Abstract
In the preclinical setting, phosphorylation and subsequent proteosomal degradation of the proapoptotic protein BIM confers resistance to paclitaxel in solid tumors with RAS/RAF/MAPK pathway activation. Concurrent administration of the proteasome inhibitor bortezomib enables paclitaxel-induced BIM accumulation, restoring cancer cell apoptosis in vitro and producing tumor regression in mice in vivo. A Phase I study was conducted to determine the MTD of paclitaxel and bortezomib combinatorial treatment. Sixteen patients with refractory solid tumors commonly exhibiting MAPK pathway activation were treated with weekly paclitaxel and bortezomib. Starting doses were 40 mg/m2 for paclitaxel and 0.7 mg/m2 for bortezomib. A modified continual reassessment method (MCRM) adapted for 2-drug escalation was used for MTD determination with 3-patient cohorts treated at each dose level. MTD was reached at 60 mg/m2 paclitaxel and 1.0 mg/m2 bortezomib, the recommended phase II dose. Therapy was overall well tolerated. Most frequently observed toxicities included anemia (in 43.75% of patients, one Grade 3 event), fatigue (in 43.75% of patients, one Grade 3 event beyond cycle 1) and neuropathy (in 31.25% of patients, one Grade 3 event after cycle 1). Of 15 evaluable patients, one NSCLC patient with paclitaxel exposure at the adjuvant setting had a PR and five patients had SD; median disease stabilization was 143.5 days; three NSCLC patients had SD lasting 165 days or longer. Thus, rationally designed weekly treatment with paclitaxel and bortezomib in solid tumors with MAPK pathway activation, including previously taxane-treated malignancies, is a tolerable regimen with preliminary signals of antitumor activity worthy of further investigation.
Keywords: MAPK, paclitaxel, bortezomib, BIM, apoptosis
Introduction
Paclitaxel, a microtubule-stabilizing agent causing late G2-M cell cycle arrest followed by apoptotic cell death, is commonly used for solid malignancy treatment. Similar to other chemotherapeutic agents, cancer cell resistance to paclitaxel occurs frequently (1), many times due to acquired apoptosis resistance, which provides malignant cells with a survival advantage, thus, compromising cancer therapy (2, 3). Understanding the mechanisms of paclitaxel-induced programmed cell death and by which tumors evade this process is critical for pharmacologic reactivation of cancer cell apoptosis and clinical benefit. Apoptosis is controlled by anti-apoptotic BCL-2 family proteins and proapoptotic BAX, BAK and BH3-only proteins. Highly stressful stimuli initiate apoptosis through BH3-only proteins, such as BIM, by inhibiting BCL-2-like proteins or activating BAX and BAK, resulting in cell death by mitochondrial outer membrane permeabilization (4). Upregulation of anti-apoptotic and/or downregulation of proapoptotic proteins influences tumorigenesis and treatment response; thus, deciphering involved mechanisms may guide rational therapy design.
Earlier in vitro and in vivo animal studies indicated that epithelial tumor resistance to paclitaxel was conferred by RAS/RAF/mitogen-activated protein kinase (MAPK) pathway activation causing phosphorylation, proteosomal degradation and thus inactivation of the BH3-only proapoptotic protein BIM, which is a major determinant of cell sensitivity to paclitaxel (5). In a baby mouse kidney (BMK) epithelial cell model (5), paclitaxel induced selective BIM accumulation, in turn promoting cancer cell apoptosis in vitro and in allograft tumors in mice in vivo. Responsiveness to paclitaxel specifically depended on BIM, and not other proapoptotic proteins, as demonstrated by BIM-deficient, but not wild type or other BH3-only-deficient, cell resistance to paclitaxel. Constitutive RAS/RAF/MAPK pathway activation suppressed BIM induction by phosphorylating BIM and targeting it to proteasomes for degradation, thus causing cancer cell resistance paclitaxel-induced apoptosis. In cancer cells and tumors with RAS/RAF/MAPK pathway activation treated with paclitaxel and a proteasome inhibitor combination, BIM degradation was blocked and paclitaxel-dependent BIM accumulation and apoptosis were restored, as manifested by cancer cell death in vitro and tumor regression in animal studies.
The preclinical data described above suggested that paclitaxel and proteasome inhibitor combination may be therapeutically beneficial against malignancies with activated RAS/RAF/MAPK pathway, such as pancreas, colon, lung, ovarian, thyroid, breast and prostate cancers (6–8), as well as malignancies traditionally treated with paclitaxel and those exhibiting paclitaxel resistance (9, 10). Independent preclinical studies confirmed the importance of BIM accumulation for drug-induced apoptosis in different tumor types (11–14). We, thus, decided to clinically investigate combining paclitaxel with the proteasome inhibitor bortezomib for restoration of BIM levels in tumors with activated RAS/RAF/MAPK pathway (5) and potentiation of paclitaxel-induced cancer cell apoptosis to enhance tumor regression and improve clinical response.
Paclitaxel and bortezomib combination was previously explored in several small studies. In an NCI-sponsored, multicenter Phase I clinical trial, bortezomib was escalated in combination with paclitaxel at 100 mg/m2. Paclitaxel given on days 1 and 8 in combination with bortezomib at 1.8 mg/m2 on days 2 and 9 showed acceptable toxicity and disease stabilization in 7/44 (16%) patients, including three patients with advanced pancreatic cancer (15). An earlier study compared two different administration schedules with schedule A patients receiving paclitaxel and carboplatin on day 1 followed by bortezomib on days 2, 5, and 8, and schedule B patients receiving bortezomib on days 1, 4, and 8 with paclitaxel and carboplatin combination administered on day 2. Toxicities were primarily hematologic and schedule B patients showed improved responses. A phase II dose of bortezomib at 1.3 mg/m2, carboplatin at AUC 6 and paclitaxel at 135 mg/m2 was recommended (16); however, a subsequent phase II trial of the same drug combination and schedule (B), but using a higher paclitaxel dose, was terminated due to insufficient clinical activity and significant associated toxicity (17). A third study escalated both bortezomib (on days 1, 4, 8, and 11 of a 3-week cycle, starting at 0.7 mg/m2) and paclitaxel (on days 1 and 8, starting at 80 mg/m2) and resulted in a 30% partial response rate, which was considered similar to that of single agent paclitaxel and was accompanied by significant peripheral neuropathy (76% of patients; Grade 3–4 in 9%) that precluded further clinical development (18).
