Abstract
Although the oral route of drug administration is the most acceptable way of self-medication with a high degree of patient compliance, the intestinal absorption of many drugs is severely hampered by different biological barriers. These barriers comprise of biochemical and physical components. The biochemical barrier includes enzymatic degradation in the gastrointestinal lumen, brush border and in the cytoplasm of the epithelial cells as well as efflux transporters that pump drug molecules from inside the epithelial cell back to the gastrointestinal lumen. The physical barrier consists of the epithelial cell membranes, tight junctions and mucus layer. Different strategies have been applied to improve the absorption of drugs after oral administration, which range from chemical modification of drug molecules and formulation technologies to the targeting of receptors, transporters and specialized cells such as the gut-associated lymphoid tissues. This review focuses specifically on the targeting of receptor-mediated endocytosis, transporters and the absorption-site as methods of optimizing intestinal drug absorption. Intestinal epithelial cells express several nutrient transporters that can be targeted by modifying the drug molecule in such a way that it is recognized as a substrate. Receptor-mediated endocytosis is a transport mechanism that can be targeted for instance by linking a receptor substrate to the drug molecule of interest. Many formulation strategies exist for enhancing drug absorption of which one is to deliver drugs at a specific site in the gastrointestinal tract where optimum drug absorption takes place.
Keywords: Oral drug delivery, absorption enhancement, receptor-mediated endocytosis, active transporters, site-specific drug delivery
Introduction
Oral delivery remains the most favorable and preferred route for drug administration. Currently more than 60% of drugs are marketed as oral products (Masaoka et al. 2006). However, many drugs cannot be effectively delivered by the oral route of administration in their original form due to reasons of instability, low membrane permeability, poor solubility and efflux transport mechanisms. Overcoming these barriers is currently one of the most challenging goals in oral drug delivery (Majumdar and Mitra, 2006; Leonard et al. 2006; Hamman et al. 2005; Ghilzai, 2004).
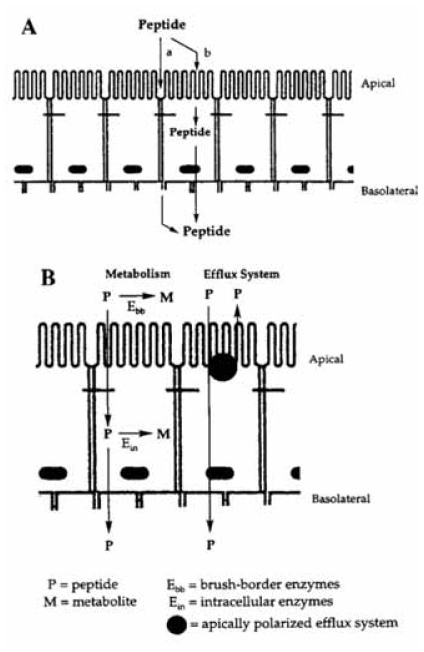
The main function of the gastrointestinal tract is to digest and absorb nutrients and fluids. In addition, it also has to prevent the invasion of toxins, antigens and pathogens. The barriers that exist to fulfill this protective task are also responsible for hampering the absorption of drug molecules after oral administration. The physical barrier of the gastrointestinal tract can be attributed to the cell membranes, the tight junctions between adjacent epithelial cells and the mucus layer, while the biochemical barrier comprises of the catabolic enzymes and efflux systems that pump molecules back into the gastrointestinal lumen (Hunter and Hirst, 1997; Lennernäs, 1998; Gabor et al. 2004). The barrier function of the gastrointestinal tract is schematically illustrated in Figure 1.
Figure 1.
Schematical illustration of the barrier properties of the intestinal mucosa. A) The physical barrier includes the tight junctions that limit paracellular transport and the epithelial cell membrane that limits the transcellular transport and B) The biochemical barrier includes brush border and/or intracellular metabolism and apical polarized efflux (with permission from Pauletti et al. 1996).
The implications of a barrier against drug absorption from the gastrointestinal tract include low drug bioavailability after oral administration. When the bioavailability of a drug is low, it is most likely that insufficient drug will become available at the site of action and it will therefore also not produce its pharmacological effect (Aungst, 1993; Aungst, 2000).
Several strategies have been employed to improve the bioavailability of drugs after oral administration. Some strategies aim at maximizing the intestinal uptake while others focus on protecting the drug molecules from degradation, but combinations there of have also been reported. These strategies include the formation of pro-drugs and/or drug conjugates, modifying the chemical structure of the drug and formulation design approaches (Gomez-Orellana, 2005). Although some of these approaches have been demonstrated to be successful in laboratory scale research, they still present challenges in terms of long-term safety and reproducibility in the clinical situation. Morishita and Peppas (2006) suggested that the development of an effective oral delivery system for a new generation of macromolecular drugs should consider the following three approaches: modification of a physicochemical property of the drug molecule (e.g. lipophilicity and enzyme susceptibility) or addition of novel functionality (e.g. receptor recognition or cell permeability) or the use of a novel drug delivery carrier system. These strategies may be applied alone or in combination to provide a solution to the problem of poor bioavailability.
Since it is desirable that efforts do not compromise the integrity of the intestinal mucosa (both tight junctions and cell membranes), more safe and practical approaches seem to be targeting of receptors, transporters or absorption sites in the gastrointestinal tract in terms of enhancing the absorption of drugs with poor bioavailabilities. These appealing approaches for optimizing oral drug delivery will be the focus of the discussions in this review.
Receptor-Mediated Endocytosis
The pharmacological effects of a large number of macromolecules such as proteins and oligonucleotides for the treatment of several human diseases are determined by many factors, including receptor binding, cellular internalization, intracellular sorting and targeting as well as transcellular transport. Therefore, the therapeutic applications of most proteins and macromolecular drugs depend largely on their ability to be endocytosed (Shen et al. 1992). The main mechanism important in this regard is known as receptor-mediated endocytosis. Receptor-mediated endocytosis is a process of internalization of extracellular molecules during which binding occurs between these molecules and the receptors. The receptors are considered as membrane-associated proteins and the intracellular molecules which specifically bind to these receptors are known as ligands (Shen et al. 1992). Following binding to the receptor on the cell surface, the resultant ligand-receptor complex is internalized via a clathrin-dependent or a clathrin-independent endocytotic process.
