Abstract
Ageing is driven by the inexorable and stochastic accumulation of damage in biomolecules vital for proper cellular function. Although this process is fundamentally haphazard and uncontrollable, senescent decline and ageing is broadly influenced by genetic and extrinsic factors. Numerous gene mutations and treatments have been shown to extend the lifespan of diverse organisms ranging from the unicellular Saccharomyces cerevisiae to primates. It is becoming increasingly apparent that most such interventions ultimately interface with cellular stress response mechanisms, suggesting that longevity is intimately related to the ability of the organism to effectively cope with both intrinsic and extrinsic stress. Here, we survey the molecular mechanisms that link ageing to main stress response pathways, and mediate age-related changes in the effectiveness of the response to stress. We also discuss how each pathway contributes to modulate the ageing process. A better understanding of the dynamics and reciprocal interplay between stress responses and ageing is critical for the development of novel therapeutic strategies that exploit endogenous stress combat pathways against age-associated pathologies.
Keywords: autophagy, endoplasmic reticulum, heat shock, homeostasis, mitochondria
Introduction
Ageing is a complex process associated with progressive decay of physiological function and homeostasis. At the molecular level, ageing is characterized by the gradual accumulation of deleterious modifications in nucleic acids, proteins, lipids and carbohydrates. In humans, general age-related frailty is also associated with severe pathological conditions such as cancer and neurodegenerative diseases.
Over the past 20 years, ageing research has culminated in the identification of classical signalling pathways that influence ageing in a variety of species (Kenyon, 2010). Accumulating findings indicate that longevity depends on the ability of the organism to cope with extrinsic or intrinsic stressors (Kirkwood and Austad, 2000). Indeed, compromised stress responses are linked to the onset of many age-related diseases. ‘Stress’ is broadly defined as a noxious factor (physical, chemical or biological), which triggers a series of cellular and systemic events, resulting in restoration of cellular and organismal homeostasis. To cope with conditions of stress, organisms have developed a wide range of sophisticated stress response mechanisms, acting at the cellular or organelle-specific level. Notably, exposure to mild stress activates cellular homeodynamic mechanisms, without mounting a comprehensive stress response, which better prepare the organism against stronger insults and promote long-term survival. This phenomenon is known as hormesis (Calabrese et al, 1987; Rattan, 2008).
Much of our understanding of the link between activation of stress response pathways and longevity, as well as, the impact of ageing on the effectiveness of stress response mechanisms derive from studies in model organisms including yeast, worms, flies and mice. Here, we review the main cellular stress response mechanisms, focusing on the effects of ageing on the capacity of the cell to mount a successful stress response. Furthermore, we discuss the influence of stress response on the ageing process. Maintaining efficient mechanisms for counterbalancing stress is emerging as a potential strategy towards ameliorating age-associated pathologies. To this end, we highlight several open questions that need to be addressed before manipulation of stress response pathways can be considered for therapeutic intervention.
The heat shock response
Exposure of cells and organisms to unfavourable conditions such as heat, oxidative and osmotic stress, heavy metals and proteasome inhibitors induces a highly conserved programme of gene expression leading to selective transcription and translation of heat shock proteins (HSPs) (Lindquist and Craig, 1988; Morimoto, 2008). Based on their molecular weight, HSPs are categorized into the HSP100, HSP90, HSP70, HSP60 and the small HSP (sHSP) families. The heat shock response is orchestrated by a set of heat shock transcription factors (HSFs). The mammalian HSF family consists of four members (HSF1-4), while Drosophila, Caenorhabditis elegans and yeast express only one (HSF1) (Morimoto and Santoro, 1998; Anckar and Sistonen, 2007; Akerfelt et al, 2010). Activation of heat shock response is a complex process that involves trimerization and translocation of HSF1 to the nucleus, where it binds to heat shock elements within the promoters of heat shock genes (Figure 1). HSF1 undergoes multiple post-translational regulatory modifications such as phosphorylation (Sorger and Pelham, 1988; Knauf et al, 1996; Kline and Morimoto, 1997; Holmberg et al, 2001; Guettouche et al, 2005), sumoylation (Hietakangas et al, 2003; Anckar et al, 2006) and acetylation (Westerheide et al, 2009). Upon sufficient induction of the heat shock response pathway, HSF1 returns to monomeric form and interacts with HSP90, HSP70 and HSP40 chaperones (Abravaya et al, 1992; Shi et al, 1998; Zou et al, 1998). In this state, HSF1 remains inactive and the response is terminated. The tightly regulated initiation, execution and termination of the response, through complex post-translational modifications and protein interactions, underscores the requirement for precise activation of the heat shock pathway upon stressful conditions.
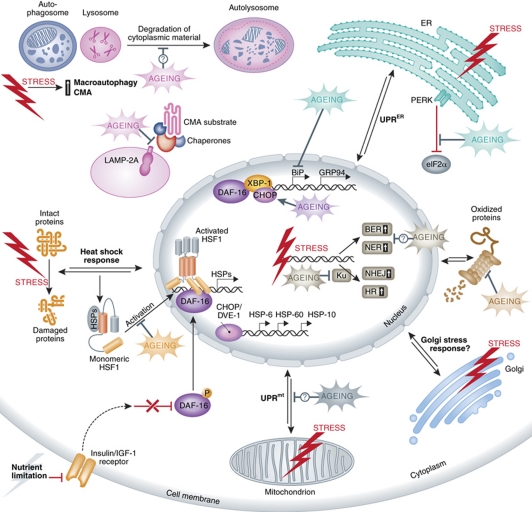
Figure 1.
General and organelle-specific stress response pathways influenced by the ageing process. Depending on the type of macromolecule and the site of damage, distinct stress response pathways, such as autophagy, heat shock response, UPRmt, UPRER, remodelled proteasome and the DNA damage response are initiated. Ageing broadly affects stress response pathways in multiple steps. For simplicity, only proteins with functions modulated by ageing are depicted. Double arrows denote bi-directional communication with the nucleus, which involves generation of stress signals in the stressed organelle or the cytoplasm, transduction of the signals to the nucleus and upregulation of stress-relieving proteins, which in turn function to ameliorate damage. Question marks denote lack of information about specific molecules mediating the effects of ageing. Although, a typical Golgi stress response pathway has not been described yet, several types of stress and also ageing may influence gene expression in the nucleus and cell homeostasis by impinging on Golgi function. BER, base-excision repair; BiP, Ig-binding protein; CHOP, C/EBP homologous protein; CMA, chaperone-mediated autophagy; DAF-16, abnormal dauer formation 16; DVE-1, defective proventriculus 1; GRP94, glucose-regulated protein 94; HSF1, heat shock factor 1; HSP, heat shock protein; HR, homologous recombination; IGF-1, insulin growth factor 1; LAMP-2A, lysosome-associated membrane protein 2A; NER, nucleotide-excision repair; NHEJ, non-homologous end joining; PERK, PKR-like ER kinase; UPRER/mt, unfolded protein response endoplasmic reticulum/mitochondrion; XBP-1, X-box-binding protein 1.
The hallmark of many age-related neurodegenerative diseases, such as Alzheimer's, Parkinson's and Huntington's disease is the formation of insoluble protein aggregates. Nevertheless, recent studies show that protein aggregation also occurs in a non-disease context during ageing (David et al, 2010). Global proteomics analysis revealed that several hundred proteins displayed increased aggregation propensity during normal ageing in C. elegans. Interestingly, mutations that reduce insulin/IGF-1 signalling prevent protein insolubility during ageing. Reduced insulin/IGF-1 signalling is also beneficial against pathology and disease caused by protein aggregation (Morley et al, 2002; Cohen et al, 2006). In the nematode, the effects of insulin/IGF-1 signalling on ageing are mediated by the transcription factor DAF-16/FOXO. Remarkably, both DAF-16 and HSF1 increase longevity partly by inducing shsp expression (Hsu et al, 2003). Identification of the subset of DAF-16 target genes, responsible for the anti-aggregation effects of low insulin/IGF-1 signalling will shed light into the molecular mechanisms defending against protein aggregation-induced cytotoxicity during ageing. It is important to note that age-related protein aggregate formation may alternatively serve as a protective function, similar to that in neurodegeneration (Ross and Poirier, 2005). It is not clear whether these aggregates contain damaged proteins, carrying modifications that render them potentially harmful for the cell. Moreover, the kinetics of protein aggregation during ageing remains unknown; is there an age threshold above which new aggregates form at a faster pace? Does this point in time coincide with extensive aggregation of critical chaperones, which consequently become inactive? Given that multiple chaperone proteins involved in the maintenance of proteostasis are themselves also prone to aggregation, it would be interesting to investigate whether the concomitant, runaway protein aggregation overwhelms proteostatic mechanisms, contributing to the collapse of general cellular homeostasis during ageing.
