Abstract
Small molecule inhibitors of Kinesin-5 (K5Is) that arrest cells in mitosis with monopolar spindles are promising anti-cancer drug candidates. Clinical trials of K5Is revealed dose-limiting neutropenia, or loss of neutrophils, for which the molecular mechanism is unclear. We investigated the effects of a K5I on HL60 cells, a human promyelocytic leukemia cell line that is often used to model dividing neutrophil progenitors in cell culture. We found K5I treatment caused unusually rapid death of HL60 cells exclusively during mitotic arrest. This mitotic death occurred via the intrinsic apoptosis pathway with molecular events that include cytochrome c leakage into the cytoplasm, caspase activation, and Parp1 cleavage. Bcl-2 overexpression protected from death. We probed mitochondrial physiology to find candidate triggers of cytochrome c release, and observed a decrease of membrane potential (ΔΨm) before mitochondrial outer membrane permeabilization (MOMP). Interestingly, this loss of ΔΨm was not blocked by overexpressing Bcl-2, suggesting it might be a cause of Bax/Bak activation, not a consequence. Taken together, these results show that K5I induces intrinsic apoptosis during mitotic arrest in HL60 with loss of ΔΨm as an upstream event of MOMP.
Keywords: Kinesin-5, mitosis, apoptosis, HL60
1. Introduction
Anti-mitotic drugs are thought to gain their selectivity largely through killing cells that enter mitosis, which is more frequent in tumors than most normal tissues [1]. Taxanes and Vinca alkaloids are classic cell-cycle specific drugs. By inhibiting microtubule polymerization dynamics they prevent satisfaction of the spindle assembly checkpoint, which then arrests cells in mitosis. This arrest causes cell death by still poorly-characterized mechanisms [2,3]. They are active against a variety of cancers, but their use is limited in part by neurotoxicity. This prompted development of more selective anti-mitotic drugs that target proteins important only during mitosis, including Polo-like kinase (PLK1), Aurora A, Aurora B, CENP-E and Kinesin-5 motor protein (KSP, Eg5) [1,2,4–7].
Here we focus on Kinesin-5 inhibitors (K5Is), which promote mitotic arrest and cell death via formation of monopolar mitotic spindles [8]. Several K5Is have been developed and introduced into Phase 1 or 2 clinical trials, including SB-715992 (Ispinesib) and SB-743921 from Cytokinetics, AZD4877 from AstraZeneca, ARRY-520 from Array BioPharma, EMD534085 from Merck Serono, and LY2523355 from Eli Lily [9–15]. If mitotic arrest is a general therapeutic strategy in cancer, K5Is should be as effective as microtubule-targeting drugs in killing cancer cells, but lack their neurotoxicity. This promise appeared to hold in tumor cell lines and mouse xenograft cancer models [16–20]. In clinic, SB-715992 showed some promising responses in a Phase-2 trial in advanced-stage breast cancer [12]. All of the K5Is tested in clinical trials caused dose-limiting neutropenia, or loss of neutrophils, but caused little toxicity to proliferating epithelial cells in hair follicles and the gut, at the dose limit [1,6,21]. It thus appears that K5Is are selectively toxic to neutrophil progenitors in the bone marrow.
Determining the mechanistic basis of this toxicity on neutrophil progenitors is important in development of more efficacious anti-mitotic strategies. To gain mechanistic clues into how a K5I induces cell death in proliferating pre-neutrophils, we investigated its effects on HL60 cells. This line, derived from an acute promyelocytic leukemia (APL) patient, readily differentiates into neutrophil-like cells, and is often used as a neutrophil precursor model [22–25]. The K5I we tested throughout this study, EMD534085, is a potent and selective compound developed by Merck Serono, which inhibits ATPase activity of Kinesin-5 [20].
2. Materials and Methods
2.1 Cell Culture
RPE-1, A549, HeLa, MCF7, TF1a, K562, and HL60 were grown in media recommended by the American Type Culture Collection. SU-DHL-8 (gift from Jing Deng and Anthony Letai, Dana Farber Cancer Institute) was grown in RPMI medium supplemented with 10% fetal bovine serum (FBS), 100 I.U./mL penicillin, and 100 µg/mL streptomycin. H7 (gift from Andrew Chess, Massachusetts General Hospital and Harvard Medical School) was grown in the same medium as SU-DHL-8 except with 15% FBS.
2.2 Reagents and Antibodies
EMD534085 was from Merck Serono. 4-hydroperoxy-cyclophosphamide (4-OOH-CY) was from Santa Cruz. RO-3306 was from Axxora. MitoTracker-Red CMXRos was from Invitrogen. Protease and phosphatase inhibitor cocktails were from Roche. PhosphoS10-Histone H3 antibody was from Millipore; Parp1, caspase-8, −9, cleaved caspase-3, −7, XIAP antibodies were from Cell Signaling Technologies Inc.; Mcl1 antibody was from Santa Cruz Biotechnology; Bim antibody was from Calbiochem; actin antibody was from Sigma. Rabbit anti-Bax and mouse anti-Bax clone 6A7 were from Santa Cruz Biotechnology and Sigma respectively.
2.3 Live-cell Time-lapse Microscopy and Analysis
Adherent cells were in growth medium or CO2-independent medium (Invitrogen) with 10% FBS, 100 I.U./mL penicillin, and 100 µg/mL streptomycin. Suspension cells were in growth medium and were immobilized on glass-bottom MatTek dishes using Cell-Tak (BD Biosciences) following the manufacturer’s instructions. A Nikon TE2000E motorized inverted microscope with incubation chamber (37°C, 5% CO2) and Hamamatsu ORCA-ER cooled CCD camera were used. Phase-contrast time-lapse microscopy was taken for adherent cells and was performed as described previously [26]. Differential interference contrast (DIC) microscopy was taken for suspension cells. Time-lapse movies were reviewed and cells were scored and analyzed in MetaMorph.
2.4 Flow Cytometry and Cell Cycle Distribution Analysis
Cells were collected and fixed in 80% ethanol at −20°C for at least 2 hrs. Fixed cells were washed in cold PBS once and stained in 25 µg/mL propidium iodide, 0.1% Triton X-100, 0.2 mg/mL RNAseA in PBS at 37°C in dark for 1 hr. The DNA content was determined using a BD FACSCalibur, and cell-cycle distribution was analyzed using CellQuest.
2.5 Cell-cycle Synchronization
Three different synchronization methods were tested for HL60 cells: G2-phase block using RO-3306, or S-phase block using single-thymidine or double-thymidine treatment. For G2-phase block, HL60 cells were treated with 10µM RO-3306 for 16 hrs, then were washed and released to warm growth medium. For single-thymidine synchronization, HL60 cells were treated with 2mM thymidine for 24 hrs, then were washed and released to warm growth medium. For double-thymidine synchronization, HL60 cells were first treated with 2mM thymidine for 24 hrs, then were released to growth medium for 5 hrs, and then were treated with 2mM thymidine again for another 16 hrs before being washed and released to growth medium.
Epithelial cell lines HeLa and MCF7 were synchronized in G2-phase using RO-3306. Cells were treated with 10µM RO-3306 for 16 hrs, and then were washed and released to either warm growth medium or medium supplemented with 500nM EMD534085.
2.6 Western Blot and Immunoprecipitation
Harvested cell pellets were lysed in lysis buffer (50mM Tris pH 7.5, 100mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 1x protease inhibitor cocktail, 50mM NaF, phosphatase inhibitor cocktail) for 30min on ice. Lysed cells were centrifuged at 14,000 RPM for 30min at 4°C. Supernatant was collected and protein concentration was determined using Pierce BCA Protein Assay Kit. Protein samples were prepared in LDS Sample Buffer and Reducing Agent (NuPAGE), and 10–40 µg of protein were loaded into each well of 4–12% Bis-Tris gels (Invitrogen). Gels were transferred onto nitrocellulose membranes. Blots were probed with commercial primary antibodies and chemiluminescent detection was done using ECL-plus (Amersham).
