Abstract
Stroke has been identified as the second leading cause of death worldwide. Stroke is a focal neurologic deficit caused by a change in cerebral circulation. The use of animal models in recent years has improved our understanding of the physiopathology of this disease. Rats and mice are the most commonly used stroke models, but the demand for larger models, such as rabbits and even nonhuman primates, is increasing so as to better understand the disease and its treatment. Although the basic mechanisms of stroke are nearly identical among mammals, we here discuss the differences between the human encephalon and various animals. In addition, we compare common surgical techniques used to induce animal models of stroke. A more complete anatomic knowledge of the cerebral vessels of various model species is needed to develop more reliable models for objective results that improve knowledge of the pathology of stroke in both human and veterinary medicine.
Abbreviations: EVA, encephalic vascular accident
The success of stroke studies in animals depends on the choice of the experimental model species. This selection must be rigorous because it is the most important aspect of experiment design. An inadequate model may lead to limitations that compromise results and analyses. Furthermore, the extrapolation of results from animal models to humans can be unreliable.14
Four basic types of animal models are referred to in the medical literature: induced, spontaneous, negative, and orphan. The first 2 types are the most important models. As the name suggests, in induced models, a diseased condition is induced experimentally, as in the induction of diabetes mellitus.54 Spontaneous models of human diseases involve animals that naturally present a disease with similar causes and symptoms.54 Several hundred breeds or strains of animals have inherited diseases that display similar conditions to those in humans and therefore have been characterized and maintained.54
Negative models involve a specific disease that inhibits growth, such as gonococcal infection in rabbits, and includes animals that are unable to react when submitted to a specific condition. The most common application of negative models involves studying the mechanism of resistance to achieve a clear understanding of the physiology.14
Orphan models of disease refer to conditions that occur naturally in nonhuman species but have not yet been described in humans. An orphan model is studied when a similar disease is identified in humans.54
Stroke
Stroke is a focal neurologic deficit caused by an alteration in circulation in the encephalon. In the last decade, this term has evolved to include injuries caused by hemodynamic disturbances and coagulation that cannot be detected in arteries or veins.70 Stroke is one of the most prevalent pathologies affecting the CNS. Recent studies indicate that stroke has become the second most common cause of death. Stroke is important for public health reasons because it is the main cause of physical and cognitive incapacities in developing countries.30,12,33 In 2001, stroke was responsible for 5.5 million deaths and 15 million nonlethal brain injuries worldwide; these figures are projected to increase to 6.3 million deaths in 2015 and 7.8 million in 2030.46,67 Stroke lethality is 11% in women and 8.4% in men and is more prevalent among blacks than whites, especially in the younger age groups.45
Of all strokes, 88% are ischemic, 9% involve an intracerebral hemorrhage, and 3% involve a subarachnoid hemorrhage. The most common type of stroke is atherothrombotic brain infarction, which accounts for approximately 61% of all strokes (excluding transient ischemic attacks). The second most common type of stroke is embolic stroke, at 22%.66 Most stroke survivors develop lasting symptoms, such as physical and intellectual limitations, leading to high social costs.
Encephalic vascular accident (EVA) is the newest terminology used to describe stroke, replacing the previous nomenclature of ‘cerebrovascular accident.’56 EVA occurs in 4 different forms: 1) ischemic and transitory, with decreased blood flow and possible recovery after 24 h; 2) ischemic and complete, with neurologic deficits caused by a vascular disturbance for one day or more that remains stable; 3) progressive, with intermittent increases in deficits caused by embolisms or thrombus and 4) hemorrhagic, with ruptured vessels and blood overflow caused by increased intracranial pressure. The main risk factors of EVA are hypertension, obesity, smoking, sedentary lifestyle, stress, and high cholesterol.1
In ischemic EVA, an interruption in cellular oxidative metabolism decreases phosphate and glucose production, liberates neurotransmitters, and decreases levels of calcium and sodium. These factors lead to a reduction in neuronal metabolism and mitochondrial function, energetic insufficiency, formation of arachidonic acid, prostaglandin and leukotrienes, vasoconstriction, plate aggregation and poor microvasculature.15,26,29,44 In hemorrhagic cerebrovascular accidents or EVA, an expansive, acute lesion forms that leads to the destruction, compression, and displacement of encephalic structures; a secondary ischemic lesion around the hematoma may also occur.15,26,29,44
The pathophysiology of cerebral ischemia has been studied in animals with various forms of ischemic lesions. These models have shown that metabolic alterations in reperfusion may lead to cellular lesions in specific brain regions, depending on the duration of the ischemia.15,26,29,44 Regional destruction of the brain is followed by alterations in motor activity.63 Recovery processes begin immediately after the lesion and last for months.63 Even though the recovery process begins gradually after development of the lesion, the motor function present before the lesion will not necessarily be recovered. However, residual functional mechanisms may adapt, demonstrating neuronal plasticity.63
The principal arteries affected by stroke and their clinical symptoms are shown in Figure 1. Clinically, several deficiencies are possible, including deficits in motor function, sensitivity, perception, and language skills. Motor deficiencies are characterized by paralysis (hemiplegia) or weakness (hemiparesis) on the side of the body opposite the lesion. Strokes vary from mild to serious, and the consequences can be either temporary or permanent.53
Figure 1.