Ageing is associated with elevated expression of HSP genes, in the absence of other external stressors, suggesting that the process of ageing generates intrinsic stress signals and/or detunes gene expression programmes (Morrow and Tanguay, 2003; Landis and Tower, 2005; Macario and Conway de Macario, 2005; Muller et al, 2007). In spite of elevated basal HSP expression, the effectiveness of the heat shock response following acute extrinsic stress deteriorates with age. Attenuation of the heat shock response is not the result of decreased levels of HSF1 in aged animals (Heydari et al, 2000; Soti and Csermely, 2000). Instead, defects in the signal transduction pathway that leads to HSF1 activation are likely the reason for suboptimal induced expression of HSPs during ageing (Heydari et al, 2000; Lu et al, 2000; Soti and Csermely, 2000). Upon stress, HSF1 is acetylated by histone acetyltransferase p300/CBP (CREB-binding protein). This modification quenches the heat shock response by triggering the dissociation of HSF1 from target heat shock response elements on DNA (HSEs). Pharmacological activation of SIRT1 by resveratrol, or overexpression of SIRT1 prolongs HSF1 binding to target promoters and enhances the heat shock response (Westerheide et al, 2009). Interestingly, reduced HSF1 DNA binding and HSP expression coincides with a decrease in SIRT1 expression during ageing. Another mechanism underlying the attenuation of stress response during ageing involves specific HSPs. Increased basal levels of HSP70 and other HSPs in old cells may retain HSF in an inactive state, as part of the heat shock response initiation control mechanism (Morimoto, 2002).
While detailed analysis of the heat shock response pathway steps affected by ageing is still incomplete, it is becoming clear that protein aggregation both in the context of heritable disorders and in a non-disease setting, is characterized by age-dependent progression. Indeed, recent studies indicate that failure of proteostasis occurs in an age-dependent manner, with the initial decline commencing early in adulthood and leading to misfolding of folding sensors (Ben-Zvi et al, 2009). Importantly, collapse of proteostasis is ameliorated by overexpression of the stress transcription factors HSF1 and DAF-16, suggesting that interventions designed to fortify cell proteostasis may successfully offset the consequences of ageing on protein aggregation pathologies.
A paradoxical trait of several neurodegenerative disorders is that, although the relevant mutant protein implicated in the pathogenesis of the disease is expressed in a wide range of neurons, only specific neuronal subtypes are prone to degeneration. Interestingly, even though the components of the heat shock response are present in all cells, different tissues show differential chaperoning capacity and induction of the pathway (Kern et al, 2010). This disparity, combined with tissue-specific alterations in the ubiquitin–proteasome system (UPS) activity during ageing (Holmberg et al, 2001; Tonoki et al, 2009), may explain the increased vulnerability of certain cell types. However, whether activation of the heat shock response or other proteostatic mechanisms is atypical in degenerating neuronal types remains to be investigated.
The ubiquitin–proteasome system
Ageing is accompanied by accumulation of damaged and modified proteins. The build up of altered proteins is the result of a gradual deterioration of cellular quality control mechanisms, decreased protein degradation or a combination of both. The UPS is the main proteolytic mechanism, responsible for the degradation of damaged proteins and the turnover of most cytosolic and nuclear proteins. The process of protein degradation by the UPS involves two steps: tagging of the protein with a polyubiquitin chain and the degradation of the tagged protein by the proteasome (Ciechanover, 2005). Polyubiquitination is a complex reaction involving ubiquitin, a highly conserved 76 amino acids protein, and three different enzymes (E1–E3). The proteasome is a multicatalytic protease complex composed of one 20S catalytic core and two 19S regulatory caps (Jung et al, 2009).
Oxidative stress has important roles during the ageing process and age-related diseases. Oxidized proteins that escape the low-molecular weight and enzymatic anti-oxidative damage defences of the cell are recognized and degraded by the proteasome. In the presence of moderate oxidant concentrations, proteasomal degradation increases, whereas higher oxidation levels lead to proteolytic inhibition (Ding et al, 2006; Farout and Friguet, 2006; Breusing and Grune, 2008). Impairment of proteasomal activity induces a proteasome stress response that ultimately results in upregulation of proteasome subunit expression (Meiners et al, 2003; Ju et al, 2004). In addition, ubiquitin depletion triggers a ubiquitin stress response in yeast (Hanna et al, 2007). Under such conditions, loading of proteasomes with Ubp6, a deubiquitinating enzyme, is increased. In turn, this results in greater recycling of ubiquitin at the proteasome. It would be interesting to test whether this stress response is maintained during ageing, ensuring the availability of sufficient ubiquitin for degradation of accumulating damaged proteins.
Proteasome activity declines with age in a variety of tissues (Conconi et al, 1996; Shibatani et al, 1996; Anselmi et al, 1998; Ponnappan et al, 1999; Keller et al, 2000b). By contrast, the ubiquitination system does not appear as affected by age (Carrard et al, 2002). It should be noted that the decrease in proteasome activity likely has an important role in the physiological control of lifespan of specific cell types. For example, proteasome capacity dramatically decreases during plasma cell differentiation (Cenci et al, 2006). This is paradoxical given that plasma cells are highly active in antibody production. Nevertheless, the decrease in abundance and activity of proteasome predisposes plasma cells to apoptosis protecting from excessive humoral response.
Multiple mechanisms have been implicated in the age-dependent decline of the proteasome. Decreased expression of proteasomal subunits has been reported in several experimental setups (Ly et al, 2000; Bulteau et al, 2002). In addition, changes in proteasomal enzymatic activities are caused by increased oxidative stress during ageing (Carrard et al, 2002). Moreover, oxidized and damaged proteins can directly inhibit the proteasome leading to depletion of active proteolytic units (Terman and Brunk, 2004). However, the impact of each of these factors on age-related loss of proteasome function is not yet fully characterized. The threshold of age-related modifications above which the proteasome is overwhelmed and becomes dysfunctional, needs to be determined. Intriguingly, many of the genes mutated in age-related diseases such as Alzheimer's and Parkinson's disease, have a role in UPS (Keller et al, 2000a; Jenner, 2001). Maintenance or enhancement of the proteolytic activity of the proteasome during ageing might provide protection against neuronal cell death documented in these diseases. It is important to note that accumulation of unfolded and damaged proteins, following proteasome inhibition, leads to activation of the heat shock and the endoplasmic reticulum (ER) stress response (Kisselev and Goldberg, 2001). Thus, the ubiquitin–proteasome system is tightly integrated into a wider and complex network of cellular proteostasis-preserving mechanisms.