Immunoprecipitation of activated Bax using anti-Bax antibody clone 6A7 (Sigma) was described previously [27]. Cell pellets were lysed in 1% CHAPS lysis buffer (150mM NaCl, 10mM HEPES (pH 7.4), 1% CHAPS) with a cocktail of protease inhibitors on ice for 30 minutes.
2.7 Immunofluorescence Microscopy and Analysis
HL60 cells were washed in PBS, immobilized on glass coverslips using Cell-Tak (BD Biosciences), and were fixed in 3.7% formaldehyde at room temperature for 20 min. Cells were permeabilized in 0.2% Triton X-100 in PBS for 10 min, blocked in 4% bovine serum albumin fraction V in PBS for 1 hr, and labeled with α-tubulin (Sigma; DM1A) and cytochrome c (Promega) antibodies for 1 hr at room temperature. Cells were washed in PBS, labeled with secondary antibodies conjugated to Alexa Fluor 488 or 594, washed in PBS, and stained for DNA with 1 µg/mL TO-PRO3 (Invitrogen) for 5 min. Coverslips were mounted on glass microscope slides using Prolong-Gold antifade reagent (Invitrogen).
For cleaved-Parp and cytochrome c co-staining, HL60 cells were processed as above, and labeled with cytochrome c antibody (Promega) for 1 hr at room temperature. Cells were washed with PBS, labeled with secondary antibody conjugated to Alexa Fluor 594, washed in PBS, and blocked in normal mouse IgG (Santa Cruz Biotechnology) for another 30min at room temperature. Cells were then labeled with DM1A conjugated to FITC and cleaved-Parp conjugated to Alexa Fluor 647 (BD Biosciences) antibodies for 1 hr at room temperature. Cells were then washed, and coverslips were mounted as above.
For MitoTracker-Red and cytochrome c co-staining, cells were incubated with 125nM MitoTracker-Red (Invitrogen) in culture for 10 min, washed in warm PBS, immobilized on Cell-Tak coated glass coverslip for HL60/Neo and HL60/Bcl-2 cells, and were fixed in 3.7% formaldehyde as before. After aspirating off the fixative, cells were permeabilized in −20°C methanol for 10 min, and were washed with PBS. Cells were blocked in 4% bovine serum albumin fraction V in PBS for 1 hr, and labeled with FITC conjugated α-tubulin (Sigma; DM1A) and Alexa Fluor 647 conjugated cytochrome c (BD Biosciences) antibodies. Cells were washed in PBS and were mounted as before. Z-planes of random fields were acquired using a Nikon Ti motorized inverted microscope, Yokagawa CSU-10 spinning disk confocal with 488nm, 568nm, and 647nm laser lines, and Hamamatsu ORCA-AG cooled CCD camera. Regions of cells were drawn in MetaMorph, in which average MitoTracker-Red fluorescent intensities of mitochondria in all z planes were analyzed in a customized Matlab program. Statistical analysis was done in Excel. A box-and-whisker plot of average MitoTracker-Red fluorescent intensities of mitochondria in normal mitotic and mitotic arrest cells of HL60/Neo, HL60/Bcl-2, HeLa, and MCF7 is generated in Matlab. (A box-and-whisker plot is a graphical display that shows the median, quartiles, and outliers of a set of data.)
2.8 shRNA Transfection and Lentiviral Transduction for Bim and Bid Knockdown
Human pLKO.1 lentiviral shRNA target gene set (4 clones) for Bim and shRNA target gene set (4 clones) for Bid were obtained from Open Biosystems. Each lentiviral shRNA construct was transfected into 293T cells using FuGENE 6 (Roche). Viral supernatants were collected 48 and 72 hrs after transfection, filtered, and used to infect HL60 cells for 4 hrs. After the last viral infection, cells were re-plated in growth medium containing 1 µg/mL puromycin. 24 hrs later, parental, Bim-, and Bid-knockdown HL60 cells were harvested for western-blot and in parallel, were treated with EMD534085.
3. Results
3.1 Cell Death Kinetics after Mitotic Arrest
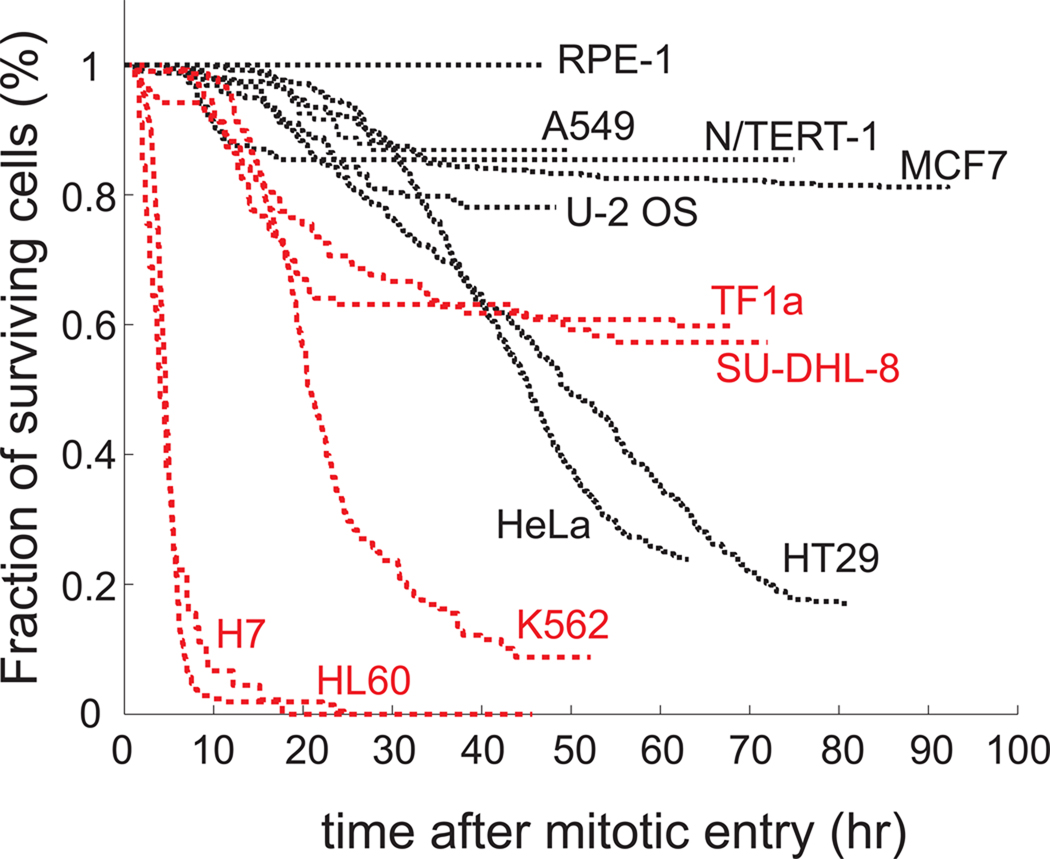
In preliminary tests, we found HL60 cells exhibited unusually rapid and complete cell death during K5I-induced mitotic arrest. To test if this effect was specific to this cell line, we tested five cell lines derived from hematological lineages. These included four lines derived from leukemias or lymphomas (TF1a, SU-DHL-8, K562, and HL60) and one non-cancer line derived from normal human lymphocytes by immortalization with Epstein-Barr virus (H7). As comparators, we chose seven lines derived from epithelial lineages, including five carcinoma-derived lines (A549, U-2 OS, MCF7, HeLa, and HT29) and two telomerase-immortalized lines derived from normal tissues (RPE-1 from retinal pigment epithelium, and N/TERT-1 from keratinocytes).
To measure the extent and kinetics of mitotic arrest and cell death in EMD534085, we performed time-lapse microscopy. This allows accurate scoring of death kinetics in single cells despite varying rates between individual cells in a population, and also measurement of time elapsed between mitotic entry and death without the need for synchronization [26,28,29]. Non-adherent hematological lineage lines were immobilized using BD Cell-Tak for imaging. Compared to cells in suspension measured by flow cytometry, immobilization had no effect on growth kinetics, or on the approximate rate and extent of apoptosis (not shown). Cells were imaged with differential interference contrast (DIC, Normaski) optics. Entry into mitosis could be scored by DIC but not by phase-contrast in small, round cells using breakdown of nucleoli and the nuclear envelope as markers. Adherent lines were imaged using standard phase-contrast methods, where mitotic entry was scored by rounding-up. For all lines, cell death was scored by vigorous blebbing followed by complete cessation of movement. Time-lapse movie analysis of HeLa, HT29, U-2 OS, MCF7, and N/TERT-1 that had been taken previously were included in this study for comparison [26].