Major human arteries and specific clinical symptoms produced after stroke.
The Use of Animal Models in Stroke Research
The use of animal models in recent years has provided a better understanding of the pathophysiologic mechanisms of strokes.60 Numerous animal species have been used to study strokes.60 Mice and rats are the most commonly used species, with a growing use of larger species, such as rabbits and even nonhuman primates, to better study the disease and its treatments.60
However, the applicability of results obtained for animals to the treatment of human diseases has been limited, as occurred with neuroprotection.60 Neuroprotection is an intervention, sometimes involving drug administration, that acts directly on the intracellular mechanisms of the ischemic cascade to affect the area around the stroke. Neuroprotection may decrease the size of the compromised area after an acute ischemic process. Several pharmacologic agents have been effective in animal models but not in humans.21,60
Most variables are tightly controlled in laboratory experiments; therefore, they may not reflect factors contributing to strokes in the human population as a whole. In the laboratory, animals are treated according to a strict protocol after induction of stroke. In contrast, a human patient experiencing an ischemic process may not notice the symptoms or seek medical assistance promptly. Precise and rapid identification of symptoms enables improved treatment options and outcomes.68
Another important difference between animal models and humans is the rigorous control of the animals used.68 Typically, young, healthy, genetically similar animals of the same sex or age groups are used, especially in studies involving rodents. However, such homogeneity does not exist in the human population. The typical stroke patient is elderly, with many risk factors, and may present additional complications, such as diabetes, hypertension, or coronary disease. Age is a primary risk factor for stroke pathologies, and this factor is often overlooked in studies on animals. The use of older animals can provide information about stroke-induced damage and facets of the recovery process that are not well-represented in younger animal models.68 For example, one study used young and old rats to assess the reduction in blood volume due to a cortical infarction after occlusion of the transient middle cerebral artery occlusion.31 Several intergroup differences emerged, including the total volume of affected tissue, edema formation, and functional consequences.31
In 3 databases (Medline, http://www.ncbi.nlm.nih.gov/pubmed/; Lilacs, http://regional.bvsalud.org; SciELO, http://www.scielo.br/), mice were the most commonly used animal model, followed by rats, rabbits, dogs, swine, and primates. Approximately 85% of the articles in Medline and 70.5% of the entries in Lilacs used mice as models.14 The success of stroke research requires parallel studies to identify the best animal model for each form of EVA. Therefore, detailed anatomic knowledge of the encephalic vessels of various species is essential for developing a reliable and useful model of the pathology.
General Encephalic Vasculature
The brain has undergone many structural evolutionary changes.48 As the complexity of the nervous system has increased throughout evolution, the encephalon and arrangement of arterial vessels have also been modified, with a correlation between the evolution of the CNS and modifications in the arrangement of encephalic vessels. The vessels that supply the encephalon constitute the circle of Willis. These arteries include the anterior and posterior cerebral arteries and the anterior and posterior communicating arteries. The vertebral arteries that unite to form the basilar artery also are important to the encephalic blood supply.42 A phylogenetic study in domestic animals demonstrated the diverse arrangements of the multiple arteries constituting the circle of Willis, but these different morphologic features do not necessarily represent evolutionary adaptations.60
In lower vertebrates, the internal carotid artery directed blood to the encephalic mass through the posterior branch without contribution from the basilar artery.7 In higher vertebrates, 2 posterior branches stemmed from a single and central branch that turned into the branch of the basilar artery. Two tiny vertebral arteries have been described, running from the bottom upward and connecting to the terminal portion of the basilar artery at the border between the pons and bulbus.7 In the third phase of evolution, the vertebral artery enlarged to feed the basilar artery, conducting blood to the internal carotid artery that is used during the development of the anterior portion of the brain.7 The basilar artery flowed from bottom to top, and its 2 branches increased in volume and continued into the corresponding posterior cerebral arteries.7 The carotid and basilar arteries are responsible for the blood supply to the brain and are connected by the posterior branches of the carotid artery, which atrophies to form the posterior communicating artery in each antimere.7
Ontogenetic studies64 have shown that the vasculature developmental process followed the evolution of a complex encephalon.7 Despite all of the changes that arterial branches have undergone during development of the encephalon, their vascular territories have remained constant throughout the evolutionary process. Encephalic metabolism requires an adequate supply of glucose and oxygen for correct function and therefore a high rate of blood flow.
