Abstract
Seaweeds are an important source of bioactive metabolites for the pharmaceutical industry in drug development. Many of these compounds are used to treat diseases like cancer, acquired immune-deficiency syndrome (AIDS), inflammation, pain, arthritis, as well as viral, bacterial, and fungal infections. This paper offers a survey of the literature for Gracilaria algae extracts with biological activity, and identifies avenues for future research. Nineteen species of this genus that were tested for antibacterial, antiviral, antifungal, antihypertensive, cytotoxic, spermicidal, embriotoxic, and anti-inflammatory activities are cited from the 121 references consulted.
Keywords: Gracilaria, macroalgae, seaweed, biological activity, natural product, review
1. Introduction
The ocean environment contains over 80% of world’s plant and animal species [1] and with more than 150,000 seaweeds found in the intertidal zones and tropical waters of the oceans, it is a primary source of natural products [2].
Seaweeds are floating and submerged plants of shallow marine meadows. They have salt tolerance because the osmolarity of cytoplasm is adjusted to match the osmolarity of the seawater so that desiccation does not occur. They lack true stems, roots and leaves; however, they possess a blade that is leaf like, a stipe that is stem like, and a holdfast that resembles roots like terrestrial plants. Seaweeds contain photosynthetic pigments and use sunlight to produce food and oxygen from carbon dioxide, and the water [3].
Marine macroalgae are important ecologically and commercially to many regions of the world, especially in Asian countries such as China, Japan and Korea [4]. They are a valuable food resource which contains low calories, and they are rich in vitamins, minerals, proteins, polysaccharides, steroids and dietary fibers [5–7]. Since as early as 3000 BC, they were also considered important as traditional remedies [4]. The Japanese and Chinese use brown algae in the treatment of hyperthyroidism and other glandular disorders [8–11]. The unsaturated lipids afford protection against cardiovascular pathogens [12].
Seaweeds have been one of the richest and most promising sources of bioactive primary and secondary metabolites [13] and their discovery has significantly expanded in the past three decades [4,14,15]. The algae synthetize a variety of compounds such as carotenoids, terpenoids, xanthophylls, chlorophyll, vitamins, saturated and polyunsaturated fatty acids, amino acids, acetogenins, antioxidants such as polyphenols, alkaloids, halogenated compounds and polysaccharides such as agar, carrageenan, proteoglycans, alginate, laminaran, rhamnan sulfate, galactosyl glycerol and fucoidan [16–25].
These compounds probably have diverse simultaneous functions for the seaweeds and can act as allelopathic, antimicrobial, antifouling, and herbivore deterrents, or as ultraviolet-screening agents [26]. They are also used by the pharmaceutical industry in drug development to treat diseases like cancer, acquired immune-deficiency syndrome (AIDS), inflammation, pain, arthritis, infection for virus, bacteria and fungus [27]. Currently, algae represent about 9% of biomedical compounds obtained from the sea [28].
Compounds with cytostatic, antiviral, antihelmintic, antifungal and antibacterial activities have been detected in green, brown and red algae [29,30]. The algae produce pure forms of the fatty acids found in human milk that appear to be building blocks for mental and visual development [31] and have been extensively screened for syntheses of new drugs [32,33].
During the 1970s, Ryther and collaborators evaluated numerous species of red, green and brown macroalgae for their potential growth rates and dry weight yields [34]. They demonstrated that the genus Gracilaria was the most attractive candidate because of its ability to achieve high yields and while producing commercially valuable extracts [35].
Gracilaria Greville genus (Gracilariales, Rhodophyta) is a macroalgae group with more than 300 species of which 160 have been accepted taxonomically. These are usually red, green or greenish brown with a three-phase cycle and can be found in tropical and subtropical seas [36,37].
The Gracilaria species are important for the industrial and biotechnological uses because they have phycocolloids, the main source of agar α-(1,4)-3,6-anhydro-l-galactose and β-(1,3)-d-galactose with little esterification in cell wall [2,38]. Among the carbohydrates, agar and other polysaccharides are present in G. confervoides [39], G. dura [40], G. chilensi and G. secundata [41,42].
These algae also produce important bioactive metabolites like the primary compound with antibiotic activity acrylic acid [43], and the eicosanoids which are derivatives C20 polyunsaturated fatty acid (PUFA) metabolism through oxidative pathways that originate mainly from arachidonic acid and eicosapentaenoic acids, the precursors of prostaglandins (PGs) [44,45]. Species such as G. asiatica and G. lichenoids contain PGE2 [46,47]. PGF2 and 15-keto-PGE2 were respectively isolated from G. lichenoids and G. asiatica [45]; G. verrucosa contains PGA2 that appears to be responsible for a gastrointestinal disorder, known as “ogonori” poisoning in Japan [48].
Lipids are abundant in this genus being mainly prostaglandins [49], steroids, such as cholesterol and clinoasterol are present in G. crassa and G. coronopifolia respectively [50–52], as well as G. longa [48,53–57] and G. dura. Other steroids such as 3-beta-hydroxy-poriferast-5-en-7-one, 3-beta-7-alpha-diol-poriferast-5-ene and 5-alpha-poriferast-9(11)-en-3-beta-ol are isolated from G. dura [50]; cholestane-3-β-5-diol,5-α:24(S)-ethyl [52], poriferastene 8 [50], poriferast-5-ene-3-β-7-β-diol [51] and poriferast-5-ene-3-β-7-α-diol [51] were identified in G. coronopifolia; G. longa also has a variety of compounds like alpha linolenic acid, gamma linolenic acid [58], glycolipids [59], 5-dehydro avenasterol, fucosterol, myristic acid, desmosterol and 5-alpha-24(S)-ethyl-cholestane-3-beta-6-beta-diol [60]. Phytochemical studies with extracts from fresh thallus of G. andersoniana showed the following isolates: oleic acid, linoleic acid, cholesterol, prostaglandin A2, prostaglandin E2, leukotriene B4 and phytol [61–63].
Studies with G. asiatica reported the diterpenes cis and trans-phytol [63]. A variety of lactones are present in Gracilaria from the Pacific Ocean, such as aplysiatoxin isolated from G. confervoides [64,65], polycavernoside B, polycavernoside B2, and polycavernoside A2 and A3 isolated from G. crassa [49,66]. Other constituents are also containedin this genus such as proteins r-phycoerythrin from G. salicornia [67] and G. longa [68], gigartinine from G. chilensis [69] and proteoglycan from G. longa [70].
The possibility of finding new molecules from natural products is immeasurable. For this reason the plants and their derivatives are major sources of all drugs, affecting about 30% of pharmaceutical market [71]. According to Newman et al. (2003), between the years 1981 and 2002, 877 new molecules were introduced into the market, with 49% of substances isolated from natural sources followed by semi-synthetic derivatives or synthesized molecules taking the structures of natural origin as models [29].
The search for new effective medicines remains a challenge for scientists. Therefore around the world, many researchers have focused on natural sources for new molecules with algae among the targets of these studies. So in this study we reviewed the literature related to bioactivities for Gracilaria algae.
2. Results and Discussion
In this review, among the 160 species of Gracilaria already identified taxonomically, only 19 of them had their extracts and fractions chemically tested for toxicity, cytotoxic, spermicidal, antiimplantation, antibacterial, antiviral, antifungal, antiprotozoa, antihypertensive, antioxidant, anti-inflammatory, analgesic, and spasmolytic effects in gastrointestinal tract (Table 1).
Table 1.
