Abstract
Trypanosomiasis and leishmaniasis are two debilitating disease groups caused by parasites of Trypanosoma and Leishmania spp. and affecting millions of people worldwide. A brief outline of the potential targets for rational drug design against these diseases are presented, with an emphasis placed on the enzyme trypanothione reductase. Trypanothione reductase was identified as unique to parasites and proposed to be an effective target against trypanosomiasis and leishmaniasis. The biochemical basis of selecting this enzyme as a target, with reference to the simile and contrast to human analogous enzyme glutathione reductase, and the structural aspects of its active site are presented. The process of designing selective inhibitors for the enzyme trypanothione reductase has been discussed. An overview of the different chemical classes of inhibitors of trypanothione reductase with their inhibitory activities against the parasites and their prospects as future chemotherapeutic agents are briefly revealed.
Keywords: Trypanothione, glutathione, Chagas disease, sleeping sickness, rational drug design
Introduction
Trypanosomiasis and leishmaniasis are among the major debilitating and devastating tropical diseases that are targets for the World Health Organization’s special program Research and Training in Tropical Diseases (TDR) (Hyde, 1990) and most recently the Drugs for Neglected Diseases initiative (DNDi, www.dndi.org). These diseases are caused by the parasites of the genus Trypanosoma and Leishmania. The present review focuses on the major human diseases caused by trypanosomal and leishmanial infections and the potential targets for designing chemotherapeutic agents against these diseases with special emphasis on trypanothione reductase. Table 1 gives an outline of the major human trypanosomiasis and leishmaniasis with their global annual disease burdens (as of 1999) in terms of disability adjusted life years (DALY).
Table 1.
The major trypanosomiasis and leishmaniasis, causative agents, their global burdens in terms of Disability Adjusted Life Years (DALY) and current treatments.
| Disease | Causative agents | DALY* (million/year) | Current Treatments |
|---|---|---|---|
| African trypanosomiasis or sleeping sickness | Trypanosoma brucei gambiense and T. b. rhodesiense | 1.2 | Suramine, pentamidine, DFMO, tryparsamide |
| American trypanosomiasis or Chagas disease | T. cruzi | 0.6 | Benznidazole, nifurtimox |
| Visceral leishmaniasis or kalazar | Leishmania donovani | 1.7 | Pentostam, glucantime, |
| Dermal leishmaniasis or tropical sore | L. major, L. tropica, L. braziliensis, L. mexicana | aminosidine |
Taken from World Health Report 1999, publ. World Health Organization Geneva (1999).
Suramine (1) and pentamidine (2) are useful drugs for treating Human African Trypanosomiasis (HAT) during early infection, but being highly charged, cannot cross the blood brain barrier and are of no use for late stage infection with involvement of central nervous system (CNS) with either Trypanosoma brucei gambiense or T. b. rhodesiense. Melarsoprol (3), a trivalent arsenical, or tryparsamide (4) is then used. Difluoromethylornithine (DFMO, 5) is effective in treatment of HAT, but is not very effective against rhodesiense sleeping sickness where large doses must be used, resulting in significant side-effects, including bone marrow suppression (Meshnick, 1984; Neva and Brown, 1994). Nifurtimox (6), a nitrofuran derivative, is the best drug currently available for treating Chagas disease but is still considered investigational. It is thought to kill trypanosomes selectively through futile cycling by the formation of hydrogen peroxide and toxic oxygen species (Docampo and Stoppani, 1979; Le Trant et al. 1983; Docampo and Moreno, 1984a and b, 1986; Neva and Brown, 1994). Benznidazole (7) is of equivalent effectiveness and is used in South America in the acute stage. Both drugs must be given for several months and are associated with severe side effects (Neva and Brown, 1994). Recently, the present and future prospects of chemotherapy of HAT, in addition to the possible mode of action and the mechanism of resistance of the current chemotherapeutic agents have been reviewed (Fairlamb, 2003a).
Pentavalent antimonials e.g. pentostam (8) is the recommended treatment for visceral leishmaniasis but are toxic and developed resistance (Croft, 1988; Grogl et al. 1992). Relapse of disease or only partial response is more common in Kenya, the Sudan, and India than in Mediterranean or Latin American kala azar, where a second or longer course of treatment is often needed.
Overall, the demand for chemotherapeutic agents for the treatment of Chagas disease, sleeping sickness and kala azar is desperate. Those needing treatment are mainly in impoverished rural and urban communities with poor housing and limited access to medical attention, and in countries where basic healthcare infrastructures have yet to be developed. Approved chemotherapies that are available were developed in the first half of the last century (suramine, pentamidine, arsenicals and antimonials); some would fail today’s more stringent standards for drug safety. Given the initial success of a largely empirical approach, progress in drug development in recent years has been poor and, undoubtedly there is great need for new less toxic treatments for human diseases by parasitic trypanosomes and leishmanias. In view of the economies of the Third World countries suffering most from these diseases, such drugs will have to be cheap and simple to administer.
Potential Chemotherapeutic Targets for Trypanosomiasis and Leishmaniasis
In this genomic, proteomic, and bioinformatics era of target identification, scores of potential targets for antitrypanosomal and antileishmanial chemotherapy will be emerging. In the pre-genomic era, basic research in molecular biology and multidimensional research initiatives has identified some biological features associated with the development of trypanosomiasis that have been well documented and studied extensively. Ergosterol biosynthesis, parasite specific proteases and reductases, purine salvage and phospholipid biosynthesis (for review see Urbina, 2003) could be turned into targets, provided that the following two requirements can be met: (a) the target must be essential for the survival of the parasites, and (b) the target must be such that a counterpart in the mammalian host either does not exist or is sufficiently different to allow selective inhibition (Wang, 1995). The technological advancements related to post-target selection are also important criteria. Because the target must be suitable for study at the molecular level that include most importantly the high throughput screening for which convenient, cheap and sensitive assay methods are highly desirable.
Glycolytic pathway
As trypanosomal cells are completely energetically dependent on glycolysis it might be used to develop new trypanocidal drugs (Michels, 1988). Compertmentation of glycolytic enzymes is generally assumed to result in an enhancement of the rate of glycolysis in bloodstream trypanosomes, approximately 50-times higher than that in mammalian cells (Brohn and Clarkson, 1980). This appears to be necessary to compensate for poor yields of energy. Trypanosomes need to replicate every 6 to 8 h in mammalian blood and Varian surface glycoproteins (VSGs) must be replaced frequently to evade a host immune response (Donelson and Rice-Ficht, 1985). Thus it has been suggested that inhibition of any one of the glycolytic enzymes inside the glycosomes may block glycolytic activity and kill bloodstream trypanosomes (Clarkson and Brohn, 1976; Michels, 1988). The three dimensional (3D) structure of T. brucei glycosomal triosephosphate isomerase (TIM), determined at 2.4 Å resolution, was found to be very similar to that of mammalian TIM (Wierenga et al. 1987). The 3D structure of glycosomal glyceraldehyde-3-phosphate dehydrogenase (GADPH) (Vellieux et al. 1993) could provide opportunities for designing selective inhibitors as it differs from the mammalian homolog (Verlinde et al. 1994; Wang, 1995). Bloodstream T. b. brucei imports glucose by facilitated diffusion and the uptake of glucose apparently represents the rate-limiting step in glycolysis. The genes encoding trypanosomal glucose transporters are tandemly arranged in a multigene family consisting of two homologous groups, trypanosome hexos transporter (THT)1 and THT2. THT1-encoded glucose transporters, preferentially expressed in a bloodstream form, have a moderate sensitivity to cytochalasin B and recognize D-fructose as substrate, thereby distinguishing them from the human erythrocyte glucose transporter. They are potential targets for antitrypanosomal chemotherapy (for review, see Wang, 1995).
DNA topoisomerases
Many of the established antiprotozoal agents are known to bind to DNA. There are two potential sites for DNA binding in members of the kinetoplastida: nuclear and kinetoplast DNA. In general, DNA binding agents would be expected to be active against protozoa, but toxicity is a major factor. It was assumed that binding to DNA leads directly to inhibition of DNA-dependent processes, but it is now generally accepted that intercalating agents induce topoisomerase II – mediated strand breaks in DNA (Brown, 1987). Trypanosomal topoisomerase II inhibitors affect both nuclear and mitochondrial DNA and may prove to be effective and safe antitrypanosomal drugs (Shapiro, 1993) as they differ structurally from mammalian topoisomerase II (Shapiro and Showalter, 1994). DNA topoisomerase I could also serve as an intracellular target, as its inhibition can cause DNA-cleavage and ultimate death of trypanosomes (Bodley et al. 1995).
