Abstract
The only way for dengue to spread in the human population is through the human-mosquito-human cycle. Most research in this field discusses the dengue-mosquito or dengue-human relationships over a particular study area, but few have explored the local spatial variations of dengue-mosquito and dengue-human relationships within a study area. This study examined whether spatial heterogeneity exists in these relationships. We used Ordinary Least Squares (OLS) and Geographically Weighted Regression (GWR) models to analyze spatial relationships and identify the geographical heterogeneities by using the information of entomology and dengue cases in the cities of Kaohsiung and Fengshan in 2002. Our findings indicate that dengue-mosquito and dengue-human relationships were significantly spatially non-stationary. This means that in some areas higher dengue incidences were associated with higher vector/host densities, but in some areas higher incidences were related to lower vector/host densities. We demonstrated that a GWR model can be used to geographically differentiate the relationships of dengue incidence with immature mosquito and human densities. This study provides more insights into spatial targeting of intervention and control programs against dengue outbreaks within the study areas.
Keywords: dengue, spatial heterogeneity, geographically weighted regression (GWR), human density, Aedes mosquitoes
1. Introduction
Dengue is the most rapidly spreading mosquito-borne viral disease in the World [1]. Its incidence has increased 30-fold in the last 50 years and has extended to new areas, across both rural and urban environments [1]. The South, South-East Asia and the Western Pacific WHO regions are among the most affected areas [1]. Dengue or dengue-like transmission has been observed in southern Taiwan since the late 19th century [2], initially as intermittent epidemics at intervals of up to 40 years [3]. However, for the past 10 years, dengue epidemics have become an annual phenomenon with the cities of Kaohsiung and Fengshan as the main foci of activity.
The dengue viruses (DENVs) are transmitted to humans by Aedes (Ae.) mosquitoes (vector), in particular Ae. aegypti and Ae. albopictus [1,4]. There is currently no effective treatment or available vaccine against dengue [5], hence current prevention and control policies mainly aim to reduce human-mosquito contact or to decrease the vector population to levels where viral transmission is unsustainable. To ensure efficient prevention policies, it is important to understand the relative impact of vector and host density on the dispersal of DENVs within an area.
The relationships of the dengue incidence–mosquito abundance and dengue incidence-human density are still not well understood. Since the density of adult mosquitoes is difficult to estimate, immature vector data were widely used for evaluating the incidence–mosquito relationship [6–14]. Some entomology studies have found no correlation between dengue incidence and immature vectors, neither temporally nor spatially [6–10]. However, a study in Trinidad recently showed that high dengue incidences were significantly related to high mosquito larval densities during certain years [11]. Other spatial studies in Cuba, Trinidad and Thailand have successfully demonstrated that the Breteau index (BI) and house index can be an indicator for incidence [12–14]. The association of dengue incidence and human density are also ambiguous. Studies in Brazil have shown no correlation between dengue incidence and human density [15], but researches in both Taiwan and Puerto Rico have proven that the spatial distribution of dengue incidence may be positively related to the population density [16,17]. Population density and urbanization are also considered as risk factors for DENV spread in Argentina and Hawaii [18,19]. Moreover, in hyperendemic areas of Thailand, DENVs transmission is more prevalent in children in localized neighborhoods [20].
Until now, most studies of dengue-mosquito or dengue-human relationships have presented a global perspective by which any relationship was assumed to be spatially constant across the whole study area, thereby ignoring local variations. However, this assumption may be inappropriate since the dengue-mosquito or dengue-human relationships could be positively correlated in some study areas, but negatively or not correlated at all in other areas. For example, a small number of female mosquitoes in a very dense area is sufficient to cause an outbreak. This study was conducted to evaluate the hypothesis that spatial heterogeneity existed for dengue-mosquito and dengue-human relationships. We demonstrated that the variation of dengue incidences among study areas was reflected by the densities of both immature vectors and hosts. By capturing the local relationships across the space, the authorities can design more effective, locally-specific strategies. This understanding is especially important where the control and prevention resources are limited.
2. Materials and Methods
2.1. Study Area
Kaohsiung city has been the epicenter where most of the dengue outbreaks have been recorded in Taiwan [3]. It is a major port and industrial metropolis with a population of some 1.5 million. Kaohsiung International Airport is an important access point for visitors as well as foreign workers, many of whom are employed in the city’s commercial harbor. The large industrial and export processing zones of Kaohsiung city also attract around 15,000 foreign workers per year, mainly citizens from neighboring countries such as Philippines, Indonesia, Vietnam and Thailand. Kaohsiung city, which covers a total area of 150 km2, is the most densely populated urban centre in Taiwan. The neighboring Fengshan city, located directly to its east, has a population of 330,000 within an area of 27 km2. Piped water is available for 99% of the city households and household waste is removed daily throughout both cities by the government. The 2002 dengue epidemic in which Kaohsiung and Fengshan cities were the major foci was the largest outbreak in recent years in Taiwan (Figure 1), with more than 15,000 reported cases, with a total of 3,786 confirmed dengue cases [3].
Figure 1.
The epidemic curve of confirmed dengue cases as cumulated by weeks of onset in Kaohsiung and Fengshan cities, 2002–2009.
