Abstract
A subset of Eph receptors and their corresponding ligands are commonly expressed in tumor cells, where they mediate biological processes such as cell migration and adhesion, while their expression in endothelial cells promotes angiogenesis. In particular, the tumor-specific upregulation of EphA2 confers properties of increased cellular motility, invasiveness, tumor angiogenesis, and tumor progression, and its overexpression correlates with poor prognosis in several cancer types. The cellular chaperone Hsp90 also plays a significant role in regulating cell migration and angiogenesis, although the full repertoire of motility driving proteins dependent upon Hsp90 function remain poorly defined. We explored the hypothesis that Hsp90 may regulate the activity of EphA2 and examined the potential relationship between EphA2 receptor signaling and chaperone function. We demonstrate that geldanamycin (GA), an Hsp90 antagonist, dramatically destabilizes newly synthesized EphA2 protein and diminishes receptor levels in a proteasome-dependent pathway. In addition, GA treatment impairs EphA2 signaling, as evidenced by a decrease in ligand-dependent receptor phosphorylation and subsequent cell rounding. Therefore, Hsp90 exerts a dual role in regulating the stability of nascent EphA2 protein, and maintaining the signaling capacity of the mature receptor. Our findings also suggest that the GA-dependent mitigation of EphA2 signaling in receptor-overexpressing cancer cells may be sufficient to recapitulate the anti-motility effects of this drug. Finally, the identification of a pharmacologic approach to suppress EphA2 expression and signaling highlights the attractive possibility that Hsp90 inhibitors may have clinical utility in antagonizing EphA2-dependent tumorigenic progression.
Keywords: Hsp90, EphA2, geldanamycin, cell migration, glioblastoma
Introduction
Cancer spread and metastasis are responsible for the majority of cancer related deaths. Cell migration is one of the rate limiting steps in metastasis (1) and targeting proteins within this pathway represents an attractive therapeutic strategy. Cell migration is a complex and highly coordinated process involving signaling through focal adhesion proteins and subsequent remodeling of the actin cytoskeleton (2). The receptor tyrosine kinase EphA2 is a key mediator of cell migration through its ability to interact with the focal adhesion kinase FAK, resulting in modulation of integrin function, rho GTPase signaling, and cytoskeletal remodeling (3). EphA2 is a representative member of a 16 member superfamily of receptor tyrosine kinases (RTKs). The corresponding ligands for Eph RTKs, called ephrins, are anchored on the plasma membrane through either a glycophosphatidylinositol (GPI) linkage (ephrin-A) or through a transmembrane domain (ephrin-B). Eph receptors and their cognate ligands are typically expressed in distinct cell types, ensuring that ligand-dependent receptor activation is a tightly regulated process occurring at sites of cell-cell contact. Ephs are distinct from other RTKs in that they are capable of mediating bi-directional signaling upon interaction with their ligands (4). Eph-dependent forward signaling is mediated by receptor expressing cells, while reverse signaling is initiated within ligand-expressing cells. Although Eph forward signaling may lead to repulsion in normal processes, such as during the development of neural growth cones, forward signaling can promote cell migration in cancers. Thus, this complex interplay of forward and reverse signaling coordinately regulates a diverse array of physiological processes and prevents the inappropriate mixing of distinct cell populations (5–7).
EphA2 is overexpressed in a variety of human malignancies, such as breast, colon, ovarian, pancreatic, colon, prostate, renal, and glioblastoma (8–12). In cancers, EphA2 has emerged as a pivotal player in angiogenesis, tumor growth, invasion, and metastasis (8, 13–15) and its expression is correlated with poor prognosis (16–19). Moreover, recent reports implicate a cooperative role for EphA2 in tumorigenesis, both in EGF-dependent cell motility (20) and ErbB2-driven mammary cancer (15). Although the kinase domain is necessary for EphA2-driven cell migration and tumorigenicity (21), the outcome of ligand-dependent receptor phosphorylation is controversial. EphA2 receptor may have inherent tyrosine kinase activity (22) and EphA2-driven tumorigenesis can be observed in the absence of ligand (11, 23). Furthermore, the activation of oncogenic signaling pathways may suppress ligand expression (24), which further disrupts the delicate balance of EphA2 bi-directional signaling.
Ligand binding stimulates receptor phosphorylation and potently suppresses EphA2-dependent cell migration and tumorigenicity in a number of cell types (9, 11, 25). It remains unclear whether the ligand-suppressive effects are due to the subsequent proteasomal depletion of receptor expression (9, 11, 26) or to the rapid ligand-initiated inhibitory effects upon FAK and integrin signaling (25, 27). However, the finding that genetic manipulation and downregulation of EphA2 leads to a decrease in tumorigenicity in several in vitro and preclinical models (9, 12, 20, 28) strongly suggests that EphA2-dependent tumorigenic properties are conferred by EphA2 expression levels within a variety of cancer cell types. Although ligand treatment may be therapeutic within some contexts, ephrin A1 ligand may also stimulate the recruitment of endothelial cells and facilitate angiogenesis and metastatic spread (29, 30). Given the cell context dependent multi-functional outcomes of ephrin-mediated receptor activation, the ability of Hsp90 inhibition to target EphA2 and to reduce receptor expression in a ligand-independent manner represents a promising strategy to attenuate EphA2-dependent signaling and diminish its pro-tumorigenic properties.
The molecular chaperone heat shock protein 90 (Hsp90) facilitates the proper folding and conformation of its clients (31, 32). The emerging picture is that Hsp90 is required for protein maturation and conversion of the client to a functionally active protein (33). Hsp90 antagonists such as geldanamycin (GA), inhibit Hsp90 ATPase activity and abrogate chaperone function (34–36), resulting in impaired client activity and subsequent proteasomal degradation. Pharmacologic inhibitors such as GA possess potent tumoricidal activity (37), in part due to their targeting of numerous clients essential for malignant signaling and progression (38). Although GA and derivatives potently inhibit cell migration, angiogenesis and metastasis in a variety of cancer types (39), the specific molecular targets involved in these processes are not well defined. Given the essential role of EphA2 in cell migration in a variety of cancers, we examined whether EphA2 signaling was dependent upon Hsp90 function. We identify EphA2 as a novel Hsp90 client protein and further show that Hsp90 is an essential mediator of EphA2 stability and function. Hsp90-dependent targeting of EphA2 may therefore represent an alternative therapeutic strategy to impair EphA2 signaling and antagonize tumor growth.