We now report the findings of a rationally designed, single center Phase I study of weekly paclitaxel and bortezomib combination in patients with advanced and refractory solid tumors commonly exhibiting MAPK pathway activation using an adaptive dose-finding approach. In our trial, the two drugs were given on the same day weekly, in a schedule different than those used in earlier studies and expected to more actively induce BIM-mediated tumor cell apoptosis, as our in vitro and in vivo preclinical data (5) argued for concurrent, rather than sequential, drug administration. Despite earlier reports of antagonistic effects and decreased prostate cancer cell apoptosis upon simultaneous exposure to bortezomib and paclitaxel (19), concurrently given drugs clearly demonstrated killing synergy in BMK cancer cell lines and allograft tumors with RAS/RAF/MAPK pathway activation (5). Therefore, a Phase I clinical trial of paclitaxel and bortezomib combination for the treatment of tumors commonly exhibiting RAS/RAF/MAPK pathway activation was rationally designed to identify the maximum tolerated dose (MTD) of weekly treatment using a modified continual reassessment method (MCRM) (20–23) adapted for 2-drug escalation. Correlative studies determined BIM protein level changes in peripheral blood mononuclear cells (PBMCs) during treatment as a surrogate marker for intratumoral treatment-induced BIM expression alterations. Cancer cell RAS/RAF/MAPK pathway activation was evaluated by phospho-p44/42 MAPK IHC on archived tumor biopsies. Finally, paclitaxel plasma concentrations in bortezomib presence were compared to historical pharmacokinetic data for this drug.
Materials and Methods
Eligibility criteria
Patients were eligible if older than 18, with ECOG performance status 0–2 and histologically confirmed malignancy that was metastatic or unresectable and for which no standard curative or palliative measures existed. Entry was restricted to patients with solid tumors commonly exhibiting RAS/RAF/MAPK pathway activation, i.e., pancreas, NSCLC, melanoma, colon, breast, prostate, ovary, and papillary thyroid cancer (6, 7). Unlimited number of prior chemotherapies and past paclitaxel or bortezomib treatment was also allowed. Adequate organ function was required: WBC ≥ 3,500/µL and ANC ≥ 1,500/µL, platelets ≥ 100,000/µL, creatinine ≤ 2X the upper limit of normal (ULN), total bilirubin ≤ 1.5× ULN, aspartate (AST) and alanine (ALT) aminotrasnferases ≤ 2.5X ULN (or ≤ 5× ULN, if tumor-involved liver). Patients with untreated or uncontrolled brain metastases, active infections, significant comorbid cardiac disease, neuropathy ≥ CTCAE Grade 1 with pain within 14 days or having received other anticancer treatment within 4 weeks prior to enrollment were excluded. Prophylactic use of antiemetics, antidiarrheals, and other supportive care measures was allowed; growth factor support was allowed after cycle 1 at treating physician’s discretion. The Cancer Institute of New Jersey (CINJ) Institutional Review Board (IRB) approved the study protocol, and all patients signed informed consent prior to treatment initiation.
Study design and treatment
This was a phase I study of paclitaxel and bortezomib given on days 1, 8 and 15 of a 21-day cycle with primary objective to identify this combination’s maximum tolerated dose (MTD). Starting paclitaxel dose (40 mg/m2) was half of the maximum FDA-approved dose for weekly paclitaxel treatment of ovarian and metastatic breast cancer. Starting bortezomib dose (0.7 mg/m2) was shown to effectively inhibit proteasome function (24) and was the lowest dose used in bortezomib monotherapy, when peripheral neuropathy symptoms necessitated dose reduction (25). Dose Limiting Toxicities (DLTs) were defined as: any Grade 3 or 4 treatment-related non-hematologic toxicity (except nausea and vomiting occuring in the absence of antiemetic regimen, and elevated ALT and/or AST that decreased to Grade 2 or lower within one week), or Grade 4 hematologic toxicity during treatment or within one week of treatment completion and 1) lasting more than 7 days, or 2) resulting in omission or delay of ≥ 2 drug doses in a cycle due to toxicity, or 3) resulting in toxicity-associated cycle initiation delay beyond 2 weeks.
MTD was determined by a modified continual reassessment method (MCRM) [20–23] adapted for 2-drug escalation and taking into account all treatment-related toxicities Grade 2 and above (Grade 1 with pain and above for neurotoxicity) that could be intolerable if sustained, rather than solely the DLTs. Forty to 80 mg/m2 and 0.7 to 1.3 mg/m2 were considered the useful paclitaxel and bortezomib ranges, respectively, when both drugs were given weekly. Dose-toxicity relationships in the paclitaxel and bortezomib ranges were assumed similar and a standardized effective dose (SED) approach was used. Actual paclitaxel and bortezomib doses ranges were standardized to a 10–100 range, i.e., linear transformations were used so that SED of 10 represented paclitaxel 40 mg/m2 or bortezomib 0.7 mg/m2 and SED of 100 represented paclitaxel 80 mg/m2 or bortezomib 1.3 mg/m2. The paclitaxel and bortezomib combination SED was the sum of paclitaxel and bortezomib SEDs. With starting doses of bortezomib at 0.7 mg/m2 and paclitaxel at 40 mg/m2 (i.e., combination SED of 20), cohorts of 3 patients were treated at each dose level. Subsequent bortezomib and paclitaxel combination SED levels were determined by the CINJ Biometrics Department using MCRM with a two-parameter logistic model adaptation. The two drugs were assumed equally important, hence both were modified by the same dose change percentage to determine subsequent SED doses, which were then reviewed and, if necessary, adjusted by the principal investigator based on clinical and safety factors. SEDs of 10 and 200 were assumed to have DLT rates of 20% and 98%, respectively, and the target toxicity level (probability of treatment-related DLT at the MTD) was set at 25%. With these assumptions, the DLT rate at the starting dose level (SED of 20) was 25%. Starting at the presumed MTD is one of the reasons that MCRM is more efficient than the traditional 3+3 design in reaching actual MTD. The above assumptions initiated the MCRM fitting process and were modified or discarded, as more outcome data became available. For subsequent SED level calculations, ordinal values of 0, 0, 0.2, 1, and 1 were used to represent Grade 0, 1, 2, 3, and 4 drug-related toxicities, respectively, except for neuropathy which was given greater weight (values of 0.5 and 1 were used for Grade 1 with pain or Grade 2 toxicities and Grade 2 with pain or Grade 3 or 4 toxicities, respectively). If no Grade 3 hematologic toxicity or a DLT was observed, the maximum dose escalation was no more than 75% of the previous SED level. Upon Grade 3 hematologic toxicity or a DLT, the maximum dose escalation was no more than 50% of the previous SED level. Process continued until 25 patients entered the study or changes in the next recommended SED were no more than 10% for two consecutive cohorts, whichever came first.