In the clathrin-dependent pathway, the formation of ligand-receptor complex is followed by concentration in clathrin-coated regions or coated pits of the plasma membrane. These coated pits invaginate from the plasma membranes and turn into coated vesicles by pinching inward from the membrane. After their formation, the coated vesicles lose the clathrin coats rapidly, and as a result, smooth membrane vesicles and tubules are formed (Morris et al. 1989; Vyas and Sihorkar, 2000). These early endosomes, which carry receptors and ligands, subsequently participate in a sequence of intracellular processing and sorting events. The clathrin-independent pathway, on the other hand, involves vesicle formation derived from the invagination of non-clathrin-coated plasma membrane. In general, this type of receptor-mediated endocytosis occurs in receptors with a low population and a slow rate of internalization, when compared to that of the clathrin-dependent pathway. The exact internalization mechanism of this pathway is still largely unknown (Shen et al. 1992).
Following receptor-mediated endocytosis process, the endocytosed material may be processed in one of four ways. Most ligands are dissociated from their receptors by the low pH (i.e. <5.5) encountered within the endosomes or the recepto-somes or the Compartment for Uncoupling of Receptors or Ligands (CURL) (Geuze et al. 1983). The receptors may either be recycled to the cell surface or degraded, while the ligand is routed to the lysosomes for degradation (Mostov et al. 1985). Alternatively, the binding between the receptor and ligand may be unaffected by acidification and the receptor-ligand complexes are directly sorted to lysosomes for degradation e.g. insulin receptor (Féger et al. 1994). The internalization of transferrin-bound iron represents a third process, in which the iron dissociates from the transferrin, which is then returned to the cell surface (Russel-Jones, 2001). The fourth case is characteristic of epithelial and enterocytic cells, and results in the endocytosed material being transcytosed across the cell. In this process, the ligands such as thyroglobulin bind to their receptors on either the apical or basolateral membrane. The complex is then endocytosed and transported to endosomes via coated vesicles. The endosomal material is uncoupled from its receptor and then transported across the cell in an as yet to be identified membrane vesicle (Simons et al. 1985).
The use of receptor-mediated endocytosis in the gut is very important for oral drug delivery because it can be used to delay intestinal transit of drugs (KilPatrick et al. 1985; King et al. 1986; Woodley and Naisbet, 1988; Lehr et al. 1992), to target drugs to the intestinal epithelial cells (Russel-Jones, 2001) and for systemic drug delivery (de Aizpurua and Russel-Jones, 1987; Pusztai, 1989; Lindner et al. 1994). Significant developments in the oral delivery of peptides and proteins have been conducted involving receptor-mediated endocytosis process in the vitamin B12 uptake system (de Aizpurua et al. 1986; Russel-Jones and de Aizpurua, 1988; Habberfield et al. 1996; Russel-Jones, 2001), folate absorption (Ward et al. 2000; Ni et al. 2002; Lu, 2002) and also system in which transferrin-receptors are activated (Qian et al. 2002; Kovar et al. 2002; Kursa et al. 2003).
Vitamin B12 (cyanocobalamine)
Vitamin B12 is a much larger molecule than other vitamins and therefore cannot enter the body through simple diffusion, facililated diffusion or active transport (Russel-Jones, 2001). During the absorption of vitamin B12, intrinsic factor (IF) produced in the stomach binds to vitamin B12 forming a complex which passes down the small intestine until it reaches the ileum. Here the complex binds to a specific IF receptor (IFR) located on the apical membrane of the villous enterocyte depending on the concentration of calcium ions. The complex is then internalized by the enterocyte via receptor-mediated endocytosis. Once inside the cell, the Vitamin B12 is released from IF following the action of cathepsin L on IF (Fyfe et al. 1991; Schohn et al. 1991; Guéant et al. 1992).
Research has demonstrated that it is possible to link chemically peptides such as luteinizing hormone-releasing hormone (LHRH), and protein such as erythropoietin (EPO), granulocyte-colony stimulating factor (G-CSF) or interferon-α to vitamin B12 in a way which is capable of shuttling these molecules across the intestinal epithelia without interfering with the ability of vitamin B12 to bind to IF (de Aizpurua et al. 1986; Russel-Jones and de Aizpurua, 1988; Russel-Jones, 1995; Habberfield et al. 1996). Vitamin B12 conjugated to an analogue of LHRH was found to be active in stimulating ovulation significantly better in experimental mice than in control mice following an oral dose (Russel-Jones, 1995). Habberfield and co-workers linked vitamin B12 to EPO and also to G-CSF and used these complexes to examine the potential of the vitamin B12 uptake system to transport these systems from the small intestine to the circulation in rats (Habberfield et al. 1996). It was shown that the vitamin B12 uptake system could deliver EPO or G-CSF to the circulation in rats at a level 4-fold higher than similar administration of EPO or G-CSF alone.
The use of receptor-mediated endocytosis for oral delivery of nanoparticles linked to vitamin B12 for systemic circulation has also been demonstrated (Russel-Jones, 1995). Nanoparticles containing a fluorochrome have been chemically linked to vitamin B12 and administered to rats orally. Upon histological examination, the fluorescent particles were initially found to be bound to the surface of the intestinal villous cells. Some time later, the nanoparticles could be found to have crossed the villous epithelial cells and were observed below the mucosal cell layer congregating in the central lacteal gland for systemic circulation (Russel-Jones, 1995).
Folate
In rapidly dividing cells such as cancer cells, receptors are up-regulated and can thus be differentially targeted in drug delivery strategies. The folate receptor is an ideal candidate for tumor-targeted drug delivery because it is upregulated in many human cancers. Access to the folate receptor in normal tissues can be severely limited due to its location on the apical membrane of polarized epithelia, and the density of folate receptors appears to increase as the stage or grade of the cancer worsens (Lu et al. 2002).