All lines were treated with 500nM EMD534085, which is saturating for all the cell lines in terms of promoting prolonged mitotic arrest [26,30]. We chose to focus on the kinetics and extent of cell death at this saturating drug concentration, i.e., Emax value for the drug (Emax is the fractional response at a saturating drug concentration), over more traditional EC50 measures (concentration at which the effect is half-maximal) because apoptosis sensitivity compared between anti-mitotic drugs across multiple cell lines correlated well for Emax measures, and poorly for EC50 measures [30]. In patients, drug concentration changes greatly during a single round of chemotherapy, but what matters most is the fraction of cells (cancer or normal tissue) that die during this round. For these reasons we believe Emax measures in cell culture are more likely than EC50 measures to predict potential drug sensitivity of different cell populations in patients to K5Is.
Treatment with 500nM EMD534085 had no effect on the cell cycle kinetics, but once cells entered mitosis, they arrested with the circular arrangement of chromosomes characteristic of mitotic arrest with monopolar spindles. Nearly 100% mitotic arrest was achieved for all the lines, confirming a general requirement of Kinesin-5 activity for bipolar spindle assembly. After an extended period of mitotic arrest, cells either slipped out of mitosis without cytokinesis, underwent cytokinesis (rare), or died without exiting mitosis. The fraction of cells that underwent each of these fates varied significantly across the lines (Supplemental Table 1). Death kinetics for hundreds of EMD534085-treated cells in each cell line are shown in Fig. 1. Since EMD534085 is a mitosis-specific drug and cells enter mitosis asynchronously, we conceptually synchronized each cell to the time of their mitotic entry, taken as t = 0. In this figure we pool death that occurred within mitosis, and after slippage, to provide a measure of general apoptosis sensitivity. From these cumulative survival curves, the initial rate of death following entry into mitotic arrest was higher in all the hematological linage lines than the epithelial lineage lines, with the exception of a small amount of fast death in N/TERT-1. The fraction of cells that died during 50–90 hr movies was also higher for most of the hematological lineage lines, though the relatively death-sensitive epithelial-lineage HeLa and HT-29 lines eventually accumulated more death than two of the hematological lineage lines TF1a and SU-DHL-8. Notably, the two non-cancer epithelial lineage lines RPE-1 and N/TERT-1 underwent relatively little death (<20% of cells), while the non-cancer lymphocyte-derived line H7 underwent >90% death in 10hrs. While these results cannot be directly extrapolated to the human body, they raise the possibility that dividing cells in the bone marrow may be more prone to cell death during K5I–induced mitotic arrest than dividing cells in epithelial tissues.
Figure 1.
Cumulative survival curves of 12 cell lines treated with EMD534085. Phase-contrast and DIC time-lapse microscopy are taken for adherent and suspension cells respectively. In general, cells from hematological lineage (red curves) are more sensitive than cells from epithelial lineage (black curves).
To test whether hematopoietic cells are in general more sensitive to chemotherapy agents, we performed the same time-lapse experiments on HL60, TF1a, HeLa, and MCF7 cells treated with two other drugs acting through mechanisms different from K5I. One is 4-hydroperoxy-cyclophosphamide (4-OOH-CY), an active form of cyclophosphamide (CY) that is a DNA-damaging agent inducing inter-strand DNA cross-links [31]. Cumulative survival curves of these four cell lines under 20µM 4-OOH-CY treatment are shown in Supplemental Fig. 1A. HL60 cells are extremely sensitive to 4-OOH-CY, with all cells being dead after 2 hrs of drug treatment. TF1a cells are less sensitive to 4-OOH-CY than HL60, while MCF7 and HeLa cells are completely resistant to this drug. This agrees with some of earlier studies’ conclusions about lymphocyte-derived cell lines being more sensitive to DNA-damaging agents than epithelial cell lines [32]. The other drug we tested is etoposide, a topoisomerase II inhibitor. From the cumulative survival curves of these four cell lines under 30µM etoposide treatment in Supplemental Fig. 1B, we can see that HL60, again, is the most sensitive line with the fastest death kinetics and MCF7 is negligibly affected. TF1a showed faster death kinetics than HeLa initially and both lines die completely in the end. From these results, it seems that hematopoietic cells might be in general more sensitive to chemotherapy drugs than epithelial cells.
3.2 Rapid Cell Death during Mitotic Arrest in HL60 Cells
The mechanism of cell death during mitotic arrest has been controversial, with many authors using the term “mitotic catastrophe” without a precise molecular definition. While death during mitotic arrest in some epithelial lineages occurred by the intrinsic apoptosis pathway [26,29,30,33,34], it was not clear this would be the case for the unusually rapid death in hematological lineage cells.
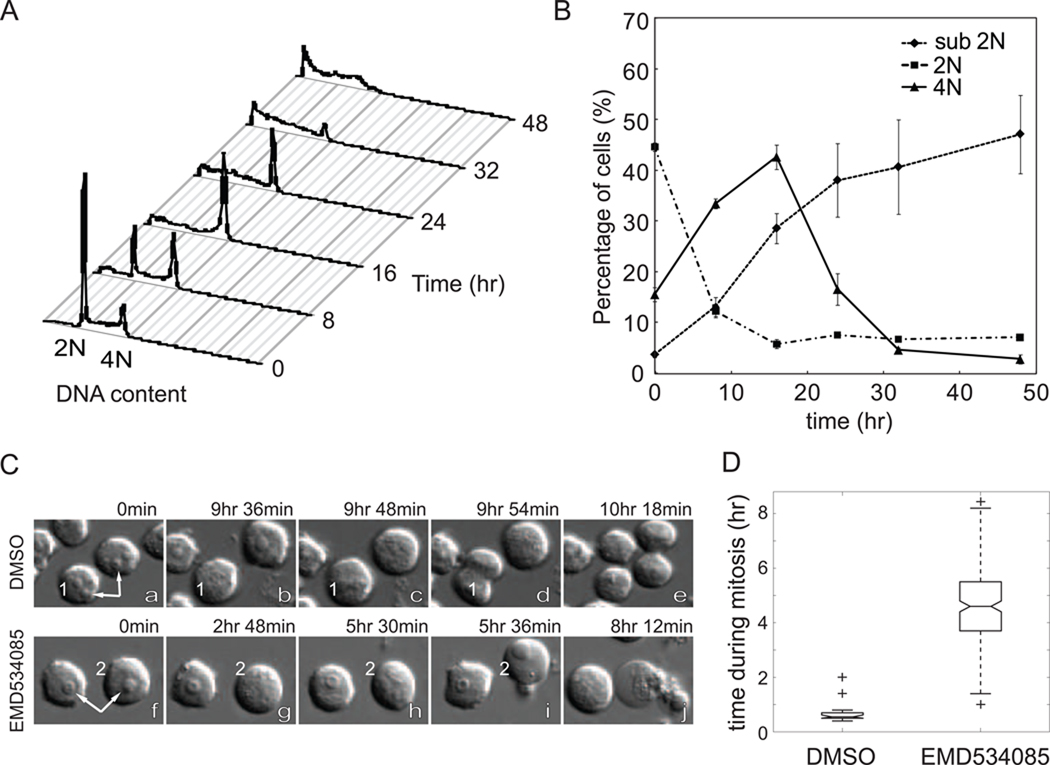
In this study, we focused on HL60 cells, which is often used to model dividing pre-neutrophils in the bone marrow [22–25]. First, we used DNA staining and flow cytometry to determine the cell cycle distribution of drug treated cells. The response of unsynchronized cells to EMD534085 is shown in Fig. 2A. Healthy control cells had FACS curves similar to t=0 at all times (data not shown). Between 0 and 8 hrs, the 4N population increased from ~15% to ~30% (Fig. 2B), indicating cell-cycle arrest in mitosis. Later, the 4N population declined, as most of the cells accumulated in the sub-2N population, indicating progressive cell death. These data are consistent with death following mitotic arrest, but do not distinguish whether or not cells slip out of mitosis before dying.
