Abstract
OBJECTIVE
We investigated participation levels and relationships among cognition, depression, and participation for people with severe congestive heart failure (CHF).
METHOD
People with severe CHF (New York Heart Association Class III or IV) awaiting heart transplantation (N = 27) completed standardized tests of cognition and self-report measures of executive dysfunction, depressive symptoms, and participation.
RESULTS
Possible depression (64%) and cognitive impairment (15%–59%) were prevalent. Participants reported significant reductions in participation across all activity domains since CHF diagnosis (ps < .001). Worse executive dysfunction and depressive symptoms were associated with reduced participation and together accounted for 35%–46% of the variance in participation (ps < .01).
CONCLUSION
Participation restrictions associated with CHF are not limited to physically demanding activities and are significantly associated with executive dysfunction and depression. Cardiac rehabilitation should address cognitive and psychological functioning in the context of all life situations instead of focusing solely on physical function and disability.
Keywords: cognition disorders, depression, executive function, heart failure, human activities
Congestive heart failure (CHF) is a chronic and progressive medical condition in which the heart is unable to provide adequate blood flow or pressure to meet the body’s demands (Mudd & Kass, 2008). It is a syndrome that can result from several cardiovascular abnormalities, including coronary artery disease, hypertension, and myocardial infarction, which damage the heart so it cannot pump blood efficiently throughout the body. CHF affects almost 6 million Americans, accounts for more than 290,000 deaths annually in the United States (Lloyd-Jones et al., 2009), and is associated with significant functional limitations and reduced quality of life (QOL; Calvert, Freemantle, & Cleland, 2005; Juenger et al., 2002; Masoudi et al., 2004). The cost of CHF in the United States for 2009 was estimated at $37.2 billion, with a large proportion attributed to rehospitalizations (Lloyd-Jones et al., 2009; Moser & Watkins, 2008). As the population ages, the prevalence and subsequent socioeconomic impact of CHF are expected to rise.
The main clinical symptoms thought to limit function in people with CHF are fatigue, edema, and dyspnea (e.g., Spertus et al., 2002). CHF severity is classified according to the severity of these symptoms and the resulting severity of physical limitations in daily activities (Criteria Committee of the New York Heart Association [NYHA], 1994). In some cases, CHF symptoms can be ameliorated with aggressive medical therapy; for most clients, however, management programs aim to slow disease progression and maintain or improve physical functioning though lifestyle change (e.g., diet, smoking cessation), exercise, and medication.
Management for CHF primarily addresses physical functioning, but a growing body of research indicates that CHF is associated with a variety of “nonphysical” problems that can result in disability and reduced QOL. One of the most prevalent themes that emerged from a qualitative study investigating QOL from the perspective of people with CHF was role loss and the inability to engage fully in many of the instrumental, work, social, and family-related activities that gave them a sense of purpose, worth, and life satisfaction (Bosworth et al., 2004).
People described participation restrictions that were determined by limitations in more than just physical functioning. For instance, depression is well established in CHF, and prevalence estimates are as high as 70%, depending on the client population and measurement tool used (Joynt, Whellan, & O’Connor, 2004). Higher levels of depression in CHF are associated with increased mortality, rehospitalization, and reduced QOL (Carels, 2004; Gottlieb et al., 2004; Jiang et al., 2001) and may have a greater impact on QOL than do physical function limitations (Carels, 2004). In addition, approximately 25%–50% of people with CHF have cognitive impairment (Pressler, 2008). Cognitive impairment in CHF is associated with disability and even a fivefold increased risk of death (Zuccala et al., 2001, 2003). People with CHF often report that cognitive difficulties interfere with their everyday lives (Bennett, Sauve, & Shaw, 2005; Bosworth et al., 2004).
Despite existing literature on the prevalence and functional relevance of depression and cognitive impairment in CHF, these factors are not consistently addressed in treatment. By focusing primarily on physical functioning, clinicians are likely missing the full effect of CHF on their clients’ lives. These gaps may account for the inconsistent evidence related to the beneficial effect of existing disease management programs on QOL (e.g., Austin, Williams, Ross, Moseley, & Hutchison, 2005; Kulcu, Kurtais, Tur, Gulec, & Seckin, 2007). Although cardiac rehabilitation appears to have a net positive effect on health-related QOL (HRQOL), improvements are small and may be limited to physical domains of QOL (Cohen et al., 1999; Davies et al., 2010).