Organelle-specific stress response pathways
Endoplasmic reticulum
The ER is the organelle where newly synthesized proteins destined for secretion, integration into the plasma membrane or distribution to various organelles, are folded and post-translationally modified. The environment within the ER is highly crowded with chaperones, processing enzymes and client proteins (Stevens and Argon, 1999). In this cluttered and aggregation-prone environment, complex ER quality control mechanisms ensure the proper translation and folding of nascent proteins as well as the degradation of improperly folded polypeptides (Ellgaard and Helenius, 2003). Key chaperones and folding sensors reside in the ER, including the Ig-binding protein (BiP)/glucose-regulated protein 78 (GRP78), GRP94, calnexin, calreticulin and protein disulphide isomerase (PDI) (Naidoo, 2009). Several of these vital chaperones and enzymes show decreased mRNA, protein levels and/or enzymatic activity in various tissues during ageing (Erickson et al, 2006; Paz Gavilan et al, 2006; Hussain and Ramaiah, 2007; Naidoo et al, 2008; Nuss et al, 2008). Consequently, age-dependent decline of protein folding efficiency creates an unstable ER environment, not capable of sustaining homeostasis under steady-state or elevated stress conditions.
Conditions that elicit increased load of misfolded proteins within the ER trigger the ER stress or unfolded protein response (UPRER) (Schroder and Kaufman, 2005; Ron and Walter, 2007). UPRER helps restore the normal function of ER through upregulation of ER chaperones, halting protein translation and stimulating the degradation of misfolded proteins (Prostko et al, 1993; Kaufman, 1999; Hampton, 2000). In situations of persistent stress, failure of UPRER to restore ER homeostasis results in apoptosis (Szegezdi et al, 2006). Apart from its role in the relief from stress induced by various triggers, UPR is important for the differentiation and proper function of professional secretory cells, which have increased protein folding demands in the ER, such as antibody-secreting plasma cells, pancreatic β cells, hepatocytes and osteoblasts (Wu and Kaufman, 2006). Downstream signalling during the UPRER response is mediated by three transmembrane sensors: the inositol requiring element-1 (IRE-1), the PKR-like ER kinase (PERK) and the activating transcription factor 6 (ATF6). The molecular chaperone BiP/GRP78 retains these transmembrane receptor proteins in an inactive state. When critical level of unfolded proteins is exceeded, BiP/GRP78 dissociates from IRE-1, PERK and ATF6 to facilitate protection against the overwhelming load of misfolded proteins (Zhang and Kaufman, 2006). Activation of UPRER through titration of BiP/GRP78 away from the three sensors of ER stress is reminiscent of the mechanism by which HSF is mobilized. Activation of ER stress mechanism via direct recognition of unfolded proteins by stress transducers, as well as, a hybrid recognition model, involving both mechanisms has been proposed (Ron and Walter, 2007). Activated PERK phosphorylates the translation initiation factor eIF2α, preventing protein synthesis from further overwhelming the ER (Figure 1). Activated ATF6 translocates to the Golgi, where it is cleaved by proteases to form an active 50 kDa transcription factor, which enters the nucleus and upregulates the transcription of genes encoding ER chaperone proteins such as BiP, PDI and GRP94 (Yoshida et al, 1998). IRE-1 activation results in X-box-binding protein 1 (XBP-1) splicing and activation. The activated spliced form of XBP-1 (XBP-1s) acts as a transcription factor, which enhances the expression of genes involved in ER homeostasis as well as in export and degradation of misfolded proteins (Yoshida et al, 2001, 2003; Calfon et al, 2002; Lee et al, 2003).
PERK mRNA expression is lower in aged rats compared with young animals (Paz Gavilan et al, 2006). Interestingly, in aged mice subjected to acute sleep deprivation, activation of PERK and the subsequent inhibition of mRNA translation are impaired (Naidoo et al, 2008). Such alleviation of the translation block initiates a vicious circle, where new protein synthesis further aggravates ER stress. CHOP, a transcription factor of the C/EBP family, is expressed at low levels under normal conditions and it is markedly induced upon sustained ER stress (Zinszner et al, 1998). CHOP also mediates apoptosis under conditions of extreme ER stress (McCullough et al, 2001). Contrary to PERK, CHOP levels are higher in various tissues of aged rodents (Paz Gavilan et al, 2006; Hussain and Ramaiah, 2007; Naidoo et al, 2008). Exposure of aged animals to stressors further increases the levels of CHOP, whereas young animals show no increase of the protein under similar conditions. It appears that aged animals fail to mount a timely ER stress response due to alterations in the expression of key components of the response. Moreover, aged cells display increased levels of CHOP, which further facilitates apoptosis, reducing the threshold for initiation of cell death.
Recent studies in C. elegans show that XBP-1s synergizes with DAF-16 to activate genes, which lead to enhanced ER stress resistance and also promote the longevity of mutants with reduced insulin/IGF-1 signalling (Henis-Korenblit et al, 2010). It remains to be determined whether the IRE-1/XBP-1 axis is modified during ageing, which would impede coordination with other stress response factors and consequently impair the ER stress response. ER stress activates both the ubiquitin–proteasome and the macroautophagy–lysosome proteolytic system (Yorimitsu et al, 2006; Ding and Yin, 2008). Whether ageing hinders the activation of these degradation systems, leading to overflow of damaged proteins remains to be seen. Interestingly, defective ER stress response has been associated with age-related pathologies such as diabetes, heart disease and neurodegenerative disorders (Lindholm et al, 2006; Yoshida, 2007; Lin et al, 2008). Whether ageing precipitates common alterations of the UPRER components that underlie these diverse diseases is unclear.
Mitochondria
Mitochondrial dysfunction has been associated with oxidative stress, accelerated ageing, apoptosis, neurodegenerative disorders and other pathological conditions. The matrix of mitochondria contains a specific set of chaperones involved in importing, refolding and preventing aggregation of proteins encoded both by the nuclear genome and by mtDNA. The main mitochondrial stress proteins are HSP60, mtHSP70, HSP10, mtGrpE and mtDnaJ. Perturbation of the folding environment in mitochondria elicits the mitochondrial unfolded response (UPRmt) by inducing expression of nuclear genes that encode mitochondrial chaperones (Zhao et al, 2002; Kuzmin et al, 2004; Yoneda et al, 2004). The mitochondrial stress response involves the transcription factors CHOP and C/EBPβ (Zhao et al, 2002). Several downstream components of the UPRmt have also been identified. Upon proteotoxic conditions, CLPP-1, a proteolytic subunit of the mitochondrial Clp protease, generates peptides that are transported to the cytosol by the ABC transporter HAF-1. Activation of UPRmt correlates with the formation of a complex between UBL-5 (a ubiquitin-like protein) and DVE-1 (a homeobox containing transcription factor) and the subsequent relocation of the complex to the nucleus. UPRmt also triggers the relocation of the bZip transcription factor ZC376.7 to the nucleus (Benedetti et al, 2006; Haynes et al, 2007, 2010).
In addition to the intrinsically stressful cellular context accompanying ageing, organisms have to also cope with external environmental challenges. Mitochondria isolated from the liver of old rats display increased susceptibility to hyperthermic conditions compared with young animals. Moreover, activation of the mitochondrial stress response is compromised in old animals, which fail to properly upregulate the mitochondrial stress proteins HSP60 and HSP10 (Haak et al, 2009). Finally, the processes of protein import and damaged protein degradation, which are mediated by mitochondrial stress proteins and are vital for mitochondrial homeostasis, become inefficient during ageing (Craig and Hood, 1997; Bulteau et al, 2006). However, the molecular mechanisms that bring about this decline are not understood.