Bioactivities of marine algae of the Gracilaria genus.
| Botanical Name | Part used | Type of extract | Bioassays models, organism, dose or route of administration | Result |
|---|---|---|---|---|
| Studies of toxicity | ||||
| Gracilaria bursa-pastoris (S.G.Gmelin) P.C.Silva | FzDTh | H2O Ext. | Cytotoxic activity-cell culture-10.0 μg/mL | Inactive [72] |
| FTh | 95% EtOH Ext. or CHCl3 Ext. | Cytotoxic activity-cell culture-10.0 μg/mL | Inactive [72] | |
| Gracilaria chorda (Holmes) | FsO | H2O Ext. | Toxicity assessment-mouse-1.2 mg/animal-i.p. | Active [48] |
| Gracilaria coronopifolia (J. Agardh) | FTh | Plant | Toxic effect-human adult-oral | Active [65] |
| Gracilaria corticata (J.Agardh) J.Agardh | Th | 50% EtOH-H2O Ext. | Toxicity assessment-mouse-DL50 1000 mg/kg-ip | Active [73] |
| Gracilaria domingensis (Kützing) Sonder ex Dickie | DO | 90% EtOH Ext. | Cytotoxity-Artemia salina L.-200 μg/mL | Active [74] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | DTh | Plant | Toxicity effect (death)-human adult-oral | Active [49] |
| SDTh | 90% EtOH Ext. | Toxicity assessment-mouse-DL50 0.825 mg/kg-i.p. | Active [75] | |
| Gracilaria foliifera (Forsskål) Borgesen | DEP | (1:1) EtOH-H2O Ext. | Cytotoxic activity-cell culture-dose: dry weight of plant | Active [76] |
| Gracilaria textorii (Suringar) De Toni | FzDO | MeOH Ext. | Cytotoxic activity-cell culture (CA 9 KB) | Inactive [77] |
| FsTh | Hexane Ext. | Cytotoxic activity-culture cell (LEUK P 388)-ED 50 > 100 μg/mL | Equivocal [78] | |
| CCl4 Ext. | Cytotoxic activity-culture cell (LEUK P 388)-ED 50 22.2 μg/mL | Equivocal [78] | ||
| CHCl3 Ext. | Cytotoxic activity-culture cell (LEUK P 388)-ED 50 32.2 μg/mL | Inactive [78] | ||
| Gracilaria verrucosa (Hudson) Papenfuss | DO | H2O Ext. | Toxicity assessment-mouse-1.2 mg/animal-i.p. | Active [48] |
| FzDO | MeOH Ext. | Cytotoxic activity-cell culture (CA 9 KB) | Inactive [77] | |
| FO | 30% EtOH Ext. | Cytotoxic activity-cell culture (CA 9 KB)-10.0 μg/mL | Inactive [79] | |
| (1:1) CHCl3-MeOH Ext. | Cytotoxic activity-cell culture (CA 9 KB)-1.0 μg/mL | Equivocal [79] | ||
| FTh | H2O Ext. and 95% EtOH Ext. | Cytotoxic activity-cell culture (LEUK P 388-P 3)-10.0 μg/μL | Inactive [72] | |
| Effects on the nervous system | ||||
| Gracilaria corticata J.Agardh | SDTh | 90% EtOH Ext. | Autonomic effects-dog-50 mg/kg-iv | Inactive [75] |
| CNS effects-mouse | Inactive [75] | |||
| Analgesic activity-mouse | Inactive [75] | |||
| Anticonvulsant activity-mouse | Inactive [75] | |||
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Autonomic effects-dog-50 mg/kg-iv | Inactive [75] |
| CNS effects-mouse | Inactive [75] | |||
| Analgesic activity-mouse | Inactive [75] | |||
| Anticonvulsant activity-mouse | Inactive [75] | |||
| Gracilaria verrucosa (Hudson) Papenfuss | SDTh | 90% EtOH Ext. | CNS effects-mouse | Inactive [75] |
| Contraception activity | ||||
| Gracilaria corticata J.Agardh | DTh | (1:1) MeOH-CH2Cl2 Ext. | Embryotoxic effect-pregnant rat-1.0 mg/kg-intragastric | Inactive [80] |
| SDTh | 90% EtOH Ext. | Antiimplantation effect-pregnant rat-100.0 mg/kg | Inactive [75] | |
| Spermicidal effect-rat-2.0 % | Inactive [75] | |||
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Antiimplantation effect-pregnant rat-100.0 mg/kg | Inactive [75] |
| Spermicidal effect-rat-2.0% | Inactive [75] | |||
| Gracilaria verrucosa (Hudson) Papenfuss | SDTh | 90% EtOH Ext. | Spermicidal effect-rat-2.0% | Inactive [75] |
| Anti-inflammatory activity | ||||
| Gracilaria textorii (Suringar) De Toni | EP | H2O Ext. | Platelet aggregation inhibition (adenosine diphosphate; arachidonic acid or collagen stimulation)-100.0 μg/mL | Inactive [81] |
| Venotonic activity (platelet aggregating factor stimulation)-100.0 μg/mL | Inactive [81] | |||
| Gracilaria verrucosa (Hudson) Papenfuss | DTh | Polysaccharide fraction | Immunostimulant activity-mouse-4.0 mg/animal-i.p. | Active [82] |
| Phagocytosis stimulation-mouse-4.0 mg/animal-i.p. | Active [82] | |||
| SDTh | 90% EtOH Ext. | Antiinflammatory activity-rat-intragastric | Inactive [75] | |
| Antioxidant activity | ||||
| Gracilaria verrucosa (Hudson) Papenfuss | Plant | MeOH Ext. | Radical scavenging effect (DPPH radicals)-IC50 480.0 μg | Active [83] |
| DTh | Polysaccharide fraction | Oxygen radical formation induction-mouse-4.0 mg/animal-i.p. | Active [82] | |
| Gastrointestinal effects | ||||
| Gracilaria chorda (Holmes) | FsO | H2O Ext. | Mouse-0.5 mg/animal-gastric intubation and dose 0.5 mg/loop-i.p. | Active [48] |
| Gracilaria verrucosa (Hudson) Papenfuss | DO | H2O Ext. | Mouse-0.5 mg/animal-gastric intubation | Active [48] |
| Cardiovascular effects | ||||
| Gracilaria corticata (J.Agardh) J.Agardh | SDTh | 90% EtOH Ext. | Cardiovascular effects-dog-50 mg/kg-iv | Inactive [75] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Cardiovascular effects-dog-50 mg/kg-iv | Inactive [75] |
| Diuretic activity-rat-intragastric | Active [75] | |||
| Gracilaria lichenoides (Greville) | EP | H2O Ext. | Antihypertensive activity-rat-iv | Active [84] |
| FsTh | H2O Ext. | Antihypertensive activity-rat-iv | Active [85] | |
| Gracilaria verrucosa (Hudson) Papenfuss | SDTh | 90%EtOH Ext. | Cardiovascular effects-dog-50 mg/kg-iv | Inactive [75] |
| Hypoglycemic activity | ||||
| Gracilaria corticata J.Agardh | SDTh | 90% EtOH Ext. | Rat-250 mg/kg – intragastric | Inactive [75] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Rat-250.0 mg/kg – intragastric | Inactive [75] |
| Anti-enzymes activity | ||||
| Gracilaria arcuata (Zanardini) | DTh | MeOH Ext. | Tyrosinase inhibition-0.33 mg/mL | Inactive [86] |
| Gracilaria corticata (J.Agardh) J.Agardh | DO | PET Ether Ext.; CHCl3 Ext. or MeOH Ext. | Penicillinase inhibition-1.0 μg/units | Inactive [87] |
| Gracilaria textorii (Suringar) De Toni | EP | H2O Ext. | Aldose reductase inhibition-10.0 μg/mL | Inactive [81] |
| FzDO | MeOH Ext. | Cyclic AMP phosphodiesterase inhibition | Inactive [77] | |
| Gracilaria verrucosa (Hudson) Papenfuss | FzDO | EtOAc Ext. | Lipase inhibition | Equivocal [88] |
| MeOH Ext. | Cyclic AMP phosphodiesterase inhibition | Inactive [77] | ||
| Respiratory effects | ||||
| Gracilaria corticata (J.Agardh) J.Agardh | SDTh | 90% EtOH Ext. | Respiratory depressant-dog-50 mg/kg-iv | Inactive [75] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Respiratory depressant-dog-50 mg/kg-iv | Inactive [75] |
| Gracilaria verrucosa (Hudson) Papenfuss | SDTh | 90% EtOH Ext. | Respiratory depressant-dog-50.0 mg/kg-iv | Inactive [75] |
| Spasmolytic activity | ||||
| Gracilaria corticata (J.Agardh) J.Agardh | SDTh | 90% EtOH Ext. | Spasmolytic activity-guinea pig | Inactive [75] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Negative chronotropic effect-dog-50.0 mg/kg-iv | Inactive [75] |
| Antibacterial activity | ||||
| Gracilaria cervicornis (Turner) J.Agardh | DEP | 95% EtOH Ext. | Agar plate-Staphylococcus aureus-5.0 mg/mL | Active [89] |
