Abstract

Hypoxia is a well-described phenomenon in the offshore waters of the Baltic Sea with both the spatial extent and intensity of hypoxia known to have increased due to anthropogenic eutrophication, however, an unknown amount of hypoxia is present in the coastal zone. Here we report on the widespread unprecedented occurrence of hypoxia across the coastal zone of the Baltic Sea. We have identified 115 sites that have experienced hypoxia during the period 1955–2009 increasing the global total to ca. 500 sites, with the Baltic Sea coastal zone containing over 20% of all known sites worldwide. Most sites experienced episodic hypoxia, which is a precursor to development of seasonal hypoxia. The Baltic Sea coastal zone displays an alarming trend with hypoxia steadily increasing with time since the 1950s effecting nutrient biogeochemical processes, ecosystem services, and coastal habitat.
Introduction
Deterioration in ecological integrity of the coastal zone due to human-induced increases in chemical nutrient runoff has produced a series of classic ecosystem changes mostly impacting primary producer communities including changes in phytoplankton species composition, increases in harmful algal blooms (HABs), and habitat losses of seagrasses. However, the lack of oxygen in bottom waters, which has increased drastically over a short period of time with anthropogenic nutrient enrichment,1,2 is one of the most deleterious effects on the coastal marine environment with immediate large-scale impacts on the entire ecosystem.
Hypoxia, oxygen concentrations less than 2 mg L–1, kills bottom-living organisms(3) altering benthic faunal communities and impairing fish habitat. In addition, the consequences of hypoxia on nutrient biogeochemical cycles are substantial4,5 with low oxygen concentrations leading to the increased release of phosphorus from sediments6,7 and often reductions in nitrogen losses.8,9 Alteration of nutrient biogeochemical cycles can further increase the negative impacts of nutrient-driven eutrophication through stimulation of phytoplankton as nutrient recycling processes are enhanced.(5)
Dead zones in the coastal zone caused by the lack of oxygen (hypoxia) in bottom waters is a growing global problem,1,2 however, the analysis of the global occurrence of hypoxia has been impeded by the lack of systematic data collection in the marine environment with inadequate data available to establish historical trends. Hypoxia in the off-shore deep waters of the Baltic Sea is well-described,10−12 but our knowledge of hypoxia in the coastal zone is lacking, despite that the Baltic Sea is one of the most data rich regions in the world. To fill this knowledge gap, we compiled coastal monitoring and research data from the coastal countries around the Baltic Sea to identify areas where hypoxia occurs and its frequency of occurrence.
Methods
Known sources of data were compiled to determine the status of oxygen in the coastal zone of the Baltic Sea (Supporting Information (SI) Figure S1 and Table S1) and are archived in the Baltic Environmental Database (BED; http://nest.su.se/models/bed.htm) of the Baltic Nest Institute, Stockholm University. The data were partitioned into different areas, largely following the regional divisions from HELCOM (SI Figure S1). The number of profiles by year and region, constituted by discrete water samples (typically 2–5 samples) or continuous CTD data, gradually increased to a maximum of ca. 6500 profiles during 1999–2005. Over 2 million records were obtained, which included 163,000 profiles at ca. 3,500 monitoring points. These monitoring points were associated with 613 coastal units or sites representing different estuaries, embayments, and coastal stretches. The average height of the bottom water sample was 0.78 m above the sediments.