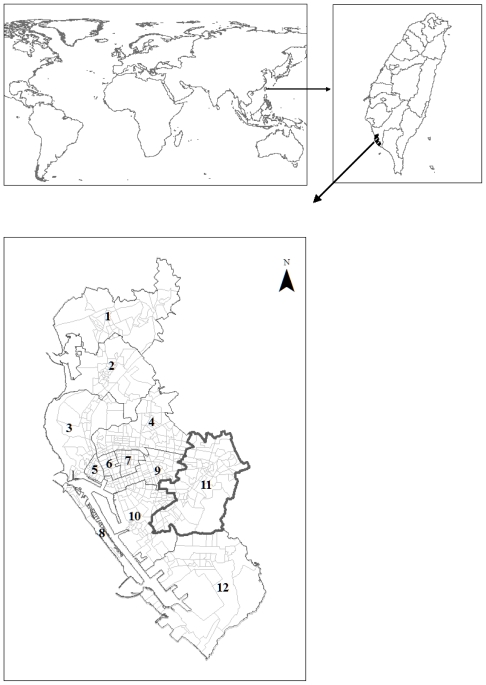
The “Li”, the lowest administrative unit in Taiwan, was used as the spatial mapping unit in this study. There were a total of 542 Lis and 12 districts in the two cities during the study period of 2002. On average each Li had a population of 3,366 and 1138.41 households in an area of 0.36 km2. The study area is shown in Figure 2.
Figure 2.
The distribution of 542 Lis and 12 districts in Kaohsiung (district 1–10 and 12) and Fengshan cities (district 11) in Taiwan. Each small polygon represents each Li.
2.2. Dengue Data
All information on dengue cases was provided by the Centers for Disease Control-Taiwan (Taiwan CDC). Laboratory confirmation was obtained for all suspected cases identified through passive, active and passive-based active surveillance activities. Passive surveillance involved the mandatory referral of suspected dengue cases within 24 h of presentation at any of 231 health clinics or hospitals (both private and public), school-based reports of absence due to fever as well as individual self-reporting [3]. Self-reporting refers to any requests for a free dengue test by patients presenting at any public health center. Active surveillances were maintained through fever checkpoints at the Kaohsiung airport. Passive-based active method was systematic screening of contacts (family members, colleagues and neighbors) of confirmed cases [3]. Laboratory diagnosis of suspected cases was conducted by the fifth branch office of Taiwan CDC in Kaohsiung City. Confirmation of dengue infection using patient serum was obtained by: (1) detection of DENV-specific IgM or IgG by capturing enzyme linked immune-sorbent assay (ELISA) in single sample or fourfold IgG titer increase in paired acute and convalescent samples, or (2) detection of DENV RNA by reverse transcriptase polymerase chain reaction (RT-PCR) [3]. All test expenses were covered by the National Health Insurance.
2.3. Immature Mosquito and Human Density
Larval habitats of Ae. aegypti and Ae. albopictus were observed on a routine basis by trained personnel from the Kaohsiung city Health Bureau and county governments during the study period. All personnel had received training in mosquito species distinction, mosquito habitat recognition techniques and sampling methods. According to the control and prevention protocol of Taiwan CDC, 50 households in each Li were randomly selected for inspection, which covered indoor and outdoor areas of the selected premise. On average, each Li was surveyed once per month. Containers with immature Ae. mosquitoes (larvae/pupae) were considered as positive containers [3]. For habitats with low water volume (<30 L) the larvae/pupae would be strained off and transferred into white bowls for visualization and counting. For habitats containing high water volume, as many larvae/pupae would be collected as possible [3]. The stage of larval maturation (1–4 instars) was not documented. The mosquito species was determined following adult emergence from collected specimen reared at the laboratory facilities of the health bureau of Kaohsiung city and county governments.
Breteau index was used to estimate the density of immature Ae. mosquitoes in the study. BI is defined as number of positive containers per 100 houses [1], and was estimated on a monthly basis in each Li. The average number of people per unit area (people/km2) was taken from 2002 census data as an indicator of human population density (POPden) estimated for each Li.
2.4. Statistical Analysis
In this study, the dengue annual cumulative incidence (IR), given as cases per 100,000 populations, was used as the measure of disease severity, and as the dependent variable; independent variables were POPden and the monthly maximum BI detected in each Li in 2002 (BImax). A summary of the variables in both Ordinary Least Squares (OLS) and Geographically Weighted Regression (GWR) models are shown in Table 1. We first applied OLS regression, in an attempt to explain the global relations between dependent and independent variables. The model was set as: IR = β0 + β1 BImax+ β2 POPden + ɛ. β0 and β1 were the regression coefficients whereas ɛ was the model random error.
Table 1.
Summary of dependent and independent variables used in OLS and GWR.
| Variable | Numerator | Denominator | |
|---|---|---|---|
| Dependent: | IR | 100,000 × number of cases | Populations |
| Independent: | BImax | 100 × number of positive containers | Number of premises inspected |
| POPden | Populations | The area of Li (km2) | |
IR: cumulative incidence of dengue; BImax: Maximum Breteau index; POPden: Population density.