The conjugation of folic acid via its γ-carboxylic group has resulted in drug binding to cells expressing the folate receptor and consequently endocytosis taking place (Lu et al. 2002). Folic acid has been linked to both drugs of low molecular weight and the macromolecular complexes as a means of targeting the attached molecules to malignant cells (Lu et al. 2002). Although this conjugation has been shown to enhance the delivery of macromolecules to folate receptor-expressing cancer cells in almost all in vitro situations tested, mixed effects have however, been observed when conducting similar studies in vivo conditions. Despite these mixed effects, prominent examples do exist where folate targeting has significantly improved the outcome of a macromolecule-based therapy, leading to complete remission of established tumors (Ward, 2000; Lu et al. 2002). For example, folate receptor-targeted delivery of liposomal daunorubicin to folate receptor expressing cells was found to have significantly increased drug cellular uptake and cytotoxicity compared to other cells (Ni et al. 2002).
Transferrin
The use of the transferrin receptor for targeted drug delivery has also been receiving attention in literature in recent years (Xu et al. 2001; Qian et al. 2002; Kovar et al. 2002; Kursa et al. 2003). High levels of transferrin receptors are expressed on the surface of actively metabolizing cells (Iacopetta et al. 1982; Banerjee et al. 1986), certain tumors (Faulk et al. 1980), and the brain capillary endothelium (Jefferies et al. 1984). The expression of high levels of transferrin receptor in the brain capillary endothelium is particularly important because there is a possibility of delivering drugs across the blood-brain barrier. This was demonstrated using a conjugate of methotrexate with anti-transferrin receptor antibody which was shown to bind and traverse the blood-brain barrier (Frieden et al. 1980). Apart from delivering drugs across the blood-brain barrier, conjugates of transferrin have been successfully used to selectively kill cell lines expressing the transferrin receptor in certain cancers (Cawley et al. 1981; Raso and Basala, 1984). Furthermore, polylysine conjugates of transferrin in particular have been used to deliver DNA sequences to cell lines in culture for the development of gene therapy (Wagner et al. 1990).
The potential problem with manipulation of transferrin uptake as a means of drug delivery across cells is the recyling pathway through which both transferrin and its receptor undergo (Dautryvarsat, 1986). However, research findings have now suggested possible means of modulating the recycling pathway to achieve greater transport across the cells. This was demonstrated with Brefeldin A, a drug that causes the disruption of transport of secretory proteins from the endoplasmic reticulum to Golgi cisternae (Wan et al. 1991). This drug showed a capacity to cause a missorting of the transferrin receptor from the basal to the apical membrane and a consequent 30- and 100-fold increase in the transcytosis of transferrin in the basal-to-apical and the apical-to-basal direction, respectively (Wan et al. 1991). Similarly, monensin which is a drug with reversible disrupting activity on the Golgi apparatus (Wan et al. 1990) has been shown to increase the transcotysis of transferrin and its conjugates in the basal-to-apical direction by up to 26-fold (Wan et al. 1991).
Membrane Transporters
Many organic solutes such as nutrients (i.e. amino acids, sugars, vitamins and bile acids) and neurotransmitters are transferred across cell membranes by means of specialized transporters. These carrier systems comprise integral membrane proteins that are capable of transferring substrates across cell membranes by means of a passive process (i.e. through channels or facilitated transporters) or an active process (i.e. with carriers). Carrier-mediated active transport requires energy obtained by adenosine tri-phosphate (ATP) hydrolysis or by coupling to the co-transport of a counterion down its electrochemical gradient (e.g. Na+, H+, Cl−). Several drugs and pro-drugs share this transport pathway with nutrients and it has been shown that targeting drugs to these transporter carriers can influence their bioavailability as well as their distribution (Zhang et al. 2002; Steffansen, 2004).
Targeting drug delivery to intestinal nutrient transporters has emerged as an important strategy to improve oral bioavailability of poorly permeating therapeutic agents. This approach usually entails linking the drug molecule to a natural ligand in order to be recognized as a substrate by a specific nutrient transporter in the apical membrane of the epithelial cell. Alternatively, the drug molecule can be designed or changed (e.g. formation of derivatives or pro-drugs) in such a way that it mimics the three-dimensional features of natural ligands. These pro-moieties are then either cleaved within the intracellular environment of the epithelial cells or elsewhere in the body to free the active drug (Zhang et al. 2002; Majumdar et al. 2004). In general, transporter proteins that can be targeted for this purpose are those that provide transport mechanisms for amino acids, dipeptides, monosaccharides, monocarboxylic acids, organic cations, phosphates, nucleosides and water-soluble vitamins (Lee, 2000).
Peptide transporters
Exogenic peptides are rapidly metabolized in the gastrointestinal tract by proteolytic enzymes into smaller oligopeptides, tripeptides, dipeptides as well as amino acids. While the absorption of larger peptides across intestinal epithelial cells is restricted, large amounts of amino acids and di/tripeptides cross the enterocytic membrane by means of transporter systems (Steffansen et al. 2005).
Human intestinal membrane transporters involved in the uptake of di/tripeptides include the peptide transporter PepT1, the peptide/histidin transporters PHT1, PHT2 and the peptide transporter PT1. The peptide transporter PepT2 is found in other types of tissue than the small intestine and only limited information is available on PHT1 and PT1, but PepT1 is widely described in the literature (Steffansen et al. 2004). PepT1 is an H+-coupled, active transport system with a broad substrate specificity, which may range from natural substrates in food such as di- and tripeptides to peptide-like therapeutic agents such as β-lactam antibiotics and angiotensin-converting enzyme (ACE) inhibitors (Zhang et al. 2002). Even substances without an obvious peptide bond such as δ-amino-levulinic acid and ω-amino fatty acids are substrates for this transporter (Lee, 2000).