Seasonal windows, where hypoxia occurs, were identified from monthly means of oxygen in the bottom layer (SI, Table S2). The frequency of hypoxia was calculated within these region-specific seasonal windows (only for sites represented by monitoring points ≥10 profiles during the seasonal window) and used to partition sites into 4 distinct groups: (1) no hypoxia when <1% of profiles were hypoxic, (2) episodic hypoxia when ≥1% to ≤50% of profiles were hypoxic, (3) seasonal hypoxia when >50% to <80% of profiles were hypoxic, and (4) persistent hypoxia when ≥80% of profiles were hypoxic. Trend analyses in bottom water oxygen concentrations during the region-specific seasonal window were carried out for sites with hypoxia (episodic, seasonal, and persistent) and at least 10 years of data (118 sites in total) by means of a generalized linear mixed model (GLMM) with year as a linear fixed effect, month as a fixed factor, and monitoring points within sites as a random factor. Residuals from the GLMM analyses were approximately normally distributed with a slight tendency to right skewness (skewness = 0.3317) and heavy tails (kurtosis = 0.2197). However, given the relatively large sample sizes the strict normality assumption is less critical for the test statistics (c.f., Central Limit Theorem). To assess the generality of the site-specific trend the interaction between year and monitoring points was included as a random factor, however this was possible for only 20 sites, but none of these sites showed significant random variation in the trend across monitoring points within the same site. Therefore, trend inference was made for entire coastal units summarized across monitoring points to sites.
Results
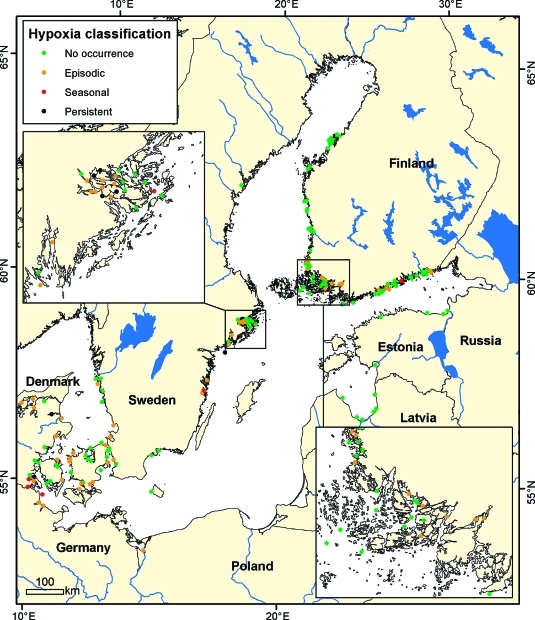
Hypoxia occurred in at least 115 sites during the period of record from 1955 to 2009 (Figure 1). Of the 326 sites with sufficient data (≥ 10 profiles), 65% had not experienced hypoxia, 30% experienced episodic hypoxia, 4.0% were seasonally hypoxic, and 1.5% were persistently hypoxic (Table 1). During the past decade (2000–2009) there were a reduced number of sites with sufficient data available (169 sites) with a similar proportion of observations of hypoxia type, e.g., 63% of the sites have not experienced hypoxia, 30% have experienced episodic periods of hypoxia, 3.6% are seasonally hypoxic, with 4.1% persistently hypoxic (Figure 2; Table 1). There are six regional areas of the Baltic Sea coastal zone (SI Figure S1) where hypoxia is recorded in at least 5% of the profiles in decreasing order: the Western Gotland Basin < Belt Seas < Finnish Archipelago Sea< The Kattegat < Limfjorden < Stockholm Archipelago, with frequencies ranging from 5% to 15% (SI Figure S2).
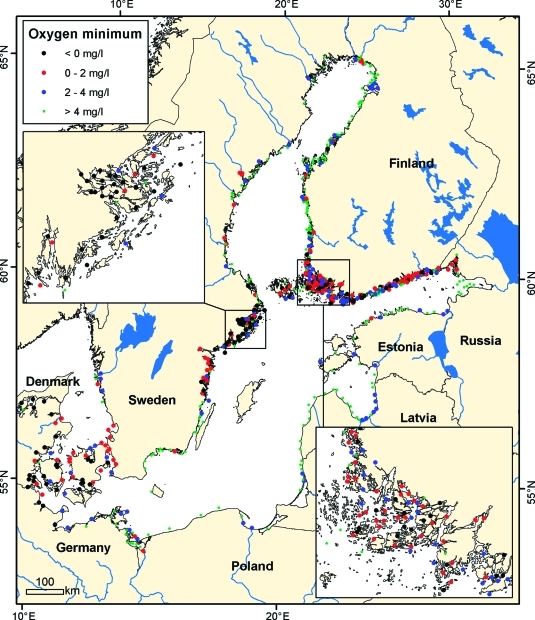
Figure 1.