The diagnoses of an OLS model were approached by assessing multicollinearity and the residuals. The multicollinearity was assessed through variance inflation factor (VIF) values, and if VIFs were greater than 10, this indicated multicollinearity existed [21]. The spatial independency of residuals was evaluated by the spatial autocorrelation coefficient, Moran’s I, which was expressed as:
where n was the total number of Li in the study [19]. i and j represented different Lis. yi was the residual of i, and ȳ was the mean of residuals. wij was a measure of spatial proximity pairs of i and j [22]. We used the inverse of the distance between i and j for specifying the relationship between them.
The values of Moran's I would be approximately between +1 (positive autocorrelation) and −1 (negative autocorrelation), and the expected value in the absence of autocorrelation was (−1)/(n−1). Positive spatial autocorrelation meant similar values tended to occur in adjacent areas, while negative autocorrelation implied nearby locations tended to have dissimilar values. If no spatial autocorrelation was found, then the spatial arrangement would be completely random [23].
A GWR local model was applied to analyze how the IR-BImax and POPden relationships changed from one Li to another. It was a localized multivariate regression that allowed the parameters of a regression estimation to change locally. Unlike conventional regression, which produced a single regression equation to summarize global relationships among the independent and dependent variables, GWR detected spatial variation of relationships in a model and produced maps for exploring and interpreting spatial non-stationarity [24]. GWR was calibrated by multiplying the geographically weighted matrix w(g) consisting of geo-referenced data [24,25]. The w(g) was defined by the spatial neighboring relations between points, which can be presented as:
Within the matrix, wgn referred to the impact between position g and position n in which the values range between 0 and 1. This study presumed the degree of impact had an inverse ratio to the square distance of different Lis. In other words, the larger the wgn was, the closer geographically data points were, and the stronger impact they had on each other.
The spatial variability of an estimated local regression coefficient was examined to determine whether the underlying process exhibited spatial heterogeneity [24,25]. The regression model can be rewritten as IRi(g) = β0i(g) + β1i BImaxi(g) + β2i POPdeni(g) + ɛi, where (g) indicated the parameters that were estimated at each Li in which the coordinates were given by vector g; i represented each Li. By applying GWR modeling, the spatial influences among neighborhoods could be assessed, which was not able to be achieved through traditional OLS methods [25].
We also examined the local collinearity as well as the independency and normality of residuals of GWR model to evaluate the fit of the model. The local collinearity was assessed by scatter plots of the local coefficient estimates for BImax and POPden and condition number. The condition number is the square root of the largest eigenvalue divided by the smallest eigenvalue. If the condition numbers are greater than 30, multicollinearity would be a very serious concern. The adjusted coefficient of determination (Adjusted R2), and ANOVA were used for comparing OLS and GWR models. Akaike Information Criterion (AIC) generated for OLS and corrected Akaike Information Criterion (AICc) calculated for GWR were also used for model comparison [24]. The concept here is to determine which model could interpret data better.
Our analysis in this article was based on Li-level data. All analyses were implemented using ESRI®ArcGISTM9.3 and GWR 3.0 with 0.05 significance level. In the GWR model, the adaptive kernel with AICc estimated bandwidth was chosen. The adaptive kernel was chosen because the distribution of Li was inhomogeneous in the study area (Figure 2). The data set from the 2002 dengue outbreak in Kaohsiung and Fengshan cities was provided by Taiwan CDC. We aggregated the confirmed dengue cases with home address to each Li for regression analyses.
3. Results
3.1. OLS Regression
The spatial distributions of the IR, BImax and POPden were mapped in Figure 3. The map of cumulative IR showed high values clustered in some border areas. The northern areas generally had lower IR values than middle and southern areas in the cities. The pattern of maximum Breteau index was less obvious. High population density was found in both city centers.
Figure 3.
Spatial distributions of (a) dengue incidence (IR); (b) maximum Breteau index (BImax); and (c) population density (POPden) in each Li in Kaohsiung and Fengshan cities, 2002. IR was based on 2002 census with the unit of case per 100,000 population. The unit of POPden was populations per km2. Li was the basic administrative unit in Taiwan, and there were 542 Lis in the study area.
The results of applying OLS regression showed that holding the variable of population density fixed, ceteris paribus, one BImax increase is significantly associated with 947.93 increase of average IR (Table 2). The VIF values indicated OLS estimations were not biased from multicollinearity. However, this global regression model explained only 4 percent of the total variance of IR with the AIC 7,902.12. We further examined the residuals of the OLS model, and found the residuals had positive spatial autocorrelation (Moran’s I = 0.28, p < 0.01). Since the existence of dependent residuals violates the assumptions of OLS estimation, we employed a GWR model to fit the data. We used GWR to present the spatial diversities of the IR-BImax and POPden relationships.
Table 2.
Ordinary Least Squares (OLS) results.
| Parameter | Estimated Value | Standard Error | p-value | VIF |
|---|---|---|---|---|
| Intercept | 115.52 | 34.73 | 0.003 | |
| BImax | 947.93 | 202.48 | 0.013 | 1.02 |
| POPden | 0.00 | 0.00 | 0.111 | 1.02 |
| Adjusted R2 | 0.04 | |||
| AIC | 7,902.12 |
3.2. GWR Model and Spatial Variations
The summary results of GWR are listed in Table 3 and showed the GWR was more suitable than the OLS model since GWR could explain 59 percent of the total model variation with the decreased AICc. Moreover, the ANOVA comparison results also showed the GWR local model was significantly more appropriate than the OLS global model (F = 5.36, p < 0.001).