The encephalon has a peculiar vasculature; vessels enter at several points and are divided into different circulation territories and return to the bilateral carotid arteries and vertebrobasilar system. These 2 systems have a mutual anastomosis, which is not always functional, through the posterior communicating artery, which connects the internal carotid artery (anterior circulation) and posterior cerebral artery (posterior circulation).9 In the anterior circulation, the internal carotid artery has a larger caliber and more distal branches compared with the anterior cerebral, middle cerebral, posterior communicating, anterior choroidal, and ophthalmic arteries. In the posterior circulation, blood bilaterally reaches the brain by way of the vertebral arteries, which originate on each side of the posterior inferior cerebellar artery and join the groove-level bulbopontine to form the single and medial basilar arteries. These arteries run superior and rostral to the pons to form the bilateral anterior inferior cerebellar, superior cerebellar, and posterior cerebral arteries. By failing to maintain a significant level of anaerobic metabolism, the brain is subject to injury from brief interruptions in the blood supply.9
Particularities of Encephalic Vasculature in Animal Models
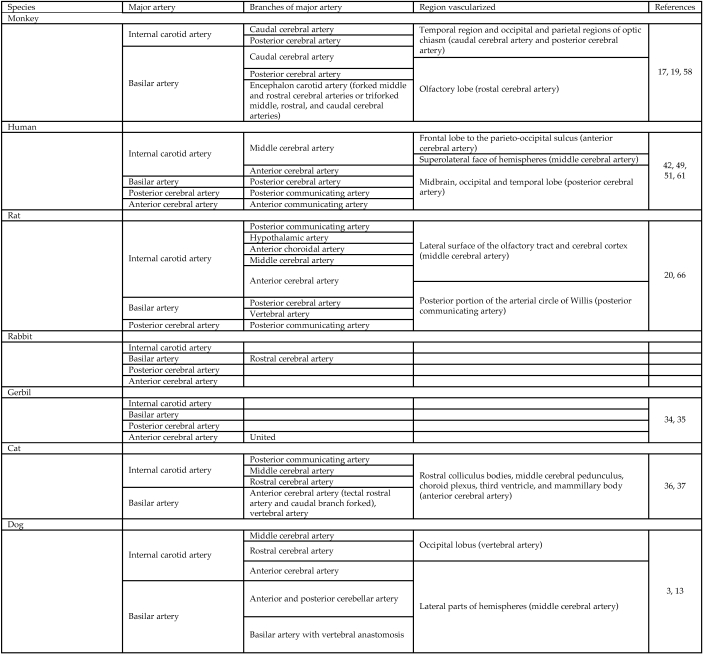
Among various species, the general arrangement of encephalic arteries is conserved with some particularities (Figures 2 and 3). In humans, the internal carotid artery provides the major blood supply to the encephalon. In dogs, the vertebral artery assumes this role. This different model of blood supply with numerous intra- and extracranial anastomoses protects the encephalon of the dog from the effects of cerebral arterial occlusions. These differences of blood supply in the dog encephalon explain the low use of this model in ischemia studies.24
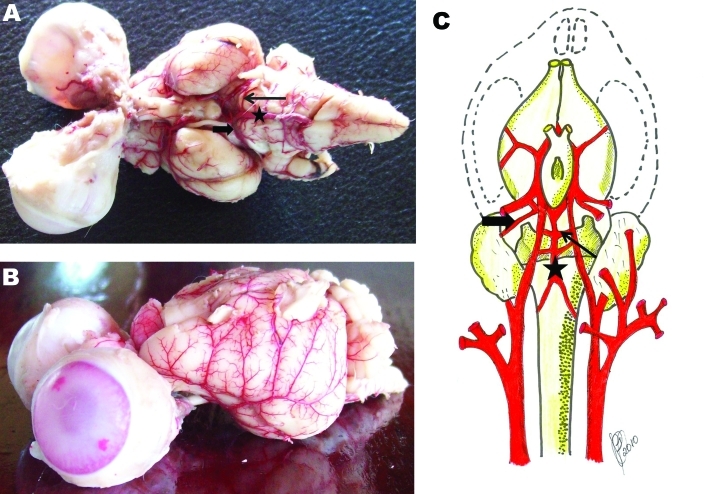
Figure 2.
(A) Ventral and (B) lateral views of rabbit encephalon injected with latex to show arterial vasculature and (C) a schema of the same vasculature. The origin of the inferior cerebellar artery is the vertebral artery in humans in primates but is the basilar artery (star) in rabbits. Andrade (1983) noted that rodents, like rabbits, have a lower anterior cerebellar artery (solid arrow) and superior cerebellar artery (open arrow).