Lowest recorded oxygen concentration at all monitoring locations throughout the period (1955–2009) in the entire Baltic Sea with insets for the Stockholm Archipelago (upper left) and the Finnish Archipelago Sea (lower right). Oxygen concentrations in bottom waters was divided into four categories (<0 mg L–1, 0–2 mg L–1, 2–4 mg L–1, >4 mg L–1). Oxygen concentrations <0 mg L–1 are anoxic, 0–2 mg L–1 are considered hypoxic by definition, and 2–4 mg L–1 are considered oxygen stressed.(26)
Table 1. Number of Cases for Monitoring Points Observed for Different Categoriesa.
| all data | 2000–2009 | all data (%) | 2000–2009 (%) | |
|---|---|---|---|---|
| no occurrence | 211 | 106 | 65 | 63 |
| episodic | 97 | 50 | 30 | 30 |
| seasonal | 13 | 6 | 4.0 | 3.6 |
| persistent | 5 | 7 | 1.5 | 4.1 |
No occurrences: <1% probability of hypoxia at any time. Episodic: ≥1% and ≤50% probability of hypoxia. Seasonal: >50% probability of hypoxia. Persistent: >80% probability. All frequencies of hypoxia calculated within region-specific seasonal windows (SI Table S2).
Figure 2.
Classification of monitoring locations into different hypoxia categories after 2000 in the Baltic Sea with insets for the Stockholm Archipelago (upper left) and the Finnish Archipelago Sea (lower right). Different categories included the following: Never, e.g., where <1% of the observations in the data were hypoxic, persistent hypoxia where 80% or greater of all observations were hypoxic, seasonal hypoxia where 50% to 80% of observations during defined periods were hypoxic (see seasonal window definition SI, Table S2), and episodic hypoxia that comprise the remainder of the observations.
Seasonal patterns in water column oxygen concentrations were observed across the entire coastal zone (SI Figure S3) with the peak months for the occurrence of hypoxia in August and September in all regions. Hypoxia occurred primarily at the deepest depths in the coastal zone of the Bothnian Sea, the Belt Seas, and Limfjorden, whereas in the Finnish Archipelago Sea and the Western Gotland Basin the peak hypoxic areas occurred at depths ranging from 10 to 40 m (SI Figure S4) and not at the deepest sites.(6) In the shallow and isolated basins of the Stockholm Archipelago nearly 50% of the hypoxic profiles occurred at depths of 10–15 m.
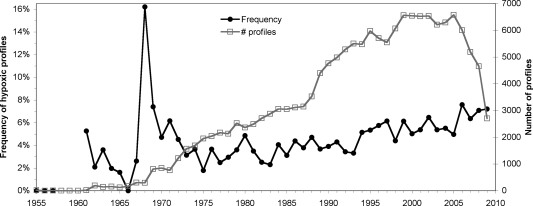
The total numbers of hypoxic profiles by year and region were variable, but overall there was a significant long-term increase in hypoxic profiles. The overall frequency of hypoxia increased, as determined from the number of profiles that have recorded occurrences of hypoxia over time, with ca. 5% of all profiles measured in 2009 hypoxic (Figure 3). The peak occurrence in 1968 was due to the addition of many hypoxic profiles into the database from the hypoxia-prone Stockholm Archipelago. Most regions do not show long-term trends in oxygen concentrations with time, however, the coastal zone of the Bothnian Sea and the Gulf of Finland both show decreases in oxygen concentrations from the 1970s to the present (SI Figure S5). By contrast, the offshore waters of the Baltic Sea have shown large annual variations in hypoxic volume through the last 50 years partly related to salt water inputs4,10 with no long-term trends in hypoxic volume.
Figure 3.
Number of profiles over time for the entire Baltic Sea and frequency of hypoxia calculated as the number of profiles with recorded hypoxia (<2 mg L–1) relative to the total number of profiles.