| Agar plate-Proteus vulgaris; Escherichia coli; Aspergillus fumigates; Candida albicans; Pseudomonas aeruginosa; Streptococcus pyogenes- 50.0 mg/mL | Inactive [89] | |||
| Gracilaria corticata (J.Agardh) J.Agardh | DO | PET Ether Ext.; CHCl3 Ext. or MeOH Ext. | Agar plate-Staphylococcus aureus; Escherichia coli-MIC >200 μg/mL | Inactive [90] |
| FsO | MeOH Ext. | Agar plate-Escherichia coli; Salmonella paratyphi A; Salmonella paratyphi B; Shigella sonnei | Inactive [91] | |
| Agar plate-Bacillus subtilis; Staphylococcus aureus; Bacillus megaterium; Streptococcus viridans | Active [91] | |||
| SDTh | 90% EtOH Ext. | Agar plate-Klebsiella pneumonia; Pseudomonas aeruginosa; Staphylococcus aureus; Escherichia coli; Streptococcus faecalis | Inactive [75] | |
| Gracilaria debilis (Forsskål) Borgesen | DO | 95% EtOH Ext. | Agar plate-Escherichia coli; Staphylococcus aureus | Active [92] |
| Agar plate-Mycobacterium smegmatis Inactive | [92] | |||
| Gracilaria domingensis (Kützing) Sonder ex Dickie | DO | 95% EtOH Ext. | Agar plate-Escherichia coli; Staphylococcus aureus | Active [92] |
| Acetone Ext. or Ether Ext. | Agar plate-Escherichia coli; Staphylococcus aureus | Inactive [92] | ||
| 95% EtOH Ext. or Acetone Ext. | Agar plate-Mycobacterium smegmatis | Active [92] | ||
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Agar plate-Escherichia coli; Streptococcus faecalis; Staphylococcus aureus; Pseudomonas aeruginosa; Klebsiella pneumoniae | Inactive [75] |
| Gracilaria pygmea (Borgesen) | FsO | MeOH Ext. | Agar plate-Bacillus subtilis; Staphylococcus aureus; Escherichia coli; Salmonella paratyphi A; Streptococcus viridans; Shigella sonnei; Salmonella paratyphi B | Inactive [91] |
| Agar plate-Bacillus megaterium | Active [91] | |||
| Gracilaria sjoestedii (Kylin) | DO | 95% EtOH Ext. | Agar plate-Escherichia coli; Staphylococcus aureus | Active [92] |
| Agar plate-Mycobacterium smegmatis | Inactive [92] | |||
| Gracilaria tikvahiae McLachlan | DEP | CHCl3 Ext. or EtOH Ext. | Agar plate -Staphylococcus aureus | Active [93] |
| Agar plate-Streptococcus faecalis; Pseudomonas aeruginosa | Inactive [93] | |||
| Gracilaria verrucosa (Hudson) Papenfuss | FTh | ** | Agar plate-Vibrio marinofulvis; Micrococcus imfimus; Pseudomonas atlantica-40.0 μg/μL | Inactive [94] |
| Th | 70% EtOH Ext. | Antiphage activity-agar plate-Bacteriophage T 1; Bacteriophage T 2; Bacteriophage T 4; Bacteriophage T 7; Bacteriophage MS 2; Bacteriophage PHI-CHI 174-0.50 μg/mL | Inactive [95] | |
| Antifungal activity | ||||
| Gracilaria corticata (J.Agardh) J.Agardh | FsO | MeOH Ext. | Agar plate-Aspergillus niger; Fusarium solani; Alternaria solani; Penicillium funiculosum | Inactive [91] |
| SDTh | 90% EtOH Ext. | Agar plate-Sporotrichum schenckii; Cryptococcun neoformans; Candida albicans; Trichophyton mentagrophytes; Aspergillus fumigates | Inactive [75] | |
| Gracilaria debilis (Forsskål) Borgesen | DO | 95% EtOH Ext. | Agar plate-Candida albicans | Active [92] |
| Agar plate-Neurospora crassa | Inactive [92] | |||
| Gracilaria domingensis (Kützing) Sonder ex Dickie | DO | 95% EtOH Ext. and Acetone Ext. | Agar plate-Candida albicans; Neurospora crassa | Active [92] |
| Ether Ext. | Agar plate-Candida albicans | Inactive [92] | ||
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Agar plate-Sporotrichum schenckii; Candida albicans; Cryptococcus neoformans; Trichophyton mentagrophytes; Aspergillus fumigates | Inactive [75] |
| Gracilaria pygmea (Borgesen) | FsO | MeOH Ext. | Agar plate-Aspergillus niger; Fusarium solani; Alternaria solani; Penicillium funiculosum | Inactive [91] |
| Gracilaria sjoestedii (Kylin) | DO | 95% EtOH Ext. | Agar plate-Candida albicans | Active [92] |
| Agar plate-Neurospora crassa | Inactive [92] | |||
| Gracilaria tikvahiae McLachlan | DEP | CHCl3 Ext. and EtOH Ext. | Agar plate-Candida albicans | Active [93] |
| Antiviral activity | ||||
| Gracilaria bursa-pastoris (S.G.Gmelin) P.C.Silva | FzDTh | ** | Cell culture-Herpes simplex 1 and HIV Virus | Inactive [96] |
| Gracilaria corticata (J.Agardh) J.Agardh | Th | 50% EtOH-H2O Ext. | Cell culture-Semlicki-forest Virus-0.05 mg/mL | Equivocal [73] |
| Cell culture-Ranikhet and Vaccinia Virus-0.05 mg/mL | Inactive [73] | |||
| SDTh | 90% EtOH Ext. | Cell culture-Ranikhet Virus | Inactive [75] | |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Cell culture-Semlicki-forest and Ranikhet Virus | Inactive [75] |
| Gracilaria pacifica (I. A. Abbott) | DO | MeOH Ext. | Cell culture-Herpes simplex 1 Virus-400.0 μg/mL | Inactive [97] |
| Cell culture-Virus sindbis-200.0 μg/mL | Active [97] | |||
| Gracilaria species | FzDTh | ** | Cell culture-Herpes simplex 1 and HIV Virus | Inactive [96] |
| Gracilaria textorii (Suringar) De Toni | FzDO | MeOH Ext. | Cell culture-Herpes simplex 1 Virus | Inactive [77] |
| Th | H2O Ext. | Cell culture-HIV Virus-MIC > 1000 μg/mL | Inactive [98] | |
| Fresh | MeOH Ext. | Epstein-Barr virus early antigen activation inhibition (telocidin b-4 induced | ** [99] | |
| Th | Epstein-Barr virus induced activation)-4.0 μg/mL | |||
| Gracilaria verrucosa (Hudson) Papenfuss | FzDO | MeOH Ext. | Cell culture-Herpes simplex 1 Virus | Inactive [77] |
| Antiprotozoal activity | ||||
| Gracilaria corticata (J.Agardh) J.Agardh | SDTh | 90% EtOH Ext. | Agar plate-Entamoeba histolytica; Plasmodium berghei | Inactive [75] |
| Gracilaria edulis (S.G.Gmelin) P.C.Silva | SDTh | 90% EtOH Ext. | Agar plate-Entamoeba histolytica; Plasmodium berghei | Inactive [75] |
| Allelophatic activity | ||||
| Gracilaria compressa (C.Agardh) Greville | DEP | 95% EtOH Ext. | Agar plate-Helianthus tuberosus-dose: dry weight of plant | Active [76] |
| Gracilaria foliifera (Forsskål) Borgesen | DEP | H2O Ext. | Agar plate-Helianthus tuberosus-dose: dry weight of plant | Active [76] |
| Gracilaria verrucosa (Hudson) Papenfuss | DEP | 95% EtOH Ext. | Agar plate-Helianthus tuberosus-dose: dry weight of plant | Active [76] |
Legend: DEP = Dried entire plant; DO = Dried organism; DTh = Dried thallus; EP = Entire plant; FO = Frozen organism; FsO = Fresh organism; FsTh = Fresh thallus; FTh = Frozen thallus; FzDO = Freeze dried organism; FzDTh = Freeze Dried thallus; SDTh = Shade dried thallus; Th = Thallus; PET Ether);
= Not stated.
These biological studies were mainly developed in Japan and Brazil. This fact is justified by the extensive coastlines and marine biodiversity and is influenced by several factors for the development of these species, such as temperature, radiation, salinity, metal ions and other chemically fundamental components. Australia and Guam have recently become interested in the study of algae and diverse marine species. The consumption of algae has increased in European countries in recent decades with 15 to 20 species of algae being marketed in Italy, France and Greece. In western countries like Venezuela, USA and Canada, the macroalgae are industrially used as a source of hydrocolloids agar, carrageenan and alginate [100]. Carrageenan has been found to be useful in ulcer therapy and alginates are known to prolong the period of activity of certain drugs [8–11].
