6:1922–9. doi: 10.1111/j.1600-6143.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Ghany MG, Kleiner DE, Doo E, Heller T, Promrat K, Ong J, Khokhar F, Soza A, Herion D, Park Y, Everhart JE, Liang TJ. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology. 2003;38:66–74. doi: 10.1053/jhep.2003.50258. [DOI] [PubMed] [Google Scholar]
- Jeong DH, Jang JJ, Lee SJ, Lee JH, Lim IK, Lee MJ, Lee YS. Expression patterns of cell cycle-related proteins in a rat cirrhotic model induced by CCl4 or thioacetamide. J Gastroenterol. 2001;36:24–32. doi: 10.1007/s005350170150. [DOI] [PubMed] [Google Scholar]
- Kamar N, Sandres-Saune K, Selves J, Ribes D, Cointault O, Durand D, Izopet J, Rostaing L. Long-term ribavirin therapy in hepatitis C virus-positive renal transplant patients: effects on renal function and liver histology. Am J Kidney Dis. 2003;42:184–192. doi: 10.1016/s0272-6386(03)00422-0. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Weber FL, Jr, Brass EP. Decreased hepatic glycogen content and accelerated response to starvation in rats with carbon tetrachloride-induced cirrhosis. Hepatology. 1991;14:1189–95. [PubMed] [Google Scholar]
- Lee JH, von_Wagner M, Roth WK, Teuber G, Sarrazin C, Zeuzem S. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C virus. J Hepatol. 1998;29:29–35. doi: 10.1016/s0168-8278(98)80175-x. [DOI] [PubMed] [Google Scholar]
- McCullough AJ, Tavill AS. Disordered energy and protein metabolism in liver disease. Semin Liver Dis. 1991;11:265–277. doi: 10.1055/s-2008-1040445. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Poynard T. Combination therapy with interferon plus ribavirin for the initial treatment of chronic hepatitis C. Semin Liver. Dis. 1999;19(Suppl 1):57–65. [PubMed] [Google Scholar]
- Meier V, Burger E, Mihm S, Saile B, Ramadori G. Ribavirin inhibits DNA, RNA, and protein synthesis in PHA-stimulated human peripheral blood mononuclear cells: possible explanation for therapeutic efficacy in patients with chronic HCV infection. J Med Virol. 2003;69:50–58. doi: 10.1002/jmv.10264. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lyden E. Impact of pegylated interferon alpha-2B and ribavirin on hepatic fibrosis in liver transplant patients with recurrent hepatitis C: an open-label series. 1. Liver. Int. 2006;26:529–535. doi: 10.1111/j.1478-3231.2006.01261.x. [DOI] [PubMed] [Google Scholar]
- Muriel P, Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol. 1990;10:275–279. doi: 10.1002/jat.2550100408. [DOI] [PubMed] [Google Scholar]
- Muriel P, Alba N, Perez-Alvarez VM, Shibayama M, Tsutsumi VK. Kupffer cells inhibition prevents hepatic lipid peroxidation and damage induced by carbon tetrachloride. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:219–26. doi: 10.1016/s1532-0456(01)00237-x. [DOI] [PubMed] [Google Scholar]
- Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding JW, Liu MF, Rotstein O, Phillips MJ, Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–93. [PubMed] [Google Scholar]
- Orfila C, Lepert JC, Alric L, Carrera G, Beraud M, Vinel JP, Pipy B. Expression of TNF-alpha and immunohistochemical distribution of hepatic macrophage surface markers in carbon tetrachloride-induced chronic liver injury in rats. Histochem J. 1999;31:677–685. doi: 10.1023/a:1003851821487. [DOI] [PubMed] [Google Scholar]
- Orfila C, Lepert JC, Alric L, Carrera G, Beraud M, Pipy B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem Cell Biol. 2005;123:585–593. doi: 10.1007/s00418-005-0785-2. [DOI] [PubMed] [Google Scholar]
- Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, Rigau J, Santos J, Rodes J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–21. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- Pianko S, McHutchison JG. Treatment of hepatitis C with inter-feron and ribavirin. J Gastroenterol Hepatol. 2000;15:581–586. doi: 10.1046/j.1440-1746.2000.02082.x. [DOI] [PubMed] [Google Scholar]
- Quadri R, Giostra E, Roskams T, Pawlotsky JM, Mentha G, Rubbia-Brandt L, Perrin L, Hadengue A, Negro F. Immunological and virological effects of ribavirin in hepatitis C after liver transplantation. Transplantation. 2002;73:373–378. doi: 10.1097/00007890-200202150-00010. [DOI] [PubMed] [Google Scholar]
- Querenghi F, Yu Q, Billaud G, Maertens G, Trepo C, Zoulim F. Evolution of hepatitis C virus genome in chronically infected patients receiving ribavirin monotherapy. J Viral Hepat. 2001;8:120–131. doi: 10.1046/j.1365-2893.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Sheikh N, Batusic DS, Dudas J, Tron K, Neubauer K, Saile B, Ramadori G. Hepcidin and Hemojuvelin gene-expression in rat liver damage: In vivo and in vitro studies. Am J Physiol. 2006 doi: 10.1152/ajpgi.00586.2005. [DOI] [PubMed] [Google Scholar]
- Shiina H, Igawa M, Nagami H, Yagi H, Urakami S, Yoneda T, Shirakawa H, Ishibe T, Kawanishi M. Immunohistochemical analysis of proliferating cell nuclear antigen, p53 protein and nm23 protein, and nuclear DNA content in transitional cell carcinoma of the bladder. Cancer. 1996;78:1762–1774. doi: 10.1002/(sici)1097-0142(19961015)78:8<1762::aid-cncr17>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Stanimirovic V, Nikolic D, Stanimirovic B, Nikolic A, Cucak S, Galenika R&D Institute, Belgrade. Evaluation of ribavirin efficacy and tolerance in subjects with chronic hepatitis C virus infection. Vojnosanit Pregl. 2002;59:479–484. doi: 10.2298/vsp0205479s. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Takeuchi T, Nakatsuka R, Watanabe T, Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539–1549. doi: 10.1016/j.lfs.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Trinchet JC, Coste T, Levy VG, Vivet F, Duchatelle V, Legendre C, Gotheil C, Beaugrand M. Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients. Gastroenterol Clin Biol. 1989;13:120–124. [PubMed] [Google Scholar]
- Ulrich RG, Bacon JA, Brass EP, Cramer CT, Petrella DK, Sun EL. Metabolic, idiosyncratic toxicity of drugs: overview of the hepatic toxicity induced by the anxiolytic, panadiplon. Chemico-Biological Interactions. 2001;134:251–270. doi: 10.1016/s0009-2797(01)00161-2. [DOI] [PubMed] [Google Scholar]
- Wang-Ni Tian, Leigh D, Braunstein Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydro-genase activity in cell death. Am J Physiol. 1999;276:C1121–31. doi: 10.1152/ajpcell.1999.276.5.C1121. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei W, Wang NP, Gui SY, Wu L, Sun WY, Xu SY. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77:1902–1915. doi: 10.1016/j.lfs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Wartelle-Bladou C, Arpurt JP, Renou C, Pariente A, Pillon D, Nalet B, Picon M, Glibert A, Chousterman M, Grasset D, Morin T, Bernard P, Fischer D, Ramdani M, Lagier E, Rotily M. Viral Hepatitis Group of the ANGH. High dose daily interferon-alpha induction and secondary adjunction of ribavirin in treatment-naive patients with chronic hepatitis C. A multicentric, randomised, controlled trial. Gastro-enterol Clin Biol. 2006;30:525–532. doi: 10.1016/s0399-8320(06)73221-3. [DOI] [PubMed] [Google Scholar]
- Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Liu J, Ye LB, Yang F, Ye L, Gao JR, Wu ZH. In vitro and in vivo protective effects of proteoglycan isolated from mycelia of Ganoderma lucidum on carbon tetrachloride-induced liver injury. World J Gastroenterol. 2006;12:1379–1385. doi: 10.3748/wjg.v12.i9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]