Due to the wide substrate specificity of PepT1, various approaches with pro-drugs that are aimed at targeting this transporter have been attempted. One approach is to form dipeptidyl based pro-drugs by linking dipeptides with intrinsic affinity for PepT1 to the drug molecule such as Asp-Sar and Glu-Sar. It was shown that a variety of derivatized dipeptides target PepT1 to improve bioavailability, for example the dipeptidyl derivatives of α-methyl-Dopa as well as p-Glu-l-Dopa-Pro and l-Dopa-Phe showed enhanced permeability as compared to the parent drugs respectively. Another approach to target PepT1 is to form amino acid pro-drugs, for example the l-valyl ester pro-drug of acyclovir increased its oral bioavailability 3–5 times (Steffansen et al. 2004). Enalapril is an ester pro-drug of the ACE-inhibitor, enalaprilat and is a substrate for PepT1. Formation of this pro-drug of enalaprilat resulted in an increase of the oral bioavailability from 3–12% to 60–70% (Zhang et al. 2002).
Although PepT1 targeted amino acid and dipeptidyl pro-drugs show potential for the effective delivery of di/tripeptidomimetics and small drug molecules, it seems to be limited for the delivery of larger peptides or macromolecules (Steffansen et al. 2005).
Amino acid transporters
Amino acid transporters are widely expressed by almost all living cells and are responsible for the absorption of amino acids from the gastrointestinal tract into the systemic circulation and distribution into tissues. Seven amino acid transport systems have been identified in the brush border of the small intestine of which some exhibit overlapping substrate specificities (Hidalgo and Li, 1996). These amino acid transporters present a potential target for improving the absorption of drugs and numerous studies have investigated the possibility of targeting pro-drugs and derivatives to be absorbed via these carriers. The absorption of Gabapentin from the small intestine, for example, is mediated by the large neutral amino acid transporter (Majumdar and Mitra, 2006).
Nucleoside transporters
Although nucleosides and nucleotides are essential precursors for the synthesis of nucleic acids, they are not required to be taken up by most cells because these compounds are synthesized intracellularly. However, the synthesis of purines and pyrimidines in enterocytes is insufficient to support their rapid division. These building blocks of nucleic acids are therefore absorbed from the intestinal lumen through equilibrative (facilitated diffusion) transport systems and Na+-dependent concentrative (energy-dependent active transport) mechanisms (Hidalgo and Li, 1996; Lee, 2000). Examples of drugs that are absorbed by equilibrative nucleoside transporters include s-adneo-sylmethionine, fludarabine, arabinosylcytosine and azidothymidine (Majumdar et al. 2004).
Bile acid transporters
Bile acids are synthesized in the liver and secreted into the duodenum after ingestion of a meal, to facilitate the digestion and absorption of fats. Approximately 90% of the bile acids that are secreted into the small intestinal lumen are recycled back to the liver to prevent the continuous re-synthesis of large amounts of bile acids. Re-absorption of bile acids occurs by passive diffusion in the jejunum, but by active transport in the ileum. The Na+ -dependent bile acid active transport carriers are located in the apical membrane of the epithelial cells of the ileum and the Na+ gradient required is provided by Na+/K+ ATPase located in the basolateral cell membrane. The strategy to enhance drug absorption via this active transporter system involves formation of bile acid-drug conjugates. It was shown that the size of the molecule conjugated to the bile acid plays an important role in its ability to be absorbed via the bile acid transporters. Another challenge to be overcome, before this strategy can be used for drug absorption, is to avoid biliary secretion of the conjugates back into the gastrointestinal lumen. It seems that if the drug is not released from the conjugate before reaching the liver, it will most probably be secreted into the bile (Hidalgo and Li, 1996).
Monocarboxylic acid transporters
The transport of lactic acid, which is produced during the metabolic reactions to generate ATP, into and out of cells is mediated by H+ -dependent monocarboxylic acid transporter family. In addition, monocarboxylate drugs such as valproic acid, salicylic acid and pravastatin have been shown to be transported by monocarboxylic acid transporters in the intestine. However, because the retinal pigmented epithelium expresses a monocarboxylic acid transporter, pro-drugs targeted at these transporters may be useful in enhanced retinal drug permeation to achieve higher drug concentrations in the deeper layers of the cornea and aqueous humor (Lee, 2000; Majumdar et al. 2004).
Miscellaneous (glucose, fatty acid, vitamin, organic cation and phosphate) transporters
Two types of transporters exist for the transport of monosaccharides across biological membranes, these include the sodium-dependent Na+/glucose co-transporters (SGLT) and sodium-independent glucose transporters (GLUT). Because SGLT1 exhibits a high capacity and broad substrate specificity, targeting this receptor for drug delivery offers an exciting opportunity to improve the bioavailability of drugs (Steffansen et al. 2004).
Three types of fatty acid transporter proteins have been identified of which FATP4 is located in the apical membrane of the small intestine with long chain fatty acids as substrates (e.g. myristate, oleate and palmitate). Not much information is currently available on these transporters and further investigation is needed to determine their usefulness in drug delivery (Steffansen et al. 2004).
Although transporters for uptake of water-soluble vitamins are expressed in the intestine such as those for vitamin C (ascorbic acid) and biotin, their general low capacities make them poor transporter candidates to target for enhancement of drug absorption (Steffansen et al. 2004).
Many drugs that carry a positive charge at physiological pH values (e.g. antihistamines) are transported by the organic cation transporters. Phosphate transporters hold some potential for the delivery of drugs and fosfomycin as well as foscarnet have been shown to be substrates for these transport carrier systems (Majumdar et al. 2004).
P-glycoprotein efflux transporters
P-glycoprotein (P-gp), an MDR1 gene product, is the most extensively studied member of the superfamily of ATP-binding cassette (ABC) transporters. P-gp is associated with multi-drug resistance (MDR) in cancer cells, which is responsible for failure of chemotherapy with many drugs. Although P-gp is over-expressed in tumors, it is also localized in several tissues, particularly in the columnar epithelial cells of the lower gastrointestinal tract, capillary endothelial cells of the brain and testis, canalicular surface of the hepatocytes and on the apical surface of the proximal tubules in the kidney. Clinically, this efflux transporter plays an important role in the absorption, disposition, metabolism and excretion of a variety of drugs. It constitutes a formidable barrier against drug absorption by limiting drug uptake from the intestinal lumen into the systemic circulation. Furthermore, it pumps drug molecules out from hepatocytes into the canalicular system, prevents distribution of drugs to the brain and restricts re-absorption of drug into the systemic circulation from renal tubules (Katragadda et al. 2005; Ambudkar et al. 2006; Varma et al. 2006).