2.1. Studies of Toxicity
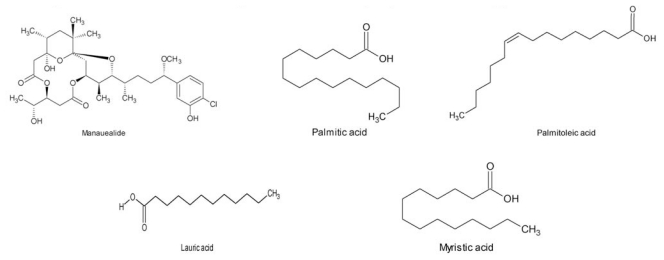
In France, extract studies with ethanol/water draw up from dried entire plant of G. foliifera showed toxicity in humans when treated with oral dose and cytotoxicity studies [75,76]. G. coronopifolia and G. edulis were also toxic to humans [65,49] (See Table 1). Carbohydrate, heparin [97], agar [101], manauealide A, manauealide B [64], manauealide C [102], palmitic, palmitoleic, oleic, lauric and myristic acids [103], steroids and alkaloids malyngamide [104] were found in these species (Figure 1).
Figure 1.
Structure of the compounds found in G. foliifera, G. coronopifolia and G. edulis.
There is currently a tendency to substitute the use of laboratory animals in toxicological tests with alternative methods to reduce their numbers in experiments, or refine the existing methodology in order to minimize pain and stress [105]. A rapid and effective alternative to realize primary toxicity and biological action screening of compounds is the estimation of the 50% lethal concentration (LC50) through brine shrimp assay using Artemia salina L. [106]. A 90% ethanol extract of G. domingensis had LC50 of 200 μg/mL against A. salina [74].
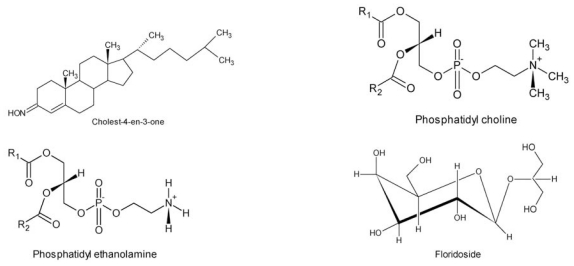
Another method to evaluate toxicity is determining cytotoxic activity. In this context aqueous extract from dried thallus of G. bursa-pastoris (10.0 μg/mL), chloroform and methanol extracts from G. textorii, which was isolated steroid cholest-4-en-3-one [107], and ethanol extract from G. verrucosa were not toxic in cell culture. However, aqueous extract from G. verrucosa at a dose of 1.2 mg/animal showed toxicity to mice [48], according to Table 1. In this seaweed, lipids were indentified, such as PGFα [84,85], glycerol, ethanolamine-phosphatidyl [58], choline-phosphatidyl [58,108], ethanol-phosphatidyl [58], floridoside [109], and carbohydrates, such as agar [110–113] (Figure 2).
Figure 2.
Structure composed of species of Gracilaria tested in cytotoxicity.
2.2. Effects on the Nervous System
Studies related to nervous system are important to understanding and treat complex degenerative and behavioral diseases. 90% ethanol extracts from G. corticata, G. edulis and G. verrucosa did not cause central or periphery effects for mice or dogs (50 mg/kg), and did not show analgesic or anticonvulsant activities for mice [75] (Table 1).
2.3. Contraception Activity
The researchers have also investigated new molecules with anticonceptive action; the post-coital contraceptive action of marine seaweeds was also evaluated in animals. Methanol: methylene chloride (1:1) extract from G. corticata was orally administered at 500 or 1000 mg/kg/day to female rats from day 1 to day 7 of their pregnancies. Higher doses produced significant post-coital contraceptive activity due to enhanced pre-implantation without any marked side effects. These findings indicate that red marine algae are a potential source for post-coital contraceptive drugs [80].
90% Ethanol extracts from G. edulis (100 mg/kg) and G. corticata were inactivated before the antiimplantation effect when they tested in pregnant rats [75,80]. Ethanol extracts from shade dried thallus of G. edulis and G. verrucosa were inactive in spermicidal bioassays [75]. Extracts from G. edulis showed 100% inhibition of sperm motility and this effect was related to disruption of the plasma membrane by spermicidal compounds [3] (Table 1).
2.4. Anti-Inflammatory and Antioxidant Activities
The anti-inflammatory activity of seaweeds has been studied. Polysaccharide fractions from G. verrucosa at a dose of 4.0 mg/animal were orally and intraperitoneally administered to mice and showed immunopotentiating activity stimulating phagocytosis [82]. Methanol extract and polysaccharide fractions from G. verrucosa were also antioxidant [82,83]. Aqueous extract from G. textorii at a dose of 100 μg/mL did not inhibit platelet aggregation induced by adenosine diphosphate, arachidonic acid or collagen [81]. G. verrucosa, G. asiatica, G. lichenoides and others species contain PGE2 [47,85], that have physiological effects including hyperthermia, hypotension, smooth muscle dilatation, hyperalgesia and gastric secretion inhibition [114,115] (Table 1).
2.5. Gastrointestinal Effects
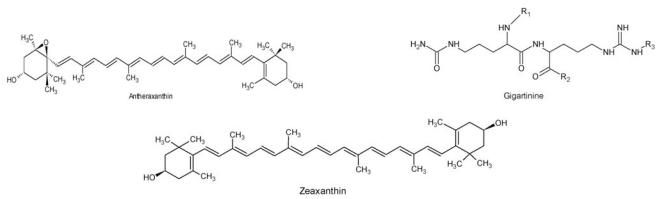
Aqueous extract from dried G. verrucosa algae or fresh G. chorda algae at a dose of 0.5 mg/animal controlled gastrointestinal disorders in mice [48] (Table 1), resulting from zeaxanthin and antheraxanthin [116], carotenoids, pyrimidine 2-amino-4-carboxy, non-alkaloid nitrogen heterocycle [90], steroids, 5-alpha-poriferastane, 3-beta-6-alpha-diol poriferastane, 5-alpha-3-beta-6-beta-diol [51] and gigatinine [85] (Figure 3).
Figure 3.
Structure of the compounds found in G. verrucosa and G. chorda.
2.6. Cardiovascular Effects
90% Ethanol extracts from G. corticata, G. edulis and G. verrucosa showed no cardiovascular effects in dogs (50 mg/kg) [75]. 90 % ethanol extract from G. edulis showed diuretic activity [75]. Aqueous extract from G. lichenoides was administered intravenously in rats and it was antihypertensive [84]. Tyrosinase inhibition was not induced by methanol extract from G. arcuata [86] and aqueous extract from G. textorii, 10 μg/mL, was negligable on aldose reductase [83] (Table 1).
2.7. Antibiotic Activity
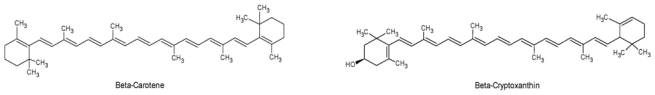
Extracts or ingredients from various algae have shown antibacterial activity in vitro against gram-positive and gram-negative bacteria [117]. The agar disc diffusion method for antibacterial susceptibility was used for evaluation and 6 mm discs were impregnated with 20 μL of the extracts and placed in inoculated Muller Hinton agar. Antibacterial activity from chloroform extract of G. edulis (Gmelin) Silva was tested against bacterial strains of Vibrio cholera, Staphylococcus aureus, Shigella dysenteriae, Shigella bodii, Salmonella paratyphi, Pseudomonas aeruginosa and Klebsiella pneumonia (Table 1). We observed higher activity for G. edulis extract than S. aureus extract [12]. Yet it was inactive for Sporotrichum schenckii, Candida albicans and Cryptococcus neoformans [75]. In the present investigation, the chemical compounds isolated from the species were steroids (carotenoids, β-cryptoxanthin and β-carotene) [118] and carbohydrates [84,85,119] (Figure 4).
Figure 4.
Chemical structure of the steroids isolated from G. edulis.
Mahasneh et al. (1995) demonstrated activity of organic extracts from algae against multi-resistant bacteria to antibiotics [120]. Ethanol extract from G. debilis showed antibacterial activity against S. aureus but was inactive against Mycobacterium smegmatis [92].
95% ethanol extract from whole dried G. cervicornis algae was active against S. aureus at a concentration of 5.0 mg/mL [89]. Methanol extract from fresh G. corticata was active against Bacillus subtilis, Bacillus megaterium, S. aureus and Streptococcus viridians [91].