Occupational therapists consider all of a client’s strengths and weaknesses in the context of his or her environment and occupations to guide treatment aimed at maximizing participation in meaningful activities and roles. Disease management programs for people with CHF may be enhanced by incorporating this holistic perspective rather than focusing solely on physiological health and physical functioning. The first step in developing more comprehensive intervention approaches for CHF is to better understand the effect of CHF on participation in the broad range of activities that give people’s lives meaning and purpose and the potential impact of nonphysical disease manifestations on this participation. Therefore, the purposes of this study were to describe the participation levels of people with severe CHF and to explore relationships among cognitive impairment, depressive symptoms, and participation for this population. This work will inform the development of client-centered interventions that may be more effective than current approaches for improving or maintaining daily function, participation, and QOL.
Method
Participants and Procedure
This article presents a cross-sectional analysis of the baseline (pretransplant) assessment data from a longitudinal study on the effects of heart transplantation on cerebral metabolism, neuropsychological functioning, participation, and HRQOL in severe CHF. This study was approved by the Human Research Protection Office at Washington University in St. Louis, and all participants provided written informed consent.
Participants with CHF (N = 27) were recruited from the Congestive Heart Failure Center at Barnes–Jewish Hospital in St. Louis, Missouri, and met the following inclusion criteria: NYHA Class III or IV (indicating severe CHF), currently awaiting heart transplantation, and age ≥18 yr. Exclusionary criteria included history of cardiac arrest, inability to comply with the requirements of a positron emission tomography study, or pregnancy.
All study procedures took place in the hospital. Each participant underwent a neurological examination by a neurologist and completed a 90-min battery designed to measure cognitive and psychological functioning, participation, and HRQOL. The battery was administered in a quiet room by a trained research assistant with experience in neuropsychological testing. Basic demographic information and CHF-related clinical characteristics were obtained through interview and medical records. The Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) was used to measure global cognitive functioning, the Wechsler Test of Adult Reading (Wechsler, 2001) was used to estimate intelligence, and the FIM™ was used to assess independence in basic activities of daily living (ADLs). Details of the key measures reported in this article are described in the following paragraphs.
Cognition
Specific aspects of cognitive functioning were assessed with a battery of standardized neuropsychological tests. The Logical Memory Immediate and Delayed recall (LM I and II) test assessed verbal memory (Wechsler, 1997b), the Digit Span and Digit Symbol tests assessed attention (Wechsler, 1997a), and the Letter Fluency and Category Fluency tests assessed a component of executive functioning (Delis, Kaplan, & Kramer, 2001).
Executive functioning consists of the cognitive processes that orchestrate the planning, execution, and regulation of complex, goal-directed behavior (Lezak, 1995). Age-scaled standardized scores were derived for each test using published normative data (mean [M] = 10 ± 3). The 20-item Dysexecutive Questionnaire (DEX; Burgess, Alderman, Evans, Emslie, & Wilson, 1998) was administered as a measure of self-reported everyday executive function. Scores on the DEX range from 0 to 80, and higher scores indicate more frequent everyday executive function problems. Most information on the psychometric properties of the DEX comes from studies of brain-injured or other neurological populations, where internal consistency is adequate (α > .74; Chaytor & Schmitter-Edgecombe, 2007). However, inconsistencies regarding its latent structure, association with objective executive function measures, and validity as a measure of executive function exist (e.g., Bennett, Ong, & Ponsford, 2005; Burgess et al., 1998; Chaytor & Schmitter-Edgecombe, 2007), likely because of the broader problem of ambiguity in the definition of executive functioning itself.