Ergosterol biosynthesis
Ergosterol biosynthesis is a novel metabolic pathway essential for parasitic survival lacking a counterpart in the host. Several enzymes of this pathway, e.g. squalene synthase, fernesylpyrophosphate synthase are capable of depleting endogenous sterols, and therefore represent viable chemotherapeutic targets (for review, see Linares et al. 2006).
Purine salvage pathway
Some striking differences between parasites and their mammalian host are apparent in purine metabolism. Unlike their mammalian host, most parasites lack the de novo purine biosynthetic mechanisms and rely on salvage pathways to meet their purine needs. There are sufficient distinctions between enzymes of the purine salvage pathway in host and parasite that can be exploited to design specific inhibitors or “subversive substrates” for the parasitic enzymes. Furthermore, the specificities of purine transport, the first step in purine salvage, differ significantly between parasites and their mammalian host to allow selective inhibitor design (for review see El Kouni, 2003).
Polyamine biosynthesis
The ability to synthesize polyamines (Fig. 2) is vitally important for the proliferation of bloodstream HAT in an environment deficient in polyamines. As shown in Figure 2, ornithine decarboxylase (ODC), S-adenosyl-L-methionine decarboxylase (SAMDC) and spermidine synthetase in trypanosomes serve crucial functions (Fairlamb and Bowman, 1980) and may be potential targets for antitrypanosomal chemotherapy. Little is known about trypanosomal SAMDC except that it did not cross-react with human SAMDC antiserum (Tekwani et al. 1992). Detailed comparison of mammalian and trypanosomal SAMDCs have not yet been done nor have crystal structure and amino acid sequence been determined, steps important for designing drugs active against this enzyme.
Figure 2.
Metabolism and function of trypanothione, showing possible sites of action of trypanocidal compounds. The insert above illustrates the futile redox cycling by nitro compounds (RNO2) to form hydrogen peroxide (H2O2) and hydroxyl radicals (OH•). Abbreviations: BSO, buthionine sulfoximine; DFMO, difluoromethylornithine; R-As=O, melarsen oxide; Mel T, melarsen trypanothione adduct; PUT, putrescine; SPD, spermidine; dSAM, decarboxylated S-adenosylmethionine; MTA, methylthioadenosine (modified from Krauth-Siegel et al. 1987).
Trypanothione is a conjugate of glutathione and the polyamine spermidine. This polyamine component of the structure of trypanothione disulfide (T[S]2) rationalized the actions of several antitrypanosomal and antileishmanial drugs. For example, DFMO (5), the first new drug licensed to treat HAT for over 50 years, inhibits ODC, which catalyzes the initial step in polyamine biosynthesis (Fig. 2), decreasing the trypanothione pool. The origin of the trypanocidal effect of DFMO has not been established (Fairlamb and Cerami, 1992).
Trypanothione reductase (TR) and the synthetic enzyme of trypanothione metabolism found in trypanosomatids exemplify unique features of the organisms. As shown in Figure 2, trypanothione synthesis proceeds from glutathione; the similar redox potentials allow for nonenzymatic thiol-disulfide exchange reactions to occur (Fairlamb and Cerami, 1992). Considerable advances have been made in characterization and inhibitor design against TR. The key enzyme of the two-step trypanothione biosynthesis, glutathionyl spermidine synthetase, was isolated, partially sequenced and kinetically analyzed (Koenig et al. 1997). The utilization of trypanothione for the reduction of hydroperoxides has remained a matter of debate. A trypanothione peroxide activity, presumed to substitute for the glutathione peroxide activity typical of host metabolism (Flohé, 1989), was observed in crude extracts of various trypanosomatids (Henderson et al. 1987a). Consistent failures to isolate putative trypanothione peroxidases, and substantial spontaneous reaction rates between trypanothione and hydrogen peroxide, led to the conclusion that trypanothione-dependent peroxide metabolism may represent a non-enzymatic event (Carnieri et al. 1993). Recent discoveries have demonstrated (Nogoceke et al. 1997) that the reduction of peroxides by trypanothione is an enzymatic process, but in contrast to previous expectations, is catalyzed by two distinct proteins in concert (Flohé, 1998).
Lipoamide dehydrogenase (LipDH) is another flavoprotein dependent enzyme, which has been discussed as a target molecule for antitrypanosomal therapy. In T. cruzi, an organism highly susceptible to oxidative stress, LipDH participates in the redox cycling of nifurtimox, one of the most effective anti-Chagas agents (Krauth-Siegel and Schöneck, 1995).
Trypanothione Reductase as a Chemotherapeutic Target
In the pre-genomic era investigation of mode of action of arsenical drugs and glutathione biosynthesis inhibitor, buthionine sulfoximine (BSO), gave rise to the discovery of trypanothione, unique to trypanosomes and absent from the mammalian cells (Fairlamb et al. 1985). In mammals, potential redox damage meets the glutathione (GSH)-based system as a first defense, during the course of which glutathione disulfide (GSSG) is formed (Equation 1).
Regeneration of protective GSH from GSSG is catalyzed by GR. In trypanosomes and leishmanias an analogous system has evolved (Fairlamb and Cerami, 1992) insofar as the disulfide (T[SH]2) differs from GSSG by the presence of a spermidine cross-link between the two glycyl carboxyl groups (compare GSSG and T[S]2). The enzyme TR reduces T[S]2 to dithiol T[SH]2 in a manner analogous to GR (Fig. 3). With this discovery of a fundamental metabolic difference, TR was proposed and amplified further as a target for the rational design of antitrypanosomal and antileishmanial drugs (Fairlamb et al. 1985; Shames et al. 1986; Benson et al. 1992; Hunter et al. 1992; Schirmer et al. 1995).
Figure 3.
Outline of glutathione and trypanothione based redox defences.
Unlike human hosts, trypanosomes contain TR instead of analogous enzyme GR to process their cognate substrates trypanothione and glutathione, respectively. Parasite TR does not process GSSG and host GR does not reduce T[S]2 (Shames et al. 1986; Krauth-Siegel et al. 1987). Selective inhibitor design is probable, due to the mutually exclusive recognition and rejection of cognate substrates between host and parasite (Shames et al. 1986; Krauth-Siegel et al. 1987; Schirmer et al. 1995). Efficient selective blockade of TR would be expected to compromise the redox defences of the parasites, increasing their sensitivity to redox-damage based drugs, e.g. nifurtimox. A TR inhibitor might be expected to be drug in its own right or for co-administration with a redox-active drug e.g. nifurtimox. The later case may even provide synergy, allowing use of lowered doses of the redox drug (Chan et al. 1998).
TR is a member of the large well-characterized protein family of FAD-dependent NADPH oxido-reductases (reviewed in Williams, 1992) and share close structural and mechanistic similarities with that of GR (summarized in Table 2). It is a dimeric protein of monomer molecular mass 52kDa, providing FAD-binding, NADPH-binding, central and interface domains. There are two identical active sites, formed by residues of the FAD, NADPH, and central domains of one monomer and the interface domain of the other (Fig. 4).
Table 2.
Properties of trypanothione reductase and glutathione reductase; taken from literature (Williams et al. 1992).
| Property | Trypanothione reductase | Glutathione reductase | ||||
|---|---|---|---|---|---|---|
| C. fasciculata | L. donovani | T. cruzi | T. congolense | E.coli | Human | |
| Flavin | FAD | n.d. | FAD | FAD | FAD | FAD |
| Pyridine dinucleotide | NADPH | NADPH | NADPH | NADPH | NADPH | NADPH |
| Subunit Mr (Da) | 54000 | 52940 | 53900 | 53400 | 48700 | 52500 |
| Amino acids/subunit | 491 | 491 | 492 | 492 | 450 | 478 |
| Oligomeric structure | dimer | dimer | dimer | dimer | dimer | dimer |
| Eox, λmax (nm) | 464 | 463 | 461 | 464 | 462 | 460 |
| ɛ0 at λmax (mM−1cm−1) | 11.3 | 11.5 | 11.2 | 10.6 | - | 11.3 |
| charge transfer in EH2 | yes | yes | yes | yes | yes | yes |
| ɛ0 at λ530 (mM−1cm−1) | 3.63 | 4.2 | 4.9 | 3.7 | - | 4.5 |
| Km (μM) trypanothione | 53 (51, 58) | 36 | 45 (55, 50) | 31 (18) | 2000 | |
| Glutathione | - | - | - | - | 66 (61, 70) | 65 |
| kcat (min−1) trypanothione | 31000 (286000) | 10760 | 14299 | 9600 | 6100 | 9.6 |
| glutathione | 3.1 | <2 | - | - | 44000 | 12600 |
| kcat/Km (M−1sec−1) | 9.8 × 106 | 5.0 × 106 | 5.3 × 106 | 5.2 × 106 | 6.2 × 106 | 3.1 × 106 |
| Kmapp (NADPH) | 7 | 9 | 5 | 5 | 16 (25) | 9 |
Figure 4.