The lysosome
Autophagy is one of the main processes mediating both bulk and specific degradation of cellular components, including whole organelles and protein aggregates. Cargoes destined for degradation are delivered to lysosomes, where they are recycled. Three main types of autophagy have been defined on the basis of lysosomal delivery mechanisms: macroautophagy, microautophagy and chaperone-mediate autophagy (CMA) (Cuervo, 2004; Mizushima et al, 2008). Macroautophagy entails the sequestration of portions of the cytoplasm within a double-membrane autophagic vacuole, called autophagosome. The autophagosome fuses with secondary lysosomes to form an autolysosome, where hydrolases degrade the sequestered material (Yorimitsu and Klionsky, 2005). In microautophagy, which is less well characterized, the lysosomal membrane itself invaginates to engulf cytosolic components (Marzella et al, 1981). CMA is a highly selective form of autophagy that requires unfolding of the protein before internalization into the lysosome for degradation (Dice, 2007; Cuervo, 2010). In addition to turnover of cellular material, autophagy is involved in development, differentiation and tissue remodelling. Although a basal level of macroautophagy and CMA is observed in various cell types, these pathways are maximally activated under conditions of stress (Figure 1). Analysis of mice harbouring tissue-specific, conditional knockout alleles of autophagy genes demonstrates that the capacity to modulate the rate of intracellular content degradation in response to stress or a nutrient-depleted environment is vital both for cell and organismal survival (Komatsu et al, 2005, 2006; Hara et al, 2006; Nakai et al, 2007). The decreased lysosomal-mediated degradation observed in rodent livers during ageing is attributed to defects both in the clearance of autophagic vacuoles and in the hormonal regulation of macroautophagy (Terman, 1995; Vittorini et al, 1999; Donati et al, 2001, 2008; Brunk and Terman, 2002). Lysosomes isolated from livers of old rats show lower rates of CMA compared with young animals (Cuervo and Dice, 2000). This decline in the efficiency of CMA is the result of altered dynamics and stability of LAMP-2A, the lysosomal receptor that recognizes substrates targeted for CMA (Kiffin et al, 2007).
Autophagy is a complex process with multiple steps that could potentially be altered by ageing. However, age-induced modifications of specific components of macroautophagy have yet to be studied systematically. Is it possible to restore the function of the entire pathway by manipulating the levels of one key protein? Interestingly, recent findings show that modulation of the amount of LAMP-2A in a transgenic mouse model is sufficient to maintain CMA activity until advanced age (Zhang and Cuervo, 2008). Livers from these transgenic animals display improved cellular homeostasis and resistance to toxic compounds. Furthermore, restoration of normal levels of Atg8, whose expression is impaired during ageing, extends lifespan (Simonsen et al, 2008). In addition, autophagy is activated when proteasome capacity is exceeded, in an effort to compensate during excessive demands for cellular proteolysis (Ding et al, 2007; Ding and Yin, 2008). It would be interesting to investigate whether activation of this backup proteolytic mechanism is impaired during ageing, leading to accumulation of misfolded and/or damaged proteins.
Activation of macroautophagy or CMA through pharmacological interventions is potentially an effective approach to maintain efficient clearance mechanisms in a damage-prone environment. Indeed, pharmacological upregulation of macroautophagy slows down the progression of disease in fly and mouse models of neurodegeneration (Ravikumar et al, 2004). The importance of maintaining an efficient autophagic response is also demonstrated by the fact that all long-lived C. elegans mutants display increased macroautophagy (Melendez et al, 2003; Hars et al, 2007; Hansen et al, 2008; Toth et al, 2008). Nonetheless, excessive activation of autophagy may lead to depletion of the essential autophagic components and failure of proper response to stress. Consistent with this notion, premature ageing in a mouse model of progeria is accompanied with extensive basal activation of autophagy (Marino et al, 2008). Moreover, examination of Alzheimer's disease patient brains revealed increased autophagy (Lipinski et al, 2010). In addition, treatment of healthy cells with Aβ both increased initiation of autophagy and decreased the rate of autophagosome clearance due to reduced lysosomal function. Therefore, autophagy upregulation may have adverse effects if initiated when cellular degradation mechanisms are already overwhelmed. Activation of autophagy above a crucial threshold, may also lead to cell demise due to interference with pro-survival mechanisms and digestion of anti-apoptotic molecules (Kourtis and Tavernarakis, 2009). Evaluation of the degradation capacity of cells through lifespan, and genetic manipulation of autophagic processes in model organisms during ageing will provide significant insights into the role of autophagy in senescent decline, and may contribute to the development of intervention strategies targeting age-associated neurodegenerative disorders.
The nuclear DNA damage response
In contrast to most biomolecules such as proteins and lipids, which can be recycled several times over the lifespan of a cell, DNA cannot be resynthesized afresh to eliminate damage. Instead, cells maintain elaborate genome maintenance machinery to mend their genetic material. DNA damage can be induced by exogenous hazards, such as UV radiation, by endogenous toxic by-products of cellular metabolism, such as reactive oxygen species (ROS) or by spontaneous chemical reactions, such as hydrolysis (Lindahl, 1993; De Bont and van Larebeke, 2004; Hoeijmakers, 2009). Defects in genome maintenance mechanisms underlie the pathology of the vast majority of progeroid syndromes, suggesting that DNA damage, which also accumulates during normal ageing, contributes to age-related deterioration (Dolle et al, 1997; Sedelnikova et al, 2004; Garinis et al, 2008). In addition, DNA repair mechanisms are subject to modifications throughout the lifespan of a cell, leading to gradual loss of repair accuracy and efficiency.
The genome maintenance apparatus of the cell consists of multiple complex repair pathways, each targeting a specific category of DNA lesion (Hoeijmakers, 2009). Base-excision repair (BER) removes subtle lesions of DNA that affect only one DNA strand, such as oxidized bases (Barnes and Lindahl, 2004; Caldecott, 2008). The complementary strand is used as a template for repair. Ageing has a negative impact on BER mechanisms (Figure 1). Both a drop in the activity of BER enzymes and a reduction in the inducibility of the pathway has been observed in aged mice (Cabelof et al, 2002, 2006; Chen et al, 2002; Intano et al, 2003; Lu et al, 2004; Krishna et al, 2005; Imam et al, 2006; Wilson and Bohr, 2007).
Nucleotide-excision repair (NER) is a multistep process involving numerous proteins that target helix-distorting lesions, resulting from UV exposure and carcinogenic compounds (Hanawalt, 2002). NER comprises two subpathways: the global-genome NER (GG-NER), which scans the genome for helix distortions (Gillet and Scharer, 2006; Sugasawa, 2006), and the transcription-coupled NER (TC-NER), which removes lesions that block transcription elongation (Fousteri and Mullenders, 2008). Studies of NER both in vitro and in young versus old animals show that NER efficiency declines with age (Vijg et al, 1985; Wei et al, 1993; Goukassian et al, 2000, 2002; Xu et al, 2000; Yamada et al, 2006). It is not clear whether this decrease is caused by diminished activity of NER enzymes in old animals or by defects in the induction of the DNA damage response.
Double-strand breaks (DSBs) are the most severe form of DNA damage. DSBs are repaired through non-homologous end joining (NHEJ), which merely joins two loose DNA ends with a risk of mutagenesis and information loss, or through homologous recombination, which uses the intact sister chromatid as a template to copy the missing information and seal the broken ends in an error-free manner. The main detrimental outcome of age-related alterations in DSB repair is the increase in cancer incidence, accompanied by genome rearrangements and loss of heterozygosity, which are characteristics of erroneous NHEJ (Fuscoe et al, 1994; DePinho, 2000). Notably, the availability of the Ku protein, which recognizes and binds DSBs is reduced with age (Frasca et al, 1999; Um et al, 2003; Doria et al, 2004; Ju et al, 2006; Seluanov et al, 2007).
Several recent studies converge to indicate that weakened DNA surveillance mechanisms are responsible for increased frequency of DNA damage in old animals. For example, point mutations and genomic rearrangements accumulate with age in mice (Curtis and Crowley, 1963; Martin et al, 1985; Dolle et al, 1997, 2000; Tucker et al, 1999; Stuart et al, 2000; Vijg and Dolle, 2002). However, the net impact of age-related alterations of DNA repair mechanisms on the overall genome integrity and organism survival is not clear. Moreover, the mechanisms by which ageing impinges on the DNA damage repair pathways are not fully understood. An additional caveat is that excessive DNA damage response triggers apoptosis, which may cause harmful loss of functional cells in the context of ageing tissues, where self-renewal is limited.
Hormesis: an anti-ageing strategy?