Figure 2.
EMD534085 induces rapid cell death in HL60 during mitotic arrest. A. Histograms of DNA contents in HL60 cells after EMD534085 treatment for 0, 8, 16, 24, 32, and 48 hrs. Cells are fixed, stained with propidium iodide (PI), and analyzed on BD FACSCalibur. B. Sub-2N population increases as 4N population accumulates during drug treatment. C. DIC time-lapse movie frames of DMSO (a – e) and EMD534085 treated (f – j) HL60 cells. Arrows point at nucleoi in interphase cells, which disappear when cells enter mitosis. Drug treated HL60 cells die during mitotic arrest. D. HL60 cells finish normal mitosis in less than 1 hr, whereas EMD534085 treated cells get arrested in mitosis for an average of 5 hrs.
Time-lapse microscopy images revealed further details of cell death (Fig. 2C). Entry into mitosis was evident from breakdown of nucleoli (white arrows) and also nuclear envelope breakdown, which is difficult to visualize in single images. In cells treated with DMSO vehicle alone, this was followed in a few minutes by formation of a metaphase plate, and then by cytokinesis (cell 1 in Fig. 2C, a – e, and Supplemental Movie 1). In EMD534085 treated cells, breakdown of the nucleoli and nuclear envelope were the same, but instead of lining up at the metaphase plate, chromosomes were monopolar. This mitotic arrest with monopolar spindles persisted for several hours, followed by vigorous blebbing, clearing of the cytoplasm, and eventually lysis and cessation of all movement (cell 2 in Fig. 2C, g – i, and Supplemental Movie 2). Cells never underwent these death events unless they entered mitosis and arrested (e.g. cell on left in Fig. 2C, f – j). By careful inspection of DIC movies, we were able to determine whether cells slipped out of mitosis, as assayed by nuclear envelope and nucleolar reassembly, before dying. In >95% of cases, HL60 cells died while arrested in mitosis. The durations of mitotic arrest for vehicle and EMD534085 treated HL60 cells are quantified in Fig. 2D. Normal mitosis is typically completed in <1 hr, whereas EMD534085 treated cells spent on average ~5hrs in mitosis before dying. The duration of mitotic arrest in HL60 (and H7, see Fig. 1) was unusually short (~5 hrs) compared to epithelial cell lines. Even HeLa, the most death sensitive epithelial cell line we have studied, spent an average ~20 hrs in mitosis before dying [26,28].
3.3 Death during Mitotic Arrest Occurs by the Intrinsic Apoptosis Pathway
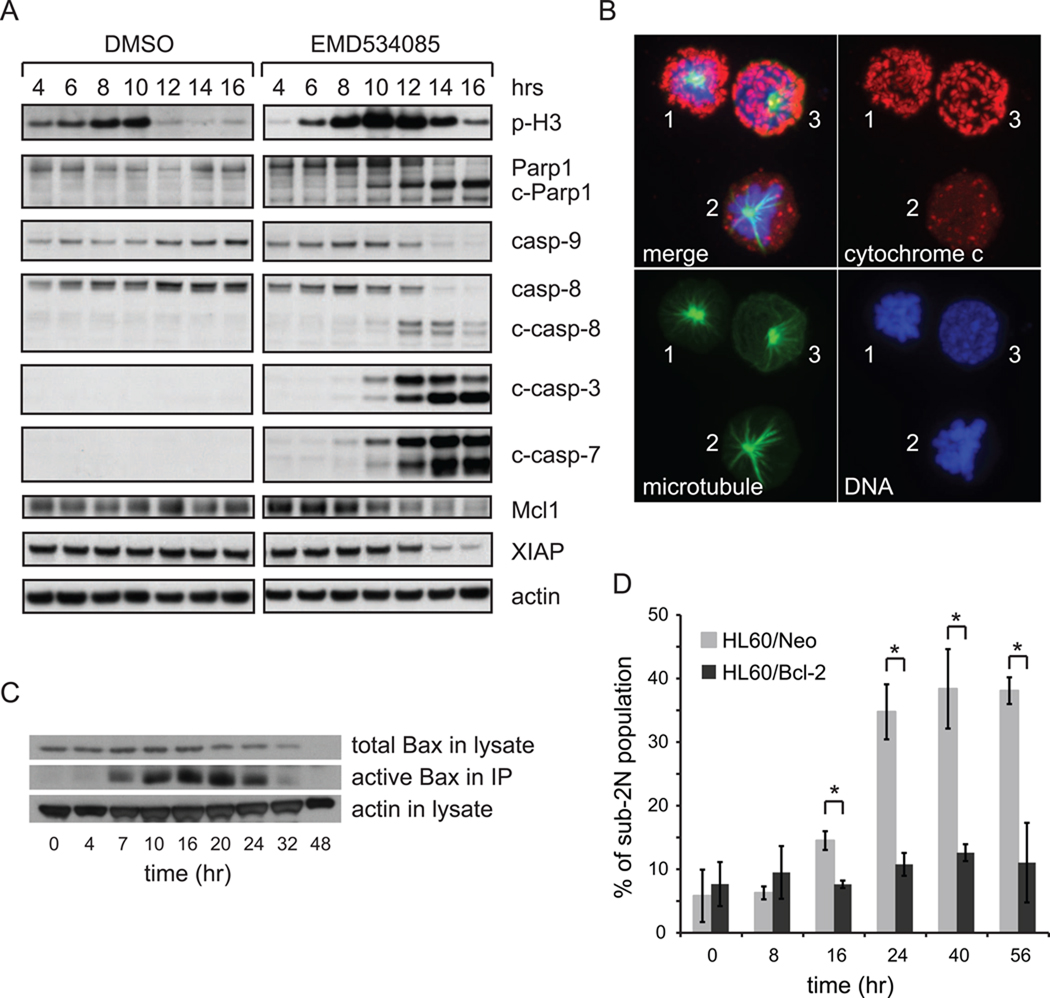
To measure death pathway biochemistry on immunoblots, it was necessary to synchronize the cells. We tested several synchronization methods including arresting cells in G2 phase using RO-3306 (RO) ([35]) and arresting cells in S phase by single-thymidine and double-thymidine treatments. Cell-cycle distributions before and after 16hrs of 10µM RO treatment revealed good G2 block, but RO drug alone induces stress on HL60 cells, evidenced from the increase of sub-2N population after RO treatment (data not shown). By contrast, we achieved equally effective cell-cycle synchronization by either single-thymidine or double-thymidine block without inducing cellular stress (Supplemental Fig. 2&3). Since double-thymidine block is not significantly more effective than single-thymidine block, and might potentially introduce more stress on cells, we chose to use single-thymidine block to synchronize HL60 cells. After being treated with 2mM thymidine for 24 hrs, HL60 cells were released into either DMSO vehicle alone, or 500nM EMD534085 supplemented growth medium. Cell lysates were collected at 4, 6, 8, 10, 12, 14, and 16 hrs post thymidine release and were immunoblotted for phosphoS10-Histone H3 (mitosis marker), Parp1, caspase-3, −7, −8 −9, Mcl1, XIAP, and actin as a loading control (Fig. 3A).
Figure 3.