Figure 3.
Encephalic regions supplied by the major encephalic arteries and their branches.
The branches of arteries that form the arterial circle of the pig encephalon may constitute 2 vascular networks: the basal and cortical vascular networks.16 The branches of the cerebral arteries have been grouped into 3 classes:16 the arteries at the base of the brain nuclei, the ventricular arteries, and the arteries of the convolutions of the cortical gray layer. The initial portion of the anterior cerebral artery has small branches that supply the basal ganglia.
The distribution of the internal carotid and vertebral arteries in human primates, especially monkeys,64 is similar to that found in humans. The anterior cerebral arteries fuse into a unique median branch, which surrounds the genu corporis callosi and bifurcates distally. The basilar artery bifurcates into the posterior cerebral arteries, which are connected to the internal carotid posterior communicating artery. In adults, the encephalon is supplied by both the internal carotid and vertebral arteries, but in embryos, blood is supplied only by the internal carotid arteries. The internal carotid artery branches from the internal ophthalmic artery, which pierces the dura mater and stems from 2 terminal branches with different calibers. A posterior branch of the small arm, the posterior communicating artery, and the anterior branch all branch into the choroidal artery and middle cerebral artery and finish as the anterior cerebral artery. In the rostral region of the anastomotic arterial circle at the base of the brain, the interhemispheric artery proceeds into the longitudinal fissure of the brain dorsal to the genu of the corpus callosum. The collateral branches are issued from the medial sides of each cerebral hemisphere from the frontal lobe, and the arteries bifurcate at the corpus callosum to produce the right and left callosum arteries.17
At the base of the brain of Cebus apella (capuchin), the inferior cerebellar and posterior inferior cerebellar arteries supply blood to the lower portion of the cerebellum and the lateral surface of the bulbus. Before the bifurcation of the basilar artery to the right and left superior cerebellar arteries, the superior cerebellar satellites spread to the midbrain, upper stem, and cerebellum.57
The encephalic arteries of monkeys are represented by 3 branches of the vascular pedicle:18 the basilar artery, right internal carotid artery, and left internal carotid artery. The basilar artery results from anastomosis by convergence of the right and left vertebral arteries. The arterial segments belonging to this system form a closed circuit with 2 distinct sectors: caudal (basilar) and carotid (cranial).
The encephalic circulation of carnivores can be classified into 3 groups:64 one in which the encephalic blood supply is provided by the internal carotid arteries, one in which the encephalic blood supply is provided by the vertebral arteries, and the last with characteristics of both of these groups. However, these 3 groups do not show an evolutionary pattern because similar classifications exist in distant phylogenetic groups.64
The surfaces of the arteries of the dog and cat encephalons are similar and follow the general model of carnivores.37 The intrinsic arteries follow the model described for submammals and primates. The extrinsic arteries of dogs and cats have some unusual modifications and thus resemble those of some ungulates. Most importantly, a single major source of arterial blood supplies the encephalon of cats and dogs. This vessel originates from the maxillary artery and acts as an anastomotic branch for the rete admirable.
The arteries at the base of the brain in cats are dependent on the carotid and vertebrobasilar systems and are responsible for the formation of the arterial circuit of the encephalon.37 The rostral portion of the arterial circuit of the encephalon lies across the base, resembling an ellipse, and is closed by the rostral communicating artery. The caudal portion of this circuit had a characteristic morphologic asymmetry and was closed by the caudal branches of the carotid arteries of the brain and terminal branches of the basilar artery on both sides. In addition, the presence of a training network prepared within this circuit was observed.37
Studies of the circle of Willis in Canis familiaris have revealed that the caudal communicating artery usually branches into 2 branches and flows laterally from rostral to caudal by means of the caudal artery of the brain and rostral cerebellar artery.10 The first of these 2 communicating branches divides the flow to serve 2 distinct regions: the proximal and distal portions of the midbrain.