Depressive Symptoms
The Center for Epidemiological Studies–Depression Scale (CES–D) was used to measure reported number and frequency of depressive symptoms (Radloff, 1977). Total scores range from 0 to 60. Higher scores indicate more depressive symptoms, and scores of ≥16 indicate possible depression. The CES–D has undergone extensive evaluation in different populations, age groups, and cultures and has acceptable internal consistency, test–retest reliability, construct validity, and discriminant validity (Devins et al., 1988; Radloff, 1977).
Participation
The Activity Card Sort (ACS; Baum & Edwards, 2001) was used to assess perceived amount of participation in 80 instrumental, low-physical-demand leisure, high-physical-demand leisure, and social activities. Participants were instructed to think of their participation in the various activities now and before their CHF diagnosis. To quantify current participation, participants sorted activities into the following categories with the corresponding numerical values: never done = 0, given up = 0, do now = 1, and do less = 0.5. Activities sorted into the given-up, do-now, and do-less categories received 1 point each for previous participation. This scoring system allowed for the calculation of each participant’s activity retention since CHF diagnosis: % activity retention = current participation / previous participation. The ACS was originally developed for older adults with Alzheimer’s disease but has since been used and deemed reliable and valid for various populations and across a broad adult age range (e.g., Baum & Edwards, 2008; Katz, Karpin, Lak, Furman, & Hartman-Maeir, 2003).
The Reintegration to Normal Living Scale (RNL; Wood-Dauphinee, Opzoomer, Williams, Marchand, & Spitzer, 1988) was used to assess satisfaction with participation in the areas of mobility, self-care, interpersonal relationships, family roles, and daily activity. Total scores range from 11 to 55. The RNL is well developed, has sound content and construct validity, and demonstrates high internal consistency in various acute-hospital, rehabilitation, and community-dwelling samples (α > .90; Stark, Edwards, Hollingsworth, & Gray, 2005; Wood-Dauphinee et al., 1988). Higher scores on both the ACS and the RNL indicate higher levels of perceived participation.
Analysis
Data were analyzed using SPSS for Windows Version 16.0 (SPSS, Inc., Chicago). Descriptive statistics were calculated for all variables. For parsimony and to reduce the number of statistical comparisons, we averaged the standardized scores of the tests in each cognitive domain to derive composite indexes of Memory (LM I and II), Executive Function (Letter and Category Fluency), and Attention (Digit Span and Digit Symbol) for each participant. Participants were then categorized as impaired in a cognitive domain if their composite index was <7 (i.e., >1 standard deviation [SD] below the published normative mean). We chose a sensitive cutoff because we believe it is clinically important to identify even subtle cognitive deficits in people with CHF because they are expected to meet the added cognitive challenge of managing a complex and chronic disease. The primary variables (cognitive measures, CES–D, ACS, and RNL) were normally distributed, so parametric tests were used (t tests, general linear models, Pearson r, linear regression). All tests were two-tailed, and p < .05 was considered significant.
Results
Sample Characteristics
Table 1 shows the basic demographic and clinical characteristics of the sample and the descriptive statistics for the cognitive measures and the CES–D. Seventeen participants were classified as NYHA III, and 10 were classified as NYHA IV. Etiology of CHF in the sample included ischemic cardiomyopathy (n = 13), idiopathic (n = 11), or other (1 each with myocarditis, postpartum cardiomyopathy, and congenital cardiomyopathy). At the time of the study, 6 participants were receiving continuous intravenous infusions of inotropic medication (dobutamine or milrinone) to improve cardiac contractility. Four participants had past neurological events consistent with transient ischemic attack (n = 3) or stroke (n = 1), but none had residual neurological deficits or physical disability from these events at the time of study. Only 1 participant had neurological deficits according to the baseline neurological examination (mild left leg weakness associated with a prior back injury), and all were independent in their basic activities of daily living. Four participants had some high school education or less, 6 had high school diplomas (or equivalent), 13 had some college education or vocational college degrees, and 4 had bachelor’s degrees or higher.