EMD534085-induced cell death in HL60 during mitotic arrest occurs by the intrinsic pathway. A. Caspase-8, −9, −3, −7 are activated; Parp1 is cleaved; Mcl1 and XIAP are degraded in EMD534085-treated HL60 cells. Cells were synchronized using single thymidine block, and then were released to either vehicle alone or 500nM EMD534085 supplemented growth medium. Cells were harvested 4, 6, 8, 10, 12, 14, and 16 hrs after thymidine release for immunoblotting. B. Cytochrome c translocates from mitochondrial inter-membrane space to the cytoplasm during mitotic arrest. cytochrome c – red; microtubule – green; DNA – blue. C. Bax is activated in HL60 during EMD534085 treatment. D. Bcl-2 overexpression protects HL60 cells from EMD534085 treatment. (*: p-value < 0.05)
Based on the phosphoS10-Histone H3 blot of the control, most HL60 cells passed through normal mitosis between 8 and 10 hrs after thymidine release. EMD534085 treated HL60 cells also showed significantly accumulated phospho-Histone H3 level starting at 6 hrs post thymidine release, and the level stayed high until the 12-hr time point, when cells started to die. Starting at ~12hrs, i.e., ~4hrs into mitotic arrest, massive Parp1 cleavage, caspase activation, and loss of Mcl1 and XIAP were evident. These data suggest activation of the intrinsic apoptosis pathway.
The hallmark event in the intrinsic apoptosis pathway is leakage of cytochrome c into the cytoplasm by mitochondrial outer membrane permeabilization (MOMP) [36–38]. To test for this, we stained EMD534085-treated HL60 cells for cytochrome c, and co-stained the cells for DNA and microtubules, which allowed us to evaluate cell-cycle state and MOMP status in the same cell (Fig. 3B). From the microtubules and DNA, cells 1&2 are arrested in mitosis with monopolar spindles and condensed DNA, while cell 3 is in interphase. Cells 1&3 exhibit normal, punctate cytochrome c staining which resembles that observed in control populations (not shown). In cell 2 this punctate staining has been largely lost, and most of the remaining signal is diffuse, indicating this cell has undergone MOMP. An unbiased quantification of the standard deviation of the pixel fluorescent intensity of cytochrome c staining revealed that it was ~2 fold smaller in cell 2 than in cells 1&3. Furthermore, we co-stained HL60 cells after 16 hrs of EMD534085 treatment for microtubule, cytochrome c, and cleaved-Parp1 (Supplemental Fig. 4). We analyzed more than 50 mitotic arrested cells at random fields and 2 random fields are shown in Supplemental Fig. 4. Notice that cell 1 in Supplemental Fig. 4 has already gone through MOMP during mitotic arrest from its diffuse cytochrome c staining and monopolar spindles, but Parp1 has not been cleaved. Cell 2 in Supplemental Fig. 4 has also gone through MOMP during mitotic arrest, and it’s cleaved-Parp1 positive. Since cleaved-Parp1 only appears in a portion of randomly scored mitotic arrested cells that have gone through MOMP, it suggests that Parp1 cleavage is triggered downstream of cytochrome c translocation to the cytoplasm, and both MOMP and Parp1 cleavage occur during mitotic arrest.
MOMP is triggered by aggregation of Bax and/or Bak that then form pores in the mitochondrial outer membrane [38,39]. To test for this, we used an antibody that recognizes only the activated form of Bax in an immunoprecipitation experiment on unsynchronized cells [27]. From the immunoblot in Fig. 3C, there was a significant increase of activated Bax in HL60 cells 7 hrs after drug treatment, which correlates with increase of mitotic and sub-2N populations evident in Fig. 2B. To test for functional importance of MOMP, which can be blocked by overexpression of Bcl-2 in most cell lines [37], we used two HL60 derivatives, one overexpressing Bcl-2 (HL60/Bcl-2) and the other expressing the same vector but with only Neo-resistant gene sequence (HL60/Neo). Cell death in EMD534085 was scored by flow cytometry of DNA content, and average values for sub-2N populations are shown in Fig. 3D. DMSO vehicle alone control showed healthy proliferating cells at all time points (data not shown). Bcl-2 overexpression substantially protected cells, consistent with a causal role of MOMP in cell death.
3.4 Bim and Bid are Not Required for Apoptosis Induction
The mechanism by which mitotic arrest triggers MOMP and the intrinsic apoptosis is not known in any cells. Investigating the upstream event of MOMP during the rapid apoptosis induction during mitotic arrest in HL60 cells will be very informative about the drug action of K5Is.
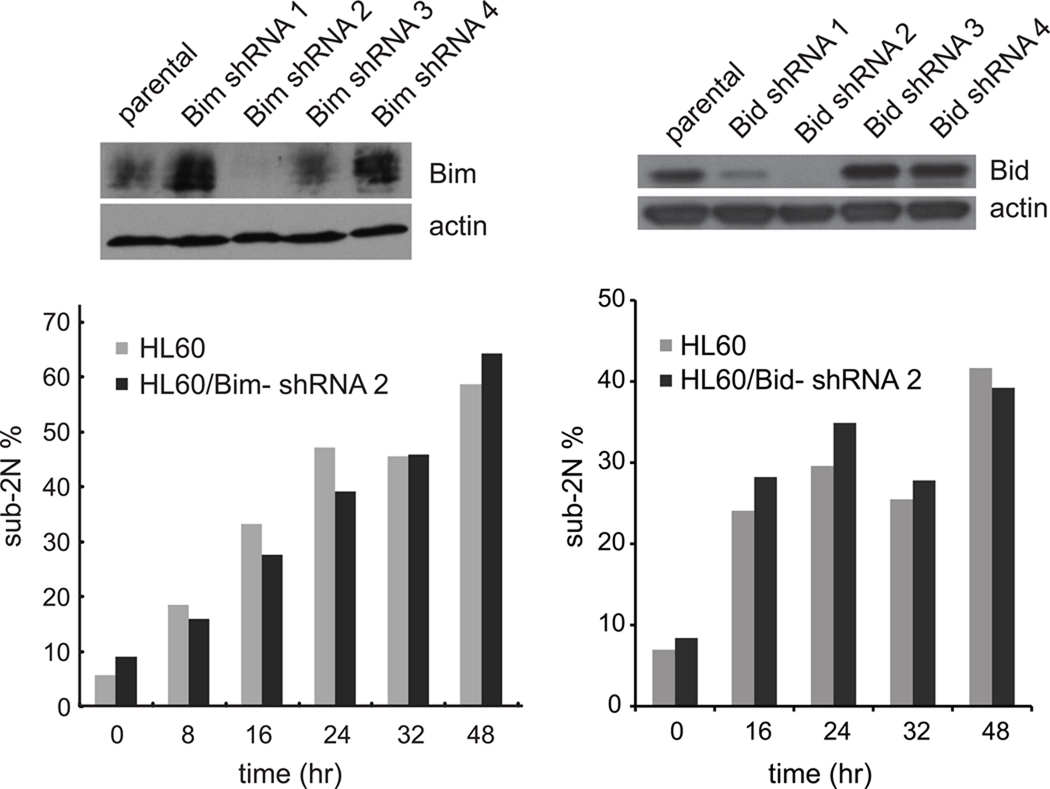
Based on the observation of Bax activation in our study, we first looked at regulators of Bax activation since Bax is one of the major effectors for triggering MOMP [37,39]. Bax aggregation can be driven by interaction with a pro-apoptotic BH3 family member that has “inducer” activity, among which Bim and Bid are the best characterized [37,40]. Bim was also previously implicated in response to anti-mitotic Paclitaxel [41–43]. We tested four different Bim shRNAs and four different Bid shRNAs to knockdown Bim and Bid respectively by lentiviral infection of HL60 cells. Lentivirus produced from Bim shRNA #2 and Bid shRNA #2 gave the most efficient knock-down of Bim and Bid respectively, but this made little difference on the accumulation of sub-2N populations in EMD534085-treated cells in each case (Fig. 4). While these data do not completely eliminate possible roles for Bim and Bid, it seems more likely that EMD534085-induced mitotic arrest activates Bax by a different pathway during mitotic arrest in HL60 cells.
Figure 4.
Neither Bim nor Bid knock-down affects HL60’s apoptotic response to EMD534085 treatment. Lentiviruses produced from 4 different shRNA against Bim and 4 different shRNA against Bid were used to infect HL60 cells. Flow cytometry over time shows the extent and timing of death (sub-2N) after K5I in either Bim- or Bid-knockdown cells is unchanged compared to parental lines.