Clinical Assessments
Toxicities were evaluated and graded using National Cancer Institute Common Terminology Criteria version 3.0. A history, physical examination, chest X-ray, electrocardiogram, and laboratory tests, including a complete blood count (CBC) with differential, chemistry, and urinalysis, were obtained at baseline. CBC was drawn weekly during cycle 1. In subsequent cycles, CBC and chemistry were obtained prior to cycle initiation. Baseline and restaging imaging studies were done within 4 weeks prior to study enrollment and every two cycles thereafter, respectively. Response was assessed using Response Evaluation Criteria in Solid Tumors (26).
Pharmacokinetic (PK) sampling, assay and analysis
Blood specimens (5 mL) were collected in heparinized tubes immediately before and at 1, 2, 3, 4, 5, 6, 7, 8, 27, 51 and 75 hours after the start of paclitaxel infusion on day 1 of cycle 1; centrifuged at 1,000 × g for 10 min at 4°C; plasma was separated and stored at −80°C until further analysis. Paclitaxel concentration in plasma was determined by HPLC using modifications of a previously described method (27). Calibration plasma standards and patient samples (0.5 mL each) were spiked with Baccatin III (100 ng), extracted in acetonitrile (5 mL) and evaporated to dryness. Extracts were reconstituted in acetonitrile:water (80:20, 1 mL), transferred to microfuge tubes and dried under nitrogen. Residues were reconstituted in water:acetonitrile (1:1, 400 µl), centrifuged at 18,000 × g for 12 min at 4°C. Clear supernatant (250 µl) was injected into an HPLC system consisting of Hitachi L-7000 series UV detector, autosampler and quaternary gradient solvent delivery pump. Analytes were isolated using Luna PFP column (4.6 × 250 mm, 5 µ; Phenomenex, Torrance, CA, USA), maintained at 37°C and analyzed in a mobile phase gradient of water and acetonitrile at the following ratios and times: 61:39, 0–12 min; 61:39 to 53:47, 12–30 min; 53:47 to 61:39, 30–45 min, at a flow rate of 0.8 mL/min, detector set at 229 nm. Paclitaxel concentrations in standards and samples were calculated using least-square linear regression analysis of paclitaxel and internal standard peak height ratios over nominal concentrations. The PK parameters, area under the concentration-time curve (AUC), total body clearance (CL), peak plasma concentration (Cmax), time to maximum concentration (Tmax), half-life (T½) and volume of distribution (Vz), were determined using a non-compartmental model with WinNonlin 2.1 software (Pharsight Corp. Mountain View, CA, USA). AUC was estimated by the log-linear trapezoidal method up to the last measurable concentration (Clast). CL was calculated by dividing dose by AUC.
Pharmacodynamic studies: treatment-induced BIM expression changes in PBMCs
Blood samples (8 mL) prior to and at 27 and 51 hours after paclitaxel infusion start on day 1 of cycle 1 were collected in CPT tubes (BD-Vacutainer Cell Preparation Tubes, BD, Franklin Lakes, NJ, USA), and mononuclear cells were prepared according to manufacturer’s instructions. Cells were washed with phosphate buffered saline and stored at −80°C. Upon request, cells were thawed, lysed in 0.15 M NaCl, 0.01 M Tris-HCl, pH 7.4, (with pepstatin, leupeptin, DTT, protease inhibitors and phosphatase inhibitor cocktails 1 and 2), mixed with Laemmli sample buffer, boiled at 95°C for 5 min and loaded on 12% SDS-PAGE followed by transfer to nitrocellulose membranes. As a positive control, MCF-7 breast cancer cells were prepared and lysed similarly to PBMCs. BIM immunoblotting was performed using a rabbit monoclonal antibody (C34C5, Cell Signaling Technology, Danvers, MA) (11, 28).
Tumor evaluation for RAS/RAF/MAPK pathway activation
RAS/RAF/MAPK pathway activation was evaluated in paraffin-embedded archived tumors by phospho-p44/42 MAPK IHC using a rabbit polyclonal antibody (#4377; Cell Signaling Technology, Beverly, MA), as previously described (29). Protein expression was manually and independently evaluated by two study investigators, Drs. White and Karantza, and was considered ‘positive’ when more than 5% of tumor specimen exhibited at least low (1+) to moderate (2+) staining intensity.
Results
Patient Characteristics
Between April 2007 and March 2008, sixteen patients, more commonly with NSCLC and colon cancer, were enrolled in study (Table 1). In total, 44 treatment cycles were administered with a median number of 3.3 cycles per patient. Seven patients had been previously treated with taxane-containing regimen (6 with paclitaxel; 1 with docetaxel). No patient had prior bortezomib exposure. Patient study participation ended due to disease progression (15) or consent withdrawal (1).
Table 1.
Patient (n=16) characteristics
| Median (range) age, y | 63 (47–81) |
|---|---|
| Gender | |
| Male | 7 |
| Female | 9 |
| Race | |
| Caucasian | 15 |
| Hispanic | 1 |
| ECOG PS | |
| 0 | 4 |
| 1 | 11 |
| 2 | 1 |
| Tumor Type | |
| NSCLC | 6 |
| Colon | 4 |
| Pancreas | 2 |
| Melanoma | 2 |
| Ovarian | 1 |
| Breast | 1 |
| # prior chemotherapy regimens | |
| 0 | 1 |
| 1 | 3 |
| 2 | 2 |
| > 2 | 10 |
| # prior targeted therapies | |
| 0 | 13 |
| 1 | 3 |
| # patients treated with paclitaxel in the past | 6 |
| # patients treated with bortezomib in the past | 0 |
Treatment-related toxicities and MTD
Table 2 summarizes drug doses and toxicities observed at all dose levels during cycle 1. The initial cohort was expanded to include 4 patients, as one patient died of disease progression during cycle 1 and was thus non-evaluable for tumor response; however, toxicity data was included in MCRM. At the first dose level, treatment-related Grade 2 anemia (2 events) and Grade 2 fatigue (1 event) were reported, but no DLTs, and combination SED was increased by 10% to paclitaxel 45 mg/m2 and bortezomib 0.75 mg/m2. At the second and third dose levels, no treatment-related Grade 2 or above toxicities were observed, hence combination SED was increased by 14% and 12%, respectively, to paclitaxel 60 mg/m2 and bortezomib 1.0 mg/m2. At the fourth dose level, treatment-related Grade 3 myalgias lasting for 7 days prior to resolution (1 event) and Grade 2 anemia (1 event) were reported and a combination SED decrement by 4% was recommended. However, paclitaxel and bortezomib doses were kept unchanged, as a 4% dose decrease was considered clinically non-significant for both drugs. Finally, at the fifth dose level, treatment-related Grade 2 and 3 anemia (1 event each) occurred and combination SED decrease by 6.3% was recommended. Since the recommended change in combination SED was less than 10% at two consecutive (fourth and fifth) dose levels, MCRM was terminated and MTD was reached at the fifth dose level (paclitaxel 60 mg/m2 and bortezomib 1.0 mg/m2). In summary, using MCRM, paclitaxel was escalated from 40 to 45, 51 and 60 mg/m2, while bortezomib was escalated from 0.70 to 0.75, 0.86 and finally 1.0 mg/m2.