Structure of trypanothione reductase with FAD, NADPH and trypanothione bound (modified from Bond et al. 1999).
Validating suitability of trypanothione reductase as a target
To validate TR as a viable target for rational drug design, inhibition of TR in vitro should correlate with an observable effect in vivo. This is a difficult test to satisfy unambiguously; biochemical and molecular biological attempts had been made to probe TR as a reasonable drug target. In absolute terms, disruption or deletion of the TR genes in parasites should be lethal. Over-expression of TR in transfected L. donovani promastigotes was not found to alter the sensitivity to hydrogen peroxide indicating that the regeneration of T[SH]2 from its disulfide after oxidative challenge is not the rate-determining feature of the defense system (Kelly et al. 1993). Over-expression of TR in T. cruzi led to gene rearrangements when antisense modulation of TR expression was attempted (Tovar and Fairlamb, 1996). Disruption of two of the TR alleles of Leishmania failed to produce a null mutant, and actually produced a third copy of TR gene by genomic rearrangement to a larger chromosome (Dumas et al. 1997). Down-regulation of TR using a trans-dominant mutation strategy demonstrated that even with only 15% residual TR activity, promastigote growth is still supported in culture (Tovar et al. 1998a). It was proposed that any rationally designed inhibitor of TR must attain >85% inhibition for activity as an antileishmanial species, but it was pointed out that such cells are more sensitive to oxidative insult, such as the oxidative burst of macrophages, and that clinically usable TR inhibitors may need to be as effective in vivo (Tovar et al. 1998a). Later it had been demonstrated that TR absence is incompatible with parasite survival and validated TR as a bonafide drug target. As it was not possible to obtain viable Leishmania devoid of TR catalytic activity, specific inhibitors of this enzyme are likely to be useful antileishmanial agents for chemotherapeutic use (Tovar et al. 1998b).
Sequence alignment of TRs and human GRs (hGRs) showed that relative to TR hGR has an N-terminal extension, a C-terminal truncation and several deletion and insertions throughout the sequence. The most striking regions of homology are 14-residue sequence containing the redox-active cysteine residues and the 10-residue sequence containing the active site histidine. Although the disulfide-binding site in TR in general rather closely resembles that for GSSG in GR, they have over 1000-fold selectivity for their cognate substrates, the molecular basis for which involves size, charge and hydrophobicity of their active sites. TR binding site is much wider in the outer region (~22 Å × 20 Å × 28 Å) due to different orientations of two helices in the FAD domains (Kuriyan et al. 1991). The TR active site is more hydrophobic and has an overall negative charge to attract its positively charged and more hydrophobic polyamine containing cognate substrate trypanothione and repel the negatively charged smaller host substrate glutathione. Conversely, the GR active site is smaller and positively charged, repelling the larger and positively charged trypanothione. The X-ray crystal structures of TRs from Crithedia fasciculata (Kuriyan et al. 1991; Hunter et al. 1992), and T. cruzi have been solved (Lantwin et al. 1994; Zhang et al. 1996). The crystal structure of TR in complex with trypanothione was solved (Bond et al. 1999) as was the crystal structure of TR complexed with the alternative substrate glutathionyl spermidine (Bailey et al. 1993) and with the weak, but selective, inhibitor mepacrine (Jacoby et al. 1996). As already pointed out, a convenient screening technology is highly desirable for a suitable substrate to expedite the drug discovery process. To this end, a convenient colorimetric plate assay suitable for automated high-throughput screening has been developed by Hamilton et al. (2003).
One disadvantage of selecting TR as a drug target for the development of broad-spectrum antiparasitics is that intracellular trypanothione concentrations vary from 0.3 mM in African trypanosomes to ~3.0 mM in Leishmania spp., >99% of which is in the reduced form. As soon as TR is inhibited, T[S]2 will start to accumulate due to continuing intracellular oxidant process. It is not currently known what T[S]2/T[SH]2 ratio is lethal to the parasite. It has been suggested that the design of competitive inhibitors with Ki-values in nM range will be required, and the design of irreversible inhibitors may represent a better strategy for drug development (Fairlamb, 2003b).
The following section will focus on recent discoveries of different classes of TR inhibitors, which demonstrated antiparasitic activity and promise of development of newer chemotherapeutic agents.
Rational drug design based on trypanothione reductase as a target
The homology modeling of TR (Benson et al. 1992) with that of hGR shed light on the structural aspects of the enzyme also in addition to its substrate-binding mode from a theoretical perspective. Studies with various alternative peptide substrates demonstrated that the spermidine moiety of trypanothione could be conveniently replaced with N,N-dimethylaminopropylamide (dmapa) without loss of substrate activity. Subsequently, a series of alternative γ-glutamyl-modified trypanothione substrates were synthesized. The discovery that a benzyloxy-carbonyl (Z) group represented a suitable replacement resulted in (Z.Cys.Gly.dmapa)2 as a convenient alternative assay substrate in which the Z group occupies a hydrophobic pocket near Phe396’ of TR (Henderson et al. 1987b; El-Waer et al. 1991, 1993a and b; Marsh and Bradley, 1997). This pocket was since named the Z-site, approximately enclosed by Phe396’, Pro398’ and Leu399’. This initial advancement in structural aspects of TR active site prompted the discovery of peptide inhibitors (Garforth et al. 1994; McKie et al. 2001; Chan et al. 2002). The first rationally designed non-peptide inhibitors are represented by the tricyclic ring structures that are competitive versus trypanothione (Benson et al. 1992). Progress has been made in discovering large classes of inhibitors of TR over recent years, which are also found to be lethal to parasites in vitro.
The tricyclic compounds and congeners
Molecular modeling approaches were useful in identifying tricyclic antidepressants including phenothiazines and related structures (e.g. 9, 10) and mepacrine (11) (Fig. 5) as competitive inhibitors of TR but not GR (Benson et al. 1992; Jacoby et al. 1996; Chan et al. 1998). The tricyclic moiety of these compounds were shown to lodge against the hydrophobic wall of TR active site formed by Trp21 and Met113, with the aminopropyl side chain pointing towards the Glu466’ and Glu467’ residues. The synthesis and biological evaluation of a series of dibenzazepine analogs (Garforth et al. 1997), N-acylpromazines, 2-substituted phenothiazines, and trisubstituted promazines failed to show any improvements over the parent leads (Chan et al. 1998).
Figure 5.
Structures and activities of tricyclic inhibitors of trypanothione reductase.
The antimalarial drug mepacrine (11), an acridine derivative, is also a competitive inhibitor of TR. The acridine ring also aligns to the hydrophobic wall of TR formed by Trp21 and Met113, but the alkylamino side chain point towards Glu18, unlike the tricyclics described above (Bonse et al. 1999). Sulfonamides and urea derivatives of mepacrine with varying methylene spacer lengths (e.g. 12, 13) were found to be superior inhibitors of TR relative to mepacrine with the best inhibitors being 40-fold more potent (Chibale et al. 2001). A series of synthetic 9,9-dimethylxanthene derivatives (e.g. 14) were shown to be competitive inhibitors of TR which are comparable to known tricyclic inhibitors of TR (Chibale et al. 2003).
It is important to note that as required in principle for an ideal drug target, inhibition of TR in vitro should also correlate with antiparasitic activity in vitro against T. brucei, T. cruzi and L. donovani. However, no strong correlation between TR-inhibitory activity and in vitro anti-parasitic activity was observed indicative of unfavorable pharmacokinetic profiles.
2-Aminodiphenylsulfides and its congeners
Modification of the central ring of the phenothiazines furnished the 2-aminodiphenylsulfides, termed ‘open chain chlorpromazines’. Using a microplate assay to screen TR inhibitors, this group of compounds was discovered to be potent competitive inhibitors (Fig. 6). Compound 15 was the best inhibitor in the preliminary series (Fernandez-Gomez et al. 1995). Based on these results and molecular modeling studies, a series of bis (2-aminodiphenylsulfides) were synthesized and compound 16 was shown to be the most potent in this series (Girault et al. 1997). Further improvement was evident with a newer generation of bis(2-aminodiphenylsulfides) (e.g. 17) corresponding to attachment of an additional hydrophobic side to an analog of compound 16. The large volume of the TR active site justified the introduction of an additional hydrophobic group to the end of the side chain (Girault et al. 2001).
Figure 6.