Animal Models Compared with Methods of Occlusion Induction
The use of diverse experimental models is useful for experimental studies on ischemia, preventing the development of a standard surgical model. The ideal model has the characteristics of clinical relevance, ease of experimental execution, reproducibility, and absence of collateral effects unrelated to ischemia.6 Several methods of ischemia induction have been described, including craniotomy, arterial embolism, and occlusion of 3 or 4 cervical vessels.6 Variation in time of ischemia contributes to the diversity of the experimental models used. The most used method for inducing ischemia is thrombosis by middle cerebral artery occlusion.6 In tests of motor behavior, animals presented different degrees of functional defects on the contralateral side of the ischemia. Histologically, middle cerebral artery occlusion produces small necrotic central and apoptotic peripheral regions.4,8,43
Occlusion of the middle cerebral artery is the most commonly used surgical method of producing stroke.50 By first damaging subcortical structures and then damaging cortical structures, this occlusion mimics human striatocapsular infarcts in terms of size and the structures affected. Striatocapsular infarcts affect the majority of the basal ganglia or adjacent white matter.50 These lesions are caused by occlusion of the transient middle cerebral artery with early reperfusion or, if the occlusion persists, with good collateral flow from the anterior or posterior arteries to the cortical middle cerebral artery territory.50
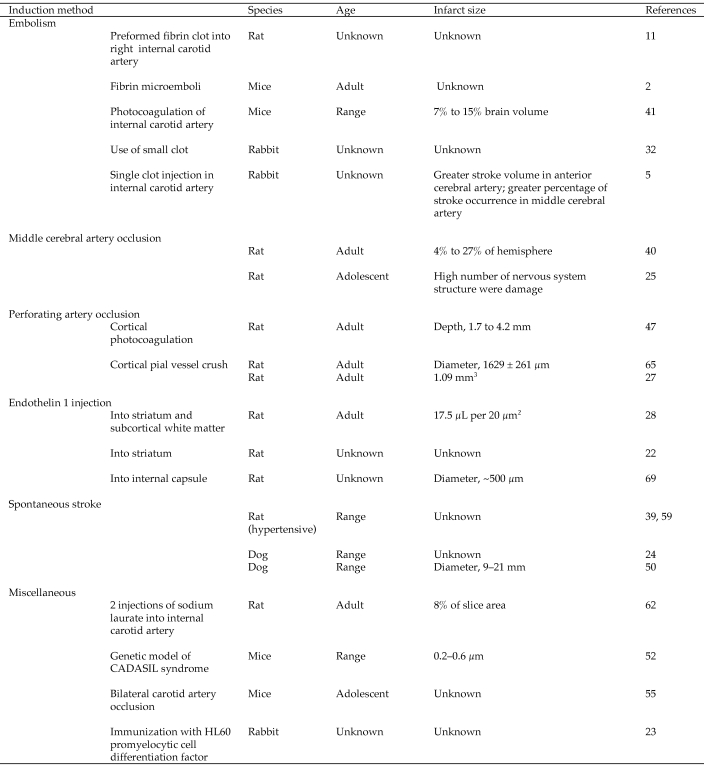
Techniques other than occlusion of the middle cerebral artery can also be used to induce stroke in animals (Figure 4). Injection of endothelin 1 (a powerful vasoconstrictor) affects microvessels, causing ischemic lesions. In gray matter, endothelin 1 causes small lesions with neuronal and astrocyte losses and a delayed macrophagic–microglial response. In white matter, endothelin 1 causes axonal and oligodendrocyte disruption followed by myelin damage and increased astrocyte reactivity.
Figure 4.
Major methods of stroke induction in different animal models.
Embolism caused by injecting different amounts and sizes of emboli (microspheres, black beads, silicone rubber cylinders, and preformed clots) into the internal carotid artery produced multiple, unpredictable infarcts.3 These infarcts were mostly cortical, with a few in the basal ganglia and caudate nucleus.3 However, the subcortical lesions were poorly documented, making their relevance to lacunar infarction uncertain.3
Perforating artery occlusion causes small cortical infarcts in rats after using forceps or photochemical irradiation to occlude a pial artery on the surface of the brain. Perforating artery occlusions mimic lacunar infarction because they show “cavitation caused specifically by ischemia of smaller vessels.”47 This type of occlusion may result in occlusion of the lenticulostriate artery by eosinophilic thrombus accompanied by brain tissue softening, necrosis, and cyst formation.27 These lenticulostriate occlusion models all produced striatocapsularsized rather than laccunar-sized lesions.27
Another method involves spontaneous lesion formation in transgenic or spontaneously hypertensive stroke-prone animals.39
Conclusions
The ideal model for stroke research incorporates several factors. The ideal model should have a sufficient number of features that are similar to those in humans to allow the study of the biologic, behavioral, and physiologic factors of the pathology so that, after the induction of the pathologic process, the outcomes can be investigated and treated with minimal limitations. The most applicable animal models for research related to stroke are rodents and lagomorphs. These models satisfy all of the basic requirements needed to induce, manipulate, and treat diseases that affect humans. However, other models should still be explored through similar studies.