Table 2.
Treatment-related toxicities observed at different dose levels during cycle 1
| Dose Level (# patients) |
Paclitaxel mg/m2 |
Bortezomib mg/m2 |
Study drug-related toxicities, # events |
Dose modification |
|---|---|---|---|---|
| 1 (4) | 40 | 0.7 | Grade 2 anemia, 2 Grade 2 fatigue, 1 |
10% increment |
| 2 (3) | 45 | 0.75 | ≥ Grade 2, 0 | 14% increment |
| 3 (3) | 51 (rounded from 51.4 mg/m2) | 0.86 (rounded from 0.856 mg/m2) | ≥ Grade 2, 0 | 12% increment |
| 4 (3) | 60 (rounded from 58 mg/m2) | 1.0 (rounded from 0.96 mg/m2) | Grade 3 myalgias lasting 7 days prior to resolution, 1 Grade 2 anemia, 1 |
4% decrement |
| 5 (3) | 60 (rounded from 58 mg/m2) | 1.0 (rounded from 0.96 mg/m2) | Grade 3 anemia, 1 Grade 2 anemia, 1 |
6.3% decrement |
Weekly paclitaxel and bortezomib combination was overall well tolerated. The most serious treatment-related toxicities during cycle 1 included fatigue (one Grade 2 event), myalgias (one Grade 3 event), and anemia (two Grade 2 and one Grade 3 event), but no event satisfied DLT criteria. Thus, MTD was reached with only 5 dose levels and no DLTs, indicating that our initial MCRM assumptions were near-target and quite efficiently utilized.
Adverse events
During all treatment cycles, five patients experienced a total of 9 serious AEs (SAEs) that resulted in patient hospitalization, but only one event was treatment-related (non-neutropenic fever accompanied by mucositis, observed beyond cycle 1). Two NSCLC patients died of progressive disease during cycle 1; in both cases, death was associated with new brain metastases diagnosed shortly after study initiation.
During the entire study, treatment-related adverse events were mostly Grade 1 and 2 (Table 3). For all adverse events, predominant hematologic toxicity was anemia (observed in seven patients or 43.75%; one Grade 3 event), but did not result in any delayed, reduced or missed drug doses. Six patients were given erythropoietin while on trial, but three of them had required this support even prior to study participation. The most commonly observed non-hematologic toxicities included neuropathy (in 31.25% of patients; one Grade 3 event after cycle 1) and fatigue (in 43.75% of patients; one Grade 3 event, also beyond cycle 1). Grade 3 dyspnea (1 event after cycle 1) was noted in a patient with NSCLC and COPD in the setting of non-neutropenic fever and was initially considered possibly treatment-related; however, symptoms resolved with antibiotics, oxygen and supportive care within 7 days, thus most likely representing COPD exacerbation.
Table 3.
Treatment-related adverse events observed during entire study
| Grade, # events | ||||
|---|---|---|---|---|
| Category | Dose Level 1 |
Dose Level 2 |
Dose Level 3 |
Dose Level 4 |
| Auditory | ||||
| Tinnitus | Grade 1, 1 | |||
| Blood/Bone Marrow | ||||
| Hemoglobin | Grade 2, 2 | Grade 2, 1 | Grade 2, 1 | Grade 2, 2 Grade 3, 1 |
| Constitutional | ||||
| Fatigue | Grade 1, 2 Grade 2, 1 |
Grade 2, 1 | Grade 1, 1 | Grade 1, 1 Grade 3, 1 |
| Fever (non-neutropenic) | Grade 1, 1 | Grade 2, 1 | ||
| Rigors/chills | Grade 1, 1 | Grade 1, 1 | ||
| Gastrointestinal | ||||
| Anorexia | Grade 1, 1 | |||
| Constipation | Grade 1, 1 | |||
| Nausea | Grade 1, 1 | Grade 1, 1 | Grade 1, 1 | |
| Vomiting | Grade 1, 1 | |||
| Metabolic/Laboratory | ||||
| Hypoalbuminemia | Grade 2, 1 | |||
| Musculoskeletal | ||||
| Muscle Weakness | Grade 2, 1 | |||
| Neurologic | ||||
| Neuropathy: sensory | Grade 1, 1 | Grade 3, 1 | Grade 1, 2 | |
| Neuropathy: motor | Grade 2, 1 | |||
| Dizziness | Grade 1, 1 | |||
| Pain | ||||
| Limb | Grade 1, 1 | |||
| Myalgias | Grade 3, 1 | |||
PK studies
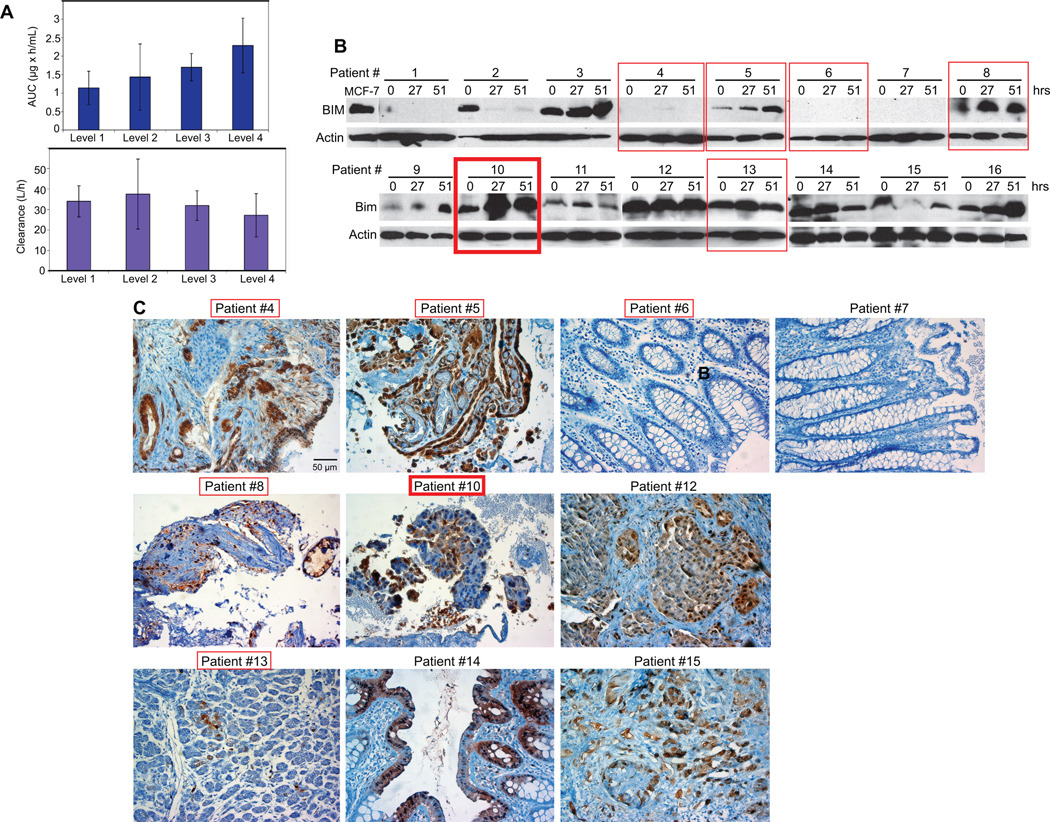