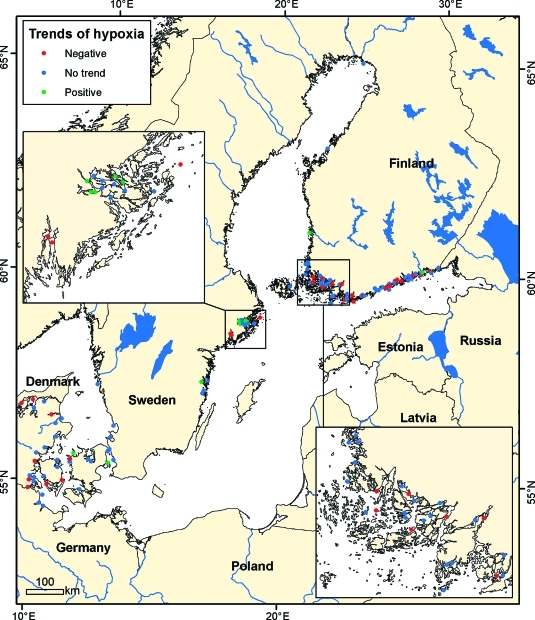
Improvements in oxygen status have also been observed in 10 sites out of a total of 118 sites with at least 10 years of data, whereas oxygen concentrations have declined in 32 sites, and 76 sites showed no trend (Figure 4). The occurrence of hypoxia during the earlier part of the monitoring records was often associated with oxygen demand from paper mill effluents,(13) especially in the northern Bothnian Sea and Bothnian Bay. Although upgrading of effluent treatment from paper mills began in the late 1960s onward, which improved oxygen conditions greatly in most places, degradation of sediment stores of oxygen-demanding wood fibers continued to sustain hypoxia for several decades. Oxygen concentrations in the inner Stockholm Archipelago have also improved with more than half of the sites showing significant increases (Figure 4), consistent with data on sediment laminations with marked improvements in the inner and middle Stockholm Archipelago during the past decade.(13)
Figure 4.
Trends in the hypoxia from the entire period for locations with sufficient data for classification with insets for the Stockholm Archipelago (upper left) and the Finnish Archipelago Sea (lower right).
Discussion
The two most important factors leading to the development of hypoxia are biological processes that determine the amount of organic matter available to be degraded and physical factors creating stratification. Stratification in most areas of the coastal zone of the Baltic Sea is due to seasonal temperature changes, although stratification by occasional inflowing saltier water does occur in some estuaries.(14) Hypoxia is common in estuaries located in the Danish Straits due to the large load of nutrients sustaining algal production and the strong stratification caused by large differences in surface and bottom water salinity.(5) Hypoxia in the Swedish and Finnish archipelagos are influenced by phytoplankton growth stimulated by nutrient loads from urban and agricultural sources, but also by restricted water circulation. The Finnish Archipelago Sea is impacted by drifting algal mats that consume oxygen during their decomposition when they sink to the bottom.(15) By contrast, hypoxia is rare in the northern Baltic Sea estuaries located in the Bothnian Sea coastal zone where nutrient loads are lower. Hypoxia is uncommon along the eastern shore from Estonia to Poland due to enhanced circulation of water in open areas along the coastline (Figure 1).