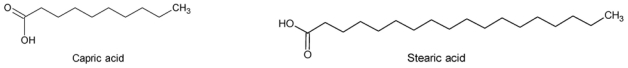
G. corticata and G. pygmea did not inhibit the growth of Aspergillus niger, Fusarium solani, Alternaria solani, or Penicillium funiculosum [91]. Petroleum ether, chloroform and methanol extracts from this seaweed at a concentration of 1.0 μg/units proved to be inactive on the inhibition of penicillinase enzyme [87]. From this specie, stearic lipids and capric acids were isolated [121] (Figure 5).
Figure 5.
Structure of compounds found in species of Gracilaria with antifungal activity.
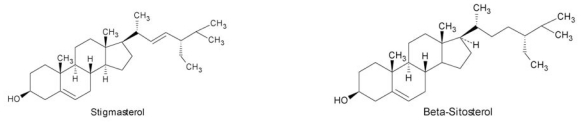
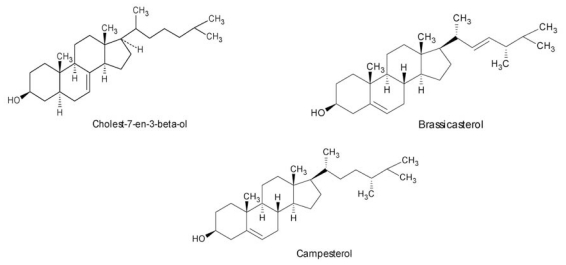
Ethanol extracts from G. domigensis and G. sjoestedii showed antibacterial activity against E. coli and S. aureus. Ethanol extracts from G. debilis, G. domingensis and G. sjoestedii were active against Candida albicans shown by agar plate method [92]; Chloroform, ether and methanol extracts from G. tikvahiae were inactive [93]. The growth of Neurospora crassa was not inhibited by extracts from G. sjoestedii and G. debilisi; ethanol extract from G. domigensis was active against Mycobacterium smegmatis and Neurospora crassa [92]. G. domigensis has as chemical constituents, polysaccharide CT-1 [122], palmitic acid and steroids (stigmasterol, sitosterol, campesterol, cholest-7-en-3-β-ol and brassicasterol) [52] (Figure 6).
Figure 6.
Structure of the steroids isolated from G. domingensis.
Some studies highlighting antiparasitic activity of seaweeds also were verified. 90 % ethanol extract from G. corticata and G. edulis were tested against Entamoeba histolytica and Plasmodium berghei and were not active [75].
2.8. Antivirial Activity
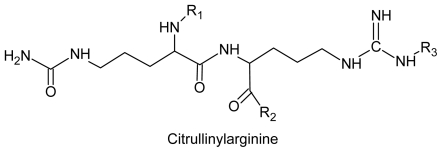
Extracts from G. bursa-pastoris and Gracilaria sp were inactive against the Herpes simplex 1 virus (HSV) and the human immunodeficiency virus (HIV) when evaluated in cell cultures [96]. Granin BP and citrullinyl-arginine proteins were isolated from these extracts [123,124]. Methanol extract from dried G. pacifica at a concentration of 200.0 μg/mL was active against Sindbis virus, but was not effective against H. simplex 1 when tested at a concentration of 400 μg/mL. Extracts and compounds obtained from Gracilaria sp with anti-HIV activity are also active against other retroviruses such as HSV. However, the pharmacodynamic mechanisms of the antiretroviral activity are still unknown because bioactive compounds from seaweed poorly investigated [9] (Table 1) (Figure 7).
Figure 7.
Structure of a compound found in Gracilaria sp and G. bursa-pastoris.
3. Material and Methods
In this article, some reports about bioactivity of Gracilaria algae were reviewed in the specialized literature published up to January 2011. The search was carried out using data banks such as; Biological Abstracts, AlgaeBase, SciFinder Scholar, Pubmed and NAPRALERT (acronym for Natural Products ALERT-University of Illinois in Chicago, USA).
4. Conclusions
Algae are abundant in the oceans and represent a rich source of as yet unknown secondary metabolites. In this review, we found only a few studies with complete chemical profiles and pharmacological potential of the Gracilaria species. Most studies raised concerns about antimicrobial activity against Staphylococcus, Streptococcus, Candida and Herpes genus. Others referenced the cytotoxicity bioassays in which these algae species were not active, but they produce various types of prostaglandins and others substances that can be toxic to humans such as gastrointestinal disorders and lethality caused by G. verrucosa and G. edulis, respectively. To research new drugs it is necessary to evaluate other bioassay models to preserve the safety, efficacy and quality of the end products. In Brazil, there is a great need for toxicological, pharmacological, preclinical and clinical studies, as recommended by the RDC 48/2004.
Finally, we conclude that algae of the Gracilaria genus are a potential source for synthesis of new natural medicines. It is important to taxonomically classify and standardize extractions, while identifying the active compounds to attenuate possible environmental interference that could undermine the pharmacochemical profile, and thus generate different pharmacologic effects. In addition, it is important to sensitize corporate researchers and financial agencies to support this cause.
Acknowledgments
The authors thank CNPq/CAPES/PRONEX-FAPESQ-PB-Brazil for financial support.
References
- 1.Jha RK, Zi-rong X. Biomedical compounds from marine organisms. Mar. Drugs. 2004;2:123–146. [Google Scholar]
- 2.Falcão VR. PhD Thesis. Institute of Chemical, University of São Paulo; São Paulo, Brazil: 2006. Aspectos moleculares de nitrato redutase da macroalga marinha Gracilaria tenuistipitata (Rhodophyta): Seqüenciamento do gene e estudo da expressão do RNA mensageiro; pp. 1–187. [Google Scholar]
- 3.Babuselvam M, Ravikumar S. Screening of Male Anti-Fertility Compounds from Marine Seaweed Macro Algae. Division of Marine Microbiology and Medicine, Manonmaniam Sundaranar University; Rajakkamangalam, India: 1993. [accessed on 14 May 2011]. pp. 1–14. Available online: http://www.scisoc.or.th/stt/32/sec_h/paper/stt32_H_H0001.pdf. [Google Scholar]
- 4.Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004;16:245–262. [Google Scholar]
- 5.Ito K, Hori K. Seaweed: Chemical composition and potential uses. Food Rev. Int. 1989;5:101–1144. [Google Scholar]
- 6.Darcy-Vrillon B. Nutritional aspects of the developing use of marine macroalgae for the human food industry. Int. J. Food Sci. Nutr. 1993;44:523–535. [Google Scholar]
- 7.Lahaye M. Marine algae as a source of dietary fibers: Determination of soluble and insoluble dietary fiber contents in some ‘sea vegetable’. J. Sci. Food Agric. 1993;54:523–535. [Google Scholar]
- 8.Elena M, Francisco Y, Erickson KL. Mailiohydrin, a cytotoxic chamigrene dibromohydrin from a Phillippine Laurencia species. J. Nat. Prod. 2001;64:790–791. doi: 10.1021/np0005053. [DOI] [PubMed] [Google Scholar]
- 9.Kim JB, Hudson AM, Huang K, Bannistes A, Jin TJ, Choi GHN, Towers YK, Wreede RE. Biological activity of seaweed extracts from British, Colombia, Canada and Korea. I. Antiviral activity. Can. J. Bot. 1997;75:1656–1660. [Google Scholar]
- 10.Okai Y, Highasi OK, Ishizaka S, Yamashita U. Enhancing effect of polysaccharides from a edible brown algae, Hijikia furiform (Hijki) on release of tumour necrosis factor alpha from macrophages of endotoxin non responder C3H/HCl mice. Nutr. Cancer. 1997;27:381–386. doi: 10.1080/01635589709514505. [DOI] [PubMed] [Google Scholar]
- 11.Premila JC, Raviraja NS, Sridhar KR. Antimicrobial activity of some marine algae of south-west coast of India. Indian J. Mar. Sci. 1996;26:201–205. [Google Scholar]
- 12.Vallinayagam K, Arumugan R, Ragupathi Raja Kannan RRR, Thirumaram G, Anantharaman P. Antibacterial activity of some selected seaweeds from Pudumadam coastal regions. Glob. J. Pharmacol. 2009;3:50–52. [Google Scholar]