Results
Eph protein expression is decreased following impairment of Hsp90 function
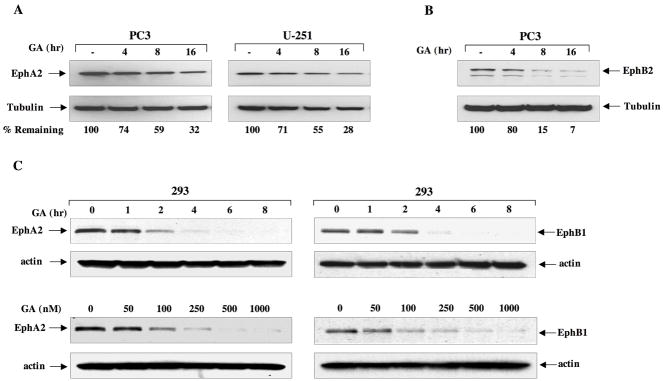
Given that Hsp90 plays an important role in cell migration and that EphA2 also has a well-documented role in this process, we considered whether EphA2 may be regulated by Hsp90. EphA2-overexpressing cancer cell lines were selected, such as PC3 prostate and U251 glioblastoma (11, 25). As shown in Fig. 1A, endogenous EphA2 levels were modestly diminished (approximately 70%) in a time dependent manner following GA treatment. We next examined whether GA similarly decreased protein expression of other Eph family members. As shown in Fig. 1B, GA treatment also significantly reduced expression of endogenous EphB2 protein in PC3 cells. It has been reported that EphB2 may be modified by glycosylation (40), which may explain the presence of multiple bands, both of which are diminished by GA. We next examined the dose and time-dependent response of EphA2 to GA inhibition. As shown in Fig. 1C, (left panels) continuous GA treatment promoted the rapid disappearance of EphA2 protein transduced into HEK293 cells. We also tested the response of EphB1 to GA treatment and, as shown in Fig. 1C (right panels), EphB1 receptor expression is also reduced by GA, and is affected by a drug dose as low as 100 nM.
Fig 1. Eph proteins are sensitive to Hsp90 inhibition.
(A) PC3 and U251 cells were seeded in 6 well plates and treated with 1 μM GA for the indicated times. Cells were then lysed and equivalent protein subjected to SDS-PAGE and immunoblot analysis with EphA2 antibody. Tubulin was used as control for protein loading. (B) PC3 cells were treated with 1 μM GA, lysed as in A, and blots were probed for EphB2. (C) HEK293 cells were transfected with plasmids encoding either HA-tagged EphA2 or EphB1. Cells were treated with 1 uM GA for the indicated times and drug concentrations and lysates were probed with anti-HA antibody. For the dose response, cells were treated for 16 h. Band intensities were quantitated with NIH Image J software and normalized to tubulin levels. The receptor levels from untreated cells were considered 100% and the percentage of remaining receptor from drug treated cells was determined relative to the control expression.
Interaction of Eph receptors with chaperone proteins is dependent upon the C-terminal domain
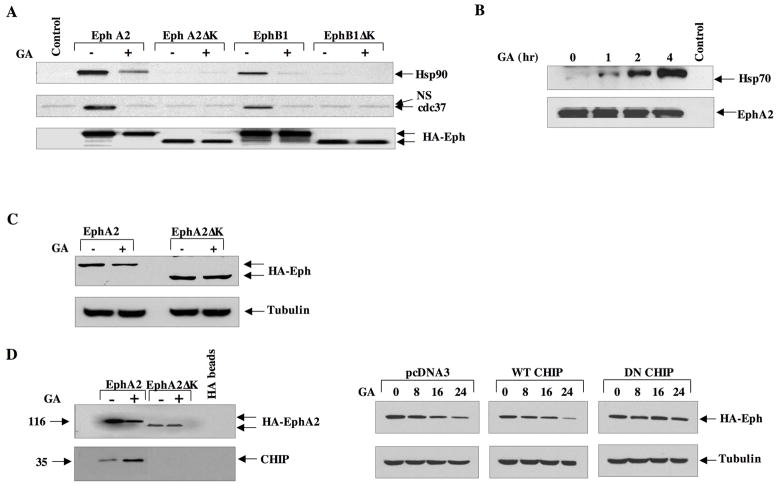
Given the apparent sensitivity of Eph receptors to Hsp90 inhibition, we sought to confirm their identity as bona fide client proteins. As shown in Fig. 2A, Hsp90 associated with HA-tagged EphA2 and EphB1 proteins expressed in 293 cells. However, Hsp90 was unable to associate with truncated versions of these Eph receptors lacking the C-terminal portion containing the kinase domain. A similar pattern was observed with the Hsp90 co-chaperone cdc37, thus highlighting the importance of this domain in facilitating chaperone protein interactions. Although the smaller PDZ and SAM domains were also deleted in this construct, the kinase domain is likely responsible for these chaperone interactions, as kinase domains have been identified as essential mediators of chaperone interactions for a vast majority of known RTK client proteins. Further, we have identified an Hsp90-interacting peptide sequence within the kinase domain (data not shown). It is well known that GA treatment remodels the Hsp90 chaperone complex (31, 41), resulting in decreased affinity of Hsp90 for its clients. As shown in Fig 2A, a brief (1 h) exposure of cells to GA facilitates the release of Hsp90 and cdc37 from both EphA2 and EphB1 receptors. The GA-dependent chaperone remodeling promotes a destabilizing complex, facilitating the recruitment of co-chaperones such as Hsp70. As shown in Fig. 2B, although no Hsp70 is associated with untreated EphA2 immunocomplexes, a 1 h drug exposure was sufficient to promote Hsp70 recruitment, coinciding with the release of EphA2 from Hsp90. This recruitment of Hsp70 increased in a time-dependent fashion. We then explored whether the C-terminal domain was essential for drug sensitivity and, as shown in Fig. 2C, while GA elicited a 70% reduction in full length EphA2 protein, the EphA2 protein lacking its C-terminal domain was insensitive to drug. GA treatment has been shown to recruit the C-terminal Hsp70 interacting protein (CHIP), a ubiquitin ligase that mediates the degradation of a subset of Hsp90 clients (42, 43). We therefore examined whether CHIP was involved in the GA-mediated depletion of EphA2. To explore this, either full length or truncated EphA2 was cotransfected with CHIP in 293 cells. As shown in Fig. 2D (left panel), although CHIP is minimally detected in EphA2 immunocomplexes in the absence of drug, it is significantly recruited following a 4 h exposure. While GA-dependent CHIP recruitment is observed with the full length receptor, drug treatment fails to recruit CHIP to the C-terminally truncated protein, implicating this domain as an essential component of this interaction. We then tested whether CHIP was involved in the Hsp90-mediated EphA2 depletion. As shown in Fig. 2D (right panel) EphA2 receptor levels decreased in HEK293 cells between 16 and 24 h in response to GA following transfection of either control or wild type CHIP plasmid. Addition of wild type CHIP did not further accelerate receptor depletion, likely due to the abundance of endogenous CHIP protein expressed in these cells (data not shown). However, transfection of a dominant negative CHIP construct rendered EphA2 insensitive to GA treatment. This construct retains the chaperone binding TPR motif, but lacks the functional U box required for ubiquitination (44). These data suggest that the GA-mediated recruitment of CHIP is required for efficient Hsp90-dependent depletion of EphA2. Although we cannot rule out the possibility that an additional ubiquitin ligase plays a supplemental role, CHIP function is essential for at least a significant proportion of this Hsp90-dependent degradative pathway.
Fig 2. The kinase domain of EphA2 is required for chaperone recruitment and drug sensitivity.