3.5 Mitochondrial Membrane Potential (ΔΨm) Drops Before Cytochrome C Release during Mitotic Arrest in Sensitive Cell Lines, Independent of Bcl-2
Next, we turned to mitochondrial physiology to further investigate upstream events of MOMP. Mitochondria generate ATP using a gradient of protons and an associated membrane potential (ΔΨm), which provides a measure of mitochondrial health. It is commonly believed that ΔΨm drops in response to MOMP in standard intrinsic apoptosis pathways, where the drop occurs at the same time as MOMP, or after it, and is sometimes used as a surrogate measure for MOMP [44]. We developed a staining procedure to measure ΔΨm, MOMP and cell-cycle state in single cells by first allowing cells to take up MitoTracker-Red, whose accumulation in cells depends on ΔΨm. We then fixed and stained for cytochrome c, microtubules and DNA to measure MOMP and cell-cycle state. We treated non-synchronized HL60/Neo and HL60/Bcl-2 for 16 hrs, and RO-3306 synchronized and released HeLa and MCF7 cells for 24hrs with EMD534085. These are informative time points at which many cells had been in mitotic arrest for a few hours, and then performed the staining. We randomly scored hundreds of cells for being in interphase, normal mitosis, or pre-MOMP mitotic arrest, and for ΔΨm by MitoTracker-Red staining intensity.
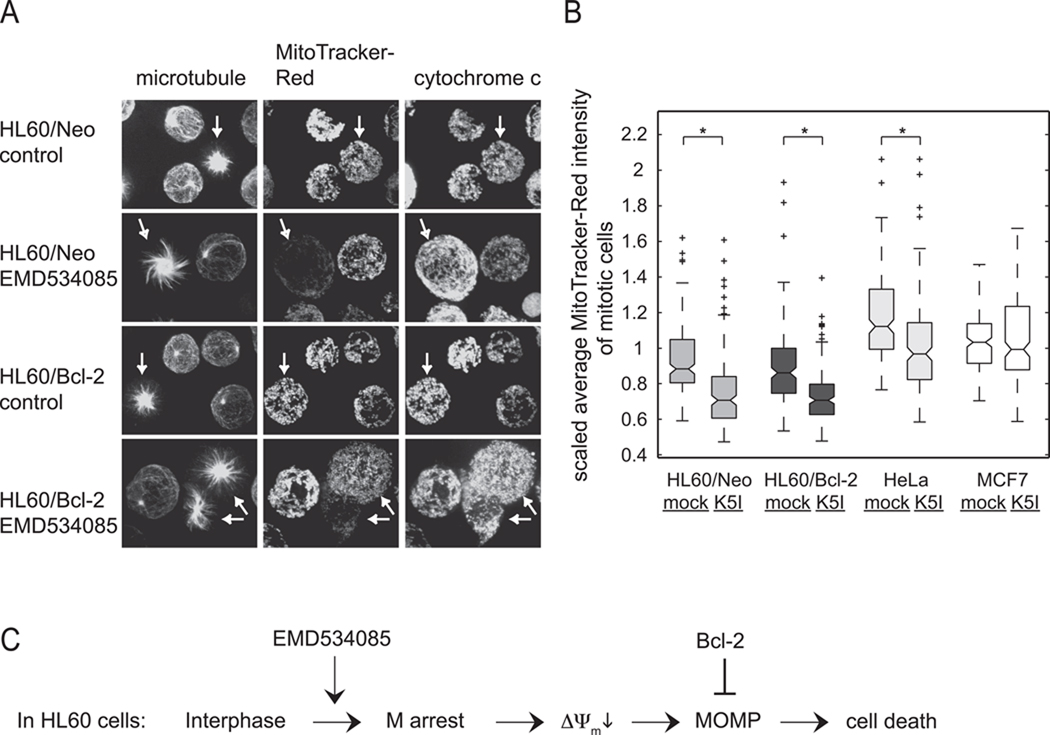
Representative fluorescence images of interphase and mitotic cells of HL60/Neo and HL60/Bcl-2 are shown in Fig. 5A. All mitotic cells in vehicle-alone treatment (arrows in the 1st and 3rd rows) have intact mitochondria that stained brightly for both MitoTracker-Red and cytochrome c, indicating that they have high ΔΨm and have not gone through MOMP – i.e., their mitochondria are healthy. In drug, we observed large number of cells in mitotic arrest with monopolar spindles (arrows in 2nd and 4th rows). In many cases, their mitochondria were only weakly stained with MitoTracker-Red, but strongly with cytochrome c, indicating low ΔΨm but no MOMP. This was seen even in cells protected against MOMP via Bcl-2 overexpression (arrow in 2nd row, lower of the two arrowed cells in 4th row). We also saw cells where the mitochondria had undergone MOMP (more frequent in HL60/Neo than in HL60/Bcl-2 as expected), and in this case the residual mitochondria also showed low MitoTracker-Red staining. These observations suggest mitochondria undergo a change to lower ΔΨm before MOMP during mitotic arrest in HL60.
Figure 5.
Mitochondrial membrane potential decreases before MOMP in EMD534085-treated HL60 cells. A. Representative immunofluorescence images of HL60/Neo and HL60/Bcl-2 cells, co-stained for microtubules, MitoTracker-Red, and cytochrome c, in the absence or presence of EMD534085. Mitochondria are normal in the DMSO vehicle-treated cells, but in some EMD534085-treated cells a significant decrease in mitochondrial membrane potential is evident. B. Box-and-whisker plot of all average MitoTracker-Red fluorescent intensities in randomly scored normal mitotic and pre-MOMP mitotic arrest cells in HL60/Neo, HL60/Bcl-2, HeLa, and MCF7 lines, in the absence or presence of EMD534085. Statistical analysis shows significantly lower ΔΨm in pre-MOMP mitotic arrest cells than normal mitotic cells in HL60/Neo, HL60/Bcl-2, and HeLa, but not in MCF7. (*: p-value < 0.0005) C. Proposed temporal events in the cell death response of HL60 cells to EMD534085.
MitoTracker-Red uptake was quite heterogeneous from cell to cell in our assay. To test the statistical significance between mitotic cells in the absence or presence of EMD534085, we scored hundreds of mitotic cells either during normal mitosis or in mitotic arrest that had not yet undergone MOMP, along with nearby interphase cells, and quantified their mitochondria’s average MitoTracker-Red intensities using a customized Matlab program that allowed for measurement of only mitochondria-based fluorescence. Fig. 5B shows a box-and-whisker plot of the average MitoTracker-Red intensity data of all mitotic cells after DMSO control or EMD534085 treatment for HL60/Neo, HL60/Bcl-2, HeLa and MCF7. We normalized all cells’ average MitoTracker-Red fluorescent intensities to the median value of interphase cells on the same coverslip, and performed t-tests between the normal mitotic and mitotic arrest cells of each line (Fig. 5B).
These data revealed that HL60/Neo, HL60/Bcl-2, and HeLa cells in drug-induced mitotic arrest that had not yet undergone MOMP had significantly lower average ΔΨm than control mitotic cells treated with DMSO. However, in the apoptosis-resistant MCF7 cell line, there was no statistical significance in ΔΨm between normal mitotic and pre-MOMP mitosis-arrested cells. Decline of ΔΨm during mitotic arrest prior to MOMP in apoptosis-sensitive HL60/Neo and HeLa cells, but not in apoptosis-resistant MCF7 cells, is consistent with it triggering, or at least contributing to MOMP. Interestingly, in a resistant HL60/Bcl-2 cell line that is protected against MOMP and apoptosis through Bcl-2 overexpression, loss of ΔΨm still occurred. This indicates that although Bcl-2 overexpression protects HL60 from MOMP and cell death, it does not inhibit loss of ΔΨm.
Taken together with previous findings, we propose the temporal events in EMD534085-induced cell death in HL60 in Fig. 5C. After cells are arrested in mitosis under K5I treatment, ΔΨm in these cells decreases before MOMP, which is the no-return point of cell death. Although Bcl-2 overexpression prevents MOMP, it does not prevent loss of ΔΨm.