Structures of 2-aminodiphenylsulfides with their anti-TR activities.
All the compounds demonstrated antiparasitic activity in vitro at low micromolar ranges. However, no correlations between TR inhibition and in vitro antiparasitic activity were apparent.
Quaternary alkylammonium compounds
The quaternization of tertiary alkylamine ω-nitrogen atom of chlorpromazine by substituted benzyl and other aromatic or heterocyclic groups resulted the discovery of quaternary alkylammonium phenothiazines as a new class of linear competitive inhibitors of TR (Fig. 7) (Khan et al. 2000). The permanent positive charge on the distal nitrogen atom of the tricyclic’s side chain contributed to binding was estimated as ≥5.6 kcal.mol−1 by comparison with the analog with cationic nitrogen atom of the quaternary (18) replaced by an ether oxygen atom (19). The major contribution to improving Ki value and inhibition strength was accomplished by incorporating the hydrophobic N-benzyl substituents. The best inhibitor identified was compound 19, containing a 3, 4-dichlorobenzyl substituent (~2 orders of magnitude >chlorpromazine).
Figure 7.
Structures of quaternary alkylammonium compounds with their anti-TR activities.
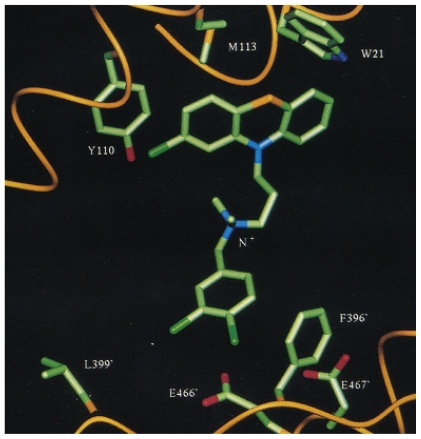
Detailed molecular modeling studies helped to establish docking orientations and energies by revealing involvement of: (i) the major hydrophobic pocket (Trp21, Met113, Tyr110), (ii) the “Z”-site (Phe396’, Pro398’, Leu399’) and (iii) the ionic interactions possible for the quaternary cationic nitrogen with nearby side chains of Glu466’ and Glu467’ (Fig. 8) (Austin et al. 1999, Khan et al. 2000). This “three point attachment”, a concept initially designed by Ogston (1948) to explain chiral specificity of enzymes, has allowed on average a 30-fold improvement of Ki values against TR (Khan et al. 2000).
Figure 8.
Compound 18 docked into active site of TR to show major interactions (taken from Austin et al. 1999).
Similarly, the quaternization of the side chain tertiary nitrogen atom of the ‘open chain chlorpromazines’ also showed improvements in inhibition up to 40-fold. The most potent compound synthesized from this series was the 3, 4-dichlorobenzyl analog (20) (Parveen et al. 2005). All analogs demonstrated strong inhibition, some in lower nM ranges, against the blood stream form of T. brucei. Antiparasitic activity was not solely determined by the inhibition strength against TR; a strong contribution from hydrophobicity was also observed. Although active against L. donovani, none showed major improvement in this activity relative to their parent compounds. Some of the analogs also showed improved inhibition against the amastigote stage of T. cruzi with ED50 values <1 μM (Khan et al. 2000; Parveen et al. 2005).
Polyamine derivatives
As shown in Scheme 1, the natural disulfide substrate of TR, T[S]2, differs from the analogous host’s GSSG only by the presence of a spermidine (a polyamine) cross-link between the two glycyl carboxyl groups. This discrimination between parasite and host substrate served as a criterion for developing polyamine derivatives as selective inhibitors of TR (O’Sullivan and Zhau, 1995; O’Sullivan et al. 1996, 1997; Baillet et al. 1996; Li et al. 2001; Bi et al. 2006). This approach led to the discovery of series of potent selective competitive inhibitors of TR, several selected compounds are included in Figure 9 (compounds 21, 22).
Scheme 1.
Glutathion and trypanothione.
Figure 9.
Structures and trypanothione reductase inhibitory activities of polyamine derivatives.
Solid phase synthesis approaches allowed for the expedient synthesis of polyamine-based focused libraries as potential TR inhibitors. Several potent inhibitors were identified with low nM activity (Orain and Bradley, 2001; De Luca et al. 2003).
Most of these compounds also displayed potent in vitro antiparasitic activity against T. brucei with ED50 values <1 μM (O’Sullivan et al. 1997; Li et al. 2001; Bi et al. 2006). These preliminary antiparasitic activity in vitro are suggestive of their TR involvement although well correlation between TR-inhibition and trypanocidal activity were not evident.
Bisbenzylisoquinoline alkaloids
The bisbenzylisoquinoline alkaloids were also shown to be potent inhibitors of TR and trypanocidal agents (Fournet et al. 1998, 2000). Six of the alkaloids evaluated displayed potent trypanocidal activity in vitro against T. cruzi with ED50 values <100 μM. The best TR inhibitor, cepharanthine (23), had an I50 value of 15 μM which is the same order of magnitude as its ED50 against the parasites, suggesting that inhibition of TR could be the mechanism of their trypanocidal activities.
Natural products
Natural products may be considered as “nature’s medicine chest” and have served as a potential source of TR-inhibitors. The natural antihypertensive agent, kukoamine A (24), a bis (trihydro-cinnamoyl) spermidine derivative, first isolated from Lycium chinense, was identified as a mixed type of inhibitor of TR (Ponasik et al. 1995).
Virtual screening of a large number of chemical databases, using the TR active site as the targeted template, identified the natural spermine-based macrocyclic alkaloid lunarin (25) (originally isolated from Lunaria biennis) as TR-inhibitor, which was shown to inhibit the enzyme in a time-dependent manner (Bond et al. 1999). Further investigation with synthetic derivatives confirmed the importance of the unique structure of the tricyclic core as a motif for inhibitor design and revealed that the non-natural enantiomer may be a more suitable scaffold upon which thiophilic groups may be presented (Hamilton et al. 2006). Ajoen (26) a garlic derived natural sulfur-containing compound was established as an irreversible inhibitor and subversive substrate of both TR and GR (Gallwitz et al. 1999). Most of these natural products also inhibited the parasites in vitro in one way or another.
Irreversible inhibitors
The anticancer nitrosourea drug carmustin was the first ligand to display irreversible inactivation of TR, but non-specifically as also inactivated hGR, by carbamoylating an active site cystinyl residue, which becomes accessible after being reduced by NADPH (Schirmer et al. 1995). Ajoen (26), as previously mentioned as a natural product inhibitor, is an irreversible non-specific inhibitor of TR (Gallwitz et al. 1999). The first rationally designed and selective irreversible inhibitors of TR include the Pt-complexes of terpyridine derivatives (e.g. 27), which inhibit the reduced TR, presumably through occupying the Cys52 by replacing its fourth pyridine ligand (Bonse et al. 2000). Coupling the irreversible ligand (terpyridine)Pt2+ complex with an 9-aminoacridine derivative (a competitive inhibitor) furnished mixed-type inhibitors (e.g. 28) (Inhoff et al. 2002). An important advancement was made with the quinacrine mustard (29), which was shown to selectively and irreversibly inactivate TR in a time-dependent manner with a stoichiometry of two inhibitors bound per monomer. The rate of inactivation was dependent upon the oxidative states of TR, with NADPH-reduced TR form being inactivated faster. The structure of TR-quinacrine mustard-adduct solved to 2.7Å revealed that two molecules of ligand are bound in the trypanothione binding site of TR. Each acridine moiety interacts through π-stacking, while only one of the acridine groups interacts with a trypanothione residue in a similar fashion (Saravanamuthu et al. 2004).
Recently, several Mannic bases (e.g. 30) were shown by Lee et al. (2005) to be irreversible inhibitors of TR. HPLC, NMR and MS analyses were performed to delineate their mechanism of action and found that divinyl ketone are the key intermediates to irreversibly inhibit TR. ESI- and MALDI-TOF-MS of TR, modified by Mannic base or corresponding divinyl ketone, demonstrated specific alkylation of Cys52 in a manner as shown in Figure 13 (Lee et al. 2005).
Figure 13.
Proposed reaction mechanisms for the modification of protein thiol (RSH), e.g. Cys52 in T. cruzi trypanothione reductase by an unsaturated Mannic base such as 30 (Modified from Lee et al. 2005).
Sixteen novel Pd-complexes of the bioactive nitrofuryl thiosemicarbazones were synthesized and tested for their in vitro activity (Otero et al. 2006). Most complexes showed higher in vitro trypanocidal activity against T. cruzi than the standard drug nifurtimox. Overall, the activities of Pd complexes ≥ their parent compounds. It has been suggested that main trypanocidal mechanism was the production of oxidative stress as a result of their bioreduction with the reductive enzymes, although strong DNA-adduct formation was also evident (Otero et al. 2006).