Table 1. Descriptive Statistics of the Sample (N = 27).
| Variable | n | M | SD | Range |
|---|---|---|---|---|
| Age (yr) | 27 | 49.1 | 12.2 | 24–64 |
| Sex: Male/female | 21/6 | — | — | — |
| Race: White/Black | 15/12 | — | — | — |
| Duration of cardiac symptoms (years) |
27 | 6.8 | 4.8 | 1–16 |
| LVEF (%) | 27 | 20.7 | 9.5 | 5–39 |
| MMSE | 27 | 28.6 | 2.6 | 20–30 |
| WTAR | 24 | 90.1 | 16.9 | 54–124 |
| LM I | 27 | 6.2 | 2.9 | 1–11 |
| LM II | 27 | 7.8 | 2.8 | 3–14 |
| Letter fluency | 27 | 8.4 | 3.7 | 2–18 |
| Category fluency | 27 | 8.7 | 3.2 | 3–17 |
| Digit span | 24 | 9.6 | 2.0 | 5–13 |
| Digit symbol | 24 | 9.3 | 3.8 | 4–18 |
| DEX | 24 | 23.0 | 15.9 | 3–68 |
| CES–D | 23 | 18.5 | 12.3 | 1–55 |
Note. CES–D = Center for Epidemiological Studies–Depression Scale; DEX = Dysexecutive Questionnaire; LM I = Logical Memory Immediate Recall; LM II = Logical Memory Delayed Recall; LVEF = left ventricular ejection fraction; M = mean; MMSE = Mini-Mental State Examination; SD = standard deviation; WTAR = Weschler Test of Adult Reading. Data presented for WTAR, LM I and II, Letter and Category Fluency, Digit Span, and Digit Symbol are age-scaled standardized scores. Data presented for DEX, CES–D, and MMSE are raw total scores.
For most of the neuropsychological tests, the group’s performance was slightly below the published normative mean but still in the range of what would be considered “normal” performance (Lezak, 1995); however, the sample performed “below normal” on LM I (Table 1). In terms of our composite indexes, 59% of our sample had impaired memory, 33% had impaired executive functioning, and 15% (of those who completed the Digit Span and Digit Symbol tests) had impaired attention. In addition, 64% of participants who completed the CES–D scored above the cutoff for possible depression. DEX and verbal fluency scores were moderately but significantly correlated with each other (r = −.44, p = .03).
Quantification of Participation
Participants reported significant reductions in participation since CHF diagnosis in all four activity domains (all t[23] > 4.58, all p < .001; see Figure 1). There was a main effect of activity domain on Activity Retention, F(3, 81) = 58.78, p < .001. Pairwise comparisons revealed that the highest percentage of activities retained was in low-demand leisure activities, followed by instrumental and social activities (not significantly different from each other), and then high-demand leisure activities.1 We summed the previous and current participation scores across the four activity domains to derive total previous and current participation scores for each participant (total previous: M = 56.7, SD = 12.5; total current: M = 35.9, SD = 10.5, t[23] = 9.49, p < .001). On average, participants had retained 64% (SD = 14%) of their total activities since being diagnosed with CHF. The sample’s mean score on the RNL was 41.1 (SD = 7.7, n = 23) indicating that, in general, people felt neutral or satisfied with their level of integration; however, participants had a wide range of scores on this measure (22–53).
Figure 1.
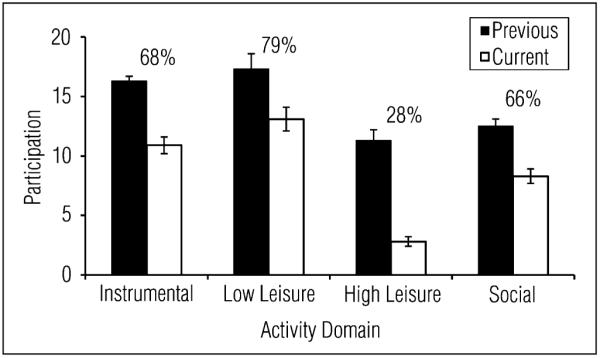
Previous and current participation scores for each domain of the Activity Card Sort (n = 24). Percentage of activity retention for each domain is also displayed. Error bars represent standard error of the mean.