4. Discussion
K5Is cause dose-limiting neutropenia that likely limits their efficacy [1,6,21]. Understanding the mechanistic basis of this effect might help us develop better anti-mitotic strategies. This prompted us to invest considerable effort in studying the mechanism of K5I–induced cell death in HL60 cells, which are often used as a neutrophil precursor model [22–25]. The non-adherent nature of this cell line necessitated development of new methods for long-term time-lapse imaging. We solved this problem by using Cell-Tak, a non-toxic cell and tissue adhesive, to glue the cells to the bottom of the dish during imaging. The small, round morphology of these cells also made it impossible to differentiate mitotic cells from interphase cells via the traditional approach of phase contrast imaging. To overcome this hurdle, we used DIC imaging to visualize the morphological changes associated with the breakdown of nucleoli and nuclear envelope that signal entry into mitosis. These novel methods are generally applicable to all small, round cells. Using these methods, we found that K5I–treated HL60 cells undergo cell death during mitotic arrest much more rapidly than epithelial cancer lines. Rapid death seems to be characteristic of hematological lineage cells, in that other leukocyte-derived lines, both cancer and non-cancer, also underwent relatively fast death during mitotic arrest (Fig. 1). Extrapolation of these data to the human body is speculative, but faster death of mitosis-arrested pre-neutrophils compared to epithelial lineages might help explain why K5Is cause neutropenia, by killing mitotic pre-neutrophils in the bone marrow, while sparing dividing cells in the hair follicles and gut mucosa [1,21]. Higher sensitivity to apoptosis in proliferating cells from hematological lineages compared to epithelial lineages may be rather general for chemotherapy drugs, as indicated by responses to two DNA-damaging agents (Supplemental Fig. 1).
The mechanistic origin of differences in average apoptosis sensitivity between cell lines, and between individual cells in a clonal line, remains obscure. Letai and co-workers have emphasized the role of “priming”, i.e., addiction of cells to anti-apoptotic Bcl-2 family members, which can be measured through BH3 profiling, in predicting cells’ sensitivity to MOMP-inducing agents [40,45,46]. We compared the rank order of priming status of 6 cell lines (A549, MCF7, SU-DHL-8, HT29, K562, and HL60) and the rank order of sensitivities to death in K5I–induced mitotic arrest, and found little correlation. An interesting possibility is that priming status changes during the cell cycle. Priming status was measured in interphase cells, while the critical period for K5I–induced death is during mitosis. The idea that mitosis predisposes mitochondria to MOMP has been discussed in the literature [47], and it would be very interesting to quantify priming in interphase vs. mitosis.
Using standard molecular tool and cytology, we found that the rapid mitotic death of HL60 cells had all the hallmarks of intrinsic apoptosis [37–40]: Bax aggregation, cytochrome c leakage from mitochondria into the cytoplasm, caspase activation, Parp1 cleavage, and protection by Bcl-2 overexpression. Death was not prevented by the pan-caspase inhibitor zVAD-FMK, though it may be somewhat slowed (data not shown). zVAD-FMK blocks caspase activation in this pathway, but not MOMP, and we suspect HL60 cells cannot survive with damaged mitochondria.
The question of what lies downstream of mitotic arrest, and upstream of MOMP, is central to understanding the action of anti-mitotic drugs. In our study, we made a provocative new observation that will open new lines of mechanistic investigation. That is, mitochondrial membrane potential (ΔΨm) declined during mitotic arrest before MOMP in apoptosis-sensitive HL60/Neo and HeLa cell lines, showing that some kind of mitochondrial dysfunction precedes MOMP during prolonged mitotic arrest. Decline of ΔΨm during mitotic arrest was not observed in an apoptosis-resistant MCF7 cell line, which rarely induces MOMP under K5I treatment (Fig. 1). The observation of loss of ΔΨm during mitotic arrest and before MOMP in sensitive cell lines but not in a resistant cell line may explain the different death responses between these lines during mitotic arrest. Interestingly, over-expression of Bcl-2 in HL60 protected against MOMP and apoptosis through Bcl-2 overexpression, but did not protect from the decrease in ΔΨm during mitotic arrest (Fig. 3D and Fig. 5B). This indicates that the loss-of-ΔΨm-related mitochondrial dysfunction occurs prior to and independent of Bax/Bak activation.
The cause of decreased ΔΨm during mitotic arrest is unclear. One important change in mitochondrial physiology when cells enter mitosis is an increase in fission relative to fusion, which is caused by phosphorylation of the fission protein Drp1 by Cdk1/cyclin B [48]. Drp1 was recently implicated as a Bax-oligomerizing factor [49], and it will be interesting to test if it is involved in perturbing mitochondrial function during mitotic arrest. Another possible change in mitochondrial physiology would be increased activity of permeability transition pores (PTPs). These still mysterious channels are known to decrease ΔΨm, and might pre-dispose mitochondria to MOMP [50,51]. Voltage-dependent anion channel (VDAC), an important component of PTPs, plays a critical role in mitochondria-mediated apoptosis. It was reported that VDAC oligomerization occurs in different stimuli-triggered apoptosis in multiple cell lines, with different mechanisms all funneling through mitochondria [52]. It would be interesting to determine whether or not VDAC oligomerization also occurs in K5I–induced mitotic arrest in HL60 cells and before MOMP.
In summary, K5I induces unusually rapid apoptosis during mitotic arrest in HL60 cells via a Bim-and-Bid-independent mitochondrial pathway, during which Bax is activated. We found loss of ΔΨm to be an upstream event of MOMP in the pathway. Bcl-2 overexpression significantly prevents cells from MOMP and apoptosis, but does not affect the upstream loss of ΔΨm. This pathway may account for the pronounced neutropenia caused by K5Is and other experimental drugs with targets in the mitotic spindles [1,6,21].
Supplementary Material
Acknowledgements
We thank Andrew Chess, Kapil Bhalla, and Anthony Letai for cell lines, Seungmin Lee and Hyeseong Cho for advice on immunofluorescence experiment, Jennifer Waters, Lara Petrak, and Cassandra Rogers (The Nikon Imaging Center at Harvard Medical School) for help on microscopy, and the members of the Mitchison laboratory. The work was supported by National Cancer Institute grant CA139980.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared.