The hypothesis that inhibition of P-gp improves the bioavailability of drugs that are substrates for this efflux transporter is gaining widespread recognition. Moreover, the pharmacokinetic advantages of P-gp inhibition includes improved efficacy of chemotherapeutic agents, enhanced intestinal absorption and reduced clearance. Oral co-administration of the P-gp inhibitor, verapamil, has demonstrated an increase in the peak plasma level and volume of distribution as well as a prolonged half-life of doxorubicin. However, these first generation P-gp inhibitors pose a pharmacological effect themselves and therefore possible toxic and or other unwanted effects may occur. This has led to the design of second and third generation P-gp inhibitors with the potential to enhance the absorption of P-gp substrates without undesirable pharmacologic or toxic effects (Varma et al. 2003).
Examples of pro-drugs that target an active transporter and simultaneously decrease the substrate’s interaction with P-gp are the dipeptides derivatives of saquinavir, namely l-valine-l-valine-saquinavir and l-glycine-l-valine-saquinavir. These dipeptides pro-drugs that target peptide transporters and diminish interaction with P-gp exhibited an overall increased transport from the apical to basolateral side in Caco-2 cell monolayers. This example shows the potential of rational pro-drug design to decrease P-gp mediated efflux and thereby increase the absorption of drugs that are substrates for this efflux pump (Majumdar and Mitra, 2006).
Site of Absorption
Site-specific absorption occurs in the gastrointestinal tract because of differences in the composition and thickness of the mucus layer, pH, surface area and enzyme activity (Hamman et al. 2005). Furthermore, the physicochemical properties of the drug not only influence the site of absorption but also the mechanism of absorption. Despite these differences the most important site for intestinal drug absorption is the small intestine (Lacombe et al. 2004, Masaoka et al. 2006). In general, drug permeability is accepted to be higher in the upper region of the gastrointestinal tract compared to the lower parts (Masaoka et al. 2006). Timing of drug delivery is therefore important for optimized absorption and in diseases that are related to the circadian rhythm such as asthma and rheumatoid arthritis (Weidner, 2001).
In a recent study by Masaoke et al. (2006), various factors that may contribute to the regional absorption of drugs from the intestine were studied. They concluded that the epithelial surface area should not be a determining factor in drug absorption for highly permeable drugs in the different regions of the gastrointestinal tract. In contrast, the effects of the mucus layer and fluidity of the cell membrane of the different regions were found to contribute to dissimilarities in intestinal drug permeability. Regional membrane fluidity decreased from the upper to the lower parts of the gastrointestinal tract. Atenolol, a drug with low permeability, was observed to be absorbed in the middle and lower portions of the jejunum, while highly permeable drugs such as antipyrine and metoprolol were generally absorbed in the upper part of the intestine and also possibly in the stomach. The drug permeability of griseofulvin and naproxen was higher in the colon compared to the jejunum. It was found that removal of the mucus layer of the jejunum significantly enhances griseofulvin absorption to almost the same levels as those observed in the ileum and colon. They concluded that the main factors affecting drug absorption are membrane permeability, luminal drug concentration and residence time in the different parts of the gastrointestinal tract, while regional pH differences are specifically important for poorly permeable drugs.
Upper gastrointestinal delivery
The stomach is responsible for initial digestion, temporary food storage and controlled release of the resulting chime into the duodenum. The small surface area and short residence time in the stomach limits gastric absorption, however, gastric retentive systems can be used for local action in the stomach (e.g. antacids, misoprostol, antibiotics for Helicobacter pylori), absorption of drugs in the stomach and upper small intestine (e.g. l-DOPA, p-aminobenzoic acid, furosemide, riboflavin and flavin mononucleotide), drugs that are unstable in the intestine and colon (e.g. captopril and ranitidine) or for drugs that exhibit low solubility at high pH values (e.g. diazepam, chlordiazepoxide and verapamil). Gastric retention is not desirable when drugs cause gastric irritation (e.g. non-steroidal anti-inflammatory drugs), are unstable in the acid pH of the stomach or for drugs that exhibit significant first-pass liver metabolism (e.g. nifedipine) (Streubel et al. 2006).
Various formulation techniques have been used to achieve gastric retention, including bioadhesive systems, gastric swellable systems, density controlled systems that float or sink in gastric fluid and magnetic systems that require positioning of an external magnet. Each of the above techniques face their own challenges such as the high turnover rate of gastric mucus for bioadhesive systems, the low-density floating systems are dependent on the fluid volume in the stomach and magnetic systems require accurate external magnet positioning that patients may not be able to comply with (Bardonnet et al. 2006; Streubel et al. 2006).
Enteric coating
Enteric coating is employed to delay release of the active ingredient until it reaches the small intestine. This coating technique has been used to release drugs in the small intestine such as aspirin in order to reduce gastric irritation and erythromycin that exhibits acid degradation. Various polymers have been used as enteric polymers that become “soluble” once the pH of the environment reaches the range between 5 and 7. Polymers that degrade above a pH of 7 have been used in an attempt to target colonic drug delivery in diseases such as colitis (Gibaldi, 1984). However, the use of enteric coating to obtain colonic delivery has been reported to be less successful (Basit et al. 2004).
Magnesium chloride is an example of a compound that is prone to gastric irritation due to excessive formation of hydrochloric acid in the stomach when formulated into immediate release products. Magnesium is actively absorbed from the small intestine (Reynolds, 1993) and attempts have been made to target this area by means of enteric coating. The targeting of the proximal regions of the small intestine by enteric coating has, however, been criticized because release of the active ingredient may only occur 1–2 hours after expulsion from the stomach (Basit et al. 2004). This suggests release of the drug in the distal parts of the small intestine. Another potential drawback is that enteric coated tablets may be retained in the stomach for an extended period of time when taken with a heavy breakfast (Friend, 2005).