Table 3.
Geographical weighted regression (GWR) results.
| Parameter | Minimum | 25% quartile | 50 % quartile | 75 % quartile | Maximum |
|---|---|---|---|---|---|
| Intercept | −272.60 | 78.46 | 166.09 | 320.92 | 1,088.31 |
| BImax | −2980.43 | −262.53 | 100.40 | 838.91 | 5,797.87 |
| POPden | −0.02 | −0.00 | 0.00 | 0.00 | 0.02 |
| Condition number | 2.96 | 4.67 | 5.83 | 7.32 10.39 | |
| Adjusted R2 | 0.59 | ||||
| AICc | 7,715.17 |
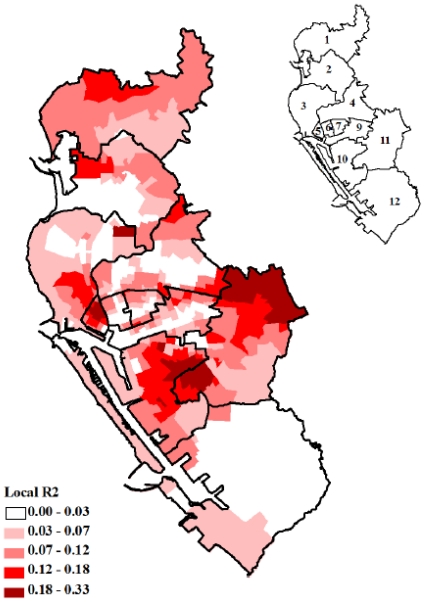
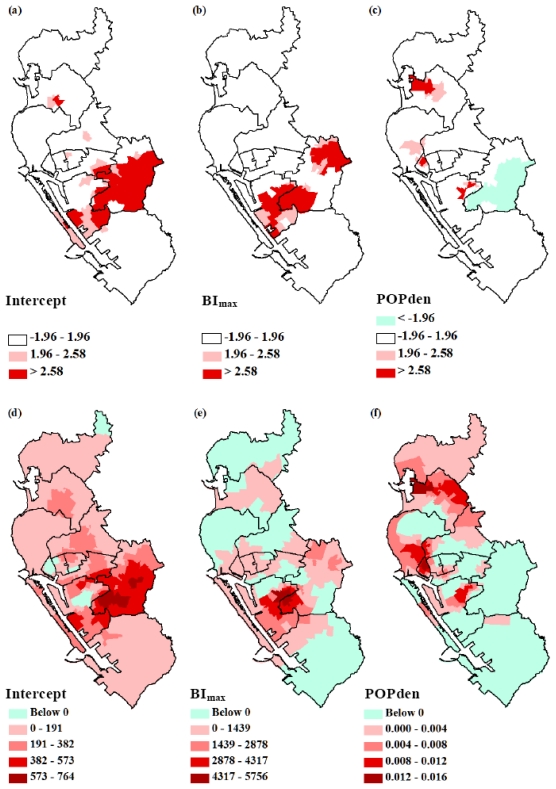
Figure 4 showed the maps of the locally weighed R2 between the observed and fitted values, which indicated how well the GWR model replicated the local IR around BImax and POPden. It was obvious that the value of R2 was not homogeneously distributed in all Lis, and the overall GWR regression fitted best in districts 1, 5, 10 and 11. This model did not fit well in district 12, and this could imply additional covariates were needed to explain the IR in district 12. Figure 4 helped us realize whether additional explanatory factors were required and where could those factors be applied. We also mapped the pseudo t values for intercept and each dependent variable to represent the fitting level for each specific variable under GWR [Figure 5(a)–(c)]. The significant t values, blue and red areas, indicated that the parameter estimations in these areas were reliable.
Figure 4.
Spatial mapping of the locally weighed coefficient of determination (R2) between the observed and fitted values by geographically weighted regression (GWR) modeling. The data presented here were the 2002 dengue incidence, the maximum Breteau index and population density in each Li in Kaohsiung and Fengshan cities.
Figure 5.
Spatial mapping of pseudo t values of regression fitting (a–c) and the coefficients (d-f) of intercept, maximum Breteau index (BImax) and population density (POPden) for each Li by geographically weighted regression (GWR) modeling. The dependent variable was dengue incidence (per 100,000 populations) taken from 2002 dengue epidemic data in Kaohsiung and Fengshan cities. Each polygon represents each district.