(A) HEK293 cells were cultured in 10 cm dishes and transfected with 5 ug of the indicated plasmids. Following lysis, 1 mg total lysate was immunoprecipitated with HA-conjugated protein G beads and the blots probed for the indicated proteins. (B) PC3 cells were treated with 1 μM GA for the indicated times, EphA2 was immunoprecipitated as in A, and resultant blots were incubated with antibodies to either Hsp70 or EphA2. C) HEK293 cells were transfected with either full length or kinase deleted HA-tagged EphA2, treated for 16 h with GA, and equivalent protein was immunoblotted with HA antibody. D) (Left panel) HEK293 cells were transfected as in C, treated with GA for 8 h, and 1 mg total protein was immunoprecipiated as in A. Resultant blots were probed for CHIP and EphA2 expression (anti-HA). (Right panel) HEK293 cells were transfected with either pcDNA3.1 control vector, wild type CHIP, or dominant negative CHIP, and EphA2 levels were immunodetected as indicated.
Hsp90 is required for EphA2 protein stability
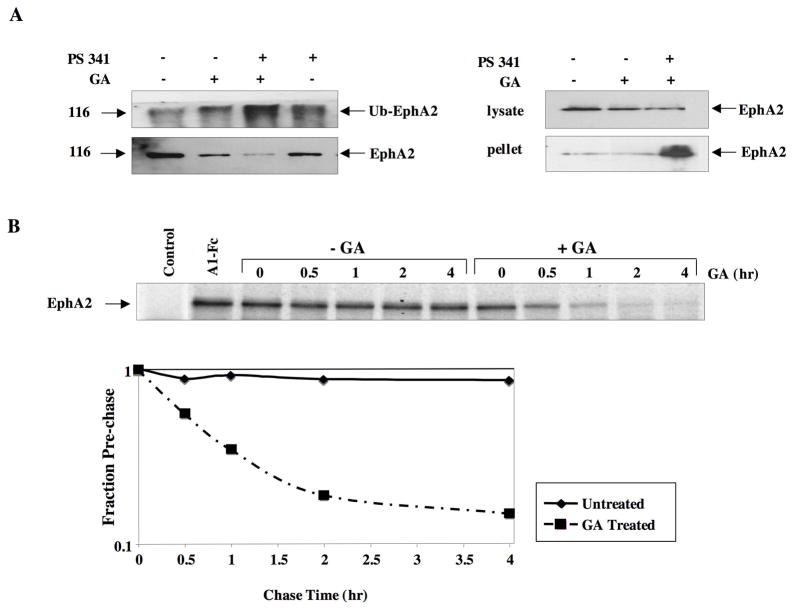
The depletion of EphA2 protein following GA, coupled with recruitment of the ubiquitin ligase CHIP, implicated an Hsp90-dependent proteasomal pathway in the regulation of EphA2 expression and stability. To examine this possibility, we examined whether GA treatment facilitated receptor ubiquitination. As shown in Fig. 3A (upper left panel), GA alone caused a moderate increase in ubiquitinated EphA2, while the combination of GA and proteasomal inhibitor (PS-341, bortezomib), significantly increased ubiquitinated species compared to either proteasome inhibitor or GA alone. As shown in the lower panel, GA decreased the amount of total EphA2, and interestingly, the combination of GA and bortezomib significantly decreased total Eph levels, although the overall ubiquitination modestly increased. We sought to explain these results by detecting EphA2 in cellular lysates following these same treatments. As shown in Fig. 3A (upper right panel), GA treatment moderately decreased total EphA2 protein; however, the combination of proteasome inhibitor and GA further decreased protein levels. Proteasomal inhibition of GA-treated cells has been shown to promote the accumulation of misfolded proteins, which appear in the insoluble pellet fraction (45). As shown in Fig 3A, lower panel, a dramatic increase of EphA2 protein was detected in the insoluble pellet fraction from the combination treatment, suggesting that Hsp90 is essential for EphA2 protein folding and that misfolded EphA2 is processed by the proteasomal pathway. We next investigated the GA-mediated depletion of EphA2 was due to receptor destabilization. To explore this, the half-life of EphA2 was determined from either GA or mock treated PC3 cells. As shown in Fig. 3B, while EphA2 is extremely stable (over 4 hr) in the absence of drug, GA treatment greatly facilitates protein turnover and shortens the half-life of the receptor from well over 4 h to less than 40 min. These data suggest that Hsp90 and the proteasome cooperatively regulate EphA2 receptor stability.
Fig 3. Hsp90 inhibition facilitates EphA2 ubiquitination and degradation.
(A) (Left panel) HEK293 cells were transfected with 5 ug HA tagged EphA2 and treated for 6 h with 1 μM GA and/or 1 μM proteasomal inhibitor PS-341 for the last 3 h. Following lysis, 1 mg of total protein was immunoprecipitated with anti-HA antibody and ubiquitinylated protein was immunodetected. The blot was then stripped and reprobed with anti-HA antibody for analysis of total EphA2 levels. (Right panel) HEK293 cells were similarly transfected and treated with GA alone or with PS341 and EphA2 levels were dected from insoluble cell pellets following centrifugation, and from soluble protein lysate. B) Logarithmically growing PC3 cells were starved in methionine-free medium 1 h, pulse labeled for 1 h in the presence of 1 uM GA, and then chased for the indicated times in nonradioactive complete medium either lacking or containing 1 uM GA. Endogenous EphA2 was immunoprecipitated from equivalent protein and resultant gels were exposed to film and subject to densitometric analysis.
Hsp90 modulates EphA2 intracellular localization
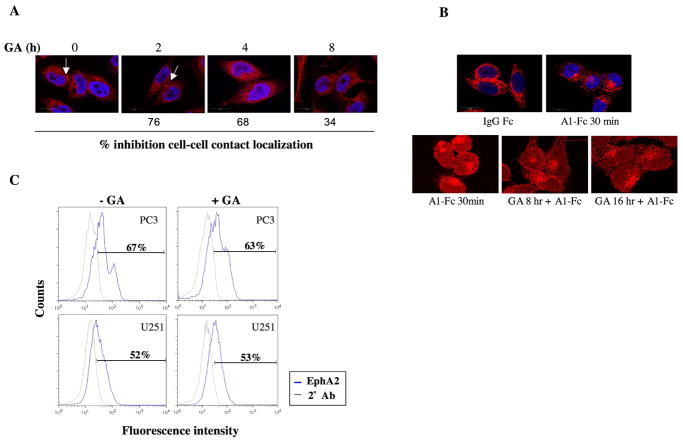
As Hsp90 can affect the localization of receptor clients, we examined whether Hsp90 modulated EphA2 cellular localization. To explore this, PC3 cells were densely plated to encourage the formation cell-cell interfaces, which have been reported to contain concentrated EphA2 expression (23). The pattern of EphA2 distribution, as determined by confocal microscopy, was represented by a mix of cytoplasmic diffuse staining and more intense signal localized to cell-cell contacts (shown by arrows, Fig. 4A). Interestingly, GA reduced the expression of EphA2 at cell-cell contacts in PC3 cells in a time-dependent manner. Less of an effect was observed in U251 cells, where EphA2 expression was primarily concentrated within membrane ruffles (data not shown). We next investigated whether Hsp90 was required for ligand-dependent internalization. It is well documented that ligand binding promotes rapid internalization of EphA2 (46), as shown in Fig. 4B, top panels. Drug pretreatment for either 8 or 16 h did not inhibit ligand-dependent internalization of EphA2 (lower panels). We next examined whether Hsp90 regulated EphA2 receptor expression at the cell surface. PC3 and U251 cells were either mock treated or treated with GA for 8 hr prior to fixation and preparation for flow cytometry. As shown in Fig. 4C, drug treatment did not reduce the surface-localized expression of EphA2.