Relationships Among Cognition, Depression, and Participation
Correlations among current levels of cognitive functioning, depressive symptoms, and participation are presented in Table 2. DEX and CES–D scores were significantly correlated with total current ACS and RNL scores, such that more reported everyday executive function problems and depressive symptoms were associated with less perceived participation. No significant correlations were found between memory or attention performance and participation (ps > .35). In addition, no significant correlations were found between participation, DEX, and CES–D and other potentially influential demographic or clinical characteristics, including age, cardiac symptom duration, or left ventricular ejection fraction (rs = −.27 to −.20, ps > .20). Moreover, participation, DEX, and CES–D scores did not differ according to NYHA class (ps > 0.15).
Table 2. Correlations (Pearson r) Between Cognition and Depressive Symptoms and Current Participation.
| Total Current Activity Card Sort | Reintegration to Normal Living Scale | |
|---|---|---|
| Memory | .20 | .13 |
| Executive function | .34 | .41* |
| Attention | .04 | .11 |
| Dysexecutive Questionnaire | −.57** | −.49* |
| Center for Epidemiological Studies–Depression Scale | −.64*** | −.68*** |
Note. For analyses with Center for Epidemiological Studies–Depression Scale and Reintegration to Normal Living Scale, n = 23. For all other analyses, n = 24.
p < .05.
p < .01.
p < .005 (Bonferroni-corrected significant p value).
To further explore the independent contributions of everyday executive function and depressive symptoms to participation, we performed hierarchical linear regression analyses. Total current ACS and RNL scores were dependent variables (two separate models), and DEX and CES–D scores were force-entered as predictors. DEX and CES–D scores each uniquely accounted for 11% of the variance in total current ACS (ps = .07) and together accounted for 35% of the variance in total current ACS (F[2, 20] = 6.68, p = .006). In the model predicting RNL, CES–D uniquely accounted for 27% of the variance (p = .004), and the unique variance accounted for by the DEX was only 3% (p = .26). Together, DEX and CES–D scores accounted for 46% of the variance in RNL (F[2, 20] = 9.84, p = .001).
Discussion
Our purpose was to examine activity participation in people with severe CHF, including the potential relevance of cognitive and psychological factors. We found that people with severe CHF experience significant reductions in instrumental, leisure, and social activity participation. Executive dysfunction and depressive symptoms, which are relatively common in this population, are independent predictors of restricted participation. Our study was limited in sample size, and several participants did not complete the entire testing session because of scheduling issues or fatigue. Despite these limitations, our results provide new insight into CHF that is relevant for occupational therapy. These results suggest that management of CHF should expand in focus from physical functioning and physically demanding activities to consider cognitive and psychological functioning in the context of all life situations.
Most investigations into the effect of CHF on people’s lives have focused primarily on physical symptoms and physical disability. Even commonly used measures of QOL in this population overemphasize physical domains of function and, in actuality, consist of items that describe health status rather than the patient’s perspective of the effect of CHF on his or her everyday life (Dunderdale, Thompson, Miles, Beer, & Furze, 2005). Bosworth et al. (2004) revealed the broad range of concerns of people with CHF and highlighted the centrality of participation in instrumental activities and work, social, and family roles to QOL. The current study extends those findings and quantifies the participation levels of people with severe CHF in these meaningful activities and roles.
The extent of restricted participation in our sample was surprising. For comparison, despite being younger, our participants had given up, on average, 20% more of their activities since diagnosis than had a sample of people after stroke (Connor, Wolf, Foster, Hildebrand, & Baum, in press). The drastic reduction in high-physical-demand leisure participation was expected, considering the effect of CHF on physical fitness. The relative preservation of low-physical-demand leisure activities may represent a shift in leisure engagement toward less strenuous pursuits. Our version of the ACS did not allow for quantification of activities gained over time, but many participants expressed increases in sedentary activities such as resting and watching TV. Although less extreme than the high-physical-demand leisure domain, participants also reported substantial reductions in instrumental and social activities. These activities do not necessarily require high levels of physical capacity, yet they are essential for health and well-being (e.g., Everard, Lach, Fisher, & Baum, 2000; Glass, de Leon, Marottoli, & Berkman, 1999).