There are very limited data available to examine how far back in time hypoxia has occurred. A key question in this ecosystem affected daily by over 85 million people could be “Is hypoxia a natural phenomenon?”.(16) The large numbers of enclosed areas, in many cases with shallow sills that restrict the exchange of bottom water and often in combination with large irregular changes in salinity, might mean that hypoxia has always been present in the coastal zone. However, sediment laminations from Stockholm Archipelago, an indicator of past hypoxia,(17) have been shown to be a recent phenomenon with very few areas with laminated sediments prior to 1900.17,18 The long-term millennial trends in coastal hypoxia remain unknown, although hypoxia has occurred intermittently throughout the last 8000 years in offshore deep waters of the Baltic Sea.(10) Further sediment studies are necessary, similar to those carried out in the open Baltic Sea,(10) to ascertain how far back in time hypoxia was observed in the coastal zone. Key uncertainties remain including how have the driving forces for hypoxia, e.g., climate(19) and nutrient loading, changed through time in the Baltic Sea coastal zone, and do they vary during the same time periods that hypoxia varies in the open waters?(16)
An important consideration in determining the number of ecosystems experiencing hypoxia is the size of the assessment unit. We combined numerous monitoring points and sites to form a coherent coastal unit when bottom waters were physically connected to each other. This means that assessment units considered hypoxic might occur in the same geographical region and could be in close proximately to each other, if their bottom waters were physically disconnected. The Baltic Sea archipelagos have a complex topography with many basins disjoined by shallow sills resulting in many distinct sites, although they are all affected by similar mechanisms. In fact, the Stockholm and Finnish archipelagos contributed 21% of the total assessment units, a relatively large share compared to the total Baltic Sea coastal zone, but >40% of the hypoxic sites. The coastal stretches of the Western Gotland Basin and Gulf of Finland that have a substantial number of hypoxic sites are also archipelagos, which are more prone to hypoxia because of the complex topography, proximity to development, and consequently more sensitive to enhanced nutrient inputs from land.
Surprisingly only 4.0% of all sites were classified as seasonally hypoxic according to our definition when >50% and <80% of profiles during the stratified period have oxygen concentrations less than 2 mg L–1. A statistically rigorous definition of what constitutes seasonal hypoxia does not currently exist and in fact should be linked to ecological consequences for it to be ecologically relevant and not just connected to absolute concentrations.(3) Currently coastal marine ecosystems that experience hypoxia for a period of days to months every year are often considered to be seasonally hypoxic, although we have categorized them here as being episodic, because we required the monitoring units to be hypoxic for most of the stratified period. However, in Limfjorden, Denmark, bottom water oxygen concentrations can vary greatly between weekly samplings (SI Figure S6), although nearly every year hypoxic conditions are observed somewhere in the Limfjorden. Our statistical estimation is that Limfjorden is episodically hypoxic. Diaz and Rosenberg(1) would classify Limfjorden as periodically hypoxic, although we have found it difficult to create a statistically robust indicator that would classify systems as periodically hypoxic. Periodic hypoxia is problematic because it never allows for re-establishment of benthic communities.(20)
Currently, there are 416 areas in the world with reported coastal hypoxia with about 30 previously reported sites in the Baltic Sea region, which includes both coastal zone hypoxia and deep water sites in the Baltic Sea.(1) We have identified an additional 96 sites that have experienced hypoxia, increasing the global total to nearly 500 sites. Of all the known sites around the world, around 20% of the sites are found in the Baltic Sea region. Most sites experienced episodic hypoxia, which is a precursor to development of seasonal hypoxia.(20) The large number of sites partially reflects the fact that the Baltic Sea is one of the most data rich regions of the world with no tidal mixing and complex bottom topography creating conditions favorable for hypoxia, although these are only the “monitored areas” and many more hypoxic sites probably exist in the Baltic Sea coastal zone. In contrast, there was no evidence of hypoxia at 95 stations in estuarine and coastal waters around Ireland due to sufficient tidal mixing.(21) If such detailed data existed for other coastal regions around the world, it is likely that the number of areas would increase globally.
Oxygen is an important parameter in water quality assessments since sufficient oxygen is essential to aquatic life and is necessary to support healthy biological communities.(3) For example, oxygen concentration is a supporting element to the biological quality elements in the European Water Framework Directive,(21) is one of the ecological objectives in the Helsinki Commission’s (HELCOM) eutrophication assessment of the Baltic Sea, and is included in the European Marine Strategy Framework Directive as an effect parameter of eutrophication. Accurate data for the concentration of dissolved oxygen are essential for documenting environmental changes in water resources resulting from natural phenomena and human activities.