Fig 4. Hsp90 inhibition impairs EphA2 localization but not ligand binding and internalization.
(A) PC3 cells were cultured on fibronectin and treated with GA for the indicated times. The cells were then fixed and prepared for EphA2 immunofluorescence staining. The numbers correspond to the percentage of cells exhibiting EphA2 localization at cell-cell contacts relative to untreated cells, as calculated from the average of 4 fields per coverslip, in duplicate. (B) PC3 cells were pretreated with GA for the indicated times and then stimulated by A1-Fc (1 ug/ml) 30 min prior to fixation. (C) PC3 and U251 cells were either untreated or treated for 8 h with 1 uM GA and subsequently processed for flow cytometry as described. Values represent percentage of cells expressing surface expression of EphA2. Representative data from duplicate experiments is shown.
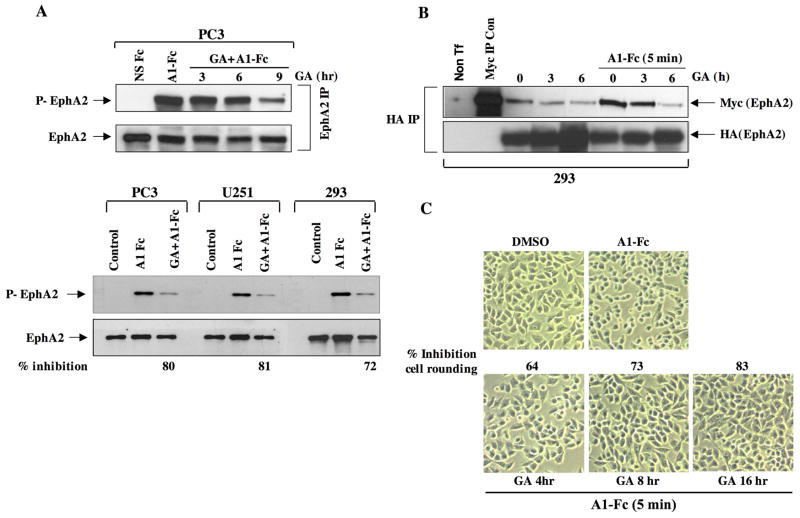
Hsp90 is required for EphA2 phosphorylation, dimerization, and cell rounding
Hsp90 plays an important role in regulating the phosphorylation of a variety of kinase clients (47, 48); and we therefore investigated whether Hsp90 may be required for the ligand-dependent receptor phosphorylation of EphA2. As shown in Fig. 5A (top panel), although ligand stimulation rapidly induced EphA2 phosphorylation in PC3 cells, receptor phosphorylation was dramatically reduced following a 9 h pretreatment with GA. We next examined whether this GA-mediated effect on receptor phosphorylation occurred in other cell types. PC3, U251, and HEK293 cells were pretreated with GA for 9 h, followed by a 5 min stimulation with ligand. As shown (lower panel), GA dramatically reduced receptor phosphorylation in U251 and HEK293 cells to a similar extent.
Fig 5. Hsp90 regulates EphA2 receptor dimerization and phosphorylation.
(A) PC3 cells were treated with GA for the indicated times, EphA2 was immunoprecipitated from 1 mg cell lysate, and resultant blots were probed for phosphotyrosine and reprobed for EphA2 to assess total receptor levels. (B) HEK 293 cells were cotransfected with myc-EphA2 and HA-EphA2 plasmids, treated as indicated, and HA-EphA2 was immunoprecipitated from lysates. Receptor dimerization was determined by visualization of myc-EphA2 co-purifying with anti-HA immunoprecipitates. A control Myc IP was also performed from myc-EphA2 transfected cells to validate signal specificity. Total levels of immunoprecipitated EphA2 were detected by anti-HA immunoblotting. (C) To assess the effects of GA on receptor phosphorylation, the indicated cell lines were treated with GA for 9 h, EphA2 was immunoprecipitated from 500 ug cell lysate, and resultant blots were probed for phosphotyrosine. The percentage decrease in phosphorylation was calculated from the quantitation of receptor phosphorylation (ImageJ), normalized to total receptor levels. (D) To assess whether GA affects ligand-dependent cell rounding, cells were grown on fibronectin coated cover slips and treated with A1-Fc for 10 min in either the presence or absence of a pretreatment with GA. The cells were then fixed with paraformaldehyde and prepared for viewing. The percentage of rounded cells in response to ligand was determined from 4 fields, in duplicate, relative to the total number of cells per field, and normalized to the percentage of rounded cells in untreated samples.
Since ligand binding triggers receptor aggregation and autophosphorylation (4), we explored whether the GA-mediated reduction in receptor phosphorylation was a consequence of reduced EphA2 dimerization. To assess receptor dimerization, HEK293 cells were transfected with both HA- and myc-tagged EphA2 expression constructs and EphA2 was immunopurified with anti-HA antibody. Dimerization was assessed by the amount of myc-tagged EphA2 co-purifying with HA-EphA2. As shown in Fig. 5B, a 5 min ligand exposure promotes rapid myc-EphA2 recruitment, indicative of receptor dimerization. However, a 3 h pretreatment with GA reduced, and a 6 hr pretreatment eliminated the ability of ligand to induce receptor dimerization. These results suggest that Hsp90 supports ligand-mediated receptor dimerization and subsequent phosphorylation.
Ligand binding elicits phenotypic changes such as cell rounding, due to receptor-mediated signaling events that impact cytoskeletal dynamics (25). Therefore, a cell rounding assay was utilized to monitor the ability of EphA2 to transduce cytoskeletal signaling events and to determine whether these events were dependent upon Hsp90 function. PC3 cells were pretreated with GA for the indicated times, followed by a brief exposure to ephrin A1-Fc, after which time approximately 90% of the cells appeared rounded. Consistent with previous reports describing the transient nature of this response (25), cells returned to their original flattened morphology 30–40 min following ligand exposure (data not shown). Pretreatment with GA reduced ligand-dependent rounding of PC3 cells in a time dependent manner. These data implicate a requirement for Hsp90 chaperone function in ligand-dependent EphA2 phosphorylation and signaling.