Ranitidine was used as a model drug to investigate differences in the bioavailability when administered in the form of immediate release, enteric coated and colon targeted delivery systems. The absolute mean bioavailability of ranitidine was found to be statistically similar for the immediate and enteric coated formulations, while it was much lower for the colonic release formulation. This was despite the fact that effective colonic release was demonstrated which was achieved by using a mixture of amylose and ethylcellose. Amylose is susceptible to degradation by amylase producing bacteria that reside in the colon (Basit et al. 2004). In this case the poor colonic bioavailability of ranitidine was ascribed to colonic bacterial metabolism (Friend, 2005).
Colonic delivery
Targeting of the colon as a site of absorption has recently received attention by various authors because of its favorable properties particularly for the absorption of peptide drugs, proteins and biotechnical molecules (Weidner, 2001, Gazzaniga et al. 2006). Some of the advantages of colonic delivery for these types of drugs include the reduced concentration of enzymes such as peptidases that degrade peptide drugs, the colon is a site with significant absorption due to the long residence time in this part of the gastrointestinal tract, it exhibits enhanced sensitivity to absorption enhancers, demonstrates natural absorptive characteristics and the abundance of lymphoid tissue follicles may be responsible for macromolecule uptake (Weidner, 2001, Hamman et al. 2005, Gazzaniga et al. 2006). The colon has been used as a target for treatment of conditions that affect this part of the gastrointestinal tract such as ulcerative colitis, Crohn’s disease and adenocarcinoma (Weidner, 2001, Gazzaniga et al. 2006).
Colonic targeting has been studied using various formulation techniques such as reservoir systems with rupturable-, erodible-, diffusive polymeric coats, release controlling polymeric plugs and osmotic systems. Currently only micro flora-, pH dependent pressure- and time controlled technologies are available on the market. Potential problems associated with some of the above systems include intra- and inter-subject intestinal pH variability, physiological fluctuation and disease conditions. Micro flora imbalances due to diet and habit changes are also a cause for concern when targeting the colon particularly when using systems based on metabolism of coatings for colonic delivery. Most of these disadvantages can be circumvented by using time-controlled systems (Gazzaniga et al. 2006), however in patients with irritable bowl syndrome intestinal transit times can vary from those observed in healthy subjects and patients with ulcerative colitis commonly experience diarrhea (Friend, 2005).
Specialized delivery systems
Superporous hydrogels and composites thereof have been described for use in specialized systems designed for the delivery of peptide drug. They swell very quickly and mechanically interact with intestinal membranes at the specific site of absorption. The lag time provided by the system enables drug release from the core to achieve optimal absorption (Dorkoosh et al. 2001).
Other specialized dosage forms include particulate systems that are designed to protect the drug against enzymatic degradation and to provide a high transfer rate of drug across the epithelial mucosa. Some particulate systems are capable of being taken up through Peyer’s patches without addition of absorption enhancers. These systems include nanoparticles, liposomes, microspheres and lipid based systems. Despite increased oral peptide delivery with modified liposomes, solid particles appear to be more effective for the delivery of hydrophilic macromolecules (Morishita and Peppas, 2006).
Nano-sized particles such as chitosan-coated nanoparticles have illustrated limited success as peptide delivery systems. These systems have also been linked to ligands to target specific absorption carriers, however, these types of systems still have serious problems with the manufacturing process and safety issues such as accumulation of the carrier in tissues (Hamman et al. 2005, Morishita and Peppas, 2006).
Examples of marketed drug products and drugs under investigation are listed in Table 1.
Table 1.
Some examples of drug products on the market associated with targeting the site of drug delivery (Lund, 1994; Friend, 2005).
| Drug | Formulation | Trade name |
|---|---|---|
| Mesalamine | Eudragit-S/-L coated tablets | Asacol®/Salofac® |
| Budesonide | Eudragit-L coated beads | Entocort® |
| Acetazolamide | Sustained release capsules | Diamox SA® |
| Aminophyline | Matrix tablets | Pecram® |
| Aspirin | Enteric coated tablets | Caprin® |
| Carbamazepine | Modified release tablet | Tegretol retard® |
| Diclofenac | Enteric coated tablets | Voltaren® |
References
- Ambudkar SV, Kim IW, Sauna ZE. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur J Pharm Sci. 2006;27:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J. Pharm. Sci. 1993;82(10):979–87. [PubMed] [Google Scholar]
- Aungst BJ. Intestinal permeation enhancers. J. Pharm. Sci. 2000;89(4):429–42. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Flanagan PR, Cluett J, et al. Transferrin receptors in the human gastrointestinal tract. Gastroenterology. 1986;91:861–9. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- Bardonnet PL, Faive V, Pugh WJ, et al. Gastroretentive dosage forms: Overview and special case of Helicobacter Pilori. J. Control Release. 2006;111:1–18. doi: 10.1016/j.jconrel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Basit AW, Podczeck F, Newton JM, et al. The use of formulation technology to assess regional gastrointestinal drug absorption in humans. Eur J Pharm Sci. 2004;21:179–89. doi: 10.1016/j.ejps.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Cawley DB, Simpson DL, Hershman HR. Asiologlycoprotein receptor mediates the toxic effects of an asiolofetuin-diptheria toxin fragment A conjugate on cultured rat hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 1981;78:3383. doi: 10.1073/pnas.78.6.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68:375–81. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- De Aizpurua HJ, Burge HJ, Howe PA, et al. Oral Delivery System PCT/AU86/0299. 1986. [Google Scholar]