Subversive substrates of trypanothione reductase
Nifurtimox (6) and related compounds exert their parasiticidal activity through acting as futile, superoxide ion producing substrates of TR. TR and GR reduce these futile, superoxide ion producing substrates in a single electron step (NADPH + 2RNO2 → 2RNO2•− + 2H+; 2RNO2•− + O2 → 2RNO2 + O2•−), a process by which NADPH and O2 are wasted and T[SH]2 is inhibited/oxidized by scores of newly formed superoxide ions causing a reduction in thiol/disulfide titer and thus producing oxidative stress (Henderson et al. 1988; Schirmer et al. 1995). This group of compounds, known as ‘subversive substrates’ or ‘turncoat inhibitors’, are best represented by nitrofurans or quinones, chemically modified to take into account the substrate specificity for TR over GR (Cenas et al. 1994a and b). Thus, chinifur (31), a nitrofuran derivative with a positively charged side chain, was discovered as a selective inhibitor and subversive substrate of TR (Cenas et al. 1994a). Recently, the anti-TR activities of a series of nitrofuran and nitroimidazole derivatives were studied to examine the mechanism of action of different types of nitro-group containing compounds. The results indicated that the nitrofurans, e.g. nifurtimox act as futile-cyclers as discussed above, whereas 5-nitroimidazoles, e.g. megazole (32) act as thiol scavengers particularly for T[SH]2 thus reducing the thiol/disulfide titer which is detrimental for the parasites’ survival (Maya et al. 2003). Vega-Teijido et al (2006) explored three possible binding sites of TR and GR, i.e. the active site, the dimer interface and the NADPH binding site to study the mechanism of action of nitrofuran and nitrothiophene analogs. It has been suggested that this class of compounds act as either uncompetitive or mixed inhibitors of TR. Moreover, it has also been indicated that the presence of an α-helix connecting the active site of TR with the interface may be crucial for charge-transfer processes.
1,4-naphthaqiuinone derivatives (e.g. 33) are examples of quinine-based subversive substrates of TR, designed after plumbagin. Compound 33 proved to be a potent subversive substrate and an effective uncompetitive inhibitor of TR versus T[SH]2 and NADPH (Salmon-Chemin et al. 2001).
Most of these compounds were potent inhibitors of the parasites in vitro. For a series of naphthaquinones, a correlation between their potency as subversive substrates in vitro and trypanocidal activity in vivo has been demonstrated (Salmon-Chemin et al. 2001). This ‘alternative approach to chemotherapy of Chagas disease’ has been considered one of the most promising advances.
Chemotherapeutic Prospects of the Trypanothione Reductase Inhibitors
For an enzyme inhibitor to become a practical drug, several criteria must be met: (i) the biochemical pathway that is inhibited must be related to the disease state in such a way that inhibition of that pathway in a patient is therapeutic; (ii) the enzyme inhibitor must be specific so that unwanted inhibition of other pathways or receptors does not occur at therapeutic doses; (iii) the compounds must have the pharmacokinetic characteristics of a practical drug, i.e. must be absorbed, must penetrate to the site of action and must have a reasonably predictable dose-response relationship and duration of action; (iv) the compound must have an acceptable toxicological profile in animals, and the results of clinical studies in humans must demonstrate an appropriate balance between benefits and risks in therapeutic use; (v) the compound must survive a long and expensive clinical development process and ultimately be approved by regulatory agencies; (vi) the compound must be economically viable in the marketplace and compete successfully with other therapeutic alternatives (Crout, 1989). The criteria (i) and (ii) have already been addressed while selecting the target, and thus, all of the above mentioned inhibitors have fulfilled them. Pharmacological and toxicological studies in animal models are needed to meet other criteria.
Although many potent inhibitors of TR have been discovered through enzyme screening, in vivo evaluation of these lead compounds are scant. Several polyamine derivatives that were shown to be potent trypanocidals in vitro (with ED50 values in submicromolar ranges) failed to prolong the lives of experimental mice infected with trypanosomes or to cause a significant decrease in bloodstream parasitemia of infected mice (O’Sullivan et al. 1997). However, none of the compounds exerted overt toxicity in mice. It has been suggested that the lack of in vivo trypanocidal activity may be due to their rapid elimination and/or metabolism. Since these compounds are reversible inhibitors of TR, concentrations of these compounds may not be maintained, and as a result, TR activity will not be significantly decreased (O’Sullivan et al. 1997). It has also been suggested that converting reversible inhibitors into irreversible inhibitors through complexation with metal ions might be a reasonable strategy for identifying improved leads with enhanced in vivo profiles. Clomipramine and thioridazine were shown to be effective in treatment of mice with experimental Chagas disease (Rivarola et al. 2001, 2002). The investigation of effects of clomipramine on T. cruzi infected mice demonstrated that clomipramine at 5 mg/kg daily doses for 30 days, or two doses of clomipramine 40 mg/kg given intraperitoneally at 1h and 7 days after infection, was not toxic for the host, but was effective against the parasite. Parasitamiasis became negative and only mild heart structural and elecctrocardiographic alterations were detected in chronic phase in the group treated with clomipramine 5 mg/kg. In mice treated with 40 mg/kg, none of these alterations was detected (Rivarola et al. 2001). Overall, it has been shown that clomipramine and thioridazine significantly modified the natural evolution of the infection. Cardiac function and survival of infected and treated animals were not different from noninfected animals. Thioridazine or clomipramine are currently registered as drugs (antipsychotics) and meet all the above mentioned criteria. However, to consider them as antitrypanosomal drugs, dose becomes an important obstacle, since they need to be active at a dose which is known to be tolerated by psychiatric patients without causing adverse events. Apart from their potent in vitro activity and a preliminary report of in vivo activity, more detail studies are needed to establish their efficacy and safety and also to improve their antiparasitic potency. Overall, it would not be overenthusiastic to consider phenothiazines and related tricyclic antidepressants and the other inhibitors of TR as important drug leads for development of future chemotherapy against trypanosomal and leishmanial infections.
Conclusion
The demand for chemotherapeutic agents for the treatment of Chagas disease, sleeping sickness and kala azar is desperate. An account of the different possible targets for rational drug design with special emphasis on trypanothione reductase has been focused. TR has been established as a potential target in several studies although it is not universally accepted. The literature, molecular graphics and other medicinal chemistry approaches have led to the development of a large class of compounds as inhibitors of TR and the parasites. Considerable progress have been made in terms of identification, validation and inhibitor design based on trypanothione reductase as a target, which will be milestones towards the goal of developing chemotherapy against these devastating diseases. This review has revealed several approaches based on TR towards the development of new drugs against the parasitic diseases. Reasonable activity against parasites living in both culture and in mouse macrophases has been demonstrated by most classes of compounds, full activity against whole animal model to be achieved. It has been suggested that the alternative approaches like subversive substrates and metal bound inhibitors would be advantageous in achieving reasonable in vivo activity against the parasite infection. Only the phenothiazines and related tricyclic antidepressants were shown to reduce parasite burden in infected mice. Development of the quaternary alkylammonium chlorpromazines and congeners, with an additional hydrophobic moiety provided, on an average, up to about 30-fold more potent inhibitors of TR. The charge on N+ is needed for interaction at the Glu466’ or Glu467’ of the enzyme active site, the tricyclic or equivalent moiety interacts with the major hydrophobic cleft and the second hydrophobic moiety may interact with Z-site. Studies with other classes of inhibitors, in addition to the TR:inhibitor complex’s X-ray crystal structure, provided similar TR:inhibitor interactive motifs. As with any rational drug design case at the current stage of computational development, the problem of bioavailability, pharmacokinetic, metabolism and targeting have not been addressed. The prodrug approach might be a reasonable step in correcting some of the aforementioned problems in developing TR inhibitors with observable effects in vivo and might open a new path towards the development of drugs against these parasitic diseases.
Figure 1.
Structures of the currently available drugs for the treatment of trypanosomiasis and leishmaniasis.
Figure 10.
Bisbenzyleisoquinoline alkaloid.
Figure 11.
The natural product inhibitors of trypanothione reductase.
Figure 12.
Structures of irreversible inhibitors of trypanothione reductase.
Figure 14.
Structures and activity of subversive substrates of trypanothione reductase.
Acknowledgements
The author wish to gratefully acknowledge Professors K. T. Douglas of Manchester, A. H. Fairlamb of Dundee and R. L. Krauth-Siegel of Heidelberg and others who are pioneering the research in the endeavor of trypanothione reductase and many of their research have been cited in this review.