Hsp90 supports cell motility in part through EphA2
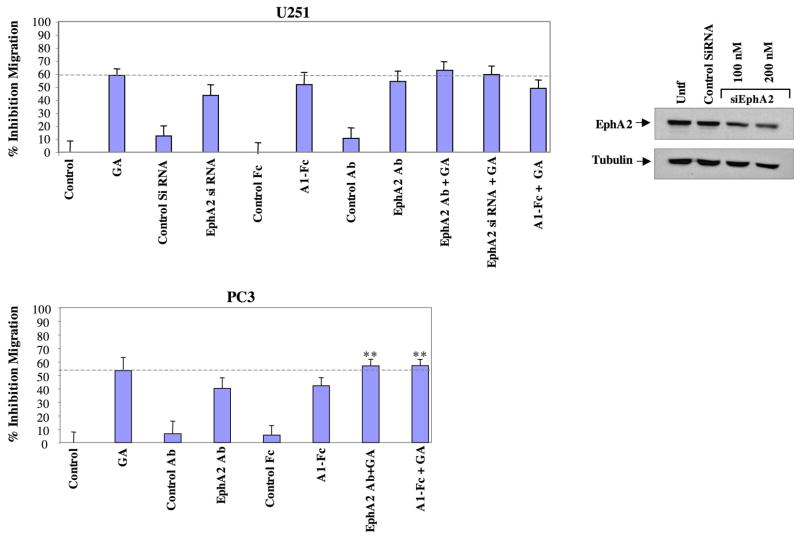
EphA2 is required for cell motility in numerous cancer types (11, 20). Hsp90 also supports cell motility in diverse cancers and Hsp90 inhibitors have potent anti-motility effects (39). Although Hsp90 regulates numerous client proteins in addition to EphA2, we investigated whether Hsp90-dependent regulation of EphA2 was an important determinant in the ability of GA to curtail cell migration. EphA2-dependent tumorigenic potential can be inhibited by a variety of approaches. EphA2 possesses tumorigenic activity in the absence of ligand (11, 22, 24), and genetic ablation of EphA2 in a mouse mammary carcinoma model reduced MAPK signaling, correlating with diminished tumorigenicity (15). Further, it has been demonstrated that siRNA-mediated reduction of EphA2 inhibits the migration of both cancer and vascular smooth muscle cells (20, 49) and reduces tumorigenicity (12). In addition to genetic reduction of EphA2, EphA2 ligand may inhibit cell migration (11, 25), although it is not clear whether this inhibition is dependent upon the ability of ligand to diminish integrin and FAK signaling (25), receptor levels (9, 11, 26), or to a combination of these events. Several groups have also shown that EphA2-targeted antibodies effectively inhibit EphA2-dependent tumorigenic activity (22, 28, 50). We therefore utilized these three modalities known to interfere with EphA2-mediated tumorigenicity (ligand, siRNA, therapeutic antibody) and evaluated the effects of these agents upon PC3 and U251 cell motility. We further compared the relative potency of these agents relative to the inhibitory effects elicited by GA. Not surprisingly, GA reduced the migration of U251 cells by approximately 60% (dashed horizontal line, Fig. 6A). Interestingly, treatment of cells with either ligand or an EphA2 specific neutralizing antibody (F32-3M) (50) reduced cell migration by approximately 50%, slightly less than the inhibitory effect observed with GA, although this difference was not statistically significant (p > .05). While ephrin ligand may affect other Eph receptors, the antibody is more specific for EphA2 and has been shown to potently neutralize EphA2 function and induce cancer cell toxicity (50, 51). Knockdown of EphA2 via siRNA inhibited cell migration by over 40%. The slightly reduced potency of siRNA upon cell migration can be explained by the modest (55%) knockdown, whereas ephrin ligand and the EphA2 antibody, both of which have nmol affinity for EphA2 (50, 52), should efficiently interact with all available EphA2 receptors and elicit more potent inhibitory effects. Inhibition of EphA2 and Hsp90 was not additive and further, inhibition of EphA2 alone exhibited an effect comparable to GA. Therefore in U251, the inhibitory effects of EphA2 and Hsp90 were not additive in curtailing cell migration, and importantly, inhibition of EphA2 alone elicited almost as potent an effect as GA. In PC3 cells (Fig. 6B), GA also inhibited cell migration approximately 60%; however, interference with EphA2 signaling was somewhat less potent in its ability to inhibit cell migration (40% inhibition). Addition of either ligand or EphA2 antibody in the presence of GA resulted in a statistically significant (P = 0.045) decrease in cell migration when compared to either agent alone. However, the combination treatments did not further inhibit cell migration above that observed with GA. These data suggest that EphA2 signaling plays a major role in Hsp90-dependent cell migration.
Fig 6. EphA2 impairment potently inhibits cell migration to an extent similar to GA.
(Upper panel) U251 cells were pretreated with EphA2 antibody (10 ug/ml) or GA (1 uM) 8 h prior to plating in Boyden chambers (ligand was added at time of plating), and cells were transfected with siRNAs 48 h prior to plating. Following a 16 h incubation period, migrating cells were counted as described and normalized to the number of migrating cells from DMSO treated cells. Values corresponding to the normalized mean were converted to percent inhibition and subjected to statistical analysis. Representative siRNA-mediated knockdown of EphA2 is shown. PC3 cells (lower panel) were treated as above and the percent migration was similarly calculated.
Discussion
The EphA2 receptor is overexpressed in numerous cancers, where it contributes to cell transformation, primary tumor initiation, progression, angiogenesis, and metastasis in a variety of cancer models (15, 30, 53). As such, therapeutic approaches to target this receptor have generated much interest. We demonstrate herein that EphA2 is a newly identified Hsp90 client protein and that pharmacologic inhibition of Hsp90 represents a promising strategy to impair EphA2 function. We show that EphA2 forms a protein complex with Hsp90 and the co-chaperone cdc37. GA treatment remodels the Hsp90 chaperone complex, resulting in the dissociation of Hsp90, with the concomitant recruitment of Hsp70 and the ubiquitin ligase CHIP, which together facilitate receptor ubiquitination and proteasome-dependent destabilization. The C-terminal portion of EphA2 is essential for chaperone and CHIP-dependent interactions, and a truncated EphA2 protein lacking this domain is refractory to GA-dependent protein degradation. Other reports demonstrate the ability of GA to recruit CHIP to the kinase domain of Hsp90 client proteins (54) and we have identified an Hsp90 binding site within the EphA2 kinase domain (data not shown), implicating the kinase domain as an important mediator of EphA2 protein stability. Our data support a model whereby Hsp90 plays a critical role in maintenance of nascent EphA2 protein stability, while the chaperone plays more of a regulatory role for the mature receptor. Thus, the heightened dependence of newly synthesized protein upon Hsp90 is reflected by its enhanced tendency to become destabilized following GA treatment. A similar differential mode of regulation has been reported for other clients, such as ErbB2 (55). This preferential sensitivity of nascent protein to drug is further strengthened by our data (Fig. 1C) showing that exogenously introduced protein is more sensitive to drug when compared to the kinetics of degradation of endogenous EphA2 in PC3 and U251. This is most likely due to the disproportionally high nascent population of protein produced by the plasmid-containing promoter. Moreover, we demonstrate that an 8 h exposure of cells to GA does not reduce the levels of surface-localized EphA2 (Fig. 4C), further supporting the notion that the mature protein is less dependent upon Hsp90 for regulation of its stability. Taken together, our findings demonstrate that Hsp90, CHIP, and the proteasome cooperatively regulate EphA2 receptor stability. Furthermore, we demonstrate that Hsp90 modulates the protein stability of EphB1 and EphB2, thereby highlighting a previously unknown role for Hsp90 in the functional regulation of additional members of the Eph receptor family.