The term hormesis describes the beneficial effects, resulting from the exposure of an organism to a low intensity stressor (Calabrese et al, 1987; Calabrese, 2004). The positive effect of hormesis is attributed to the stimulation and priming of stress response pathways by the stressor. Hormetic manipulations such as repeated mild heat shock result in increased stress-tolerance and extension in lifespan in various models (e.g. yeast, Drosophila, nematodes, rodents and human cells) (Minois, 2000; Cypser and Johnson, 2003; Rattan, 2004; Rattan et al, 2004). The molecular mechanisms by which exposure to low levels of stress confers hormetic resistance and adaptability to adverse conditions are not fully understood. Additional questions remain to be addressed before hormetic interventions and stress response mimetics can be used as an approach to influence ageing and delay or ameliorate age-related pathologies. What is the lower stress threshold for activating a hormetic response? Is there a collateral cost of repeated exposure to low stress? Which stress response pathways mediate the protective effects of hormesis?
Crosstalk between stress response pathways: a domino effect?
Accumulating findings indicate that distinct stress response pathways do not function in isolation but rather, are parts of a wider stress network with multiple hubs that serve as coordinators of various modules. The kinase target of rapamycin (TOR) is part of an evolutionary conserved signalling pathway that links extracellular stimuli with intracellular processes such as cellular growth, metabolism, translational control and proliferation. TOR also inhibits autophagy through phosphorylation of the ATG1 protein kinase (Wullschleger et al, 2006; Chan, 2009). The TOR kinase senses chaperone availability and responds differentially to mild and severe depletion of different chaperones (Qian et al, 2010). This mechanism allows continuous integration of extracellular nutrient levels and intracellular protein homeostasis. Age-dependent alterations of the heat shock response pathway and consequently, fluctuations of chaperone levels might influence other stress response pathways such as autophagy through TOR (Figure 2). Thus, TOR signalling stands at the crossroads of metabolism, protein homeostasis and ageing.
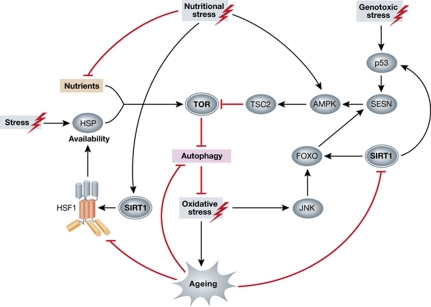
Figure 2.
Crosstalk between stress response pathways implicated in ageing. TOR and SIRT1 serve as central hubs in the stress response network connecting autophagy, DNA damage and the heat shock response. Protein misfolding, triggered by stress, depletes the pool of chaperones. Excess nutrition or reduced chaperone availability cause imbalance between mTORC1 assembly and disassembly, resulting in elevated signalling. The deacetylase SIRT1 targets multiple transcriptional regulators (p53, FOXO and HSF1), participating in distinct stress response pathways. SIRT1 activity is modulated by nutrient availability and is altered during ageing. Similarly, the efficiency of HSF1 activation and autophagosome clearance is impaired during ageing. As a consequence, heat shock protein production is perturbed. Excessive oxidative stress aggravates the symptoms of ageing and age-related pathologies. Activation of TOR increases transcription of SESN. SESN expression is dependent on ROS accumulation and involves JNK and FOXO. SESN is also the target of the tumour suppressor p53, which is activated upon genotoxic stress. Increased SESN activity inhibits TOR signalling by activating AMPK and TSC2. AMPK, adenosine monophosphate-activated protein kinase; FOXO, forkhead box O; HSF1, heat shock factor 1; HSP, heat shock protein; JNK, c-Jun N-terminal kinase; ROS, reactive oxygen species; SESN, sestrin; SIRT1, sirtuin1; TOR, target of rapamycin; TSC2, tuberous sclerosis complex 2.
Sestrins are a family of highly conserved cytoplasmic proteins, whose expression is induced by stress (Budanov et al, 2004). In mammals, sestrins 1 and 2 block mammalian TOR (mTOR) signalling in response to genotoxic stress through a pathway that involves p53, adenosine monophosphate-activated protein kinase (AMPK) and tuberous sclerosis complex 2 (TSC2) (Budanov and Karin, 2008). In Drosophila, sestrin prevents excessive activation of TOR via a negative feedback mechanism and suppresses age-related pathologies (Lee et al, 2010). Therefore, sestrins and TOR act as central nodes in the crosstalk between genotoxic stress and metabolic activity controlled by lipid/protein synthesis and autophagy (Figure 2). Another key regulator of autophagy is Beclin 1, which interacts with numerous proteins such as: Atg14L, UVRAG, Bif-1, Rubicon, Bcl-2, Ambra1, HMGB1, nPIST, VMP1, SLAM, IP(3)R, PINK and survivin (Kroemer et al, 2010). Therefore, Beclin 1 integrates signals from diverse pathways, which are themselves subjected to age-dependent fluctuations.
Both the ER and mitochondrial UPR result in elevated expression of the CHOP gene encoding a bZIP transcription factor CHOP (C/EBP Homology Protein). Although CHOP is shared by the two responses, induction of the UPRmt does not cause upregulation of stress-inducible chaperones from non-mitochondrial compartments. Identification of the additional factors that provide specificity will further illuminate the interorganelle communication of stress response pathways with the nucleus.
In addition, proteasome activity is important for DNA repair processes at various levels (Mieczkowski et al, 2000; Luo et al, 2001). Stress-induced activation of the proteasome in the nucleus declines during replicative senescence of human fibroblasts. This decline is due to ageing-dependent decrease of the expression and activity of poly-(ADP-ribose) polymerase 1 (PARP-1), which stimulates proteasome (Bakondi et al, 2011).
Sirtuins link metabolic status to the regulation of longevity. Interestingly, several transcription factors involved in cellular stress responses, including FOXO3, p53, NF-κB and HSF1, are regulated by SIRT1 (Vaziri et al, 2001; Brunet et al, 2004; Yeung et al, 2004; Westerheide et al, 2009). It appears that SIRT1 may function to orchestrate different stress response pathways during ageing. Indeed, the beneficial effects of low caloric intake are mediated by members of the sirtuin family. Given that SIRT1 directly deacetylates HSF1 and therefore regulates the heat shock response, it is possible that the positive effect of sirtuin on lifespan might be mediated through a dynamic preservation of proteostasis. It remains to be determined whether SIRT1 activity is altered in vivo during ageing and whether this coincides with the age-related decline of various stress responses. Recently, parkin has been implicated in coordinating both ER and mitochondrial stress mechanisms (Bouman et al, 2010). This is particularly interesting given the fact that a disease-associated protein is upregulated in response to distinct organelle stress. An additional important question is how the status of the response to stress in a specific organelle influences its fate. Defects in UPRER or UPRmt might signal the demise of the corresponding organelle through macroautophagy, in an effort to contain homeostasis imbalance. Accumulation of damaged organelles during ageing may stem from failure to emit or respond to such ‘eat-me’ signals. Along these lines, it is important to understand how a stress response initiated in one organelle is sensed by the cell's homeostatic network and whether compensatory mechanisms are triggered to avoid a general collapse of cellular homeostasis.
Conclusions and perspectives
The process of ageing both influences and is influenced by cellular stress responses. Studies in different organisms converge to illustrate the multifaceted nature of this bi-directional crosstalk. Both the general heat shock response pathway in the cytosol and organelle-specific stress responses (UPRER, UPRmt) are stepwise procedures depending on the detection of the damage, transmission of the stress signal to the nucleus, upregulation of stress combating proteins and translocation of these proteins to the site of damage. Ageing impinges on multiple points in this cascade to compromise the response to stress. Identification of these interference nodes will lead to a better understanding of how ageing influences stress resistance and undermines survival.