References
- Austin SE, Khan MOF, Douglas KT. Rational drug design using trypanothione reductase as a target for antitrypanosomal and anti-leishmanial drug leads. Drug Des Discov. 1999;16:5–23. [PubMed] [Google Scholar]
- Bailey S, Smith K, Fairlamb AH, et al. Substrate interactions between trypanothione reductase and N1-glutathionylspermidine disulphide at 0.28-nm resolution. Eur. J. Biochem./FEBS. 1993;213:67–75. doi: 10.1111/j.1432-1033.1993.tb17734.x. [DOI] [PubMed] [Google Scholar]
- Baillet S, Buisine E, Horvath D, et al. 2-Amino diphenylsulfides as inhibitors of trypanothione reductase: modification of the side chain. Bioorg Med Chem. 1996;4:891–9. doi: 10.1016/0968-0896(96)00083-1. [DOI] [PubMed] [Google Scholar]
- Benson TJ, McKie JH, Garforth J, et al. Rationally designed selective inhibitors of trypanothione reductase. Phenothiazines and related tricyclics as lead structures. Biochem J. 1992;286:9–11. doi: 10.1042/bj2860009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Lopez C, Bacchi CJ, et al. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg Med Chem Lett. 2006;16:3229–32. doi: 10.1016/j.bmcl.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Bodley AL, Wani MC, Wall ME, et al. Antitrypanosomal activity of camptothecin analogs. Structure-activity correlations. Biochem Pharmacol. 1995;50:937–42. doi: 10.1016/0006-2952(95)00215-l. [DOI] [PubMed] [Google Scholar]
- Bond CS, Zhang Y, Berriman M, et al. Crystal structure of Trypanosoma cruzi trypanothione reductase in complex with trypanothione, and the structure-based discovery of new natural product inhibitors. Structure. 1999;7:81–9. doi: 10.1016/s0969-2126(99)80011-2. [DOI] [PubMed] [Google Scholar]
- Bonse S, Santelli-Rouvier C, Barbe J, et al. Inhibition of Trypanosoma cruzi trypanothione reductase by acridines: kinetic studies and structure-activity relationships. J Med Chem. 1999;42:5448–54. doi: 10.1021/jm990386s. [DOI] [PubMed] [Google Scholar]
- Bonse S, Richards JM, Ross SA, et al. (2,2′:6′,2″-Terpyridine)platinum(II) complexes are irreversible inhibitors of Trypanosoma cruzi trypanothione reductase but not of human glutathione reductase. J Med Chem. 2000;43:4812–21. doi: 10.1021/jm000219o. [DOI] [PubMed] [Google Scholar]
- Brown JR. Trypanosomiasis and leishmaniasis. Critical Reports on Applied Chemistry, vol 21(Chemother Trop Dis) 1987:72–101. [Google Scholar]
- Brohn FH, Clarkson AB., Jr Trypanosoma brucei brucei: patterns of glycolysis at 37°C in vitro. Mol Biochem Parasitol. 1980;15:291–305. doi: 10.1016/0166-6851(80)90062-6. [DOI] [PubMed] [Google Scholar]
- Carnieri EG, Moreno SN, Docampo R. Trypanothione-dependent peroxide metabolism in Trypanosoma cruz different stages. Mol Biochem Parasitol. 1993;61:79–86. doi: 10.1016/0166-6851(93)90160-y. [DOI] [PubMed] [Google Scholar]
- Cenas N, Bironaite D, Dickancaite E, et al. Chinifur, a selective inhibitor and “subversive substrate” for Trypanosoma congolense trypanothione reductase. Biochem Biophys Res Comm. 1994a;204:224–9. doi: 10.1006/bbrc.1994.2448. [DOI] [PubMed] [Google Scholar]
- Cenas NK, Arscott D, Williams CH, Jr, et al. Mechanism of reduction of quinones by Trypanosoma congolense trypanothione reductase. Biochemistry. 1994b;33:2509–15. doi: 10.1021/bi00175a021. [DOI] [PubMed] [Google Scholar]
- Chan C, Yin H, Garforth J, et al. Phenothiazine inhibitors of trypanothione reductase as potential antitrypanosomal and antileishmanial drugs. J Med Chem. 1998;41:148–56. doi: 10.1021/jm960814j. [DOI] [PubMed] [Google Scholar]
- Chan C, Yin H, McKie JH, et al. Peptoid inhibition of trypanothione reductase as a potential antitrypanosomal and antileishmanial drug lead. Amino acids. 2002;22:297–308. doi: 10.1007/s007260200016. [DOI] [PubMed] [Google Scholar]
- Chibale K, Haupt H, Kendrick H, et al. Antiprotozoal and cytotoxicity evaluation of sulfonamide and urea analogues of quinacrine. Bioorg Med Chem Lett. 2001;11:2655–7. doi: 10.1016/s0960-894x(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Chibale K, Visser M, van Schalkwyk D, et al. Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents. Tetrahedron. 2003;59:2289–96. [Google Scholar]
- Clarkson AB, Jr, Brohn FH. Trypanosomiasis: an approach to chemotherapy by the inhibition of carbohydrate catabolism. Science. 1976;194(4261):204–6. doi: 10.1126/science.986688. [DOI] [PubMed] [Google Scholar]
- Croft SL. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol Sci. 1988;9:376–81. doi: 10.1016/0165-6147(88)90258-1. [DOI] [PubMed] [Google Scholar]
- Crout JR. In: Enzymes as targets for Drug Design. Palfreyman, McCann Lovenberg, et al., editors. London: Academic Press; 1989. [Google Scholar]
- De Luca S, Ulhaq S, Dixon MJ, et al. Solid phase synthesis of focused library of trypanothione reductase inhibitors. Tet Lett. 2003;44:3195–7. [Google Scholar]
- Docampo R, Stoppani AOM. Generation of superoxide anion and hydrogen peroxide induced by nifurtimox in Trypanosoma cruzi. Arch Biochem Biophys. 1979;197:317–21. doi: 10.1016/0003-9861(79)90251-0. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SNJ. Free-radical intermediates in the antiparasitic action of drugs and phagocytic cells. Free Radicals Biol. 1984a;6:243–88. [Google Scholar]
- Docampo R, Moreno SNJ. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis. 1984b;6:223–38. doi: 10.1093/clinids/6.2.223. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SNJ. Free radical metabolism of antiparasitic agents. Fed Proc. 1986;45:2471–6. [PubMed] [Google Scholar]
- Donelson JE, Rice-Ficht AC. Molecular biology of trypanosome antigenic variation. Microbiol Rev. 1985;49:107–25. doi: 10.1128/mr.49.2.107-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas C, Ouellette M, Tovar J, et al. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO, J. 1997;16:2590–8. doi: 10.1093/emboj/16.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouni MH. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol Therapeut. 2003;99:283–309. doi: 10.1016/s0163-7258(03)00071-8. [DOI] [PubMed] [Google Scholar]
- El-Waer A, Douglas KT, Smith K, et al. Synthesis of N-benzyloxycarbonyl-L-cysteinylglycine 3-dimethylaminopropylamide disulfide: a cheap and convenient new assay for trypanothione reductase. Anal Biochem. 1991;198:212–16. doi: 10.1016/0003-2697(91)90531-w. [DOI] [PubMed] [Google Scholar]
- El-Waer AF, Smith K, McKie JH, et al. The glutamyl binding site of trypanothione reductase from Crithidia fasciculata: enzyme kinetic properties of γ-glutamyl-modified substrate analogs. Biochim Biophys, Acta, Prot Struc Mol Enzymol. 1993a;1203:93–8. doi: 10.1016/0167-4838(93)90040-x. [DOI] [PubMed] [Google Scholar]
- El-Waer AF, Benson T, Douglas KT. Synthesis of substrate analogs for trypanothione reductase. Int J Pept Prot Res. 1993b;41:141–6. doi: 10.1111/j.1399-3011.1993.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Bowman IBR. Trypanosoma brucei: maintenance of concentrated suspensions of bloodstream trypomastigotes in vitro using continuous dialysis for measurement of endocytosis. Exp Parasitol. 1980;49:366–80. doi: 10.1016/0014-4894(80)90072-7. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Blackburn P, Ulrich P, et al. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985;227(4693):1485–7. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends in Parasitology. 2003a;19(11):488–494. doi: 10.1016/j.pt.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH. Target discovery and validation with special reference to trypanothione. In: Fairlamb AH, Ridley RG, Vial HJ, editors. Drugs against parasitic diseases: R&D methodologies and issues. Geneva: WHO TDR; 2003b. pp. 107–18. [Google Scholar]