Although Hsp90 does not appear to have dramatic effects upon the protein stability of the mature receptor, chaperone function was essential for multiple receptor activities. EphA2 is concentrated at cell-cell contacts, where it modulates tight junctions and paracellular permeability (56, 57). We show that GA treatment reduced EphA2 localization at cell contacts in PC3 cells (Fig 4A). Interestingly, Hsp90 has also been implicated in the regulation of tight junctions and GA has been shown to reduce cellular permeability (58), suggesting that the GA-mediated re-localization of EphA2 may play a role in this process. We further demonstrate that Hsp90 is required to maintain the mature receptor in a signaling-competent state, documented by the ability of GA to significantly attenuate ligand-mediated receptor phosphorylation (Fig. 5A). Intriguingly, while receptor phosphorylation was impaired, ligand-dependent receptor internalization appeared to proceed normally (Fig. 4B), suggesting that phosphorylation may not be essential for internalization. Therefore, although Hsp90 may modulate EphA2 cellular distribution at cell-cell junctions, chaperone activity does not appear to be necessary for ligand-dependent receptor internalization, an event that may be more dependent upon Cbl-dependent pathways (26). In addition to receptor phosphorylation, Hsp90 is also essential in supporting EphA2 signaling activities. This was demonstrated by our finding that Hsp90 inhibition prevented cell rounding in response to ligand in PC3 cells (and U251, data not shown). The kinetics of this impairment was consistent with reduced receptor dimerization and associated ligand-dependent phosphorylation (Fig. 6B). A recent report identified several phosphorylation sites in the EphA2 juxtamembrane and kinase domains important for EphA2 association with signaling intermediates (59). This finding, coupled with the ability of GA to impair ligand-dependent receptor phosphorylation and cell rounding, support the notion that Hsp90 is essential for maintaining the EphA2 receptor in a signaling-competent state. Future experiments will address the specific nature of the phosphorylation events and the protein interactions dependent upon Hsp90 chaperone function.
We demonstrate that EphA2 is essential for cell motility in prostate and GBM cell lines and that inhibition of EphA2 through a variety of targeted means (siRNA, ligand, antibody) significantly reduced cell migration in PC3 and U251, in accordance with other reports (11, 60). It is well known that Hsp90 inhibitors potently impair cell migration in a variety of cancer types, including PC3 and U251 (61, 62) and we validate the ability of GA to significantly reduce cell motility in these lines (Fig. 6). However, in U251 cells, we unexpectedly found that the targeting of EphA2 similarly and potently inhibited cell migration to an extent comparable to that of GA. This is surprising, given the large number of clients affected by Hsp90 inhibition, compared to the much smaller subset of proteins predicted to be affected by EphA2-dependent targeting. Although the targeting of EphA2 in PC3 cells less robustly inhibits cell migration when compared to GA, impairment of EphA2 in tandem with GA did not further inhibit cell migration beyond the GA effects. These data suggest that EphA2 is a major factor driving cell migration, and that, at least for these cancer cells, EphA2 comprises a majority of the cell motility supported by Hsp90 function. Although the combination of GA and either ligand or antibody was not obviously antagonistic, it may be considered that GA may inhibit ligand-mediated receptor phosphorylation, thus restraining the ability of ligand to phosphorylate the receptor and further inhibit migration within this context. Similarly, the 3F2-3M EphA2 antibody has been shown to stimulate EphA2 phosphorylation (50). However, ligand-dependent receptor phosphorylation and subsequent inhibition of downstream mediators occurs with kinetics on the order of minutes, while several hours are needed for GA to antagonize ligand-dependent phosphorylation. Further, the reduction of EphA2 expression via siRNA targeting represents an approach that is independent of receptor phosphorylation. We show that the combination of siRNA and GA did not further inhibit cell migration over that observed with GA alone, suggesting that these pathways are closely interconnected, rather than an alternate interpretation that a potentially additive effect is masked by molecular antagonism. Taken together, these data support the notion that the EphA2-dependent signaling pathway and Hsp90-mediated signaling pathways are interconnected with respect to their regulation of cell migration. Our data also highlight that, although Hsp90 supports the activity of hundreds of clients, perhaps a relatively small subset are pivotal for mediating cell motility. Although it remains an open question as to which specific downstream proteins are mutually involved in mediating Hsp90- and EphA2-dependent cell migration, one candidate may be FAK, which is an Hsp90 client protein (63) that is also a downstream mediator of EphA2 signaling and cell migration (25). We demonstrate that the Hsp90-supported EphA2 signaling module represents a main component of cell motility and that this pathway may be pharmacologically targeted with Hsp90 inhibitors.
The pharmacological targeting of EphA2 via Hsp90 inhibition is of clinical significance in malignancies such as glibolastoma (GBM), which represents one of the most invasive and aggressive diseases. Recently, three groups have demonstrated overexpression of EphA2 in a majority of glioblastoma cell lines and human GBM tissues compared to its expression in normal brain (11, 12, 64) and EphA2 is essential for GBM migration, as well as serving as a prognostic factor (11). In GBM, persistent EGFR activation simultaneously promotes EphA2 upregulation and suppresses ligand expression, the combination of which potently contributes to EphA2-driven tumorigenesis (24). Although ligand engagement can thwart tumorigenesis in several preclinical models, a number of reports illustrate that ephrin-A1 may also stimulate endothelial cell (EC) migration and assembly in vitro (65) and promotes angiogenesis in vivo (29, 30, 65). Therefore, ligand-based therapeutics to deplete EphA2 expression may not represent an ideal strategy for all tumor types and we propose that Hsp90-dependent targeting of EphA2 represents an alternative approach to reduce receptor expression. Furthermore, we demonstrate that, following GA treatment, residual surface-localized EphA2 maintains its receptiveness to ligand-dependent internalization, suggesting that a combination of Hsp90 inhibitor and ligand (or antibody) may be considered as a therapeutic approach to maximally reduce receptor expression and impair activity. This is further supported by our data indicating that GA impairs receptor dimerization and ligand-dependent phosphorylation and thus, this combination should mitigate potential ligand-mediated pro-angiogenic effects. In addition to EphA2, EphB2 has been implicated in GBM progression (66) and we demonstrate that EphB2 is similarly susceptible to Hsp90 inhibition. While the full spectrum of Eph family members regulated by Hsp90 awaits further determination, our results suggest that Hsp90 inhibition represents a rational therapeutic approach to reduce the aggressiveness of EphA2-driven cancers.
Materials and methods
Reagents and Antibodies
Geldanamycin was obtained from the Experimental Therapeutics Branch, National Cancer Institute, Bethesda, MD, PS-341 (bortezomib) was obtained from Millennium Pharmaceuticals (Cambridge, MA), ligands ephrin A1-Fc and ephrin B1-Fc were purchased from R&D Systems. The following antibodies were used: rabbit EphA2 C-20, phospho-tyrosine PY99, Hsp70 K-20 (Santa Cruz Biotechnology), Hsp90 SPA835 and cdc37 (Affinity BioReagents), HA-conjugated beads and anti-HA antibody (Covance), anti-tubulin (Sigma), goat polyclonal CHIP antibody (Chemicon) and anti-ubiquitin SPA-205 (Stressgen). The EphA2 blocking antibody 3F2-3M (67) was obtained from MedImmune (Gaithersburg, MD).