References
- 1.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nature Reviews Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 2.Weaver BAA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Rieder CL, Maiato H. Stuck in Division or Passing through: What Happens When Cells Cannot Satisfy the Spindle Assembly Checkpoint. Developmental Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Bergnes G, Brejc K, Belmont L. Mitotic kinesins: prospects for antimitotic drug discovery. Curr Top Med Chem. 2005;5:127–145. doi: 10.2174/1568026053507697. [DOI] [PubMed] [Google Scholar]
- 5.Harrison MR, Holen KD, Liu G. Beyond taxanes: a review of novel agents that target mitotic tubulin and microtubules, kinases, and kinesins. Clin Adv Hematol Oncol. 2009;7:54–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Huszar D, Theoclitou ME, Skolnik J, Herbst R. Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev. 2009;28:197–208. doi: 10.1007/s10555-009-9185-8. [DOI] [PubMed] [Google Scholar]
- 7.Sarli V, Giannis A. Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res. 2008;14:7583–7587. doi: 10.1158/1078-0432.CCR-08-0120. [DOI] [PubMed] [Google Scholar]
- 8.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 9.Lee RT, Beekman KE, Hussain M, Davis NB, Clark JI, Thomas SP, Nichols KF, Stadler WM. A University of Chicago consortium phase II trial of SB-715992 in advanced renal cell cancer. Clin Genitourin Cancer. 2008;6:21–24. doi: 10.3816/CGC.2008.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson JJ, Lewis N, Martin JC, Ho A, Li J, Wu K, Pace L, Eder JP, Schwartz GK. Phase I multicenter study to assess the safety, tolerability, and pharmacokinetics of AZD4877 administered twice weekly in adult patients with advanced solid malignancies. Proceedings of the American Society of Clinical Oncology. 2008;26:2516. [Google Scholar]
- 11.Shah JJ, Zonder JA, Cohen AD, Weber D, Thomas S, Wang M, Kaufman J, Burt SM, Freeman B, Rush S, Ptaszynski M, Orlowski RZ, Lonial S. A Phase 1/2 Trial of the KSP Inhibitor ARRY-520 in Relapsed/Refractory Multiple Myeloma. Annual Meeting of the American Society of Hematology. 2010:1959. [Google Scholar]
- 12.Miller K, Ng C, Ang P, Brufsky AM, Lee SC, Dees EC, Piccart M, Verrill M, Wardley A, Loftiss J, Bal J, Yeoh S, Hodge J, Williams D, Dar M, Kathman S, Ho PC. Phase II, Open Label Study of Ispinesib in Patients with Locally Advanced or Metastatic Breast Cancer. San Antonio Breast Cancer Symposium. 2005:1089. [Google Scholar]
- 13.Lee CW, Belanger K, Rao SC, Petrella TM, Tozer RG, Wood L, Savage KJ, Eisenhauer EA, Synold TW, Wainman N, Seymour L. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008;26:249–255. doi: 10.1007/s10637-007-9097-9. [DOI] [PubMed] [Google Scholar]
- 14.Shahin MS, Braly P, Rose P, Malpass T, Bailey H, Alvarez RD, Hodge J, Bowen C, Buller R. A phase II, open-label study of ispinesib (SB-715992) in patients with platinum/taxane refractory or resistant relapsed ovarian cancer. Proceedings of the American Society of Clinical Oncology. 2007:5562. [Google Scholar]
- 15.O’Connor OA, Goy A, Orlowski R, Hainsworth JD, Leonard JP, Afanasyev B, Osmanov D, Chen M, Wolff A. A phase I-II trial of the kinesin spindle protein (KSP) inhibitor SB-743921 on day 1 and 15 every 28 days in non-Hodgkin or Hodgkin lymphoma. Proceedings of the American Society of Clinical Oncology. 2008;26:8539. [Google Scholar]
- 16.Purcell JW, Davis J, Reddy M, Martin S, Samayoa K, Vo H, Thomsen K, Bean P, Kuo WL, Ziyad S, Billig J, Feiler HS, Gray JW, Wood KW, Cases S. Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin Cancer Res. 2010;16:566–576. doi: 10.1158/1078-0432.CCR-09-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woessner R, Tunquist B, Lemieux C, Chlipala E, Jackinsky S, Dewolf W, Jr, Voegtli W, Cox A, Rana S, Lee P, Walker D. ARRY-520, a novel KSP inhibitor with potent activity in hematological and taxane-resistant tumor models. Anticancer Res. 2009;29:4373–4380. [PubMed] [Google Scholar]
- 18.Basso AD, Liu M, Dai C, Gray K, Nale L, Tevar S, Lee S, Liang L, Ponery A, Yaremko B, Smith E, Tang H, Sheth PR, Siddiqui MA, Hicklin DJ, Kirschmeier P. SCH 2047069, a novel oral kinesin spindle protein inhibitor, shows single-agent antitumor activity and enhances the efficacy of chemotherapeutics. Mol Cancer Ther. 2010;9:2993–3002. doi: 10.1158/1535-7163.MCT-10-0548. [DOI] [PubMed] [Google Scholar]
- 19.Cox CD, Coleman PJ, Breslin MJ, Whitman DB, Garbaccio RM, Fraley ME, Buser CA, Walsh ES, Hamilton K, Schaber MD, Lobell RB, Tao W, Davide JP, Diehl RE, Abrams MT, South VJ, Huber HE, Torrent M, Prueksaritanont T, Li C, Slaughter DE, Mahan E, Fernandez-Metzler C, Yan Y, Kuo LC, Kohl NE, Hartman GD. Kinesin spindle protein (KSP) inhibitors. 9. Discovery of (2S)-4-(2,5-difluorophenyl)-n-[(3R,4S)-3-fluoro-1-methylpiperidin-4-yl]-2- (hydroxymethyl)-N-methyl-2-phenyl-2,5-dihydro-1H–pyrrole-1-carboxamide (MK-0731) for the treatment of taxane-refractory cancer. J Med Chem. 2008;51:4239–4252. doi: 10.1021/jm800386y. [DOI] [PubMed] [Google Scholar]
- 20.Schiemann K, Finsinger D, Zenke F, Amendt C, Knochel T, Bruge D, Buchstaller HP, Emde U, Stahle W, Anzali S. The discovery and optimization of hexahydro-2H–pyrano[3,2-c]quinolines (HHPQs) as potent and selective inhibitors of the mitotic kinesin-5. Bioorg Med Chem Lett. 2010;20:1491–1495. doi: 10.1016/j.bmcl.2010.01.110. [DOI] [PubMed] [Google Scholar]
- 21.Duhl DM, Renhowe PA. Inhibitors of kinesin motor proteins--research and clinical progress. Curr Opin Drug Discov Devel. 2005;8:431–436. [PubMed] [Google Scholar]
- 22.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 23.Fontana JA, Wright DG, Schiffman E, Corcoran BA, Deisseroth AB. Development of chemotactic responsiveness in myeloid precursor cells: studies with a human leukemia cell line. Proc Natl Acad Sci U S A. 1980;77:3664–3668. doi: 10.1073/pnas.77.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayilavarapu S, Kantarci A, Fredman G, Turkoglu O, Omori K, Liu H, Iwata T, Yagi M, Hasturk H, Van Dyke TE. Diabetes-induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J Immunol. 2010;184:1507–1515. doi: 10.4049/jimmunol.0901219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, Blanchard C, Zirkle D, McDonald D, Pai SY, Serhan CN, Luo HR. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orth JD, Tang Y, Shi J, Loy CT, Amendt C, Wilm C, Zenke FT, Mitchison TJ. Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate. Mol Cancer Ther. 2008;7:3480–3489. doi: 10.1158/1535-7163.MCT-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Huang H-C, Shi J, Orth JD, Mitchison TJ. Evidence that Mitotic Exit Is a Better Cancer Therapeutic Target Than Spindle Assembly. Cancer Cell. 2009;16:347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–3276. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- 31.Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5:555–560. [PubMed] [Google Scholar]
- 32.Shimizu T, Pommier Y. DNA fragmentation induced by protease activation in p53-null human leukemia HL60 cells undergoing apoptosis following treatment with the topoisomerase I inhibitor camptothecin: cell-free system studies. Exp Cell Res. 1996;226:292–301. doi: 10.1006/excr.1996.0230. [DOI] [PubMed] [Google Scholar]
- 33.Tao W, South VJ, Diehl RE, Davide JP, Sepp-Lorenzino L, Fraley ME, Arrington KL, Lobell RB. An inhibitor of the kinesin spindle protein activates the intrinsic apoptotic pathway independently of p53 and de novo protein synthesis. Mol Cell Biol. 2007;27:689–698. doi: 10.1128/MCB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijapurkar U, Wang W, Herbst R. Potentiation of kinesin spindle protein inhibitor-induced cell death by modulation of mitochondrial and death receptor apoptotic pathways. Cancer Res. 2007;67:237–245. doi: 10.1158/0008-5472.CAN-06-2406. [DOI] [PubMed] [Google Scholar]
- 35.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci U S A. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinou JC, Desagher S, Antonsson B. Cytochrome c release from mitochondria: all or nothing. Nat Cell Biol. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 37.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in Cell Biology. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyungjin K, Rafiuddin-Shah M, Ho-Chou T, Jeffers JR, Zambetti GP, Hsieh JJD, Cheng EHY. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biology. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 39.Wei MC, Zong W-X, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A Requisite Gateway to Mitochondrial Dysfunction and Death. Science. 2001;292:727. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nature Reviews Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 41.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 42.Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Moudgil T, Ross HJ, Hu HM. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12:292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- 44.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 45.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 Profiling Identifies Three Distinct Classes of Apoptotic Blocks to Predict Response to ABT-737 and Conventional Chemotherapeutic Agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 49.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 51.Zamzami N, Larochette N, Kroemer G. Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ 12 Suppl. 2005;2:1478–1480. doi: 10.1038/sj.cdd.4401682. [DOI] [PubMed] [Google Scholar]
- 52.Keinan N, Tyomkin D, Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol. 2010;30:5698–5709. doi: 10.1128/MCB.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.