By segregating chaperones to specific organelles, cells have developed efficient strategies to monitor the folding environment, prevent and neutralize damage. While considerable progress has been made in recent years towards the characterization of cellular and organelle-specific stress response pathways, the relevant molecular mechanisms at operation in some organelles remain elusive. For example, although Golgi follows the ER in the secretory pathway, a stress response similar to UPRER has not been described for this organelle, albeit the presence of signalling proteins at the Golgi membranes, which make this organelle a potent stress sensor (Preisinger and Barr, 2001; Chiu et al, 2002b; Freyberg et al, 2003; Hicks and Machamer, 2005). Interestingly, Golgi dysfunction has been implicated in several age-related diseases (Lane et al, 2002; Chiu et al, 2002a; Baloyannis et al, 2004; Fujita et al, 2006; Hu et al, 2007). Whether a bona fide Golgi stress response pathway exists and is modified during ageing remains to be elucidated.
Uncontrolled activation of stress response pathways may have undesirable effects. Tumour cells, which have lost the ability to effectively control growth, express higher levels of chaperones (Jaattela, 1999; Calderwood et al, 2006). Increased resistance to stress via enhancement of the cellular stress response pathways may promote cancer development by helping cancer cells to cope with unfavourable conditions (Dai et al, 2007). In addition, although HSPs have beneficial effects on the preservation of homeostasis, their overexpression above a certain threshold may dampen heat shock response via negative feedback on HSF activation. Moreover, given that several stress response pathways are linked to cell death, their tight regulation is imperative, since excessive activation may lead to overall loss of functional cells. Hyperactivation of stress response pathways may also lead to depletion of critical cellular resources, aggravating adverse conditions or insults.
Although the gradual deterioration of stress response mechanisms is a general feature of ageing in diverse organisms, it remains unclear whether this decline is simply a corollary of the ageing process or a significant causative factor, contributing to senescence. Studies in simple model organisms, where stress response pathways can be genetically manipulated are poised to provide significant new insights into this issue. Importantly, such studies should also yield useful information about potential targets for pharmaceutical interventions aiming to augment cellular stress response and defence mechanisms during ageing in an effort to combat age-related health hazards.
Acknowledgments
We gratefully acknowledge the contributions of numerous investigators that we could not include in this review, owing to space limitations. We wish to acknowledge funding from the EMBO Young Investigator Programme, the European Research Council (ERC) and the European Commission Framework Programmes.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abravaya K, Myers MP, Murphy SP, Morimoto RI (1992) The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6: 1153–1164 [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L (2006) Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol 26: 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594: 78–88 [DOI] [PubMed] [Google Scholar]
- Anselmi B, Conconi M, Veyrat-Durebex C, Turlin E, Biville F, Alliot J, Friguet B (1998) Dietary self-selection can compensate an age-related decrease of rat liver 20 S proteasome activity observed with standard diet. J Gerontol A Biol Sci Med Sci 53: B173–B179 [DOI] [PubMed] [Google Scholar]
- Bakondi E, Catalgol B, Bak I, Jung T, Bozaykut P, Bayramicli M, Ozer NK, Grune T (2011) Age-related loss of stress-induced nuclear proteasome activation is due to low PARP-1 activity. Free Radic Biol Med 50: 86–92 [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Costa V, Michmizos D (2004) Mitochondrial alterations in Alzheimer's disease. Am J Alzheimers Dis Other Demen 19: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Lindahl T (2004) Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 38: 445–476 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, Morimoto RI (2009) Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci USA 106: 14914–14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D (2006) Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174: 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trumbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF (2010) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18: 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breusing N, Grune T (2008) Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem 389: 203–209 [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A (2002) The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269: 1996–2002 [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304: 596–600 [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B (2002) Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys 397: 298–304 [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B (2006) Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol 41: 653–657 [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Raffoul JJ, Ge Y, Van Remmen H, Matherly LH, Heydari AR (2006) Age-related loss of the DNA repair response following exposure to oxidative stress. J Gerontol A Biol Sci Med Sci 61: 427–434 [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Raffoul JJ, Yanamadala S, Ganir C, Guo Z, Heydari AR (2002) Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat Res 500: 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ (2004) Hormesis: from marginalization to mainstream: a case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol 197: 125–136 [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, McCarthy ME, Kenyon E (1987) The occurrence of chemically induced hormesis. Health Phys 52: 531–541 [DOI] [PubMed] [Google Scholar]
- Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9: 619–631 [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31: 164–172 [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 [DOI] [PubMed] [Google Scholar]
- Carrard G, Bulteau AL, Petropoulos I, Friguet B (2002) Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol 34: 1461–1474 [DOI] [PubMed] [Google Scholar]
- Cenci S, Mezghrani A, Cascio P, Bianchi G, Cerruti F, Fra A, Lelouard H, Masciarelli S, Mattioli L, Oliva L, Orsi A, Pasqualetto E, Pierre P, Ruffato E, Tagliavacca L, Sitia R (2006) Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J 25: 1104–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EY (2009) mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal 2: pe51. [DOI] [PubMed] [Google Scholar]
- Chen D, Cao G, Hastings T, Feng Y, Pei W, O’Horo C, Chen J (2002) Age-dependent decline of DNA repair activity for oxidative lesions in rat brain mitochondria. J Neurochem 81: 1273–1284 [DOI] [PubMed] [Google Scholar]
- Chiu R, Novikov L, Mukherjee S, Shields D (2002a) A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol 159: 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL II, Cox AD, Philips MR (2002b) Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol 4: 343–350 [DOI] [PubMed] [Google Scholar]
- Ciechanover A (2005) Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol 6: 79–87 [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age-onset proteotoxicity. Science 313: 1604–1610 [DOI] [PubMed] [Google Scholar]
- Conconi M, Szweda LI, Levine RL, Stadtman ER, Friguet B (1996) Age-related decline of rat liver multicatalytic proteinase activity and protection from oxidative inactivation by heat-shock protein 90. Arch Biochem Biophys 331: 232–240 [DOI] [PubMed] [Google Scholar]
- Craig EE, Hood DA (1997) Influence of aging on protein import into cardiac mitochondria. Am J Physiol 272(6 Part 2): H2983–H2988 [DOI] [PubMed] [Google Scholar]
- Cuervo AM (2004) Autophagy: many paths to the same end. Mol Cell Biochem 263: 55–72 [DOI] [PubMed] [Google Scholar]
- Cuervo AM (2010) Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF (2000) Age-related decline in chaperone-mediated autophagy. J Biol Chem 275: 31505–31513 [DOI] [PubMed] [Google Scholar]
- Curtis H, Crowley C (1963) Chromosome aberrations in liver cells in relation to the somatic mutation theory of aging. Radiat Res 19: 337–344 [PubMed] [Google Scholar]
- Cypser JR, Johnson TE (2003) Hormesis in Caenorhabditis elegans dauer-defective mutants. Biogerontology 4: 203–214 [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S (2007) Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130: 1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C (2010) Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8: e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bont R, van Larebeke N (2004) Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19: 169–185 [DOI] [PubMed] [Google Scholar]
- DePinho RA (2000) The age of cancer. Nature 408: 248–254 [DOI] [PubMed] [Google Scholar]
- Dice JF (2007) Chaperone-mediated autophagy. Autophagy 3: 295–299 [DOI] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Keller JN (2006) Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal 8: 163–172 [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM (2007) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM (2008) Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 4: 141–150 [DOI] [PubMed] [Google Scholar]