Cell Culture and Transfection
Cells were purchased from American Type Culture Collection. PC3, U251 and HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma), supplemented with 10% fetal bovine serum 1% pencilin/streptomycin (Invitrogen) in a 5% CO2-humidified atmosphere. Transient transfections of either plasmid DNA or siRNAs were performed with Lipofectamine 2000 (Invitrogen) in serum free Opti-MEM (Invitrogen) as per the manufacturer’s instructions. Cells were routinely harvested 20 h following transfection of plasmid DNA and 48 h for siRNA transfections. For EphA2 knockdown, a validated siRNA targeting sequence (Dharmacon) was used and control siRNA (Invitrogen) was used for comparison.
Plasmids and DNA manipulation
HA-tagged EphB1 vector (in pKCH) was kindly provided by Dr. Jun-Lin Guang (Cornell University, NY) and EphA2 vector was provided by Dr. Bingcheng Wang (Case Western Reserve, Ohio). Full length EphA2 was PCR amplified and cloned into the pcDNA3.1(+) vector (Invitrogen) with HindIII and XhoI engineered sites and an HA tag was encoded by the 3′ primer. For kinase deleted mutants, EphA2 and EphB1 were PCR amplified and termination codons were engineered into the respective 3′ primers. The EphA2 and EphB1 C-terminally deleted proteins expressed their respective juxtamembrane domains and were truncated adjacent to the beginning of their kinase domains (residue 613 for EphA2 and 620 for EphB1). Wild type CHIP and dominant negative (DN) CHIP lacking the TPR domain were obtained from Wangping Xu and Len Neckers, NCI.
Immunoprecipitation and Immunoblotting
Total cellular proteins were extracted by solubilizing the cells in TNES buffer (50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 1 mM EDTA, 150 mM NaCl) containing a cocktail of protease inhibitors (Roche). Whole cell lysates were clarified by centrifugation, quantified with the BCA protein assay reagent kit (Pierce) and equivalent protein amounts were added to Laemmli sample buffer for SDS-PAGE analysis. For all experiments involving analysis of phosphorylated proteins, 5 mM sodium fluoride and 1 mM sodium orthovanadate was added to the lysis and wash buffers. For all experiments involving detection of chaperone binding, a low detergent lysis buffer was used, (20 mM HEPES, pH 7.5, 100 mM NaCl, 1 mM MgCl2, 20 mM sodium molybdate 0.1% NP40 2 mM EDTA, 10% glycerol, protease inhibitors. Unless indicated, immunoprecipitations were performed overnight, the beads (Protein G, Invitrogen) were washed with the respective lysis buffer, boiled in Laemmli buffer, and analyzed by SDS-PAGE. For detection of ubiquitinylated proteins, cells were lysed in TNES buffer containing 250 mM NaCl and 1 mg total protein was incubated with HA beads overnight at 4°C. Adsorbed proteins were washed in lysis buffer containing 500 mM NaCl, eluted, and transferred to nitrocellulose membranes. The membranes were incubated for 30 min in 20 mM Tris-HCl containing 6 M guanidine hydrochloride and 5 mM 2-mercaptoethanol, and subsequently probed with anti-ubiquitin monoclonal antibody. After incubation with primary antibodies, proteins were visualized with either horseradish peroxidase-conjugated secondary antibodies (anti-mouse or anti-rabbit, GE Healthcare), or anti-goat peroxidase conjugate (Calbiochem), followed by incubation with Super Signal West Pico peroxide solution (Pierce) and chemiluminescent detection.
Pulse-Chase Analysis
Logarithmically growing PC3 cells were starved 30 minutes in methionine and cysteine-free medium (Invitrogen) and 150 uCi/ml methionine/cysteine (Tran35S-label, ICN) was added for 1 h. For drug treated samples, 1 uM GA was added at the time of label initiation. After the labeling period, cells were washed with nonradioactive complete medium either lacking or containing drug, and incubated for the indicated times. Following lysis, 1 mg total protein was precleared with protein G beads and EphA2 was immunoprecipitated overnight. For an additional control, EphA2 was precipitated with ephrin A1-Fc. The gels were fixed, incubated with EN3Hance solution (Perkin Elmer), dried, and exposed to film. EphA2 levels were determined by densitometric scanning of the film, quantitation with ImageJ software (NIH) and values were graphed semi-logarithmically.
Immunofluorescence microscopy
Cells were cultured on coverslips coated with 10 μg/ml fibronectin (Invitrogen) and treated with either GA (1μM), ephrin A1-Fc (1μg/ml), or control IgG Fc for the indicated times at 37°C. Cells were washed with PBS and fixed in 2% paraformaldehyde in PBS for 10 min at RT and permeabilized with 0.1% Trixton X-100 for 15 min at RT. Non-specific binding was blocked with 2% BSA, followed by an overnight incubation with a 1:50 dilution of anti-EphA2 antibody clone D7 (Upstate Biotechnology). After extensive washing, cells were incubated for 1 h with a 1:400 dilution of Alexa Fluor 546 goat anti-mouse IgG (Invitrogen). Nuclear DNA was visualized with a 1:100 dilution of To-pro-3 (Invitrogen, 1:1000) and slides were mounted with SlowFade Gold antifade reagent (Invitrogen). Confocal images were acquired with an Olympus IX70 microscope (Fluoview 300). To determine whether GA affects EphA2 localization at cell-cell contacts, approximately 100 cells were randomly counted from 3–4 fields and the ratio of cells containing EphA2 localized at cell-cell contacts was quantitated relative to receptor localization in the total number of cell-cell contacts in each field.
Flow cytometry
Cells were trypsinized, washed with PBS, blocked (0.1% sodium azide, 2% BSA/PBS), and incubated with goat polyclonal EphA2 antibody (Santa Cruz) diluted 1:50 in blocking buffer. The cells were washed twice before being incubated with a 1:1000 dilution of donkey anti-goat Alexafluor 488 (Invitrogen). The cells were washed, resuspended in PBS and analyzed using a FACS Calibur4-color flow cytometer (BD Biosciences, San Jose, CA) and FlowJo software (TreeStar). A minimum of 10,000 cells was counted per experiment. Negative controls consisted of cells incubated either in the complete absence of antibody or with secondary antibody alone. We also confirmed that the H4 GBM line that does not express EphA2 (11), did not express EphA2 (data not shown).
Ligand stimulation
PC3 cells were plated on coverslips coated with 10 μg/ml fibronectin. Cultures at 80% confluency were incubated for indicated time periods with either GA (1 μM), 1 μg/ml ephrin A1-Fc or control Fc, or pretreated with GA, followed by a 5′ stimulation with ligand and immediately fixed with 3.7% formaldehyde. Cell images were collected using an Olympus IX70 microscope (Fluoview 300). For every cover slip, three fields were randomly chosen and the percentage of rounded cells was calculated from the total cells per field.