- De Aizpurua HJ, Russel-Jones GJ. Oral vaccination: Identification of classes of proteins which provoke response upon oral feeding. J Exp Med. 1987;167:440–51. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkoosh FA, Verhoef JC, Borchard G, et al. Development and characterization of a novel peroral peptide drug delivery system. J Control Release. 2001;17:307–18. doi: 10.1016/s0168-3659(01)00232-2. [DOI] [PubMed] [Google Scholar]
- Faulk WP, His BL, Stevens PJ. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980;II:390–2. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Féger J, Gil-Falcon S, Lamaze C. Cell receptors: definition, mechanisms and regulation of receptor-mediated endocytosis. Cell Mol Biol. 1994;40:1039–61. [PubMed] [Google Scholar]
- Frieden PM, Walus LR, Musso GF, et al. Anti-transferrin receptor antibody and antibody-conjugates cross the blood brain barrier. Proc Natl Acad Sci USA. 1991;88:4771–5. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DR. New oral delivery systems for the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57:247–65. doi: 10.1016/j.addr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fyfe JC, Ramanujam JC, Ramasamy KS, et al. Defective brush-border expression of Intrinsic Factor-Cobalamin receptor in canine inherited intestinal cobalamin malabsorption. J Biol Chem. 1991;266:4489–94. [PubMed] [Google Scholar]
- Gabor F, Bogner E, Weissenboeck A, et al. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv Drug Deliv Rev. 2004;56:459–80. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gazzaniga A, Marconi A, Sangalli ME, et al. Time-controlled oral delivery systems for colon targeting. Expert. Opin. Drug Deliv. 2006;3(5):583–97. doi: 10.1517/17425247.3.5.583. [DOI] [PubMed] [Google Scholar]
- Geuze JJ, Slot JW, Strou GJ. Intracellular site of asiologlycoprotein receptor-ligand uncoupling: double-label immunoelectronmicroscopy during receptor-mediated endocytosis. Cell. 1983;35:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Ghilzai MK. Advances in the delivery of large-size drug molecules. Innovations in Pharmaceutical Technology. 2004 Jun;:103–8. [Google Scholar]
- Gibaldi M. Gastrointestinal absorption—role of the dosage form in: Biopharmaceutics and clinical pharmacokinetics. 3rd ed. Lea and Febiger; Philadelphia: 1984. pp. 71–3. [Google Scholar]
- Gomez-Orellana I. Strategies to improve oral drug bioavailability. Expert Opin. Drug Deliv. 2005;2(3):419–33. doi: 10.1517/17425247.2.3.419. [DOI] [PubMed] [Google Scholar]
- Guéant JL, Masson D, Schohn H, et al. Receptor-mediated endocytosis of intrinsic factor-cobalamin complex in HT 29, a human colon carcinoma cell line. FEBS Lett. 1992;297:229–32. doi: 10.1016/0014-5793(92)80544-q. [DOI] [PubMed] [Google Scholar]
- Habberfield A, Jensen-Pippo K, Ralph L, et al. Vitamin B12-mediated uptake of recombinant therapeutic proteins from the gut. Nature Med. 1995 submitted. [Google Scholar]
- Habberfield A, Jensen-Pippo K, Ralph L, et al. Vitamin B12-mediated uptake of erythropoietin and granulocyte colony stimulating factor in vitro and in vivo. Int. J. Pharm. 1996;145:1–8. [Google Scholar]
- Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs. BioDrugs. 2005;19:165–77. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Hidalgo IJ, Li J. Carrier-mediated transport and efflux mechanisms in Caco-2 cells. Adv Drug Deliv Rev. 1996;22:53–66. [Google Scholar]
- Hunter J, Hirst BH. Intestinal secretions of drugs: the role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv Drug Deliv Rev. 1997;25:129–57. [Google Scholar]
- Iacopetta BJ, Morgan EH, Yeoh GCT. Transferrin receptors and iron uptake during erythroid cell development. Biochim Biophys Acta. 1982;687:204–10. doi: 10.1016/0005-2736(82)90547-8. [DOI] [PubMed] [Google Scholar]
- Jefferies AE, Brandon MR, Hunt SV, et al. Transferrin receptors on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Budda B, Anand A, et al. Role of efflux pumps and metabolising enzymes in drug delivery. Expert. Opin. Drug Deliv. 2005;2(4):683–705. doi: 10.1517/17425247.2.4.683. [DOI] [PubMed] [Google Scholar]
- KilPatrick DC, Pusztai A, Grant G, et al. Tomato lectin resists digestion in mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 1985;185:299–305. doi: 10.1016/0014-5793(85)80927-3. [DOI] [PubMed] [Google Scholar]
- King TP, Pusztai A, Grant G, et al. Immunogold localization of ingested kidney bean (Phaseolus vulgaries) lectin in epithelial cells of the rat small intestine. Histochem J. 1986;18:413–20. doi: 10.1007/BF01675333. [DOI] [PubMed] [Google Scholar]
- Kovar M, Strohalm J, Ulbrich K, et al. In vitro and in vivo effect of HPMA copolymer-bound doxorubicin targeted transferrin receptor to B-cell lymphoma 38C13. Drug Target. 2002;10:23–30. doi: 10.1080/10611860290007496. [DOI] [PubMed] [Google Scholar]
- Kursa M, Walker GF, Roessler V, Ogris M, et al. Novel shielded transferring-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. BioconjChem. 2003;14:222–31. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- Lacombe O, Woodley J, Solleux C, Delbos JM, et al. Localization of drug permeability along the rat small intestine, using markers of the paracellular, transcellular and some transporter routes. Eur J Pharm Sci. 2004;23:385–91. doi: 10.1016/j.ejps.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lee VHL. Membrane transporters. Eur J Pharm Sci. 2000;11:S41–50. doi: 10.1016/s0928-0987(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Lehr CM, Bouwstra JA, Kok W, et al. Bioadhesion by means of specific binding of tomato lectin. Pharmacol Res. 1992;9:547–53. doi: 10.1023/a:1015804816582. [DOI] [PubMed] [Google Scholar]
- Lennernäs H. Human intestinal permeability. J. Pharm. Sci. 1998;87(4):403–10. doi: 10.1021/js970332a. [DOI] [PubMed] [Google Scholar]