Paclitaxel PK parameters were determined in 16 patients on paclitaxel and bortezomib combination (Table 4). Peak plasma concentrations (Cmax) increased from dose level 1 to 4 reflecting paclitaxel dose escalation and were comparable to previously reported values for weekly low dose paclitaxel protocols (30, 31). Average AUC at 40, 45, 51 and 60 mg/m2 paclitaxel showed a dose-proportional linear increase (r2=0.995) (Fig. 1A). Paclitaxel clearance (CL) ranged from 17 to 48 L/h. Mean CLs at 40 and 45 mg/m2 paclitaxel were almost the same. Mean CLs at 51 and 60 mg/m2 paclitaxel were 31.9 and 27.2 L/h, respectively, also similar to each other and not significantly different from those at other dose levels (p > 0.3) (32, 33). In all cases, time to maximum paclitaxel concentration (Tmax) was 1 hour, corresponding to infusion end. Paclitaxel T½ ranged from 0.55 to 3.4 hours with mean values at different dose levels ranging from 0.69 to 1.96 hours. Paclitaxel PK parameter variation was likely due to the small patient number at each dose level.
Table 4.
Paclitaxel pharmacokinetic parameters in plasma
| Parameter | Dose Level 1 | Dose Level 2 | Dose Level 3 | Dose Level 4 |
|---|---|---|---|---|
| Cmax (µg/mL) | 1.00±0.52 | 1.25±0.90 | 1.63±0.49 | 2.04±0.73 |
| Tmax (h) | 1.0 | 1.0 | 1.0 | 1.0 |
| AUC (last) (µg × h/mL) | 1.14±0.45 | 1.43±0.90 | 1.70±0.37 | 2.29±0.74 |
| Clearance (L/h) | 33.98±7.61 | 37.50±17.06 | 31.90±7.21 | 27.16±10.55 |
| T1/2 (h) | 1.75±1.45 | 0.69±0.20 | 1.96† | 1.83±0.86 |
| Vz (L/m2) | 95.15±101.02 | 34.50±28.78 | 85.59† | 64.31±37.93 |
S.D. was not calculated due to the small number (<3) of data points.
Figure 1.
A, Paclitaxel AUC at different dose levels. B, Paclitaxel clearance at different dose levels. B, Western blots showing BIM and actin levels in PBMCs at 0, 27 and 51 hours after initiation of paclitaxel and bortezomib combinatorial treatment. C, Phospho-p44/42 MAPK IHC on paraffin-embedded archived patient tumor specimens. Red rectangles enclose data referring to patients with SD (thin line) or PR (thick line).
Antitumor Activity
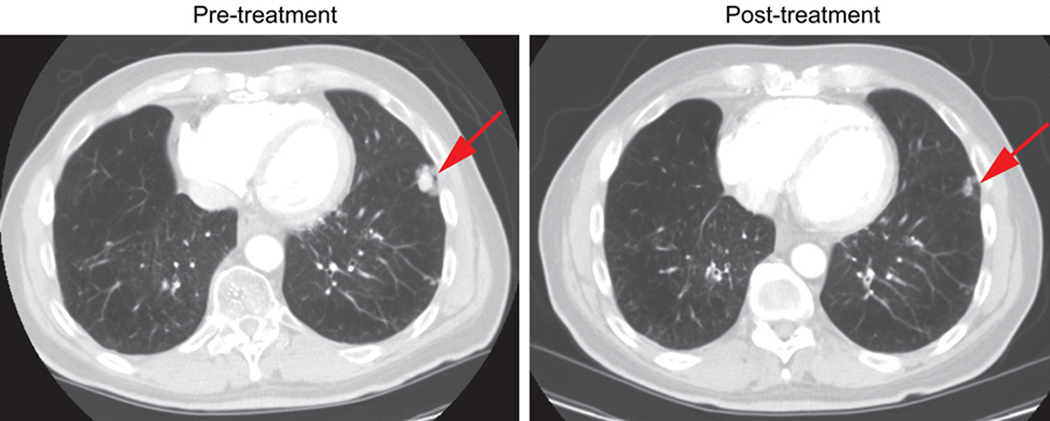
Among 15 evaluable patients (one patient withdrew consent due to Grade 3 myalgias in cycle 1 and was, thus, evaluable for toxicity, but non-evaluable for response), no complete responses (CR) were seen (Table 5). One NSCLC patient previously treated with paclitaxel during definitive chemoradiation had a confirmed partial response (PR) (Fig. 2) and remained on study for 227 days. Two other NSCLC patients showed disease stabilization as compared to progressive disease (PD) prior to study initiation, each remaining on study for 165 and 180 days. Stable disease (SD) as best response was also seen in three additional patients, one each with colon, ovarian and pancreatic tumors. Mean duration of disease stabilization was 143.5 days. Two out of 5 patients with SD had been treated with a paclitaxel-containing regimen in the past. A second NSCLC patient had received paclitaxel during definitive chemoradiation and a patient with ovarian cancer had been treated with paclitaxel in the adjuvant setting, at first recurrence, and as the most recent line of therapy prior to study participation. Despite recent disease progression on paclitaxel, tumor markers, ascitic fluid accumulation and peritoneal implants stabilized and patient remained on study for 4 cycles. Of the 9 patients with disease progression (60% of evaluable patients), three (two with lung and one with colon cancer) showed PD during cycle 1, making it unlikely that their disease trajectory was affected in any significant way by study drugs, while six other patients (two with melanoma, two with colon and one with breast cancer) completed 2 treatment cycles and were found to have PD at first restaging.