- Fernandez-Gomez R, Moutiez M, Aumercier M, et al. 2-Amino diphenylsulfides as new inhibitors of trypanothione reductase. Int J Antimicrob Agents. 1995;6:111–18. doi: 10.1016/0924-8579(95)00029-x. [DOI] [PubMed] [Google Scholar]
- Flohé L. In: Glutathione—chemical, biochemical and medical aspects. Dolphine D, Poulson R, Avramovic O, editors. New York: Wiley Interscience; 1989. [Google Scholar]
- Flohé L. The Achilles’ heel of trypanosomatids: trypanothione-mediated hydroperoxide metabolism. BioFactors. 1998;8:87–91. doi: 10.1002/biof.5520080115. [DOI] [PubMed] [Google Scholar]
- Fournet A, Inchausti A, Yaluff G, et al. Trypanocidal bisbenzylisoquinoline alkaloids are inhibitors of trypanothione reductase. J Enz Inhibit. 1998;13:1–9. doi: 10.3109/14756369809035823. [DOI] [PubMed] [Google Scholar]
- Fournet A, De Arias RA, Ferreira ME, et al. Efficacy of bisbenzylisoquinoline alkaloids in acute and chronic Trypanosoma cruzi murine model. Int J Antimicrob Agents. 2000;13:189–95. doi: 10.1016/s0924-8579(99)00117-x. [DOI] [PubMed] [Google Scholar]
- Gallwitz H, Bonse S, Martinez-Cruz A, et al. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies. J Med Chem. 1999;42:364–72. doi: 10.1021/jm980471k. [DOI] [PubMed] [Google Scholar]
- Garforth J, McKie JH, Jaouhari R, et al. Rational design of peptide-based inhibitors of trypanothione reductase as potential antitrypanosomal drugs. Amino Acids. 1994;6:295–9. doi: 10.1007/BF00813749. [DOI] [PubMed] [Google Scholar]
- Garforth J, Yin H, McKie JH, et al. Rational design of selective ligands for trypanothione reductase from Trypanosoma cruzi. structural effects on the inhibition by dibenzazepines based on imipramine. J Enz Inhibit. 1997;12:161–73. doi: 10.3109/14756369709029312. [DOI] [PubMed] [Google Scholar]
- Girault S, Baillet S, Horvath D, et al. New potent inhibitors of trypanothione reductase from Trypanosoma cruzi in the 2-aminodiphenylsulfide series. Eur J Med Chem. 1997;32:39–52. [Google Scholar]
- Girault S, Davioud-Charvet TE, Maes L, et al. Potent and specific inhibitors of trypanothione reductase from Trypanosoma cruzi: bis(2-aminodiphenylsulfides) for fluorescent labeling studies. Bioorg Med Chem. 2001;9:837–46. doi: 10.1016/s0968-0896(00)00312-6. [DOI] [PubMed] [Google Scholar]
- Grogl M, Thomason TN, Franke ED. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47:117–26. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- Hamilton CJ, Saravanamuthu A, Eggleston IM, et al. Ellman’s-reagent-mediated regeneration of trypanothione in situ: Substrate-economical microplate and time-dependent inhibition assays for trypanothione reductase. Biochem J. 2003;369:529–537. doi: 10.1042/BJ20021298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CJ, Saravanamuthu A, Poupat C, et al. Time-dependent inhibitors of trypanothione reductase: analogues of the spermidine alkaloid lunarine and related natural products. Bioorg Med Chem. 2006;14:2266–78. doi: 10.1016/j.bmc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Fairlamb AH, Cerami A. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol. Biochem. Parasitol. 1987a;24:39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Fairlamb AH, Ulrich P, et al. Substrate specificity of the flavoprotein trypanothione disulfide reductase from Crithidia fasciculata. Biochemistry. 1987b;26:3023–7. doi: 10.1021/bi00385a011. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Ulrich P, Fairlamb AH, et al. “Subversive” substrates for the enzyme trypanothione disulfide reductase: alternative approach to chemotherapy of Chagas disease. Proce Natl Acad Sci USA. 1988;85:5374–8. doi: 10.1073/pnas.85.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JE. Molecular parasitology. Milton Keynes: Open University press; 1990. [Google Scholar]
- Hunter WN, Bailey S, Habash J, et al. Active site of trypanothione reductase. A target for rational drug design. J Mol Biol. 1992;227:322–33. doi: 10.1016/0022-2836(92)90701-k. [DOI] [PubMed] [Google Scholar]
- Inhoff O, Richards JM, Briet JW, et al. Coupling of a competitive and an irreversible ligand generates mixed type inhibitors of Trypanosoma cruzi trypanothione reductase. J Med Chem. 2002;45:4524–30. doi: 10.1021/jm020885k. [DOI] [PubMed] [Google Scholar]
- Jacoby EM, Schlichting I, Lantwin CB, et al. Crystal structure of the Trypanosoma cruzi trypanothione reductase.mepacrine complex. Proteins. 1996;24:73–80. doi: 10.1002/(SICI)1097-0134(199601)24:1<73::AID-PROT5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kelly JM, Taylor MC, Smith K, et al. Phenotype of recombinant Leishmania donovani and Trypanosoma cruzi which over-express trypanothione reductase. Sensitivity towards agents that are thought to induce oxidative stress. Eur J Biochem/FEBS. 1993;218:29–37. doi: 10.1111/j.1432-1033.1993.tb18348.x. [DOI] [PubMed] [Google Scholar]
- Khan MOF, Austin SE, Chan C, et al. Use of an additional hydrophobic binding site, the Z site, in the rational drug design of a new class of stronger trypanothione reductase inhibitor, quaternary alkylammonium phenothiazines. J Med Chem. 2000;43:3148–56. doi: 10.1021/jm000156+. [DOI] [PubMed] [Google Scholar]
- Koenig K, Menge U, Kiess M, et al. Convenient isolation and kinetic mechanism of glutathionylspermidine synthetase from Crithidia fasciculata. J. Biol. Chem. 1997;272:11908–11915. doi: 10.1074/jbc.272.18.11908. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel RL, Enders B, Henderson GB, et al. Trypanothione reductase from Trypanosoma cruzi. Purification and characterization of the crystalline enzyme. Eur J Biochem. 1987;164:123–8. doi: 10.1111/j.1432-1033.1987.tb11002.x. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel RL, Schoneck R. Flavoprotein structure and mechanism. 5. Trypanothione reductase and lipoamide dehydrogenase as targets for a structure-based drug design. FASEB J. 1995;9:1138–46. doi: 10.1096/fasebj.9.12.7672506. [DOI] [PubMed] [Google Scholar]
- Kuriyan J, Kong XP, Krishna TS, et al. X-ray structure of trypanothione reductase from Crithidia fasciculata at 2.4-Å resolution. Proc Natl Acad Sci, USA. 1991;88:8764–8. doi: 10.1073/pnas.88.19.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantwin CB, Schlichting I, Kabsch W, et al. The structure of Trypanosoma cruzi trypanothione reductase in the oxidized and NADPH reduced state. Proteins. 1994;18:161–73. doi: 10.1002/prot.340180208. [DOI] [PubMed] [Google Scholar]
- Le Trant N, Meshnick SR, Kitchener K, Eaton JW, Cerami A. Iron-containing superoxide dismutase from Crithidia fasciculata. Purification, characterization, and similarity to leishmanial and trypanosomal enzymes. J Biol Chem. 1983;258:125–30. [PubMed] [Google Scholar]
- Lee B, Bauer H, Melchers J, et al. Irreversible inactivation of trypanothione reductase by unsaturated Mannich bases: a divinyl ketone as key intermediate. J Med Chem. 2005;48:7400–10. doi: 10.1021/jm0504860. [DOI] [PubMed] [Google Scholar]
- Li Z, Fennie MW, Ganem B, et al. Polyamines with N-(3-phenylpropyl) substituents are effective competitive inhibitors of trypanothione reductase and trypanocidal agents. Bioorg Med Chem Lett. 2001;11:251–4. doi: 10.1016/s0960-894x(00)00643-0. [DOI] [PubMed] [Google Scholar]
- Linares GEG, Ravaschino EL, Rodriguez JB. Progresses in the field of drug design to combat tropical protozoan parasitic diseases. Curr Med Chem. 2006;13:335–60. doi: 10.2174/092986706775476043. [DOI] [PubMed] [Google Scholar]
- Marsh IR, Bradley M. Substrate specificity of trypanothione reductase. Eur J Biochem. 1997;243:690–4. doi: 10.1111/j.1432-1033.1997.00690.x. [DOI] [PubMed] [Google Scholar]