Migration Assays
Chemotactic cell migration was assessed using modified Boyden chambers (tissue culture treated, 0.8-μm, BD Biosciences). Cells (3–5 × 103) were plated into the upper chamber in 0.1% FBS -DMEM medium and the lower chamber contained DMEM supplemented with 10% serum. For inhibitor studies, cells were pretreated for 8 h with the indicated treatments prior to plating into the chambers and these same agents were added to the chambers following cell plating. For siRNA treatments, cells were transfected with the indicated siRNAs and then plated 48 h following transfection. Following an 18 hr time after plating, the inserts were washed with PBS, the non-motile cells removed with cotton swabs, and the adherent cells attached to the filter, representing the migratory cells, were fixed in 3.7 % formaldehyde for 15 minutes and visualized with 0.1% crystal violet solution. Cells were counted using a NIKON Eclipse TS100 microscope with 20X magnification. Inhibition of cell migration in response to indicated treatments was determined by the relative numbers of cells adhering to filters following treatment, as compared to untreated controls (ie 100% migration). The mean value from 10 fields per chamber was calculated from 2 chambers per experiment and the experiment was repeated. The data presented represent the standard error of the mean (SEM). Differences in treatment groups were defined as significant if the p value, as calculcated from Student’s T test, was 0.05 or less.
Acknowledgments
We thank Bingcheng Wang (Case Western University, Cleveland, OH) for the EphA2 vector and for many helpful discussions and Jin Chen (Vanderbilt University, TN) for myc-tagged EphA2 vector. We thank Dowdy Jackson and Elizabeth Bruckheimer (MedImmune) for the gift of the EphA2 blocking antibody 3F2-3M. Many thanks to lab members for helpful suggestions, to Patrick Nasarre for assistance with data interpretation, and to Chris Parsons for assistance with flow cytometry. Partial support was provided by American Cancer Society grant IRG-97-219-05 and R01 CA135297-01.
References
- 1.Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 3.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 5.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 6.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- 8.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 9.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochem Biophys Res Commun. 2004;320:1096–1102. doi: 10.1016/j.bbrc.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 11.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 13.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 14.Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- 15.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–892. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 17.Herrem CJ, Tatsumi T, Olson KS, Shirai K, Finke JH, Bukowski RM, et al. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically treated patients with renal cell carcinoma. Clin Cancer Res. 2005;11:226–231. [PubMed] [Google Scholar]
- 18.Merritt WM, Thaker PH, Landen CN, Jr, Deavers MT, Fletcher MS, Lin YG, et al. Analysis of EphA2 expression and mutant p53 in ovarian carcinoma. Cancer Biol Ther. 2006;5:1357–1360. doi: 10.4161/cbt.5.10.3225. [DOI] [PubMed] [Google Scholar]
- 19.Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, et al. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–360. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 20.Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283–293. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- 21.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 22.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- 23.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 24.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 26.Walker-Daniels J, Riese DJ, 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res. 2002;1:79–87. [PubMed] [Google Scholar]
- 27.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal reorganization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 28.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 29.Cheng N, Brantley D, Fang WB, Liu H, Fanslow W, Cerretti DP, et al. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–456. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 32.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 33.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 34.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 36.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 37.Whitesell L, Shifrin SD, Schwab G, Neckers LM. Benzoquinonoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res. 1992;52:1721–1728. [PubMed] [Google Scholar]
- 38.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: Combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson S, Valenti M, Gowan S, Patterson L, Ahmad Z, Workman P, et al. Benzoquinone ansamycin heat shock protein 90 inhibitors modulate multiple functions required for tumor angiogenesis. Mol Cancer Ther. 2006;5:522–532. doi: 10.1158/1535-7163.MCT-05-0439. [DOI] [PubMed] [Google Scholar]
- 40.Salvucci O, de la Luz Sierra M, Martina JA, McCormick PJ, Tosato G. EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood. 2006;108:2914–2922. doi: 10.1182/blood-2006-05-023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, et al. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 45.Bonvini P, Dalla Rosa H, Vignes N, Rosolen A. Ubiquitination and proteasomal degradation of nucleophosmin-anaplastic lymphoma kinase induced by 17-allylamino-demethoxygeldanamycin: role of the co-chaperone carboxyl heat shock protein 70-interacting protein. Cancer Res. 2004;64:3256–3264. doi: 10.1158/0008-5472.can-03-3531. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J Biol Chem. 2007;282:2683–2694. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
- 47.Belova L, Brickley DR, Ky B, Sharma SK, Conzen SD. Hsp90 regulates the phosphorylation and activity of serum- and glucocorticoid-regulated kinase-1. J Biol Chem. 2008;283:18821–18831. doi: 10.1074/jbc.M803289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miao RQ, Fontana J, Fulton D, Lin MI, Harrison KD, Sessa WC. Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:105–111. doi: 10.1161/ATVBAHA.107.155499. [DOI] [PubMed] [Google Scholar]
- 49.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- 50.Bruckheimer EM, Fazenbaker CA, Gallagher S, Mulgrew K, Fuhrmann S, Coffman KT, et al. ADCC effector enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors. Neoplasia. 2009 doi: 10.1593/neo.81578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- 52.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 53.Lu C, Shahzad MM, Wang H, Landen CN, Kim SW, Allen J, et al. EphA2 Overexpression Promotes Ovarian Cancer Growth. Cancer Biol Ther. 2008;7 doi: 10.4161/cbt.7.7.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99:12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–42382. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- 57.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–368. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 58.Sabath E, Negoro H, Beaudry S, Paniagua M, Angelow S, Shah J, et al. Galpha12 regulates protein interactions within the MDCK cell tight junction and inhibits tight-junction assembly. J Cell Sci. 2008;121:814–824. doi: 10.1242/jcs.014878. [DOI] [PubMed] [Google Scholar]
- 59.Fang WB, Brantley-Sieders DM, Hwang Y, Ham AJ, Chen J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J Biol Chem. 2008;283:16017–16026. doi: 10.1074/jbc.M709934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, et al. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem. 2007;282:19619–19628. doi: 10.1074/jbc.M701319200. [DOI] [PubMed] [Google Scholar]
- 61.Zagzag D, Nomura M, Friedlander DR, Blanco CY, Gagner JP, Nomura N, et al. Geldanamycin inhibits migration of glioma cells in vitro: a potential role for hypoxia-inducible factor (HIF-1alpha) in glioma cell invasion. J Cell Physiol. 2003;196:394–402. doi: 10.1002/jcp.10306. [DOI] [PubMed] [Google Scholar]
- 62.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 63.Ochel HJ, Schulte TW, Nguyen P, Trepel J, Neckers L. The benzoquinone ansamycin geldanamycin stimulates proteolytic degradation of focal adhesion kinase. Mol Genet Metab. 1999;66:24–30. doi: 10.1006/mgme.1998.2774. [DOI] [PubMed] [Google Scholar]
- 64.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 66.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oganesyan V, Damschroder MM, Leach W, Wu H, Dall’Acqua WF. Structural characterization of a mutated, ADCC-enhanced human Fc fragment. Mol Immunol. 2008;45:1872–1882. doi: 10.1016/j.molimm.2007.10.042. [DOI] [PubMed] [Google Scholar]