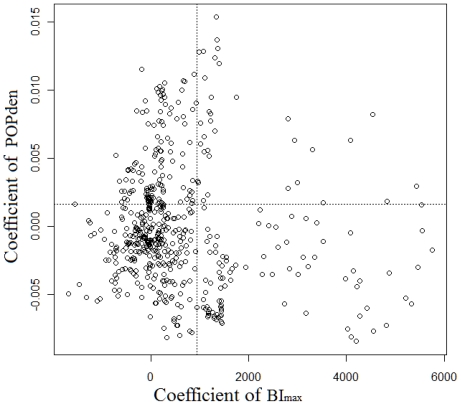
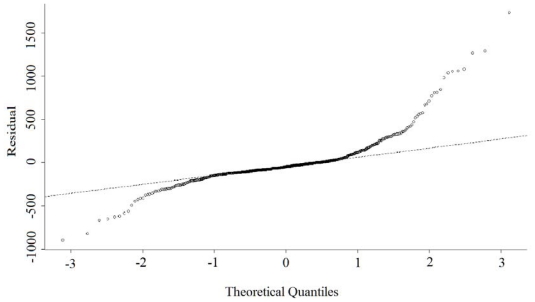
The condition number shown in Table 3 and the scatter plot of the GWR coefficients suggested multicollinearity was not serious (Figure 6). However, the local residuals deviated, since residuals showed moderate positive spatial autocorrelation (Moran’s I = 0.02, p = 0.02), and some parts failed to follow a normal distribution (Figure 7).
Figure 6.
Scatter plot of the GWR coefficients of population density (POPden) and maximum Breteau index (BImax) with R2 = 0.01. The dashed lines were the levels of the OLS estimations.
Figure 7.
Normal quantile-quantile plot of the residuals from GWR estimations.
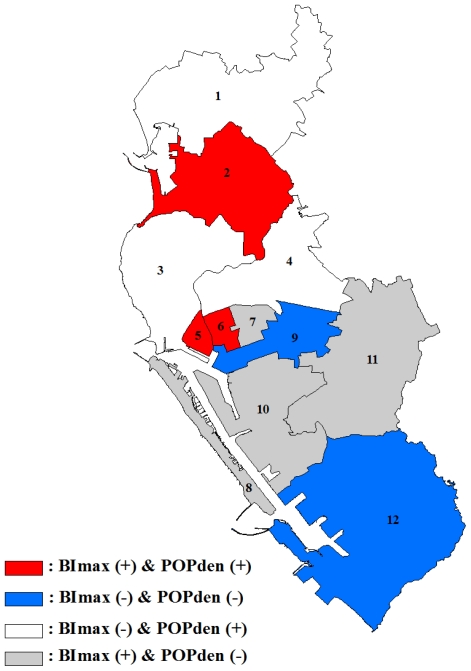
The spatial variations in parameter estimations for intercept, maximum Breteau index and population density are shown in Figure 5(d)–(f). The map of intercept term represented the distributions of IR when BImax and POPden equaled zero. It was observed that higher intercept values were located around the borders of two cities [districts 9, 10 and 11, Figure 5(d)]. This spatial heterogeneity implied that besides immature mosquito and population density, there were still other variables that would influence IR pattern. The relationship between IR and BImax shown in Figure 5(e) suggested that, ceteris paribus, in districts 2, 5, 6, 7, 8, 10 and 11, increased IR would relate to increased BImax. However, in the remaining districts, higher IR associated with lower BImax and vice versa. The distribution of population density parameter showed a more clearly spatial non-stationary pattern [Figure 5(f)]. The positive relationships that were mostly clustered in the northern areas, indicating higher population density tended to associate with higher IR. On the contrary, the impact of population density on IR was negative in the southern parts representing higher IR related to lower population density. A brief summary of these relationships is shown in Figure 8.
Figure 8.
Summary of BImax and POPden impact on incidence using GWR in each district. BImax (+) means BImax had positive impact on incidence while BImax (−) means BImax had negative impact on incidence; POPden (+) means POPden had positive impact on incidence whereas POPden (−) means POPden had negative impact on incidence.
4. Discussion
This study provides further indications that the relationships of dengue incidence-maximum BI and dengue incidence-population density were spatially non-stationary in Kaohsiung and Fengshan cities. In regression maps, it is clear that the intensity and directions of the influence of maximum BI and population density on dengue incidence were different in the study area. This result gives the policy makers more ideas how to better adopt specific control and prevention strategies to specific areas [26].
The spatial heterogeneity of intercept results in Figure 5(a) could imply that the DENVs seroprevalence was non-stationary. Our study found that the density of immature vectors was a significant predictor of dengue incidence in some areas with either positive or negative correlations. Reducing immature mosquito densities is currently the major control and prevention approach for dengue [1]. However, the results from this study suggest that this strategy may not be spatially and universally suitable for the control of dengue, especially for those areas with negative incidence-maximum BI correlations. Possible reasons could be that other than immature mosquito density, local characteristics could affect dengue transmission as well. For instance, some rural areas with high vector density may lack common exposure sites to humans, thus making outbreaks less likely to occur. On the contrary, places where crowds gather easily like markets, parks, train stations and schools may propel huge dengue outbreaks even though the mosquito density is low [17]. Human activities that promote host-vector contact increases the risks for people to be infected within a short distance [1,27,28]. This study showed that the distribution of dengue incidence-BI relationships was very similar to the distribution of districts which implied the presence of additional risk factors, such as the age distribution in human population, human activity [15,29], housing structures/patterns [30,31], environmental factors [29], and serosurveillance [32]. These other factors should also be considered, since the diversities of these factors were large among the districts.