More than three-quarters of our sample had cognitive impairment, possible depression, or both. These results are consistent with previous studies that have found high rates of concomitant cognitive and psychological dysfunction in people with CHF (Joynt et al., 2004; Pressler, 2008). There was a high degree of variability in reported dysexecutive and depressive signs in our sample, which was not related to variations in markers of heart failure severity. Whether this heterogeneity is an effect of individual differences in other personal, environmental (e.g., social support), or occupational (e.g., level of daily cognitive challenge) factors warrants further investigation. The potential for unreliable subjective reports of executive dysfunction also cannot be overlooked; however, the significant association between the subjective (DEX) and objective (verbal fluency) measures of executive function provides some support for the validity of our participants’ self-reports. In any case, our findings reinforce the notion that sensitive, individualized evaluation of cognitive function and depressive symptoms—ideally from multiple sources such as self-report, informant report, and standardized tests—should be a priority in the clinical management of this population.
Although not all participants experienced executive dysfunction or depressive symptoms in daily life, those who did tend to participate in fewer activities. Whereas executive dysfunction and depressive symptoms may contribute equally to level of participation, depressive symptoms may be more strongly related to satisfaction with that participation, as indicated by the stronger association with the RNL. That these factors share a large amount of the variance in participation is not surprising, because they are commonly associated with each other, although the exact nature of their relationship in the context of CHF remains to be determined (Bennett, Sauve, & Shaw 2005).
The clinical implications of cognitive impairment and depressive symptoms in CHF may go beyond their effects on activity participation. Health care professionals must also be aware that these issues can pose barriers to treatment. Depression could lead to reduced effort or motivation during rehabilitation, and cognitive impairment could limit a person’s capacity to learn complex dietary and medical regimens. These problems may contribute to noncompliance and an increased frequency of complications and rehospitalization (Jiang et al., 2001; Joynt et al., 2004; McLennan, Pearson, Cameron, & Stewart, 2006). Given that much of the enormous socioeconomic burden associated with CHF is caused by hospital readmissions, it is imperative that we develop enhanced intervention approaches for people with CHF who have these neuropsychological dysfunctions.
Much of the evaluation and management of CHF targets physical function and disability. However, we found that people with CHF experience drastic reductions in participation across all activity domains and that these participation restrictions may, in part, result from executive dysfunction and depressive symptoms. Although there appears to be a trend toward more comprehensive cardiac rehabilitation programs that involve occupational therapy and address psychosocial concerns (e.g., Austin et al., 2005; Balady et al., 2000), this model is not consistently used, and cognitive functioning continues to be overlooked. Occupational therapists have been challenged to expand their toolboxes beyond physical rehabilitation and to begin to address less obvious impairments, like executive dysfunction, that are prevalent in many of the clinical populations they encounter (Baum, Foster, & Wolf, 2009). This study has identified yet another clinical population that could benefit from this approach and has revealed a valuable role for occupational therapy in enhancing current disease management programs for people with CHF.
Acknowledgment
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS044885) and the James S. McDonnell Foundation (220020087).
Footnotes
In total, 10 tests were performed to quantify changes in participation since CHF diagnosis: four t tests comparing previous vs. current scores for each activity domain and six pairwise comparisons of activity retention scores among the activity domains. All comparisons (except for the difference between instrumental and social activity retention) met statistical significance after Bonferroni correction (i.e., p < .05/10, or .005).
Foster, E. R., Cunnane, K. B., Edwards, D. F., Morrison, M. T., Ewald, G. A., Geltman, E. M., et al. (2011). Executive dysfunction and depressive symptoms associated with reduced participation of people with severe congestive heart failure. American Journal of Occupational Therapy, 65, 306–313. doi: 10.5014/ajot.2011.000588
Contributor Information
Erin R. Foster, Program in Occupational Therapy, Department of Neurology and Department of Psychiatry, Washington University in St. Louis School of Medicine, 660 South Euclid Avenue, Campus Box 8111, St. Louis, MO 63110.
Kathleen B. Cunnane, The Rehabilitation Institute of St. Louis, St. Louis, MO.