- Maya JD, Bollo S, Nunez-Vergara LJ, et al. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem Pharmacol. 2003;65:999–1006. doi: 10.1016/s0006-2952(02)01663-5. [DOI] [PubMed] [Google Scholar]
- McKie JH, Garforth J, Jaouhari R, et al. Specific peptide inhibitors of trypanothione reductase with backbone structures unrelated to that of substrate: potential rational drug design lead frameworks. Amino Acids. 2001;20:145–53. doi: 10.1007/s007260170055. [DOI] [PubMed] [Google Scholar]
- Meshnick SR. In: Parasitic Diseases. Mansfield JM, editor. Vol. 2. New York: Marcel Dekker; 1984. [Google Scholar]
- Michels PM. Compartmentation of glycolysis in trypanosomes: a potential target for new trypanocidal drugs. Biol Cell. 1988;64:157–64. doi: 10.1016/0248-4900(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Neva FA, Brown HW. Basic clinical parasitology. Norwalk, Connecticut: Appleton & Lange; 1994. [Google Scholar]
- Nogoceke E, Gommel DU, Kiess M, et al. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol. Chem. 1997;378:827–36. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- Ogston AG. Interpretation of experiments on metabolic processes, using isotopic tracer elements. Nature. 1948;162:963. doi: 10.1038/162963b0. [DOI] [PubMed] [Google Scholar]
- Orain D, Bradley M. Solid phase synthesis of trypanothione reductase inhibitors—towards single bead screening. Tet Lett. 2001;42:515–8. [Google Scholar]
- O’Sullivan MC, Zhou Q. Novel polyamine derivatives as potent competitive inhibitors of Trypanosoma cruzi trypanothione reductase. Bioorg Med Chem Lett. 1995;5:1957–60. [Google Scholar]
- O’Sullivan MC, Dalrymple DM, Zhou Q. Inhibiting effects of spermidine derivatives on Trypanosoma cruzi trypanothione reductase. J Enz Inhibition. 1996;11:97–114. doi: 10.3109/14756369609036537. [DOI] [PubMed] [Google Scholar]
- O’Sullivan MC, Zhou Q, Li Z, et al. Polyamine derivatives as inhibitors of trypanothione reductase and assessment of their trypanocidal activities. Bioorg Med Chem. 1997;5:2145–55. doi: 10.1016/s0968-0896(97)00157-0. [DOI] [PubMed] [Google Scholar]
- Otero L, Vieites M, Boiani L, et al. Novel antitrypanosomal agents based on palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. J Med Chem. 2006;49:3322–31. doi: 10.1021/jm0512241. [DOI] [PubMed] [Google Scholar]
- Parveen S, Khan MOF, Austin SE, et al. Anti-trypanosomal, anti-leishmanial and anti-malarial activities of quaternary arylalkyl-ammonium 2-amino-4-chlorophenyl phenyl sulfides, a new class of trypanothione reductase inhibitor, and of N-acyl derivatives of 2-amino-4-chlorophenyl phenyl sulfide. J Med Chem. 2005;48:8087–97. doi: 10.1021/jm050819t. [DOI] [PubMed] [Google Scholar]
- Ponasik JA, Strickland C, Faerman C, et al. Kukoamine A and other hydrophobic acylpolyamines: potent and selective inhibitors of Crithidia fasciculata trypanothione reductase. Biochem J. 1995;311:371–5. doi: 10.1042/bj3110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivarola HW, Fernandez AR, Enders JE, et al. Effects of clomipramine on Trypanosoma cruzi infection in mice. Trans Royal Soc Trop Med Hyg. 2001;95:529–33. doi: 10.1016/s0035-9203(01)90029-x. [DOI] [PubMed] [Google Scholar]
- Rivarola HW, Paglini-Oliva PA. Trypanosoma cruzi trypanothione reductase inhibitors: phenothiazines and related compounds modify experimental Chagas’ disease evolution. Curr drug targets. Cardiovasc. Haematol. Disord. 2002;2:43–52. doi: 10.2174/1568006023337745. [DOI] [PubMed] [Google Scholar]
- Salmon-Chemin L, Buisine E, Yardley V, et al. 2- and 3-substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity. J Med Chem. 2001;44:548–65. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- Saravanamuthu A, Vickers TJ, Bond CS, et al. Two interacting binding sites for quinacrine derivatives in the active site of trypanothione reductase: a template for drug design. J Biol Chem. 2004;279:29493–500. doi: 10.1074/jbc.M403187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer RH, Mueller JG, Krauth-Siegel RL. Disulfide-reductase inhibitors as chemotherapeutic agents: the design of drugs for trypanosomiasis and malaria. Angew Chem Int Ed. 1995;34:141–54. [Google Scholar]
- Shames SL, Fairlamb AH, Cerami A, et al. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986;25:3519–26. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Shapiro TA. Inhibition of topoisomerases in African trypanosomes. Acta Tropica. 1993;54:251–60. doi: 10.1016/0001-706x(93)90097-u. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Showalter AF. In vivo inhibition of trypanosome mitochondrial topoisomerase II: effects on kinetoplast DNA maxicircles. Mol Cell Biol. 1994;14:5891–7. doi: 10.1128/mcb.14.9.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekwani BL, Bacchi CJ, Pegg AE. Putrescine activated S-adenosylmethionine decarboxylase from Trypanosoma brucei brucei. Mol Cell Biochem. 1992;117:53–61. doi: 10.1007/BF00230410. [DOI] [PubMed] [Google Scholar]
- Tovar J, Fairlamb AH. Extrachromosomal homologous expression of trypanothione reductase and its complementary mRNA in Trypanosoma cruzi. Nucl. Acids. Res. 1996;24:2942–9. doi: 10.1093/nar/24.15.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar J, Cunningham ML, Smith AC, et al. Down-regulation of Leishmania donovani trypanothione reductase by heterologous expression of a trans-dominant mutant homolog: effect on parasite intracellular survival. Proc Natl Acad Sci USA. 1998a;95:5311–5316. doi: 10.1073/pnas.95.9.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar J, Wilkinson S, Mottram JC, et al. Evidence that trypanothione reductase is an essential enzyme in Leishmania by targeted replacement of the tryA gene locus. Mol Microbiol. 1998b;29:653–60. doi: 10.1046/j.1365-2958.1998.00968.x. [DOI] [PubMed] [Google Scholar]
- Urbina JA. Rational approach to specific chemotherapy of Chagas disease. World Class Parasites. 2003;7:127–35. [Google Scholar]
- Vega-Teijido M, Caracelli I, Zuckerman-Schpector J, et al. Conformational analyses and docking studies of a series of 5-nitrofuran- and 5-nitrothiophene-semicarbazone derivatives in three possible binding sites of trypanothione and glutathione reductases. J Mol Graph Model. 2006;24:349–55. doi: 10.1016/j.jmgm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Vellieux FMD, Hajdu J, Verlinde CLMJ, et al. Structure of glycosomal glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma brucei determined from Laue data. Proc Natl Acad Sci USA. 1993;90:2355–9. doi: 10.1073/pnas.90.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinde CLMJ, Callens M, Van Calenbergh S, et al. Selective Inhibition of Trypanosomal Glyceraldehyde-3-phosphate Dehydrogenase by Protein Structure-Based Design: Toward New Drugs for the Treatment of Sleeping Sickness. J Med Chem. 1994;37:3605–13. doi: 10.1021/jm00047a017. [DOI] [PubMed] [Google Scholar]
- Wang CC. Molecular mechanisms and therapeutic approaches to the treatment of African trypanosomiasis. Annu Rev Pharmacol Toxicol. 1995;35:93–127. doi: 10.1146/annurev.pa.35.040195.000521. [DOI] [PubMed] [Google Scholar]
- Wierenga RK, Kalk KH, Hol WGJ. Structure determination of the glycosomal triosephosphate isomerase from Trypanosoma brucei brucei at 2.4 Å resolution. J Mol Biol. 1987;198:109–21. doi: 10.1016/0022-2836(87)90461-x. [DOI] [PubMed] [Google Scholar]
- Williams CH., Jr Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase. A family of flavoenzyme transhydrogenases. Chem Biochem Flavoenzymes. 1992;3:121–211. [Google Scholar]
- Zhang Y, Bond CS, Bailey S, et al. The crystal structure of trypanothione reductase from the human pathogen Trypanosoma cruzi at 2.3 Å resolution. Protein science. 1996;5:52–61. doi: 10.1002/pro.5560050107. [DOI] [PMC free article] [PubMed] [Google Scholar]