References
- 1.Arthur AM, Martins T, Chingui L. 2008. Efeito da estimulação elétrica sobre a plasticidade neural: um estudo em pacientes com déficit sensorial decorrente de acidente vascular encefálico. Anuário da Produção Cientifica Discente 9:79–90 [Google Scholar]
- 2.Atochin DN, Murciano JC, Gursoy-Ozdemir Y, Krasik T, Noda F, Ayata C, Dunn AK, Moskowitz MA, Huang PL, Muzykantov VR. 2004. Mouse model of microembolic stroke and reperfusion. Stroke 35:2177–2182 [DOI] [PubMed] [Google Scholar]
- 3.Bailey EL, McCulloch J, Sudlow C, Wardlaw JM. 2009. Potential animal models of lacunar stroke: a systematic review. Stroke 40:e451–e458 [DOI] [PubMed] [Google Scholar]
- 4.Bliss T, Guzman R, Daadi M, Steinberg GK. 2007. Cell transplantation therapy for stroke. Stroke 38:817–826 [DOI] [PubMed] [Google Scholar]
- 5.Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, Culp WC. 2010. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol 21:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calloni RIC. 2006Modelo experimental de isquemia cerebral em ratos por obliteração temporária da artéria cerebral média. [MS thesis]. Porto Alegre (Brazil): Universidade Federal do Rio Grande do Sul [Google Scholar]
- 7.Campos A, Prada ILS, Santos I, Jr,, Santos D. 2003. Arteries at the base of the encephalon in horses. Occipitobasilar system. Brazilian J Vet Res Anim Sci 40:107–117 [Google Scholar]
- 8.Caplan AI. 2007. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347 [DOI] [PubMed] [Google Scholar]
- 9.Carvalho SMF. 2006. Contribuição da imagem funcional por ressonância magnética para o estudo da reorganização do córtex motor pós-AVCI. Revista Medicina Ribeirão Preto 39:311–312 [Google Scholar]
- 10.Casal D, Arantes M, Casimiro M, Pais D, Pina JAE. 2005. Caracterização morfológica do polígono arterial de Willis no Canis familiaris. Revista Portuguesa de Ciências Veterinárias 100: 163–167 [Google Scholar]
- 11.Chen CX, Todd KG, Yang Y, Gordon T, Shuaib A. 2001. Patency of cerebral microvessels after focal embolic stroke in the rat. J Cereb Blood Flow Metab 21:413–421 [DOI] [PubMed] [Google Scholar]
- 12.Doyle P. 2002. Measuring health outcomes in stroke survivors. Arch Phys Med Rehabil 83:S39–S43 [DOI] [PubMed] [Google Scholar]
- 13.Dyce KM, Sack WO, Wensing CJG. 2004. O sistema cardiovascular. In: Tratado de anatomia veterinária. Rio de Janeiro (Brazil): Guanabara Koogan [Google Scholar]
- 14.Fagundes DJ, Taha MO. 2004. Animal disease model: choice's criteria and current animals specimens. Acta Cir Bras 19:59–65 [Google Scholar]
- 15.Farooqui AA, Hann SE, Horrocks LA. 2006. Ischemia and hypoxia. : Siegel GJ. Basic neurochemistry: molecular, celular and medical aspects. New York (NY): Raven Press [Google Scholar]
- 16.Ferreira CG, Prada ILS. 2005. O circuito arterial da base do encéfalo em suínos (Sus scrofa domesticus Linnaeus, 1758), formação e comportamento. Brazilian Journal of Veterinary Research and Animal Science 42:53–60 [Google Scholar]
- 17.Ferreira JR, Abreu NHL, Pires JS, Ribeiro BN. 2005. O sistema carótico do encéfalo de primata neotropical, anatomia da artéria interhemisférica (Cebus apella, Linnaeus, 1766). Ciência Animal Brasileira 6:203–212 [Google Scholar]
- 18.Ferreira JR, Prada ILS. 2001. Nomenclatura proposta para denominar as artérias da base do encéfalo do macaco-prego (Cebus apella L., 1766). Acta Scientiarum Biol Sci 23:635–643 [Google Scholar]
- 19.Ferreira JR, Prada ILS. 2009. The carotid encephalic system of the Cebus apella sp., Linnaeus, 1766. Biota Neotropica 9: 285–292 [Google Scholar]
- 20.Fox G, Gallacher D, Shevde S, Loftus J, Swayne G. 1993. Anatomic variation of the middle cerebral artery in the Sprague–Dawley rat. Stroke 24: 2087–2092 [DOI] [PubMed] [Google Scholar]
- 21.Freitas GR, Noujaim JK, Haussen SR, Yamamoto FI, Novak EM, Gagliardi RJ. 2005. [Neuroprotective agents in stroke: national opinion] Arq Neuropsiquiatr 63:889–891 [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 22.Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. 2006. An animal model of capsular infarct: endothelin 1 injections in the rat. Behav Brain Res 169:206–211 [DOI] [PubMed] [Google Scholar]