- 13.Faulkner DJ. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 14.Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C: Comp. Pharmacol. 2006;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan L, Murphy B, McLoughlin P, Duggan P, Lawlor PG, Hughes H, Gardiner GE. Prebiotics from marine macroalgae for human and animal health application. Mar. Drugs. 2010;8:2038–2064. doi: 10.3390/md8072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paniagua-Michel J, Capa-Robles W, Olmos-Soto J, Gutierrez-Millan LE. The carotenogenesis pathway via the isoprenoid-beta-carotene interference approach in a new strain of Dunaliella salina isolated from Baja California Mexico. Mar. Drugs. 2009;7:45–56. doi: 10.3390/md7010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cen-Pacheco F, Nordstrom L, Souto ML, Martin MN, Fernandez JJ, Daranas AH. Studies on polyethers produced by red algae. Mar. Drugs. 2010;8:1178–1188. doi: 10.3390/md8041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klisch M, Hader DP. Mycosporine-like amino acids and marine toxins—The common and the different. Mar. Drugs. 2008;6:147–163. doi: 10.3390/md20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallela R, Na-Young Y, Kim SK. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs. 2010;8:1189–1202. doi: 10.3390/md8041189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Ayala GG, Malinconico M, Laurienzo P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules. 2008;13:2069–2106. doi: 10.3390/molecules13092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellmann R, Stuken A, Orr RJS, Svendsen HM, Jakobsen KS. Biosynthesis and molecular genetics of polyketides in marine Dinoflagellates. Mar. Drugs. 2010;8:1011–1048. doi: 10.3390/md8041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souza ET, Lira DP, Queiroz AC, Silva DJC, Aquino AB, Mella EAC, Lorenzo VP, Miranda GEC, Araújo-Júnior JX, Chaves MCO, et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs. 2009;7:689–704. doi: 10.3390/md7040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guven KC, Percot A, Sezik E. Alkaloids in marine algae. Mar. Drugs. 2010;8:269–284. doi: 10.3390/md8020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrita MT, Vale C, Rauter AP. Halogenated compounds from marine algae. Mar. Drugs. 2010;8:2301–2317. doi: 10.3390/md8082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Barre S, Potin P, Leblanc C, Delage L. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs. 2010;8:988–1010. doi: 10.3390/md8040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ianora A, Boersma M, Casotti R, Fontana A, Harder J, Hoffmann F, Pavia H, Potin P, Poulet SA, Toth G. New trends in marine chemical ecology. Estuaries Coasts. 2006;29:531–551. [Google Scholar]
- 27.Deig EF, Ehresmann DW, Hatch MT, Riedlinger DJ. Inhibition of herpesvirus replication by marine algae extracts. Antimicrob. Agents Chemother. 1974;6:524–525. doi: 10.1128/aac.6.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jha RK, Zi-rong X. Biomedical compounds from marine organisms. Mar Drugs. 2004;2:123–146. [Google Scholar]
- 29.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 30.Lindequest U, Schweder T. Marine biotechnology. In: Rehm HJ, Reed G, editors. Biotechnology. Vol. 10. Wliey-VHC; Weinheim, Germany: 2001. pp. 441–484. [Google Scholar]
- 31.Linsert P. Revolution in infant formula brewing in Herman’s calves. DNA algae. Genet. Eng. Biotechnol. Monit. 1994;1:45–46. [Google Scholar]
- 32.Khotimchenko SV, Vaskovsky VE, Titlyanavo TV. Fatty acids of marine algae from the 12 substances from marine algae Puerto Rico. Antimicrob. Agents Chemother. 1963;161:68–72. [PubMed] [Google Scholar]
- 34.Ryther JH, Goldman JC, Gifford CE. Physical models of integrated waste recycling marine polyculture systems. Aquaculture. 1975;5:163–177. [Google Scholar]
- 35.Capo TR, Jaramillo JC, Boyd AE, Lapointe BE, Serafy JE. Sustained high yields of Gracilaria (Rodophyta) grown in intensive large-scale culture. J. Appl. Phycol. 1999;11:143–147. [Google Scholar]
- 36.Guiry MD. AlgaeBase. Martin Ryan Institute, National University of Ireland; Galway, Ireland: 1996–2011. [accessed on 14 May 2011]. Available online: http://www.algaebase.org. [Google Scholar]
- 37.Skriptsova AV, Titlyanova TV, Titlyanov EA. Red algae of the genus Gracilaria in south of the Russian far east. Russ. J. Mar. Biol. 2001;27:S38–S52. [Google Scholar]
- 38.Kain JM, Destombe C. A review of the life history, reproduction and phenology of Gracilaria. J. Appl. Phycol. 1995;7:69–281. [Google Scholar]
- 39.Misawa M. Production of natural substances by plant cell cultures described in japanese patents. In: Barz W, Reinhard E, Zenk MH, editors. Plant Tissue Culture Its Bio-Technol. Springer-Verlag; Berlin, Germany: 1977. pp. 17–26. [Google Scholar]
- 40.Murano E, Toffanin R, Paoletti S, Rizzo R. Pyruvate-rich agarose from the red alga Gracilaria dura. Planta Med. 1992;58:A588–A589. [Google Scholar]
- 41.Hemmingson JA, Furneaux RX, Murray-brown VH. Biosynthesis of agar polysaccharides in Gracilaria chilensis bird. Carbohydr. Res. 1996;287:101–115. [Google Scholar]
- 42.Brasch DJ, Chuah CT, Melton LD. Marine algal polysaccharides, Part 2. The agar-type polysaccharide from the red alga Gracilaria secundata. Carbohydr. Res. 1983;115:191–198. [Google Scholar]
- 43.Glombitza KW. In: Marine Algae in Pharmaceutical Science. Hoppe HA, Levring T, editors. Vol. 1. Walter de Gruyter; New York, NY, USA: 1979. pp. 303–342. [Google Scholar]
- 44.Glickman M. Utilisation of seaweed hydrocolloids in the food industry. Hydrobiology. 1987;151/152:31–47. [Google Scholar]
- 45.Imbs AB, Vologodskaya AV, Nevshupova NV, Khotimchenko SV, Titlyanoy EA. Response of prostaglandin content in the red alga Gracilaria verrucosa to season and solar irradiance. Phytochemistry. 2001;58:1067–1072. doi: 10.1016/s0031-9422(01)00321-1. [DOI] [PubMed] [Google Scholar]
- 46.Chapman VJ, Chapman DJ. Seaweeds and Their Uses. Springer-Verlag; Berlin, Germany: 1980. [Google Scholar]
- 47.Sajiki J. Effect of acetic acid treatment on the concentrations of arachidonic acid and prostaglandin E2 in the red algae, Gracilaria asiatica and G. rhodocaudata. Fish Sci. 1997;63:128–131. [Google Scholar]
- 48.Fusetani N, Hashimoto K. Prostaglandin E2: A candidate for causative agent of “Ogonori” poisoning. Bull. Jpn. Soc. Sci. Fish. 1984;50:465–469. [Google Scholar]
- 49.Yotsu-yamashita M, Haddock RL, Yasumoto T. Polycavernoside A: A novel glycosidic macrolide from the red alga Polycavernosa tsudai (Gracilaria edulis) J. Am. Chem. Soc. 1993;115:1147–1148. [Google Scholar]
- 50.Das B, Srinivas KVNS. Minor C29-steroids from the marine red alga, Gracilaria edulis. Phytochemistry. 1992;31:2427–2429. [Google Scholar]
- 51.Das B, Srinivas KVNS. Dihydroxysterols from the marine red alga, Gracilaria edulis. Phytochemistry. 1992;31:4731–4373. [Google Scholar]
- 52.Combres A, Bianchini JP, Gaydou EM. Fatty acid and sterol composition of red algae of the Indian ocean. Oceanol. Acta. 1986;9:339–342. [Google Scholar]
- 53.Toffanin R, Murano E, Modricky C, Kvam BJ, Paoletti S, Rizzo R, Pollesello P. Free and acylated cholesterol in the red alga Gracilaria longa: detection and quantification by 1H- and 13C-NMR on lipid extracts. Planta Med. 1992;58:A589–A590. [Google Scholar]