Dorothy F. Edwards, Department Kinesiology—Occupational Therapy Program, Department of Neurology, and Department of Medicine, University of Wisconsin, Madison.
M. Tracy Morrison, Department of Occupational Therapy, University of Kansas, Kansas City, and Assistant Research Scientist, Sister Kenny Research Center, Minneapolis, MN.
Gregory A. Ewald, Department of Medicine, Cardiovascular Division, Washington University in St. Louis School of Medicine, St. Louis, MO.
Edward M. Geltman, Department of Medicine, Cardiovascular Division, Washington University in St. Louis School of Medicine, St. Louis, MO.
Allyson R. Zazulia, Department of Neurology and Department of Radiology, Washington University in St. Louis School of Medicine, St. Louis, MO.
References
- Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. European Journal of Heart Failure. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Ades PA, Comoss P, Limacher M, Pina IL, Southard D, et al. Core components of cardiac rehabilitation/secondary prevention programs: A statement for healthcare professionals from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation Writing Group. Circulation. 2000;102:1069–1073. doi: 10.1161/01.cir.102.9.1069. [DOI] [PubMed] [Google Scholar]
- Baum CM, Edwards DF. Activity Card Sort. Program in Occupational Therapy, Washington University School of Medicine; St. Louis, MO: 2001. [Google Scholar]
- Baum CM, Edwards DF. Activity Card Sort. 2nd ed. AOTA Press; Bethesda, MD: 2008. [Google Scholar]
- Baum CM, Foster ER, Wolf TJ. Addressing performance and participation in occupational therapy: The importance of cognition. British Journal of Occupational Therapy. 2009;72:143. [Google Scholar]
- Bennett PC, Ong B, Ponsford J. Assessment of executive dysfunction following traumatic brain injury: Comparison of the BADS with other clinical neuropsychological measures. Journal of the International Neuropsychological Society. 2005;11:606–613. doi: 10.1017/S1355617705050721. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Sauve MJ, Shaw RM. A conceptual model of cognitive deficits in chronic heart failure. Journal of Nursing Scholarship. 2005;37:222–228. doi: 10.1111/j.1547-5069.2005.00039.x. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Steinhauser KE, Orr M, Lindquist JH, Grambow SC, Oddone EZ. Congestive heart failure patients’ perceptions of quality of life: The integration of physical and psychosocial factors. Aging and Mental Health. 2004;8:83–91. doi: 10.1080/13607860310001613374. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society. 1998;4:547–558. doi: 10.1017/s1355617798466037. doi: 10.1017/S1355617798466037. [DOI] [PubMed] [Google Scholar]
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE–HF study. European Journal of Heart Failure. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Carels RA. The association between disease severity, functional status, depression and daily quality of life in congestive heart failure patients. Quality of Life Research. 2004;13:63–72. doi: 10.1023/B:QURE.0000015301.58054.51. doi: 10.1023/B:QURE.0000015301.58054.51. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. Fractionation of the dysexecutive syndrome in a heterogeneous neurological sample: Comparing the Dysexecutive Questionnaire and the Brock Adaptive Functioning Questionnaire. Brain Injury. 2007;21:615–621. doi: 10.1080/02699050701426949. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Moser DJ, Clark MM, Aloia MS, Cargill BR, Stefanik S, et al. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. American Journal of Cardiology. 1999;83:1374–1378. doi: 10.1016/s0002-9149(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Connor LT, Wolf TJ, Foster ER, Hildebrand M, Baum CM. Participation and engagement in occupation in adults with disabilities. In: Pierce D, editor. Occupational science for occupational therapy. Slack; Thorofare, NJ: in press. [Google Scholar]
- Criteria Committee of the New York Heart Association . Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Little, Brown; Boston: 1994. [Google Scholar]
- Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews. 2010;4:CD003331. doi: 10.1002/14651858.CD003331.pub3. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis–Kaplan Executive Function System. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Devins GM, Orme CM, Costello CG, Binik YM, Frizzell B, Stam HJ, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES–D) Scale. Psychology and Health. 1988;2:139–156. doi: 10.1080/08870448808400349. [Google Scholar]