In the northern part of the study area, higher human densities were shown to contribute to higher dengue incidence rates. This positive relationship was expected as higher human density may lead to higher vector-host contact rates. A previous finding in Taiwan showed that the relative risk of accumulated dengue incidence for areas with more than 10,000 people/km2 was 10-fold compared to areas with less than 1,000 people/km2 [16]. Other studies in Florida and Puerto Rico showed that the human population had almost the same spatial pattern as the number of dengue cases during the study period [17]. However, the GWR results also demonstrated that in some areas higher incidence related to lower human densities and vice versa [Figure 5(f)]. One explanation could be that in scattered populated areas, mosquitoes tend to aggregate since fewer blood sources were available [33]. Human travelling behaviors should also be taken into account for the link between higher incidence and lower population density. Travelers not only could initiate new indigenous epidemics, their travelling waves could also contribute to dengue occurrence in low population and rural areas [34,35]. According to our findings and those from other studies mentioned above, the relationship between human population/density and dengue occurrence remains controversial. Further studies should take more spatial information into consideration such as dwelling density [31], type of household [36,37], socioeconomic status [15,31], age and gender distributions [31], pesticide spraying areas and frequencies, water storage habits and landscape [37,38]. Successful dengue transmission requires that the virus, vector, and host exist in the same areas and interact properly. Understanding the relationship among them is necessary and urgent for more effective disease control.
The relationship between vector abundance (both immature and mature stages) and dengue occurrence has been discussed in many studies [6,8,39]. This is a practical issue especially important for policy makers to decide the control and prevention measures. This study provides insight into the spatial heterogeneity of IR-immature mosquito density relationships at Li level; however, there were some limitations for applying entomology data. First, the traditional indicators (Stegomyia indices) such as house index, container index and Breteau index are based on the immature stages of mosquitoes, but larvae/pupae quantities have no direct link with adult abundance and thus an estimate of dengue transmission risk may not be reliable [8,40]. Moreover, these indices provide little information about the container productivity of vector. Assuming all positive habitats have equal vector contribution could lead the researchers to make false estimations of adult amount [41]. The information like number of vector per person or per unit area, which also relates to dengue transmission is disregarded in these indices as well [42]. Moreover, if we directly apply adult index for dengue risk assessment to avoid the limitations of immature stage data, the major problem would be the ratio of captured vectors to existing mosquitoes is still unknown. In this study we chose the monthly maximum BI in each Li as the measurement since we assumed the maximum BI was the best entomology indicator for the dengue cases. In addition to the vector indices problems, the susceptibility of the population to a specific dengue virus serotype is also a great contributor to the scale of epidemics. Once infected a person would acquire lifelong protective immunity to the infective serotype [43]; in other words, the incidence estimation is hindered by a lack of information concerning the overall population immunity to certain serotypes. This makes the estimation of case-vector relationship more complicated. Finally, silent DENVs transmission was not considered in this study.
To improve the understanding of incidence-vector and incidence-host relationships, the followings could be further examined. First of all, the researchers could adopt GWR space-time analyses, such as stratifying the year of 2002 into different periods, or analyzing more than one epidemic year. This approach could provide more detailed patterns of spatial autocorrelation changes of incidence-vector and incidence-host associations. Secondly, the researchers could use other BI calculations such as minimum BI or average BI to see whether different incidence-BI relationships would be generated. Threshold effect of BI could also be considered. Thirdly, categorizing human by different age groups in the GWR model could assist policy makers to determine which actions are suitable for different populations. Finally, researchers could also separate Ae. aegypti and Ae. albopictus for relationship analyses to study the incidences associated with different vector ecologies.
The geographical heterogeneity was detected by the GWR method in the relationships of dengue incidence with immature mosquito and human density (Figure 5). We used GWR since the conventional regression, OLS, cannot discriminate the spatial variation in relationships if geographical nonstationarity exists. The results of Adjusted R2, AIC/AICc and ANOVA all indicated GWR was a better model to explain this dataset. GWR approaches have been applied in a lot of areas, such as public health and demography, as an exploring method for identifying the spatial variations [44–46]. However, the GWR applications are limited for some reasons. First, the results conducted from GWR models were very sensitive to the chosen kernel type and bandwidth methods [47,48]. Next, the non-linear term cannot be added in the GWR models and the model inferences cannot be done in GWR [24]. Future research could use more advanced methods like Bayesian additive regression models, which are based on Markov chain Monte Carlo (MCMC) algorithms for parameter estimations and inferences to overcome the problems mentioned above [49].
5. Conclusions
This paper underlines the spatial variations of incidence-immature mosquito density and incidence-human density relationships in a local scale. Exploring the heterogeneity of spatial relationships could provide more insights into spatial targeting of intervention against dengue epidemics.