- 54.Castedo L, Quintela JM, Vilalta R. Sterols from red and brown algae from the Galician coast. An. Quim. Ser. C. 1985;8:113–115. [Google Scholar]
- 55.Vilalta R, Quintela JM, Riguera R, Castedo L. Natural marine products from algae and cnidaria of the Galician estuaries. Cuad. Area Cienc. Mar. 1984;2:617–625. [Google Scholar]
- 56.Henriquez P, Trucco R, Silva M, Sammes PG. Cholesterol in Iridaea laminarioides and Gracilaria verrucosa. Phytochemistry. 1972;11:1171. [Google Scholar]
- 57.Anonymous. Prostaglandin E-2. Patent-Japan Kokai Tokkyo Koho. 1982;59:73–565. [Google Scholar]
- 58.Araki S, Sakurai T, Oohusa T, Kayama M. Component fatty acid of lipid from Gracilaria verrucosa. Bull. Jpn. Soc. Sci. Fish. 1986;52:1871–1874. [Google Scholar]
- 59.Son BW. Glycolipids from Gracilaria verrucosa. Phytochemistry. 1990;29:307–309. [Google Scholar]
- 60.Das B, Srinivas KVNS. Two new sterols from the marine red alga Gracilaria edulis. Planta Med. 1993;59:572–573. doi: 10.1055/s-2006-959768. [DOI] [PubMed] [Google Scholar]
- 61.Sajiki J, Hakimi H. Identification of eicosanoids in the red algae, Gracilaria asiatica, using high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Chromatogr. 1998;795:227–237. doi: 10.1016/s0021-9673(97)00943-6. [DOI] [PubMed] [Google Scholar]
- 62.Ravi BN, Faulkner DJ. Acyclic diterpenes from the marine sponge Didiscus sp. J. Org. Chem. 1979;44:968–970. [Google Scholar]
- 63.Sims JJ, Pettus JA., Jr Isolation of free cis and trans-phytol from the red alga Gracilaria andersoniana. Phytochemistry. 1976;15:1076–1077. [Google Scholar]
- 64.Nagai H, Yasumoto T, Hokama Y. Manauealides, some of the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. J. Nat. Prod. 1997;60:925–928. doi: 10.1021/np970193c. [DOI] [PubMed] [Google Scholar]
- 65.Nagai H, Yasumoto T, Hokama Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. Toxicon. 1996;34:753–761. doi: 10.1016/0041-0101(96)00014-1. [DOI] [PubMed] [Google Scholar]
- 66.Yotsu-yamashita M, Seki T, Paul VJ, Naoki H, Yasumoto T. Four new analogs of polycavernoside A. Tetrahedron Lett. 1995;36:5563–5566. [Google Scholar]
- 67.D’agnolo E, Rizzo R, Paoletti S, Murano E. R-phycoerythrin from the red alga Gracilaria longa. Phytochemistry. 1994;35:693–696. [Google Scholar]
- 68.Talarico L, Kosovel V. Properties and ultrastructures of r-phycoerythrin from Gracilaria verrucosa. Photosynthetica. 1978;12:369–374. [Google Scholar]
- 69.Wilcox SJ, Bloor SJ, Hemmingson JA, Furneaux RH, Nelson WA. The presence of gigartinine in a New Zealand Gracilaria species. J. Appl. Phycol. 2001;13:409–413. [Google Scholar]
- 70.Kanoh H, Kitamura T, Kaboyashi Y. A sulfated proteoglycan from the red alga Gracilaria verrucosa is a hemagglutinin. Comp. Biochem. Physiol. B. 1992;102:445–449. doi: 10.1016/0305-0491(92)90034-o. [DOI] [PubMed] [Google Scholar]
- 71.Kirkpatrick P. Antibacterial drugs. Stitching together naturally. Nat. Rev. Drug Discovery. 2002;1:748–748. [Google Scholar]
- 72.Kosovel V, Avanzini A, Scarcia V, Furlani A. Algae as possible sources of antitumoural agents: Preliminary evaluation of the “in vitro” cytostatic activity of crude extracts. Pharmacol. Res. Commun. 1988;20:27–31. doi: 10.1016/s0031-6989(88)80834-8. [DOI] [PubMed] [Google Scholar]
- 73.Kamat SY, Wahidulla S, D’Souza L, Naik CG, Ambiye V, Bhakuni DS, Goel AK, Garg HS, Srimal RC. Bioactivity of marine organisms. VI. Antiviral evaluation of marine algal extracts from the Indian Coast. Bot. Mar. 1992;35:161–164. [Google Scholar]
- 74.Lhullier C, Horta PA, Falkenberg M. Avaliação de extratos de macroalgas bênticas do litoral catarinense utilizando o teste de letalidade para Artemia salina. Rev. Bras. Farmacogn. 2006;16:158–163. [Google Scholar]
- 75.Bhakuni DS, Dhawan BN, Garg HS, Goel AK, Mehrotra BN, Srimal RC, Srivastava MN. Bioactivity of marine organisms: Part VI-Screening of some marine flora from Indian coasts. Indian J. Exp. Biol. 1992;30:512–517. [PubMed] [Google Scholar]
- 76.Chenieux JC, Verbist JF, Biard JF, Clement E, Le Boterff J, Maupas P, Lecocq M. Seaweeds of French atlantic coast with antimitotic activity. Planta Med. 1980;40:152–162. [PubMed] [Google Scholar]
- 77.Arisawa M, Hayashi K, Nikaido T, Koike K, Fujita D, Nunomura N, Tanaka M, Sasaki T. Screening of some marine organism extracts for camp phosphodiesterase inhibition, cytotoxicity, and antiviral activity against HSV-1. Int. J. Pharmacogn. 1997;35:6–11. [Google Scholar]
- 78.Numata A, Kanbara S, Takahashi C, Fujiki R, Yoneda M, Fujita E, Nabeshima Y. Cytotoxic activity of marine algae and a cytotoxic principle of the brown alga Sargassum tortile. Chem. Pharm. Bull. 1991;39:2129–2131. doi: 10.1248/cpb.39.2129. [DOI] [PubMed] [Google Scholar]
- 79.Avanzini A, Kosovel V, Scarcia V, Furlani A, Ravalico L. Green, red and brown algae from North Adriatic sea as source of possible cytotoxic products. Fitoterapia. 1987;58:391–394. [Google Scholar]
- 80.Ratnasooriya WD, Premakumara GAS, Tillekeratne LMV. Post-coital contraceptive activity of crude extracts of Sri Lankan marine red algae. Contraception. 1994;50:291–299. doi: 10.1016/0010-7824(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 81.Okada Y, Miyauch N, Suzuki K, Kobayashi T, Tsutsui C, Mayuzumi K, Okuyama T. Search for naturally occurring substances for prevention against the complications of diabetes; inhibitory effect on aldose reductase and platelet aggregation. Nat. Med. 1994;48:324–329. doi: 10.1248/cpb.43.1385. [DOI] [PubMed] [Google Scholar]
- 82.Yoshizawa Y, Tsunehiro J, Nomura K, Itoh M, Fukui F, Ametani A, Kaminogawa S. In vivo macrophage-stimulation activity of the enzyme-degraded water-soluble polysaccharide fraction from a marine alga (Gracilaria verrucosa) Biosci. Biotechnol. Biochem. 1996;60:1667–1671. doi: 10.1271/bbb.60.1667. [DOI] [PubMed] [Google Scholar]
- 83.Choi JS, Lee JH, Park HJ, Kim HG, Young HS, Mun SI. Screening for antioxidant activity of plants and marine algae and its active principles from Prunus davidiana. Korean J. Pharmacogn. 1993;24:299–303. [Google Scholar]
- 84.Funayama S, Hikino H. Hypotensive principles from plants. Heterocycles. 1981;15:1239–1256. [Google Scholar]
- 85.Gregson RP, Marwood JF, Quinn RJ. The occurrence of prostaglandins PGE2 and PGF2α in a plant-the red alga Gracilaria lichenoides. Tetrahedron Lett. 1979;20:4505–4506. [Google Scholar]
- 86.Choi BW, Lee BH, Kang KJ, Lee ES, Lee NH. Screening of the tyrosinase inhibitors from marine algae and medicinal plants. Korean J. Pharmacogn. 1998;29:237–242. [Google Scholar]
- 87.Sridhar P, Lakshmi VV, Polasa H, Reddy VS, Rao CHP, Srimannarayana G. Biological activity of some marine algal extracts. Indian J. Mar. Sci. 1984;13:90–91. [Google Scholar]
- 88.Bitou N, Inomiya M, Tsujita T, Okuda H. Screenning of lipase inhibitors from marine algae. Lipids. 1999;34:441–445. doi: 10.1007/s11745-999-0383-7. [DOI] [PubMed] [Google Scholar]