Table 5.
Best response to treatment and correlative studies
| Patient # | Tumor type | Best response (duration) |
Change in PBMC BIM expression upon treatment |
Phospho-p42/44 IHC on archived tumors |
|---|---|---|---|---|
| 1 | NSCLC | PD | No expression | No tumor available |
| 2 | Melanoma | PD | Decrease | No tumor available |
| 3 | Melanoma | PD | Increase | No tumor available |
| 4 | Ovary | SD (2.5 mo) | No expression | *Positive |
| 5 | NSCLC | SD (5.5 mo) | Increase | *Positive |
| 6 | Colon | SD (3.25 mo) | No expression | Negative |
| 7 | Colon | PD | No expression | Negative |
| 8 | NSCLC | SD (6 mo) | Increase | *Positive |
| 9 | Pancreas | PD | Increase | No tumor available |
| 10 | NSCLC | PR (7.5 mo) | Increase | *Positive |
| 11 | Colon | PD | No change | No tumor available |
| 12 | NSCLC | NE | No change | *Positive |
| 13 | Pancreas | SD (4 mo) | No change | *Positive |
| 14 | Colon | PD | Decrease | *Positive |
| 15 | Breast | PD | Decrease | *Positive |
| 16 | NSCLC | PD | Increase | No tumor available |
Positive: more than 5% of tumor specimen exhibited at least low (1+) to moderate (2+) staining intensity, as manually and independently evaluated by two study investigators. NE: not evaluable.
Figure 2.
Confirmed PR in a NSCLC patient. Baseline and post-treatment (at the time of PR) spiral CT scan transverse images at the level of left pleural-based mass, which decreased from 1.9 to 1.2 cm in maximum diameter. A right lower lung mass also decreased from 1.7 to 1.1 cm, and an aortopulmonary (AP) window lymph node shrunk from 1.4 to 0.4 cm.
Pharmacodynamic and other correlative laboratory studies
PBMC pre- and post-treatment BIM expression was determined for all (16) study patients (Table 5; Fig. 1B). Archived tumors, available for 10 patients, were evaluated for RAS/RAF/MAPK pathway activation by phospho-p44/42 MAPK IHC and the majority (8/10) showed evidence of MAPK activation (Table 5; Fig. 1B). For four patients (25%), BIM was neither expressed at baseline, nor was it induced by treatment in PMBCs. From 12 patients with detectable PBMC BIM prior to treatment, six (50%) exhibited BIM upregulation, three (25%) showed stable BIM levels, and three (25%) had decreased BIM expression during threatment. Of the six patients with objective disease stabilization or regression, four had detectable BIM in PBMCs at baseline; three of them (two patients with SD for longer than 5 months and one patient with PR for 7.5 months, all with NSCLC) or 75% showed PBMC BIM upregulation at 27 and 51 hours post treatment. For these three patients, archived tumors were available and all showed intratumoral MAPK pathway activation. Of the nine patients with PD, seven had detectable BIM in PBMCs before treatment, but only two of them or 28% exhibited treatment-induced increase in PBMC BIM expression.
Thus, treatment-induced BIM upregulation in PBMCs seemed to correlate with disease stabilization or regression in patients with cancers harboring MAPK pathway activation, indicating that changes in PBMC BIM expression during treatment may be a pharmacodynamic indicator of clinical benefit that warrants further investigation.
Discussion
In this Phase I clinical trial, drug combination, dosing schedule, and correlative PK and PD studies were rationally designed based on preclinical data indicating that RAS/RAF/MAPK pathway activation confers cancer cell resistance to paclitaxel due to functional BIM inactivation. While paclitaxel generally induces BIM accumulation and BIM-dependent apoptosis in vitro and in tumors in mice in vivo, the RAS/RAF/MAPK pathway suppresses BIM induction by phosphorylating BIM and targeting it to the proteasome for degradation. Bortezomib, a proteasome inhibitor, restores BIM induction in vitro, abrogates RAS-dependent cancer cell resistance to paclitaxel, and promotes BIM-dependent tumor regression (5). We hypothesized that concurrent paclitaxel and bortezomib administration would increase intratumoral BIM levels in malignancies with MAPK pathway activation and, thus, result in enhanced cancer cell death.
Given that weekly paclitaxel and bortezomib combination was a novel treatment scheme and that an earlier trial using these agents together, but on a different schedule, showed significant toxicity (18), the primary endpoint of our study was to determine the paclitaxel and bortezomib combination MTD, when both drugs were given on the same day weekly, using a modified continual reassessment method (MCRM) adapted for two-drug escalation. As shown in this study, MCRM can be more efficient in determining MTD than a classic 3+3 study design.
Paclitaxel and bortezomib combination was quite well tolerated. Most commonly observed adverse events included neuropathy (in 31.25 % of patients), fatigue (in 43.75% of patients), and anemia (in 43.75 % of patients), which were mostly Grade 1 or 2 in nature and treatment-related only in a minority of cases. A treatment-related Grade 3 neuropathy event (observed beyond cycle 1) resulted in a two-week dose delay and subsequent dose reduction, whereas an episode of treatment-related Grade 3 fatigue resulted in dose delay, but resolved with supportive care and did not require dose reduction. Treatment-related Grade 3 myalgias (one event) and Grade 2 (six events) and Grade 3 (one event) anemia were also observed. These side effects were narrower in spectrum and overall milder than those observed in previously published studies using this drug combination on different schedules. In a study combining paclitaxel on days 1 and 8 and bortezomib on days 1, 4, 8 and 11, the recommended phase II dose was 100 mg/m2 and 1.3 mg/m2, respectively (18). One third of the patients (9/31) had partial responses, but at the expense of significant toxicities, including cumulative peripheral neuropathy (in 76% of patients; Grade 3–4 in 9%) that necessitated treatment discontinuation in six patients, followed by diarrhea (55%) and fatigue (41%). Other earlier clinical trials also showed significant hematologic toxicities (16, 17). The improved safety profile observed in our study was likely due to a MCRM-determined MTD that was lower than previously defined by other approaches (18). Paclitaxel PK parameters were comparable to earlier published values (30–33), indicating no clinically significant PK interaction between paclitaxel and bortezomib.