Acknowledgements
The research was supported by the grants of National Science Council (NSC 98-2410-H-002-168-MY2) in Taiwan. The authors also acknowledge the financial support provided by Infectious Diseases Research and Education Center, Department of Health and National Taiwan University. The funder had no role in study design, data collection and analysis, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control-New Edition. WHO; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]
- 2.Akagi K, Kojima D. Experimental studies for the pathogen of dengue fever. J Formosan Med Assoc. 1915;158:1049–1078. (in Japanese) [Google Scholar]
- 3.Taiwan Centers For Disease Control (CDC) Guidelines for Dengue Control. CDC; Taipei city Taiwan: 2009. [Google Scholar]
- 4.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman A, Istúriz RE. Update on the global spread of dengue. Int. J. Antimicrob. Agents. 2010;36:S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Wu PC, Lay JG, Guo HR, Lin CY, Lung SC, Su HJ. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009;407:2224–2233. doi: 10.1016/j.scitotenv.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Wu PC, Guo HR, Lung SC, Lin CY, Su HJ. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007;103:50–57. doi: 10.1016/j.actatropica.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Focks DA. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 9.Sulaiman S, Pawanchee ZA, Arifin Z, Wahab A. Relationship between Breteau and house indices and cases of dengue/dengue hemorrhagic fever in Kuala Lumpur, Malaysia. J. Am. Mosq. Control Assoc. 1996;12:494–496. [PubMed] [Google Scholar]
- 10.Teixeira MG, Barreto ML, Costa MC, Ferreira LD, Vasconcelos PF, Cairncross S. Dynamics of dengue virus circulation: a silent epidemic in a complex urban area. Trop. Med. Int. Health. 2002;7:757–762. doi: 10.1046/j.1365-3156.2002.00930.x. [DOI] [PubMed] [Google Scholar]
- 11.Chadee DD, Shivnauth B, Rawlins SC, Chen AA. Climate, mosquito indices and the epidemiology of dengue fever in Trinidad (2002–2004) Ann. Trop. Med. Parasitol. 2007;101:69–77. doi: 10.1179/136485907X157059. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez L, Cortinas J, Pelaez O, Gutierrez H, Concepcion D, van der Stuyft P. Breteau Index threshold levels indicating risk for dengue transmission in areas with low Aedes infestation. Trop. Med. Int. Health. 2010;15:173–175. doi: 10.1111/j.1365-3156.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 13.Thammapalo S, Chongsuvivatwong V, Geater A, Dueravee M. Environmental factors and incidence of dengue fever and dengue haemorrhagic fever in an urban area, Southern Thailand. Epidemiol. Infect. 2008;136:135–143. doi: 10.1017/S0950268807008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadee DD, Williams FLR, Kitron UD. Impact of vector control on a dengue fever outbreak in Trinidad, West Indies, in 1998. Trop. Med. Int. Health. 2005;10:748–754. doi: 10.1111/j.1365-3156.2005.01449.x. [DOI] [PubMed] [Google Scholar]
- 15.Siqueira JB, Martelli CMT, Maciel IJ, Oliveira RM, Ribeiro MG, Amorim FP, Moreira BC, Cardoso DDP, Souza WV, Andrade AL. Household survey of dengue infection in Central Brazil: Spatial point pattern analysis and risk factors assessment. Am. J. Trop. Med. Hyg. 2004;71:646–651. [PubMed] [Google Scholar]
- 16.Ko YC. Epidemiology of dengue fever in Taiwan. Kaohsiung J. Med. Sci. 1989;5:1–11. [PubMed] [Google Scholar]
- 17.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am. J. Trop. Med. Hyg. 1998;58:287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]
- 18.Carbajo AE, Schweigmann N, Curto SI, De Garin A, Bejaran R. Dengue transmission risk maps of Argentina. Trop. Med. Int. Health. 2001;6:170–183. doi: 10.1046/j.1365-3156.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- 19.Kolivras KN. Mosquito habitat and dengue risk potential in Hawaii: A conceptual framework and GIS application. Prof. Geographer. 2006;58:139–154. [Google Scholar]
- 20.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLos Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard S. Applied Logistic Regression Analysis. 2nd ed. Sage; Newbury Park, CA, USA: 2002. [Google Scholar]
- 22.Wong DWS, Lee J. Statistical Analysis of Geographic Information with ArcView GIS And ArcGIS. John Wiley and Sons; Hoboken, NJ, USA: 2005. [Google Scholar]
- 23.Moran PAP. Notes on Continuous Stochastic Phenomena. Biometrika. 1950;37:17–23. [PubMed] [Google Scholar]
- 24.Fotheringham AS, Brunsdon C, Charlton M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. Wiley; New York, NY, USA: 2002. [Google Scholar]
- 25.Fotheringham AS, Brunsdon C, Charlton M. Quantitative Geography: Perspectives on Spatial Data Analysis. Sage; Newbury Park, CA, USA: 2000. [Google Scholar]