Reports of hypoxia globally are increasing1,22 both due to the recognition of the seriousness of hypoxia on ecosystem functioning and as decades-long monitoring records become available in databases. How the large number of newly identified hypoxic areas influences nutrient biogeochemical cycles and ecosystem services in the coastal zone in the Baltic Sea is currently unknown, although the functioning of the coastal filter certainly plays an important role in how adjacent marine systems respond to changes in nutrient loading. Presently about 40% of the world’s population lives within 100 km of the coastal zone strongly impacting ocean health,(24) including increasing the occurrence of hypoxic areas.1,2 What is currently lacking is the link to the driving factors of hypoxia including nutrient enrichment, organic carbon loading and climate change,(25) and a determination of appropriate nutrient loading targets for guidance to managers to ameliorate the devastating impact of hypoxia.(24)
Acknowledgments
We thank the European Environment Agency (EEA) for providing GIS files to produce the maps. This study was supported by Baltic Sea 2020, the BONUS+ HYPER Project, the Baltic Nest Institute, the Pew Charitable Trust, and FORMAS.
Supporting Information Available
Compilation of all known data sources for oxygen concentrations in the coastal zone of the Baltic Sea (Table S1); identified seasonal windows for calculating trends in oxygen concentrations (Table S2); location of monitoring data used in the study, partitioned into different regions, largely following the regional division from HELCOM (Figure S1); the number of hypoxic profiles relative to the total number of profiles for the different regions of the Baltic Sea over the entire period (1955–2009) (Figure S2); seasonal variation in surface and bottom water oxygen concentration as well as their difference for the 14 regions delimited in Figure S1 (Figure S3); depth-distribution of profiles with hypoxia (<2 mg L–1) relative to the total number of profiles for the 14 regions delimited in Figure S1 (Figure S4); trends in surface and bottom water oxygen concentration as well as their difference for the 14 regions delimited in Figure S1 using the seasonal windows defined in Table S2 (Figure S5); short-term changes in oxygen concentrations in the Limfjorden, Denmark during the summer (Figure S6). This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Díaz R. J.; Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [DOI] [PubMed] [Google Scholar]
- Rabalais N. N.; Díaz R. J.; Levin L. A.; Turner R. E.; Gilbert D.; Zhang J. Dynamics and distribution of natural and human-caused coastal hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar]
- Vaquer-Sunyer R.; Duarte C. M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci., U.S.A. 2009, 105, 15452–15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley D. J.; Humborg C.; Rahm L.; Savchuk O. P.; Wulff F. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 2002, 36, 5315–5320. [DOI] [PubMed] [Google Scholar]
- Vahtera E.; Conley D. J.; Gustafsson B. G.; Kuosa H.; Pitkänen H.; Savchuk O. P.; Tamminen T.; Viitasalo M.; Voss M.; Wasmund N.; Wulff F. Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 2007, 36, 186–194. [DOI] [PubMed] [Google Scholar]
- Virtasalo J. J.; Kohonen T.; Vuorinen I.; Huttula T. Sea bottom anoxia in the Archipelago Sea, northern Baltic Sea - Implications for phosphorus remineralization at the sediment surface. Mar. Geol. 2005, 224, 103–122. [Google Scholar]
- Mort H. P.; Slomp C. P.; Gustafsson B. G.; Andersen T. J. Phosphorus recycling and burial in Baltic Sea sediments with contrasting redox conditions. Geochim. Cosmochim. Acta 2010, 74, 1350–1362. [Google Scholar]