- 23.Gapon MV, Dranitsyna SM, Minkevich NI, Gruden MA, Babichenko II, Kostanyan IA. 2006. Experimental model of hemorrhagic stroke: rabbit immunization with HL60 promyelocytic cell differentiation factor. Bull Exp Biol Med 141:272–274 [DOI] [PubMed] [Google Scholar]
- 24.Garosi LS, McConnell JF. 2005. Ischaemic stroke in dogs and humans: a comparative review. J Small Anim Pract 46:521–529 [DOI] [PubMed] [Google Scholar]
- 25.Gharbawie OA, Auer RN, Whishaw IQ. 2006. Subcortical middle cerebral artery ischemia abolishes the digit flexion and closing used for grasping in rat skilled reaching. Neuroscience 137:1107–1118 [DOI] [PubMed] [Google Scholar]
- 26.Homi HM, Silva BA, Jr,, Velasco IT. 2000. Fisiopatologia da isquemia cerebral. Rev Bras Anestesiol 50:405–414 [Google Scholar]
- 27.Hua R, Walz W. 2006. Minocycline treatment prevents cavitation in rats after a cortical devascularizing lesion. Brain Res 1090:172–181 [DOI] [PubMed] [Google Scholar]
- 28.Hughes PM, Anthony DC, Ruddin M, Botham MS, Rankine EL, Sablone M, Baumann D, Mir AK, Perry VH. 2003. Focal lesions in the rat central nervous system induced by endothelin 1. J Neuropathol Exp Neurol 62:1276–1286 [DOI] [PubMed] [Google Scholar]
- 29.Ito U, Spatz M, Walker JT, Jr,, Klatzo I. 1975. Experimental cerebral ischemia in Mongolian gerbils. I. Light microscopic observations. Acta Neuropathol 32:209–223 [DOI] [PubMed] [Google Scholar]
- 30.Jones F, Riazi A. 2011. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil 33:797–810 [DOI] [PubMed] [Google Scholar]
- 31.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N. 2010. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small-vessel disease. J Clin Invest 120:433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapchak PA. 2010. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Transl Stroke Res 1:96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessa I. 1999. Epidemiologia das doenças cerebrovasculares no Brasil. Revista da Sociedade de Cardiologa do Estado de São Paulo 4:509–518 [Google Scholar]
- 34.Levine S, Payan H. 1966. Effects of ischemia and other procedures on the brain and retina of the gerbil (Meriones unguiculatus). Exp Neurol 16:255–262 [DOI] [PubMed] [Google Scholar]
- 35.Levine S, Shon D. 1969. Cerebral ischemia in infant and adult gerbils. Relation to incomplete circle of Willis. Arch Pathol 87:315–317 [PubMed] [Google Scholar]
- 36.Lima EMM, Prada ILS, Silva FOC, Severino RS, Santos ALQ, Borges BO, Paim TP, Vianna ARCB. 2010. Sistematização da origem, da distribuição e dos territórios da artéria cerebral caudal na superfície do encéfalo em gatos. Cienc Rural 40:1961–1965 [Google Scholar]
- 37.Lima EMM, Prada ILS, Silva FOC, Severino RS, Santos ALQ, Drummond SS, Rodrigues GS. 2006. Estudo anatômico das artérias da base do encéfalo em gatos (Felis catus domesticus). Ars Veterinária 22:1–7 [Google Scholar]
- 38.Lima EMM, Severino RS, Silva FOC, Drummond SS, Bombonato PP, Campos DB, Rodrigues GS. 2005. Artérias da base do encéfalo em suínos da linhagem Camborough 22. Bioscience Journal 21:137–147 [Google Scholar]
- 39.Lin JX, Tomimoto H, Akiguchi I, Wakita H, Shibasaki H, Horie R. 2001. White matter lesions and alteration of vascular cell composition in the brain of spontaneously hypertensive rats. Neuroreport 12:1835–1839 [DOI] [PubMed] [Google Scholar]
- 40.Lindner MD, Gribkoff VK, Donlan NA, Jones TA. 2003. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci 23:10913–10922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lozano JD, Abulafia DP, Danton GH, Watson BD, Dietrich WD. 2007. Characterization of a thromboembolic photochemical model of repeated stroke in mice. J Neurosci Methods 162:244–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado A.Neuroanatomia funcional. Rio de Janeiro (Brazil): Atheneu . 1988. [Google Scholar]
- 43.Mendez-Otero R, Giraldi-Guimarães A, Pimentel-Coelho PM, Freitas GR. 2009. Terapia celular no acidente vascular cerebral. Revista Brasileira de Hematologia e Hemoterapia 31:99–103 [Google Scholar]
- 44.Molinari GF. 1988. Why model strokes? Stroke 19:1195–1197 [DOI] [PubMed] [Google Scholar]
- 45.O'Sullivan SB, Schimitz TJ. 2004. Fisioterapia: avaliação e tratamento. São Paulo (Brazil): Manole [Google Scholar]