- Dolle ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J (1997) Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet 17: 431–434 [DOI] [PubMed] [Google Scholar]
- Dolle ME, Snyder WK, Gossen JA, Lohman PH, Vijg J (2000) Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc Natl Acad Sci USA 97: 8403–8408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E (2001) Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J Gerontol A Biol Sci Med Sci 56: B288–B293 [DOI] [PubMed] [Google Scholar]
- Donati A, Recchia G, Cavallini G, Bergamini E (2008) Effect of aging and anti-aging caloric restriction on the endocrine regulation of rat liver autophagy. J Gerontol A Biol Sci Med Sci 63: 550–555 [DOI] [PubMed] [Google Scholar]
- Doria G, Barattini P, Scarpaci S, Puel A, Guidi L, Frasca D (2004) Role of immune responsiveness and DNA repair capacity genes in ageing. Ageing Res Rev 3: 143–151 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Erickson RR, Dunning LM, Holtzman JL (2006) The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci 61: 435–443 [DOI] [PubMed] [Google Scholar]
- Farout L, Friguet B (2006) Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal 8: 205–216 [DOI] [PubMed] [Google Scholar]
- Fousteri M, Mullenders LH (2008) Transcription-coupled nucleotide excision repair in mammalian cells: molecular mechanisms and biological effects. Cell Res 18: 73–84 [DOI] [PubMed] [Google Scholar]
- Frasca D, Barattini P, Tirindelli D, Guidi L, Bartoloni C, Errani A, Costanzo M, Tricerri A, Pierelli L, Doria G (1999) Effect of age on DNA binding of the ku protein in irradiated human peripheral blood mononuclear cells (PBMC). Exp Gerontol 34: 645–658 [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Siddhanta A, Shields D (2003) ‘Slip, sliding away’: phospholipase D and the Golgi apparatus. Trends Cell Biol 13: 540–546 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Ohama E, Takatama M, Al-Sarraj S, Okamoto K (2006) Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson's disease. Acta Neuropathol 112: 261–265 [DOI] [PubMed] [Google Scholar]
- Fuscoe JC, Zimmerman LJ, Harrington-Brock K, Moore MM (1994) Deletion mutations in the hprt gene of T-lymphocytes as a biomarker for genomic rearrangements important in human cancers. Carcinogenesis 15: 1463–1466 [DOI] [PubMed] [Google Scholar]
- Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH (2008) DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol 10: 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet LC, Scharer OD (2006) Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev 106: 253–276 [DOI] [PubMed] [Google Scholar]
- Goukassian D, Gad F, Yaar M, Eller MS, Nehal US, Gilchrest BA (2000) Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J 14: 1325–1334 [DOI] [PubMed] [Google Scholar]
- Goukassian DA, Bagheri S, el-Keeb L, Eller MS, Gilchrest BA (2002) DNA oligonucleotide treatment corrects the age-associated decline in DNA repair capacity. FASEB J 16: 754–756 [DOI] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R (2005) Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak JL, Buettner GR, Spitz DR, Kregel KC (2009) Aging augments mitochondrial susceptibility to heat stress. Am J Physiol Regul Integr Comp Physiol 296: R812–R820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RY (2000) ER stress response: getting the UPR hand on misfolded proteins. Curr Biol 10: R518–R521 [DOI] [PubMed] [Google Scholar]
- Hanawalt PC (2002) Subpathways of nucleotide excision repair and their regulation. Oncogene 21: 8949–8956 [DOI] [PubMed] [Google Scholar]
- Hanna J, Meides A, Zhang DP, Finley D (2007) A ubiquitin stress response induces altered proteasome composition. Cell 129: 747–759 [DOI] [PubMed] [Google Scholar]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889 [DOI] [PubMed] [Google Scholar]
- Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, Liu LF (2007) Autophagy regulates ageing in C. elegans.. Autophagy 3: 93–95 [DOI] [PubMed] [Google Scholar]
- Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D (2007) ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans.. Dev Cell 13: 467–480 [DOI] [PubMed] [Google Scholar]
- Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D (2010) The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37: 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C (2010) Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci USA 107: 9730–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A (2000) Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res 256: 83–93 [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE (2005) Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta 1744: 406–414 [DOI] [PubMed] [Google Scholar]
- Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L (2003) Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 23: 2953–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361: 1475–1485 [DOI] [PubMed] [Google Scholar]
- Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI, Eriksson JE, Sistonen L (2001) Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J 20: 3800–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145 [DOI] [PubMed] [Google Scholar]
- Hu Z, Zeng L, Huang Z, Zhang J, Li T (2007) The study of Golgi apparatus in Alzheimer's disease. Neurochem Res 32: 1265–1277 [DOI] [PubMed] [Google Scholar]
- Hussain SG, Ramaiah KV (2007) Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem Biophys Res Commun 355: 365–370 [DOI] [PubMed] [Google Scholar]
- Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA (2006) Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol Aging 27: 1129–1136 [DOI] [PubMed] [Google Scholar]
- Intano GW, Cho EJ, McMahan CA, Walter CA (2003) Age-related base excision repair activity in mouse brain and liver nuclear extracts. J Gerontol A Biol Sci Med Sci 58: 205–211 [DOI] [PubMed] [Google Scholar]
- Jaattela M (1999) Heat shock proteins as cellular lifeguards. Ann Med 31: 261–271 [DOI] [PubMed] [Google Scholar]
- Jenner P (2001) Parkinson's disease, pesticides and mitochondrial dysfunction. Trends Neurosci 24: 245–247 [DOI] [PubMed] [Google Scholar]
- Ju D, Wang L, Mao X, Xie Y (2004) Homeostatic regulation of the proteasome via an Rpn4-dependent feedback circuit. Biochem Biophys Res Commun 321: 51–57 [DOI] [PubMed] [Google Scholar]
- Ju YJ, Lee KH, Park JE, Yi YS, Yun MY, Ham YH, Kim TJ, Choi HM, Han GJ, Lee JH, Lee J, Han JS, Lee KM, Park GH (2006) Decreased expression of DNA repair proteins Ku70 and Mre11 is associated with aging and may contribute to the cellular senescence. Exp Mol Med 38: 686–693 [DOI] [PubMed] [Google Scholar]
- Jung T, Catalgol B, Grune T (2009) The proteasomal system. Mol Aspects Med 30: 191–296 [DOI] [PubMed] [Google Scholar]
- Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233 [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR (2000a) Impaired proteasome function in Alzheimer's disease. J Neurochem 75: 436–439 [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR (2000b) Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev 113: 61–70 [DOI] [PubMed] [Google Scholar]
- Kenyon CJ (2010) The genetics of ageing. Nature 464: 504–512 [DOI] [PubMed] [Google Scholar]
- Kern A, Ackermann B, Clement AM, Duerk H, Behl C (2010) HSF1-controlled and age-associated chaperone capacity in neurons and muscle cells of C. elegans. PLoS One 5: e8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM (2007) Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci 120(Part 5): 782–791 [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN (2000) Why do we age? Nature 408: 233–238 [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8: 739–758 [DOI] [PubMed] [Google Scholar]
- Kline MP, Morimoto RI (1997) Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol 17: 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U, Newton EM, Kyriakis J, Kingston RE (1996) Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev 10: 2782–2793 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880–884 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis N, Tavernarakis N (2009) Autophagy and cell death in model organisms. Cell Death Differ 16: 21–30 [DOI] [PubMed] [Google Scholar]
- Krishna TH, Mahipal S, Sudhakar A, Sugimoto H, Kalluri R, Rao KS (2005) Reduced DNA gap repair in aging rat neuronal extracts and its restoration by DNA polymerase beta and DNA-ligase. J Neurochem 92: 818–823 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin EV, Karpova OV, Elthon TE, Newton KJ (2004) Mitochondrial respiratory deficiencies signal up-regulation of genes for heat shock proteins. J Biol Chem 279: 20672–20677 [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J (2005) Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 126: 365–379 [DOI] [PubMed] [Google Scholar]
- Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M (2002) Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol 156: 495–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M (2010) Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327: 1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS (2008) Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 3: 399–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362: 709–715 [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13: 385–392 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22: 631–677 [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci USA 107: 14164–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Park JH, Liu AY, Chen KY (2000) Activation of heat shock factor 1 by hyperosmotic or hypo-osmotic stress is drastically attenuated in normal human fibroblasts during senescence. J Cell Physiol 184: 183–190 [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429: 883–891 [DOI] [PubMed] [Google Scholar]