- Leonard TW, Lynch J, McKenna MJ, et al. Promoting absorption of drugs in humans medium chain fatty acid-based solid dosage forms: GIPET™. Expert. Opin. Drug. Deliv. 2006;3(5):685–92. doi: 10.1517/17425247.3.5.685. [DOI] [PubMed] [Google Scholar]
- Lindner J, Geczy AF, Russel-Jones GJ. Identification of the site of uptake of the E-coli heat labile enterotoxin, LTB. Scand J Immunol. 1994;40:564–72. doi: 10.1111/j.1365-3083.1994.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–93. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Lund W. The Pharmaceutical Codex. London: The Pharmaceutical Press; 1994. [Google Scholar]
- Majumdar S, Duvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv Drug Deliv Rev. 2004;56:1437–52. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Mitra A. Chemical modification and formulation approaches to elevated drug transport across cell membranes. Expert Opin. Drug Deliv. 2006;3(4):511–27. doi: 10.1517/17425247.3.4.511. [DOI] [PubMed] [Google Scholar]
- Masaoka Y, Tanaka Y, Kataoke M, et al. Site of absorption after oral administration: Assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur J Pharm Sci. 2006 doi: 10.1016/j.ejps.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery. Drug Discov Today. 2006;11:905–10. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Morris SA, Ahle S, Ungewickell E. Clathrin-coated vesicles. Curr Opin Cell Biol. 1989;1:684–90. doi: 10.1016/0955-0674(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Simister NE. Transcytosis. Cell. 1985;43:389–90. doi: 10.1016/0092-8674(85)90166-7. [DOI] [PubMed] [Google Scholar]
- Ni S, Stephenson SM, RJ Folate receptor targeted delivery of liposomal daunorubicin into tumor cells. Anticancer Res. 2002;22:2131–2135. [PubMed] [Google Scholar]
- Pauletti GM, Gangwar S, Knipp GT, et al. Structural requirements for intestinal absorption of peptide drugs. J Control Release. 1996;41:3–17. [Google Scholar]
- Pusztai A. Transport of proteins through the membranes of the adult gastro-intestinal tract — a potential for drug delivery. Adv Drug Deliv Rev. 1989;3:215–28. [Google Scholar]
- Qian ZM, Li H, Sun H, et al. Targeted drug delivery via transferring receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- Raso V, Basala M. A highly cytotoxic human transferrin-ricin A chain conjugate used to select receptor-modified cells. J Biol Chem. 1984;259:1143–49. [PubMed] [Google Scholar]
- Reynolds JEF. Martindale the extra pharmacopoeia. 30th ed. The Pharmaceutical press; London: 1993. Magnesium; p. 857. [Google Scholar]
- Russel-Jones GJ, De Aizpurua HJ. Vitamin B12: A novel carrier for orally presented antigens. Proc Int Symp Control Rel Bioact Mater. 1988;15:142–3. [Google Scholar]
- Russel-Jones GJ. Oral delivery of therapeutic proteins and peptides by the Vitamin B12 uptake system. In: Taylor MD, Amidon G, editors. Peptide-based drug design: Controlling transport and metabolism. ACS Books; Washington, DC: 1995. pp. 181–98. [Google Scholar]
- Russell-Jones GJ. The potential use of receptor-mediated endocytosis for oral drug delivery. Adv Drug Deliv Rev. 2001;46:59–73. doi: 10.1016/s0169-409x(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Schohn H, Guéant JL, Girr M, et al. Synthesis and secretion of a cobalamin-binding protein by HT 29 cell line. Biochem J. 1991;280:427–30. doi: 10.1042/bj2800427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Wan J, Ekrami H. Enhancement of polypeptide and protein absorption by macromolecular carriers via endocytosis and transcytosis. Adv Drug Deliv Rev. 1992;8:93–113. [Google Scholar]
- Simons K, Fuller SD. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–48. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Steffansen B, Nielsen CU, Brodin B, et al. Intestinal solute carriers: an overview of trends and strategies for improving oral drug absorption. Eur J Pharm Biopharm. 2004;21:3–16. doi: 10.1016/j.ejps.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Steffansen B, Nielsen CU, Frokjaer S. Delivery aspects of small peptides and substrates for peptide transporters. Eur J Pharm Biopharm. 2005;60:241–5. doi: 10.1016/j.ejpb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Steubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 2006;3(2):217–33. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- Varma MVS, Perumal OP, Panchagnula R. Functional role of P-glycoprotein in limiting peroral drug absorption: optimizing drug delivery. Curr Opin Chem Biol. 2006;10:367–73. doi: 10.1016/j.cbpa.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Varma MVS, Ashokraj Y, Dey CS, et al. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Vyas SP, Sihorkar V. Endogenous carriers and ligands in non-immunogenic site-specific drug delivery. Adv Drug Deliv Rev. 2000;43:101–164. doi: 10.1016/s0169-409x(00)00067-3. [DOI] [PubMed] [Google Scholar]
- Wagner E, Zenke M, Cotten M, et al. Transferrin-polycation conjugates as carriers for DNA uptake into cells. used to select receptor-modified cells. Proc Natl Acad Sci USA. 1990;87:3410–14. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Shen WC. Transcytosis of transferring is enhanced by monensin in filter grown MDCK epithelial cells. J. Cell Biol. 1990;111:83a. [Google Scholar]
- Wan J, Shah D, Shen WC. Brefeldin A (BFA) induces missorting of transferrin receptors (TfR) and increases receptor-mediated transcytosis of transferrin (Tf) in filter grown MDCK cells. J. Cell Biol. 1991;115:259a. [Google Scholar]
- Ward CM. Folate-targeted non-viral DNA vectors for cancer gene therapy. Curr Opin Mol Ther. 2000;2:182–187. [PubMed] [Google Scholar]
- Weidner J. Drug delivery. Drug Discov. Today. 2001;6(19):1028–9. doi: 10.1016/s1359-6446(01)01940-7. [DOI] [PubMed] [Google Scholar]
- Woodley JF, Naisbett B. The potential for delaying the gastro-intestinal transit of drugs. Proc Int Sym Control Rel Bioact Mater. 1988;15:125–126. [Google Scholar]
- Xu L, Tang WH, Huang CC. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFz. Mol Med. 2001;7:723–34. [PMC free article] [PubMed] [Google Scholar]
- Zhang EY, Phelps MA, Cheng C, et al. Modeling of active transport systems. Adv Drug Deliv Rev. 2002;54:329–54. doi: 10.1016/s0169-409x(02)00007-8. [DOI] [PubMed] [Google Scholar]