- 46.Pereira AB, Alvarenga H, Pereira RS, Jr,, Barbosa MT. 2009. [Stroke prevalence among the elderly in Vassouras, Rio de Janeiro State, Brazil, according to data from the Family Health Program] Cad Saude Publica 25:1929–1936 [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 47.Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA. 2001. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods 45:227–233 [DOI] [PubMed] [Google Scholar]
- 48.Prada ILS. 1997. A alma dos animais. Campos do Jordão (Brazil): Mantiqueira [Google Scholar]
- 49.Rogers L. 1947. The function of the circulus arteriosus of Willis. Brain 70:171–178 [DOI] [PubMed] [Google Scholar]
- 50.Rossmeisl JH, Jr, Rohleder JJ, Pickett JP, Duncan R, Herring IP. 2007. Presumed and confirmed striatocapsular brain infarctions in 6 dogs. Vet Ophthalmol 10:23–36 [DOI] [PubMed] [Google Scholar]
- 51.Rowland LP. 2002. Merritt: tratado de neurologia. Rio de Janeiro (Brazil): Guanabara Koogan [Google Scholar]
- 52.Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, Joutel A. 2003. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol 162:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryerson SD. Hemiplegia. : Umphred DA. Neurological rehabilitation. Rio de Janeiro (Brazil): Guanabara Koogan [Google Scholar]
- 54.Salén JCW. Animal models: principles and problems. : Rollin BE, Kesel ML. The experimental animal in biomedical research: care, husbandry and well-being: an overview by species. Boston (MA): CRC Press [Google Scholar]
- 55.Shibata M, Ohtani R, Ihara M, Tomimoto H. 2004. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 35:2598–2603 [DOI] [PubMed] [Google Scholar]
- 56.Silva LLM, Moura CEM, Godoy JRP. 2005. Fatores de risco para o acidente vascular encefálico. Universitas Ciências da Saúde 3:145–160 [Google Scholar]
- 57.Silva RA, Ferreira JR. 2002. Estudo das artérias cerebelares do macaco-prego. Considerações sobre a nomenclatura (Cebus apella, L.1766). Brazilian J Vet Res Anim Sci 39:296–300 [Google Scholar]
- 58.Siqueira Neto EGB, Ferreira JR. 2002. Estudo anatômico da origem e distribuição dos ramos corticais das artérias cerebrais caudais do encéfalo do macaco prego. Acta Scientiarum. Biological Sciences 2002:639–646 [Google Scholar]
- 59.Sironi L, Guerrini U, Tremoli E, Miller I, Gelosa P, Lascialfari A, Zucca I, Eberini I, Gemeiner M, Paoletti R, Gianazza E. 2004. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res 78:115–122 [DOI] [PubMed] [Google Scholar]
- 60.Teocchi MA. [Internet] 2009. Modelos animais no estudo de AVC. [Cited 10 August 2010]. Available at: http://www.comciencia.br/comciencia/?section=8&edicao=47&id=561
- 61.Testut L. 1911. Traité d'anatomie humaine: anatomie descriptive, histologie, dévelopement. Paris (France): Octave Doin [Google Scholar]
- 62.Toshima Y, Satoh S, Ikegaki I, Asano T. 2000. A new model of cerebral microthrombosis in rats and the neuroprotective effect of a Rho kinase inhibitor. Stroke 31:2245–2250 [DOI] [PubMed] [Google Scholar]
- 63.Villar FAS. 1997. Alterações centrais e periféricas após lesão do sistema nervoso central. Considerações e implicações para a prática da fisioterapia. Rev Bras Fisioter 2:19–34 [Google Scholar]
- 64.Vriese B. 1904. Sur la signification morfologique des artères cérébrales. Arch Biol (Liege) 21:357–457 [Google Scholar]
- 65.Wang K, Walz W. 2003. Unusual topographical pattern of proximal astrogliosis around a cortical devascularizing lesion. J Neurosci Res 73:497–506 [DOI] [PubMed] [Google Scholar]
- 66.Wang-Fischer Y. 2009. Manual of stroke models in rat. Boca Raton (FL): CRC Press [Google Scholar]
- 67.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. 2003. Stroke. Lancet 362:1211–1224 [DOI] [PubMed] [Google Scholar]
- 68.Wessmann A, Chandler K, Garosi L. 2009. Ischaemic and haemorrhagic stroke in the dog. Vet J 180:290–303 [DOI] [PubMed] [Google Scholar]
- 69.Whitehead S, Cheng G, Hachinski V, Cechetto DF. 2005. Interaction between a rat model of cerebral ischemia and beta-amyloid toxicity: II. Effects of triflusal. Stroke 36:1782–1789 [DOI] [PubMed] [Google Scholar]
- 70.Winikates J. Doenças vasculares. : Rolak L. Segredos em neurologia. Porto Alegre (Brazil): Artmed [Google Scholar]