- Luo Z, Zheng J, Lu Y, Bregman DB (2001) Ultraviolet radiation alters the phosphorylation of RNA polymerase II large subunit and accelerates its proteasome-dependent degradation. Mutat Res 486: 259–274 [DOI] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG (2000) Mitotic misregulation and human aging. Science 287: 2486–2492 [DOI] [PubMed] [Google Scholar]
- Macario AJ, Conway de Macario E (2005) Sick chaperones, cellular stress, and disease. N Engl J Med 353: 1489–1501 [DOI] [PubMed] [Google Scholar]
- Marino G, Ugalde AP, Salvador-Montoliu N, Varela I, Quiros PM, Cadinanos J, van der Pluijm I, Freije JM, Lopez-Otin C (2008) Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet 17: 2196–2211 [DOI] [PubMed] [Google Scholar]
- Martin GM, Smith AC, Ketterer DJ, Ogburn CE, Disteche CM (1985) Increased chromosomal aberrations in first metaphases of cells isolated from the kidneys of aged mice. Isr J Med Sci 21: 296–301 [PubMed] [Google Scholar]
- Marzella L, Ahlberg J, Glaumann H (1981) Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch B Cell Pathol Incl Mol Pathol 36: 219–234 [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Kruger E (2003) Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem 278: 21517–21525 [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B (2003) Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301: 1387–1391 [DOI] [PubMed] [Google Scholar]
- Mieczkowski P, Dajewski W, Podlaska A, Skoneczna A, Ciesla Z, Sledziewska-Gojska E (2000) Expression of UMP1 is inducible by DNA damage and required for resistance of S. cerevisiae cells to UV light. Curr Genet 38: 53–59 [DOI] [PubMed] [Google Scholar]
- Minois N (2000) Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology 1: 15–29 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI (2002) Dynamic remodeling of transcription complexes by molecular chaperones. Cell 110: 281–284 [DOI] [PubMed] [Google Scholar]
- Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22: 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG (1998) Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol 16: 833–838 [DOI] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA 99: 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM (2003) Heat shock proteins and aging in Drosophila melanogaster. Semin Cell Dev Biol 14: 291–299 [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43: 477–503 [DOI] [PubMed] [Google Scholar]
- Naidoo N (2009) ER and aging-Protein folding and the ER stress response. Ageing Res Rev 8: 150–159 [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI (2008) Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28: 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624 [DOI] [PubMed] [Google Scholar]
- Nuss JE, Choksi KB, DeFord JH, Papaconstantinou J (2008) Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem Biophys Res Commun 365: 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D (2006) Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging 27: 973–982 [DOI] [PubMed] [Google Scholar]
- Ponnappan U, Zhong M, Trebilcock GU (1999) Decreased proteasome-mediated degradation in T cells from the elderly: a role in immune senescence. Cell Immunol 192: 167–174 [DOI] [PubMed] [Google Scholar]
- Preisinger C, Barr FA (2001) Signaling pathways regulating Golgi structure and function. Sci STKE 2001: pe38. [DOI] [PubMed] [Google Scholar]
- Prostko CR, Brostrom MA, Brostrom CO (1993) Reversible phosphorylation of eukaryotic initiation factor 2 alpha in response to endoplasmic reticular signaling. Mol Cell Biochem 127–128: 255–265 [DOI] [PubMed] [Google Scholar]
- Qian SB, Zhang X, Sun J, Bennink JR, Yewdell JW, Patterson C (2010) mTORC1 links protein quality and quantity control by sensing chaperone availability. J Biol Chem 285: 27385–27395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SI (2004) Aging intervention, prevention, and therapy through hormesis. J Gerontol A Biol Sci Med Sci 59: 705–709 [DOI] [PubMed] [Google Scholar]
- Rattan SI (2008) Hormesis in aging. Ageing Res Rev 7: 63–78 [DOI] [PubMed] [Google Scholar]
- Rattan SI, Gonzalez-Dosal R, Nielsen ER, Kraft DC, Weibel J, Kahns S (2004) Slowing down aging from within: mechanistic aspects of anti-aging hormetic effects of mild heat stress on human cells. Acta Biochim Pol 51: 481–492 [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36: 585–595 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA (2005) Opinion: what is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol 6: 891–898 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC (2004) Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 6: 168–170 [DOI] [PubMed] [Google Scholar]
- Seluanov A, Danek J, Hause N, Gorbunova V (2007) Changes in the level and distribution of Ku proteins during cellular senescence. DNA Repair (Amst) 6: 1740–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 12: 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibatani T, Nazir M, Ward WF (1996) Alteration of rat liver 20S proteasome activities by age and food restriction. J Gerontol A Biol Sci Med Sci 51: B316–B322 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4: 176–184 [DOI] [PubMed] [Google Scholar]
- Sorger PK, Pelham HR (1988) Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54: 855–864 [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P (2000) Molecular chaperones and the aging process. Biogerontology 1: 225–233 [DOI] [PubMed] [Google Scholar]
- Stevens FJ, Argon Y (1999) Protein folding in the ER. Semin Cell Dev Biol 10: 443–454 [DOI] [PubMed] [Google Scholar]
- Stuart GR, Oda Y, de Boer JG, Glickman BW (2000) Mutation frequency and specificity with age in liver, bladder and brain of lacI transgenic mice. Genetics 154: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K (2006) UV-induced ubiquitylation of XPC complex, the UV-DDB-ubiquitin ligase complex, and DNA repair. J Mol Histol 37: 189–202 [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A (1995) The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41 (Suppl 2): 319–326 [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT (2004) Myocyte aging and mitochondrial turnover. Exp Gerontol 39: 701–705 [DOI] [PubMed] [Google Scholar]
- Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol 29: 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T (2008) Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4: 330–338 [DOI] [PubMed] [Google Scholar]
- Tucker JD, Spruill MD, Ramsey MJ, Director AD, Nath J (1999) Frequency of spontaneous chromosome aberrations in mice: effects of age. Mutat Res 425: 135–141 [DOI] [PubMed] [Google Scholar]
- Um JH, Kim SJ, Kim DW, Ha MY, Jang JH, Kim DW, Chung BS, Kang CD, Kim SH (2003) Tissue-specific changes of DNA repair protein Ku and mtHSP70 in aging rats and their retardation by caloric restriction. Mech Ageing Dev 124: 967–975 [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159 [DOI] [PubMed] [Google Scholar]
- Vijg J, Dolle ME (2002) Large genome rearrangements as a primary cause of aging. Mech Ageing Dev 123: 907–915 [DOI] [PubMed] [Google Scholar]
- Vijg J, Mullaart E, Lohman PH, Knook DL (1985) UV-induced unscheduled DNA synthesis in fibroblasts of aging inbred rats. Mutat Res 146: 197–204 [DOI] [PubMed] [Google Scholar]
- Vittorini S, Paradiso C, Donati A, Cavallini G, Masini M, Gori Z, Pollera M, Bergamini E (1999) The age-related accumulation of protein carbonyl in rat liver correlates with the age-related decline in liver proteolytic activities. J Gerontol A Biol Sci Med Sci 54: B318–B323 [DOI] [PubMed] [Google Scholar]
- Wei Q, Matanoski GM, Farmer ER, Hedayati MA, Grossman L (1993) DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci USA 90: 1614–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM Jr, Sistonen L, Morimoto RI (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323: 1063–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM III, Bohr VA (2007) The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 6: 544–559 [DOI] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13: 374–384 [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484 [DOI] [PubMed] [Google Scholar]
- Xu G, Snellman E, Bykov VJ, Jansen CT, Hemminki K (2000) Effect of age on the formation and repair of UV photoproducts in human skin in situ. Mutat Res 459: 195–202 [DOI] [PubMed] [Google Scholar]
- Yamada M, Udono MU, Hori M, Hirose R, Sato S, Mori T, Nikaido O (2006) Aged human skin removes UVB-induced pyrimidine dimers from the epidermis more slowly than younger adult skin in vivo. Arch Dermatol Res 297: 294–302 [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D (2004) Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci 117 (Part 18): 4055–4066 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12(Suppl 2): 1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281: 30299–30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H (2007) ER stress and diseases. FEBS J 274: 630–658 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273: 33741–33749 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4: 265–271 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891 [DOI] [PubMed] [Google Scholar]
- Zhang C, Cuervo AM (2008) Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ (2006) Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol 172: 69–91 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21: 4411–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94: 471–480 [DOI] [PubMed] [Google Scholar]