- Kemp W. M.; Testa J.; Conley D. J.; Gilbert D.; Hagy J. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 2009, 6, 2985–3008. [Google Scholar]
- Conley D. J.; Carstensen J.; Ærtebjerg G.; Christensen P. B.; Dalsgaard T.; Hansen J. L. S.; Josefson A. B. Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol. Appl. 2007, 17, S165–S184. [Google Scholar]
- Zillén L.; Conley D. J.; Andrén T.; Andrén E.; Björck S. Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth Sci. Rev. 2008, 91, 77–92. [Google Scholar]
- Conley D. J.; Björck S.; Bonsdorff E.; Carstensen J.; Destouni G.; Gustafsson B. G.; Hietanen S.; Kortekaas M.; Kuosa H.; Meier M.; Müller-Karulis B.; Nordberg K.; Nürnberg G.; Norkko A.; Pitkänen H.; Rabalais N. N.; Rosenberg R.; Savchuk O. P.; Slomp C. P.; Voss M.; Wulff F.; Zillén L. Hypoxia-related processes in the Baltic Sea. Environ. Sci. Technol. 2009, 43, 3412–3420. [DOI] [PubMed] [Google Scholar]
- Fonselius S.; Valderrama J. One hundred years of hydrographic measurements in the Baltic Sea. J. Sea Res. 2003, 49, 229–241. [Google Scholar]
- Karlsson O. M.; Jonsson P.; Lindgren D.; Malmaeus J. M.; Stehn A. Indications of recovery of hypoxia in the Inner Stockholm Archipelago. Ambio 2010, 39, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipsson H.; Nordberg K. Climate variations, an overlooked factor influencing the recent marine environment. An example from Gullmar Fjord, Sweden, illustrated by benthic foraminifera and hydrographic data. Estuaries 2005, 27, 867–881. [Google Scholar]
- Norkko A.; Bonsdorff E. Rapid zoobenthic community responses to accumulations of drifting algae. Mar. Ecol.: Prog. Ser. 1996, 131, 143–157. [Google Scholar]
- Zillén L.; Conley D. J. Hypoxia and cyanobacteria blooms - are they really natural features of the late Holocene history of the Baltic Sea?. Biogeosciences 2010, 7, 2567–2580. [Google Scholar]
- Persson J.; Jonsson P. Historical development of laminated sediments - An approach to detect soft sediment ecosystem changes in the Baltic Sea. Mar. Pollut. Bull. 2000, 40, 122–134. [Google Scholar]
- Jonsson P.Skärgårdens Bottnar; Naturvårdsverket: Stockholm, 2003; Report 5212. [Google Scholar]
- Keeling R. F.; Garcia H. E. The change in oceanic O2 inventory associated with recent global warming. Proc. Natl. Acad. Sci., U.S.A. 2002, 99, 7848–7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz R. J.; Rosenberg R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Ann. Rev. 1995, 33, 245–303. [Google Scholar]
- O’Boyle S.; McDermott G.; Wilkes R. Dissolved oxygen levels in estuarine and coastal waters around Ireland. Mar. Pollut. Bull. 2009, 34, 1657–1663. [DOI] [PubMed] [Google Scholar]
- Rabalais N. N.; Turner R. E.; Díaz R. J.; Justic’ D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar]
- Halpern B. S.; Walbridge S.; Selkoe K. A.; Kappel C. V.; Micheli F.; D’Agrosa C.; Bruno J. F.; Casey K. S.; Ebert C.; Fox H. E.; Fujita R.; Heinemann D.; Lenihan H. S.; Madin E. M. P.; Perry M. T.; Selig E. R.; Spalding M.; Steneck R.; Watson R. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [DOI] [PubMed] [Google Scholar]
- Grantham B. A.; Chan F.; Nielsen K. J.; Fox D. S.; Barth J. A.; Huyer A.; Lubchenco J.; Menge B. A. Upwelling-driven nearshore hypoxia signals ecosystem and oceanographic changes in the northeast Pacific. Nature 2004, 429, 749–754. [DOI] [PubMed] [Google Scholar]
- Scavia D.; Liu Y. Exploring estuarine nutrient susceptibility. Environ. Sci. Technol. 2009, 43, 3474–3479. [DOI] [PubMed] [Google Scholar]
- Ærtebjerg G.; Andersen J. H.; Hansen O. S.. Nutrients and Eutrophication in Danish Marine Waters: A Challenge for Science and Management; Ministry of the Environment: Copenhagen, Denmark, 2005; ISBN 89-7772-728-2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.