- 89.Perez RM, Avila JG, Perez S, Martinez A, Martinez G. Antimicrobial activity of some American algae. J. Ethnopharmacol. 1990;29:111–116. doi: 10.1016/0378-8741(90)90104-2. [DOI] [PubMed] [Google Scholar]
- 90.Salleh A, Wati Haron N, Mahmud N, Mohammad J. Distribution of pyrimidine derivatives in algae. Biochem. Syst. Ecol. 1994;22:860. [Google Scholar]
- 91.Usmanghani K, Shameel M, Sualeh M, Khan KH, Mahmood ZA. Antibacterial and antifungal activities of marine algae from Karachi seashore of Pakistan. Fitoterapia. 1984;55:73–77. [Google Scholar]
- 92.Albuquerque MR, Campos-Takaki, Koening ML. Detection of antimicrobial activity in marine seaweeds. Rev. Inst. Antibiot. Univ. Fed Pernambuco Recife. 1983;21:127–138. [Google Scholar]
- 93.Oranday MA, Verde MSJ, Martínez-Lozano NH, Waksman J. Active fractions from four species of marine algae. Phyton. 2004;73:165–170. [Google Scholar]
- 94.Lustigman B, Lee LH, Thees N, Masucci J. Production of antibacterial substances by macroalgae of the New York/New Jersey coast, USA. Bull. Environ. Contam. Toxicol. 1992;49:743–749. doi: 10.1007/BF00200789. [DOI] [PubMed] [Google Scholar]
- 95.Couladis M, Vagias C, Roussis V, Verykokidou E, Skaltsa H. Antiphage properties of some Greek algae extracts. Phytomedicine. 1998;5:479–483. doi: 10.1016/S0944-7113(98)80046-9. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi K, Hamada J, Hayashi T. A screening strategy for selection of anti-HSV-1 and anti- HIV extracts from algae. Phytother. Res. 1996;10:233–237. [Google Scholar]
- 97.Kamat SY, Wahidulla S, D’Souza L, Naik CG, Ambiye V, Bhakuni DS, Goel AK, Garg HS, Srimal RC. Bioactivity of marine organisms. VI. Antiviral evaluation of marine algal extracts from the Indian Coast. Bot. Mar. 1992;35:161–164. [Google Scholar]
- 98.Nakamura H, Ohnuki N, Sadamasu K, Sekine H, Tanaka J, Okada Y, Okuyama T. Anti-human immunodeficiency virus (HIV) activities of aqueous extracts from marine algae. Nat. Med. 1994;48:173–179. [Google Scholar]
- 99.Ohigashi H, Sakai Y, Yamaguchi K, Umezaki I, Koshimizu K. Possible anti-tumor promoting properties of marine algae and in vivo activity of wakame seaweed extract. Biosci. Biotechnol. Biochem. 1992;56:994–995. doi: 10.1271/bbb.56.994. [DOI] [PubMed] [Google Scholar]
- 100.Hsu B-Y, Tsao C-Y, Chiou T-K, Hwang D-F. Factors affeecting PGE2 production in seaweed Gracilaria tenuistipitata. J. Food Drug Anal. 2008;16:59–65. [Google Scholar]
- 101.Laserna EC, Veroy RL, Luistro AH, Cajipe GJB. Extracts from some red and brown seaweeds of the Philippines. In: Levring T, editor. Tenth International Seaweed Symposium. Walter de Gruyter & Co; Berlin, Germany: 1980. pp. 443–448. [Google Scholar]
- 102.Nagai H, Kan Y, Fujita T, Sakamoto B, Kokama Y. Manauealide C and anhydrodebromoaplysiatoxin, toxic constituents of the hawaiian red alga, Gracilaria coronopifolia. Biosci. Biotechnol. Biochem. 1998;62:1011–1013. doi: 10.1271/bbb.62.1011. [DOI] [PubMed] [Google Scholar]
- 103.Parekh KS, Parekh HH, Rao PS. Fatty acid content of some Indian marine algae. Indian J. Mar. Sci. 1984;13:45–46. [Google Scholar]
- 104.Kan Y, Fujita T, Nagai H, Sakamoto B, Hokama Y. Malyngamides M and N from the hawaiian red alga Gracilaria coronopifolia. J. Nat. Prod. 1998;61:152–155. doi: 10.1021/np970423n. [DOI] [PubMed] [Google Scholar]
- 105.Parra AL, Yhebra RS, Sardiñas IG, Buela LI. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 106.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituints. Planta Med. 1982;45:31–34. [PubMed] [Google Scholar]
- 107.Kanazawa A, Yoshioka M. Occurrence of Cholest-4-en-3-one in the Red Alga. Gracilaria textorii Proceedings of the Seventh International Seaweed Symposium; Sapporo, Japan. 1–12 August 1971; New York, NY, USA: Wiley; 1972. pp. 502–505. [Google Scholar]
- 108.Kunzler K, Eichenberger W. Betaine lipids and zwitterionic phospholipids in plants and fungi. Phytochemistry. 1997;46:883–892. doi: 10.1016/s0031-9422(97)81274-5. [DOI] [PubMed] [Google Scholar]
- 109.Roh YS, Son W, Im KS, Choi HD. Structure of floridoside, a glycerol glycoside from the marine red alga Gracilaria verrucosa. Korean J. Pharmacogn. 1994;25:117–120. [Google Scholar]
- 110.Laserna EC, Veroy RL, Luistro AH, Cajipe GJB. Extracts from some red and brown seaweeds of the Philippines. In: Levring T, editor. Tenth International Seaweed Symposium. Walter de Gruyter & Co; Berlin, Germany: 1980. pp. 443–448. [Google Scholar]
- 111.Matsuhashi T. Effects of the freezing and drying method and the mechanical dehydration method by pressure on gel properties of dried agar. Reito. 1974;49:756–760. [Google Scholar]
- 112.Shi SY, Zhang YX, Liu WQ, Li z. Seasonal variation in yield, physical properties and chemical composition of agar from Gracilaria verrucosa. Oceanol. Limnol. Sin. 1983;14:272–278. [Google Scholar]
- 113.Friedlander M, Lipkin Y, Yaphe W. Composition of agars from Gracilaria verrucosa and Pterocladia capillacea. Bot. Mar. 1981;24:595–598. [Google Scholar]
- 114.Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog. Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 115.Nishihara I, Minami T, Uda R, Ito S, Hyodo M, Hayaishi O. Effect of NMDA receptor antagonists on prostaglandin E2-induced hyperalgesia in conscious mice. Brain Res. 1995;677:138–144. doi: 10.1016/0006-8993(95)00133-b. [DOI] [PubMed] [Google Scholar]
- 116.Aihara MS, Yamamoto H. Occurrence of antheraxanthin in two Rhodophyceae Acanthophora spicifera and Gracilaria lichenoides. Phytochemistry. 1968;7:497–499. [Google Scholar]
- 117.Tuney I, Çadirci BH, Unal D, Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla. Turk. J. Biol. 2006;30:171–175. [Google Scholar]
- 118.Aihara MS, Yamamoto H. Occurrence of antheraxanthin in two Rhodophyceae Acanthophora spicifera and Gracilaria lichenoides. Phytochemistry. 1968;7:497–499. [Google Scholar]
- 119.Panossian AG. Search of prostaglandins and related compounds in plants: A review of the occurrence of prostaglandins and prostaglandin-like compounds in plants. prostaglandins. 1987;33:363–381. doi: 10.1016/0090-6980(87)90019-0. [DOI] [PubMed] [Google Scholar]
- 120.Mahasneh I, Jamal M, Kashashneh M, Zibdeh M. Antibiotic-activity of marine-algae against multi-antibiotic resistant-bacteria. Microbios. 1995;83:23–26. [PubMed] [Google Scholar]
- 121.Parekh KS, Parekh HH, Rao PS. Fatty acid content of some Indian marine algae. Indian J. Mar. Sci. 1984;13:45–46. [Google Scholar]
- 122.Fernandez LE, Valiente OG, Mainardi V, Bello JL, Velez H, Rosado A. Isolation and characterization of an antitumor active agar-type polysaccharide of Gracilaria dominguensis. Carbohydr. Res. 1989;190:77–83. doi: 10.1016/0008-6215(89)84148-5. [DOI] [PubMed] [Google Scholar]
- 123.Okamoto R, Hori K, Miyazawa K, Ito K. Isolation and charcterization of a new hemagglutinin from the red alga Gracilaria bursa-pastoris. Experientia. 1990;46:975–977. doi: 10.1007/BF01939393. [DOI] [PubMed] [Google Scholar]
- 124.Laycock MV, Craigie JS. The occurrence and seasonal variation of gigartinine and l-citrullinyl-l-arginine in Chondrus crispus stackh. Can. J. Biochem. 1977;55:27–30. doi: 10.1139/o77-004. [DOI] [PubMed] [Google Scholar]