- 26.Wen TH, Lin NH, Chao DY, Hwang KP, Kan CC, Lin KCM, Wu JTS, Huang SYJ, Fan IC, King CC. Spatial-temporal patterns of dengue in areas at risk of dengue hemorrhagic fever in Kaohsiung, Taiwan, 2002. Int. J. Infect. Dis. 2010;14:e334–e343. doi: 10.1016/j.ijid.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LC, Scott TW, Lerdthusnee KRIA, Coleman RC, Costero ADRI, Clark GG, Jones JJ, Kitthawee SANG, Kittayapong PATT, Sithiprasasna RATA, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 28.Favier C, Schmit D, Müller-Graf CDM, Cazelles B, Degallier N, Mondet B, Dubois MA. Influence of spatial heterogeneity on an emerging infectious disease: the case of dengue epidemics. Proc. R. Soc B. 2005;272:1171–1177. doi: 10.1098/rspb.2004.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanwambeke SO, van Benthem BH, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin E, Somboon P. Multi-level analyses of spatial and temporal determinants for dengue infection. Int. J. Health Geogr. 2006;5:5. doi: 10.1186/1476-072X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh BK, Ng LC, Kita Y, Tang CS, Li Ang LW, Wong KY, James L, Goh KT. The 2005 Dengue epidemic in Singapore-epidemiology, prevention and control. Ann. Acad. Med. Singapore. 2008;37:538–545. [PubMed] [Google Scholar]
- 31.Almeida MCD, Caiaffa WT, Assuncao RM, Proietti FA. Spatial vulnerability to dengue in a Brazilian urban area during a 7-year surveillance. J. Urban Health. 2007;84:334–345. doi: 10.1007/s11524-006-9154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thai KTD, Nagelkerke N, Phuong HL, Nga TTT, Giao PT, Hung LQ, Binh TQ, Nam NV, de Vries PJ. Geographical heterogeneity of dengue transmission in two villages in southern Vietnam. Epidemiol. Infect. 2010;138:585–591. doi: 10.1017/S095026880999046X. [DOI] [PubMed] [Google Scholar]
- 33.Smith DL, Dushoff J, McKenzie FE. The risk of a mosquito-borne infection in a heterogeneous environment. Plos Biol. 2004;2:1957–1964. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings DAT, Irizarry RA, Huang NE, Endy TP, Nisalak A, Ungchusak K, Burke DS. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature. 2004;427:344–347. doi: 10.1038/nature02225. [DOI] [PubMed] [Google Scholar]
- 35.Shang CS, Fang CT, Liu CM, Wen TH, Tsai KH, King CC. The role of imported cases and favorable meteorological conditions in the onset of dengue epidemics. PLoS Neglected Trop. Dis. 2010;4:e775. doi: 10.1371/journal.pntd.0000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pai HH, Lu YL, Hong YJ, Hsu EL. The differences of dengue vectors and human behavior between families with and without members having dengue fever/dengue hemorrhagic fever. Int. J. Environ. Health Res. 2005;15:263–269. doi: 10.1080/09603120500155732. [DOI] [PubMed] [Google Scholar]
- 37.Bohra A, Andrianasolo H. Application of GIS in modeling of dengue risk based on sociocultural data: Case of Jalore, Rajasthan, India. Dengue Bull. 2001;25:92–102. [Google Scholar]
- 38.Chowell G, Torre CA, Munayco-Escate C, Suarez-Ognio L, Lopez-Cruz R, Hyman JM, Castillo-Chavez C. Spatial and temporal dynamics of dengue fever in Peru: 1994–2006. Epidemiol. Infect. 2008;136:1667–1677. doi: 10.1017/S0950268808000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, et al. Texas lifestyle limits transmission of dengue virus. Emerging Infect. Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor ME, Monroe WM. Stegomyia Indices and Their Value in Yellow Fever Control. Am J Trop Med Hyg. 1923:S1–3. 9–19. [Google Scholar]
- 41.Sauthowo TR, Tonn RJ, Yasuno M, Reader PM, Murdie G. Studies on Life Budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull. W. H. O. 1972;46:211–226. [PMC free article] [PubMed] [Google Scholar]
- 42.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- 43.Halstead SB. Etiologies of the Experimental Dengues of Siler and Simmons. Am. J. Trop. Med. Hyg. 1974;23:974–982. doi: 10.4269/ajtmh.1974.23.974. [DOI] [PubMed] [Google Scholar]
- 44.Isik O, Pinarcioglu MM. Geographies of a silent transition: A geographically weighted regression approach to regional fertility differences in Turkey. Eur. J. Popul. 2006;22:399–421. [Google Scholar]
- 45.Mennis J. Mapping the results of geographically weighted regression. Cartogr. J. 2006;43:171–179. [Google Scholar]
- 46.Wen TH, Chen DR, Tsai MJ. Identifying geographical variations in poverty-obesity relationships: empirical evidence from Taiwan. Geospat. Health. 2010;4:257–265. doi: 10.4081/gh.2010.205. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler D, Tiefelsdorf M. Multicollinearity and correlation among local regression coefficients in geographically weighted regression. J. Geogr. Syst. 2005;7:161–187. [Google Scholar]
- 48.Qiu X, Wu SS. Global and Local Regression Analysis of Factors of American College Test (ACT) Score for Public High Schools in the State of Missouri. Ann. Assoc. Am. Geogr. 2011;101:63–83. [Google Scholar]
- 49.Waller LA, Zhu L, Gotway CA, Gorman DM, Gruenewald PJ. Quantifying geographic variations in associations between alcohol distribution and violence: A comparison of geographically weighted regression and spatially varying coefficient models. Stoch. Environ. Res. Risk Assess. 2007;21:573–588. [Google Scholar]