- Dunderdale K, Thompson DR, Miles JN, Beer SF, Furze G. Quality-of-life measurement in chronic heart failure: Do we take account of the patient perspective? European Journal of Heart Failure. 2005;7:572–582. doi: 10.1016/j.ejheart.2004.06.006. doi: 10.1016/j.ejheart.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Everard KM, Lach HW, Fisher EB, Baum MC. Relationship of activity and social support to the functional health of older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2000;55B:S208–S212. doi: 10.1093/geronb/55.4.s208. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glass TA, de Leon CM, Marottoli RA, Berkman LF. Population-based study of social and productive activities as predictors of survival among elderly Americans. BMJ. 1999;319:478–483. doi: 10.1136/bmj.319.7208.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. Journal of the American College of Cardiology. 2004;43:1542–1549. doi: 10.1016/j.jacc.2003.10.064. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Whellan DJ, O’Connor CM. Why is depression bad for the failing heart? A review of the mechanistic relationship between depression and heart failure. Journal of Cardiac Failure. 2004;10:258–271. doi: 10.1016/j.cardfail.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, et al. Health related quality of life in patients with congestive heart failure: Comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N, Karpin H, Lak A, Furman T, Hartman-Maeir A. Participation in occupational performance: Reliability and validity of the Activity Card Sort. OTJR: Occupation, Participation and Health. 2003;23:10–17. [Google Scholar]
- Kulcu DG, Kurtais Y, Tur BS, Gulec S, Seckin B. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure: A randomized controlled trial, short-term results. Europa Medicophysica. 2007;43:489–497. [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. Oxford University Press; New York: 1995. [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Masoudi FA, Rumsfeld JS, Havranek EP, House JA, Peterson ED, Krumholz HM, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. Journal of Cardiac Failure. 2004;10:368–373. doi: 10.1016/j.cardfail.2004.01.009. [DOI] [PubMed] [Google Scholar]
- McLennan SN, Pearson SA, Cameron J, Stewart S. Prognostic importance of cognitive impairment in chronic heart failure patients: Does specialist management make a difference? European Journal of Heart Failure. 2006;8:494–501. doi: 10.1016/j.ejheart.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Moser DK, Watkins JF. Conceptualizing self-care in heart failure: A life course model of patient characteristics. Journal of Cardiovascular Nursing. 2008;23:205–218. doi: 10.1097/01.JCN.0000305097.09710.a5. quiz 219–220. [DOI] [PubMed] [Google Scholar]
- Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- Pressler SJ. Cognitive functioning and chronic heart failure: A review of the literature (2002–July 2007) Journal of Cardiovascular Nursing. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES–D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–410. [Google Scholar]
- Spertus JA, Tooley J, Jones P, Poston C, Mahoney E, Deedwania P, et al. Expanding the outcomes in clinical trials of heart failure: The quality of life and economic components of EPHESUS (EPlerenone’s neuroHormonal Efficacy and SUrvival Study) American Heart Journal. 2002;143:636–642. doi: 10.1067/mhj.2002.120775. [DOI] [PubMed] [Google Scholar]
- Stark SL, Edwards EF, Hollingsworth H, Gray DB. Validation of the Reintegration to Normal Living Index in a population of community-dwelling people with mobility limitations. Archives of Physical Medicine and Rehabilitation. 2005;86:344–345. doi: 10.1016/j.apmr.2004.03.020. doi: 10.1016/j.apmr.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd ed. Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading manual. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Wood-Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: The Reintegration to Normal Living Index. Archives of Physical Medicine and Rehabilitation. 1988;69:583–590. [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Cocchi A, Carosella L, Cattel C, et al. GIFA (SIGG–ONLUS) Investigators Cognitive dysfunction as a major determinant of disability in patients with heart failure: Results from a multicentre survey. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:109–112. doi: 10.1136/jnnp.70.1.109. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. American Journal of Medicine. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. doi: 10.1016/S0002-9343(03)00264-X. [DOI] [PubMed] [Google Scholar]