Recommending combination paclitaxel and bortezomib doses that are lower than those commonly employed in monotherapy raises the concern that drug activity may be compromised at the gain of reduced toxicity. However, the proposed doses produce plasma paclitaxel and bortezomib concentrations known to be sufficient for microtubule and proteasome modulation, respectively. Furthermore, weekly paclitaxel at 60 mg/m2 has been used for disease control as singe agent (34) and in novel combinatorial therapy trials (35–39). Concern for significant neurologic toxicities, as both paclitaxel and bortezomib can cause neuropathy, also mandated lower initial drug doses. We are pleased to report clinical benefit, mostly as disease stabilization along with one PR case, in a third of all evaluable -generally heavily pretreated- patients. Interestingly, three out of five NSCLC patients demonstrated durable responses lasting 165 days or longer, including a confirmed PR. Antitumor activity was also observed in a recent phase I trial of neoadjuvant carboplatin, paclitaxel and bortezomib combination with concurrent radiotherapy in Stage III lung cancer, where complete pathologic complete responses were noted, but trial was terminated due to delayed, unpredictable and severe toxicities (40). Of note, two of the three NSCLC patients that showed clinical benefit on our trial had already been treated with paclitaxel in the adjuvant setting. Quite importantly, all three patients had tumors exhibiting MAPK pathway activation and showed treatment-induced BIM upregulation in PBMCs as a surrogate marker for intratumoral BIM activity, i.e. perfectly fitted the patient profile with an expected response, as predicted by our earlier preclinical studies (5). While our sample size is very small, these results clearly indicate that weekly paclitaxel and bortezomib combination is worthy of further investigation and may show clinical efficacy in carefully designed Phase II trials.
Determination of treatment-dependent PBMC BIM level changes as a pharmacodynamic parameter is another novelty of our trial. Baseline BIM levels and treatment-induced BIM expression alterations in PBMCs cannot provide quantitative information on intratumoral BIM status and its modifications, if any, during treatment, since somatic oncogenic mutations should only affect BIM expression in tumors. However, BIM monitoring in easily accessible PBMCs may be a qualitative indicator of how study drugs affect BIM expression in all tissues, including tumor site(s), as indicated by the correlation between treatment-induced PBMC BIM upregulation and clinical benefit in the small patient number treated in our study. A Phase II study for patients with documented intratumoral RAS/RAF/MAPK activation is, thus, recommended to further explore treatment-dependent PBMC BIM expression changes as a clinical response predictor. Baseline intratumoral BIM levels will also be determined and readily accessible tumor sites, such as metastatic skin lesions, will be serially biopsied for assessment of cancer cell BIM expression changes and apoptosis induction during treatment.
In conclusion, MTD for weekly paclitaxel and bortezomib combination was determined to be 60 mg/m2 and 1.0 mg/m2, respectively, using MCRM adapted for 2-drug escalation. Treatment was generally well tolerated and produced disease stabilization and/or regression in one third of evaluable patients, several of whom had received prior taxane exposure. Paclitaxel PK parameters were similar to those determined in previous studies, indicating no significant pharmacokinetic interactions between paclitaxel and bortezomib. A phase II trial combining paclitaxel and bortezomib as second line treatment for NSCLC patients is under development to more rigorously test the antitumor activity of this regimen and explore whether PBMC BIM expression changes during treatment may serve as a pharmacodynamic marker and a clinical response predictor.
Acknowledgments
We thank Millennium Pharmaceuticals, the Takeda Oncology Company, for generously providing bortezomib for this trial; the Cancer Institute of New Jersey (CINJ) Office of Human Research Services, Pharmacy, Tissue Analytical Services, and Phase I Clinic APNs Stephanie Beers, Phaedra Kirin and Cecilia Thomas for their hard work and commitment to making clinical trials happen; Dr. William N. Hait (Johnson & Jonhson) for his support and mentoring; and, above all, the patients who participated in the study.
Financial support: ECOG Paul Carbonne, MD Fellowship Award and Americal Society of Clinical Oncology Young Investigator Award (V.K.); NIH grant R37CA53370 (E.W.)
Abbreviations list
- ANC
Absolute neutrophil count
- AUC
Area under the curve
- BMK
Baby mouse kidney
- CBC
Complete blood count
- CL
Total body clearance
- Clast
last measurable concentration
- Cmax
Peak plasma concentration
- COPD
Chronic obstructive pulmonary disease
- CTCAE
Common terminology criteria for adverse events
- DLT
Dose-limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- IHC
Immunohistochemistry
- HPLC
High performance liquid chromatography
- MAPK
Mitogen-activated protein kinase
- MCRM
Modified continual reassessment method
- MTD
Maximum tolerated dose
- NSCLC
Non-small cell lung cancer
- PBMC
Peripheral blood mononuclear cell
- PD
Progressive disease
- PK
Pharmacokinetic
- PR
Partial remission
- SD
Stable disease
- SED
Standardized effective dose
- Tmax
Time to maximum concentration
- TR
Treatment-related
- ULN
Upper limit of normal
- Vz
Volume of distribution
- WBC
White blood cell
Footnotes
Potential conflicts of interest: None
References
- 1.Einzig AI, Neuberg D, Wiernik PH, Grochow LB, Ramirez G, O'Dwyer PJ, et al. Phase II Trial of Paclitaxel in Patients with Advanced Colon Cancer Previously Untreated with Cytotoxic Chemotherapy: An Eastern Cooperative Oncology Group Trial (PA286) Am J Ther. 1996;3:750–754. doi: 10.1097/00045391-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 7.Burger M, Denzinger S, Hammerschmied C, Tannapfel A, Maderstorfer A, Wieland WF, et al. Mitogen-activated protein kinase signaling is activated in prostate tumors but not mediated by B-RAF mutations. Eur Urol. 2006;50:1102–1109. doi: 10.1016/j.eururo.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury J. Genotypic guidance for chemotherapy choices. Drug Discov Today. 2005;10:608. doi: 10.1016/S1359-6446(05)03435-5. [DOI] [PubMed] [Google Scholar]
- 10.Finnberg N, El-Deiry WS. Paclitaxel and velcade: the rationale for a combo. Cancer Biol Ther. 2005;4:631–634. [Google Scholar]
- 11.Li R, Moudgil T, Ross HJ, Hu HM. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12:292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- 12.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 13.Moore PS, Barbi S, Donadelli M, Costanzo C, Bassi C, Palmieri M, et al. Gene expression profiling after treatment with the histone deacetylase inhibitor trichostatin A reveals altered expression of both pro- and anti-apoptotic genes in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2004;1693:167–176. doi: 10.1016/j.bbamcr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Qin JZ, Stennett L, Bacon P, Bodner B, Hendrix MJ, Seftor RE, et al. p53-independent NOXA induction overcomes apoptotic resistance of malignant melanomas. Mol Cancer Ther. 2004;3:895–902. [PubMed] [Google Scholar]
- 15.Ramaswamy B, Bekaii-Saab T, Schaaf LJ, Lesinski GB, Lucas DM, Young DC, et al. A dose-finding and pharmacodynamic study of bortezomib in combination with weekly paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2010;66:151–158. doi: 10.1007/s00280-009-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma C, Mandrekar SJ, Alberts SR, Croghan GA, Jatoi A, Reid JM, et al. A phase I and pharmacologic study of sequences of the proteasome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59:207–215. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 17.Croghan GA, Suman VJ, Maples WJ, Albertini M, Linette G, Flaherty L, et al. A study of paclitaxel, carboplatin, and bortezomib in the treatment of metastatic malignant melanoma: a phase 2 consortium study. Cancer. 2010;116:3463–3468. doi: 10.1002/cncr.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cresta S, Sessa C, Catapano CV, Gallerani E, Passalacqua D, Rinaldi A, et al. Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2008;44:1829–1834. doi: 10.1016/j.ejca.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Williams SA, Papandreou C, McConkey D. Preclinical effects of proteasome inhibitor PS-341 in combination chemotherapy for prostate cancer. Proc Am Soc Clin Oncol. 2001;20:169b. [Google Scholar]
- 20.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 21.Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41:429–436. doi: 10.1007/s002800050763. [DOI] [PubMed] [Google Scholar]
- 22.Faries D. Practical modifications of the continual reassessment method for phase I cancer clinical trials. J Biopharm Stat. 1994;4:147–164. doi: 10.1080/10543409408835079. [DOI] [PubMed] [Google Scholar]
- 23.O'Quigley J, Shen LZ. Continual reassessment method: a likelihood approach. Biometrics. 1996;52:673–684. [PubMed] [Google Scholar]
- 24.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology. 2004;18:14–21. [PubMed] [Google Scholar]
- 25.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Toppmeyer DL, Gounder M, Much J, Musanti R, Vyas V, Medina M, et al. A phase I and pharmacologic study of the combination of marimastat and paclitaxel in patients with advanced malignancy. Med Sci Monit. 2003;9:PI99–PI104. [PubMed] [Google Scholar]
- 28.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 30.Naoki K, Kunikane H, Fujii T, Tsujimura S, Hida N, Okamoto H, et al. Dose-escalating and pharmacokinetic study of a weekly combination of paclitaxel and carboplatin for inoperable non-small cell lung cancer: JCOG 9910-DI. Jpn J Clin Oncol. 2009;39:569–575. doi: 10.1093/jjco/hyp059. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Pandya KJ, Feins R, Johnstone DW, Watson T, Smudzin T, et al. Toxicity profile and pharmacokinetic study of a phase I low-dose schedule-dependent radiosensitizing paclitaxel chemoradiation regimen for inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;71:407–413. doi: 10.1016/j.ijrobp.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiser AL, Maluf FC, Chi DS, Sabbatini P, Hensley ML, Schwartz L, et al. A phase I study evaluating the safety and pharmacokinetics of weekly paclitaxel and carboplatin in relapsed ovarian cancer. Int J Gynecol Cancer. 2007;17:379–386. doi: 10.1111/j.1525-1438.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 33.Schneider B, Fukunaga A, Murry D, Yoder C, Fife K, Foster A, et al. A phase I, pharmacokinetic and pharmacodynamic dose escalation trial of weekly paclitaxel with interferon-alpha2b in patients with solid tumors. Cancer Chemother Pharmacol. 2007;59:261–268. doi: 10.1007/s00280-006-0264-z. [DOI] [PubMed] [Google Scholar]
- 34.Gadducci A, Katsaros D, Zola P, Scambia G, Ballardini M, Pasquini E, et al. Weekly low-dose paclitaxel as maintenance treatment in patients with advanced ovarian cancer who had microscopic residual disease at second-look surgery after 6 cycles of paclitaxel/platinum-based chemotherapy: results of an open noncomparative phase 2 multicenter Italian study (After-6 Protocol 2) Int J Gynecol Cancer. 2009;19:615–619. doi: 10.1111/IGC.0b013e3181a4476b. [DOI] [PubMed] [Google Scholar]
- 35.Hess V, Verrill MW, Bomphray CC, Vaughan MM, Allen M, Gore ME. Phase I study of carboplatin, doxorubicin and weekly paclitaxel in patients with advanced ovarian carcinoma. Ann Oncol. 2003;14:638–642. doi: 10.1093/annonc/mdg176. [DOI] [PubMed] [Google Scholar]
- 36.Inada S, Tomidokoro T, Fukunari H, Sato T, Hatano T, Nishimura A, et al. Phase I/II trial of combination therapy with S-1 and weekly paclitaxel in patients with unresectable or recurrent gastric cancer. Cancer Chemother Pharmacol. 2009;63:267–273. doi: 10.1007/s00280-008-0736-4. [DOI] [PubMed] [Google Scholar]
- 37.Wu CH, Yang CH, Lee JN, Hsu SC, Tsai EM. Weekly and monthly regimens of paclitaxel and carboplatin in the management of advanced ovarian cancer. A preliminary report on side effects. Int J Gynecol Cancer. 2001;11:295–299. doi: 10.1046/j.1525-1438.2001.011004295.x. [DOI] [PubMed] [Google Scholar]
- 38.Uhlmann C, Ballabeni P, Rijken N, Brauchli P, Mingrone W, Rauch D, et al. Capecitabine with weekly paclitaxel for advanced breast cancer: a phase I dose-finding trial. Oncology. 2004;67:117–122. doi: 10.1159/000080997. [DOI] [PubMed] [Google Scholar]
- 39.Rushing DA. Phase I/II study of weekly irinotecan and paclitaxel in patients with SCLC. Oncology. 2000;14:63–66. [PubMed] [Google Scholar]
- 40.Edelman MJ, Burrows W, Krasna MJ, Bedor M, Smith R, Suntharalingam M. Phase I trial of carboplatin/paclitaxel/bortezomib and concurrent radiotherapy followed by surgical resection in Stage III non-small cell lung cancer. Lung Cancer. 2010;68:84–88. doi: 10.1016/j.lungcan.2009.05.003. [DOI] [PubMed] [Google Scholar]