Abstract
Osteoarthritis is a common joint disease that currently lacks disease-modifying treatments. Development of therapeutic agents for osteoarthritis requires better understanding of the disease and cost-effective in vivo models that mimic the human disease. Here, we analyzed the joints of STR/ort mice, a model for spontaneous osteoarthritis, for levels of inflammatory and oxidative stress markers and measured serum cytokines to characterize the local and systemic inflammatory status of these mice. Markers of low-grade inflammatory and oxidative stress—RAGE, AGE, S100A4, and HMGB1—were evaluated through immunohistochemistry. Of these, AGE and HMGB1 levels were elevated strongly in hyperplastic synovium, cartilage, meniscus, and ligaments in the joints of STR/ort mice compared with CBA mice, an osteoarthritis-resistant mouse strain. These increases (particularly in the synovium, meniscus, and ligaments) correlated with increased histopathologic changes in the cartilage. Serum analysis showed higher concentrations of several cytokines including IL1β, IL12p70, MIP1β, and IL5 in STR/ort mice, and these changes correlated with worsened joint morphology. These results indicate that STR/ort mice exhibited local and systemic proinflammatory conditions, both of which are present in human osteoarthritis. Therefore, the STR/ort mouse model appears to be a clinically relevant and cost-effective small animal model for testing osteoarthritis therapeutics.
Abbreviations: HMGB1, high-mobility group box chromosomal protein 1; AGE, advanced glycation endproducts; RAGE, receptor for advanced glycation endproducts
Human osteoarthritis is a painful joint disease characterized by cartilage degradation and changes in bone and ligaments. The etiology is unknown and thought to be influenced by aging, genetics, trauma, and obesity.2,5,36 As a result, current osteoarthritis treatment options mostly target pain management; no disease-modifying therapeutic agents are available at present.2,36
The role of synovial changes and particularly inflammation in human osteoarthritis is unclear, and the disease generally is considered to exhibit only local intermittent synovial inflammation. However, inflammatory cytokines including IL6 and C reactive protein frequently are elevated in both synovial fluids and serum, and their correlation with osteoarthritis severity has been reported in patients with the disease.24,27 However, in addition to factors such as these cytokines, which typically are elevated in response to acute inflammation or adiposity, markers reflective of oxidative stress and low-grade inflammation are needed. Recently, receptor for advanced glycation endproducts (RAGE), a cell receptor involved in innate immunity and amplification of inflammation, has emerged as such a marker due to its elevation in many inflammatory conditions, such as atherosclerosis, diabetes, and rheumatoid arthritis.4,14,32,37 Similarly, its 3 unrelated ligands have inflammatory properties and include advanced glycation endproducts (AGE; nonenzymatic glycation products formed during oxidative stress), S100A4 (a member of the calcium-binding S100–calgranulin family of proteins), and high-mobility group box chromosomal protein 1 (HMGB1 or amphoterin; a nonhistone DNA chromatin-associated protein with inflammatory properties).20,21,30,32,37,40,41 All of these proteins are increased in human osteoarthritic joints, enhance oxidative stress, and result in production of matrix metalloproteases and cytokines from human joint cells in vitro.12,16,17,25,39,47
Currently, the majority of experimental testing for osteoarthritis is performed in surgically or chemically induced animal models. How well these models reflect human osteoarthritis is controversial.2,36 Therefore, clinically relevant and cost-effective animal models that mimic human osteoarthritis pathology are needed to evaluate new osteoarthritis drugs. In contrast to joint-injury–induced osteoarthritis models, STR/ort mice develop osteoarthritis spontaneously and exhibit human-like cartilage lesions at approximately 12 to 20 wk.26 The etiology of osteoarthritis development in these mice is unclear despite extensive genetic and microarray analyses.1,19,46 Osteoarthritis develops in male STR/ort mice preferentially but is not dependent on sex hormones.19,44 In addition, STR/ort mice are heavier than most laboratory strains on standard diet, although no correlation between obesity and joint changes has been shown.1,19,46 STR/ort mice show a tendency for patella displacement as potential disease-inducing damage, but whether this luxation is a primary or secondary event is unknown.26,45 So far, detailed joint analyses of arthritic STR/ort mice have demonstrated increased levels of matrix metalloproteases; inflammatory cytokines IL1α, IL1β, and IL6; and oxidative stress in areas of osteoarthritis lesions, similar to findings in human osteoarthritis.1,2,9,10,15,26,33 Synovial proliferation is generally mild, similar to what is seen with the human disease, with little synovial inflammatory infiltrate reported.26
The objective of the current study was to examine whether newly identified markers of low-grade inflammation in human osteoarthritis are increased in the joints of STR/ort mice and contribute to spontaneous development of osteoarthritis. In addition, we evaluated serum cytokine levels and their correlation with cartilage damage to better understand potential systemic contributors to the osteoarthritis in these mice.
Materials and Methods
Animal experiment.
Male STR/ort and CBA mice (Harlan Laboratories, Indianapolis, IN) were housed in cages (room temperature, 70 °C; 12:12-h light:dark cycle; relative humidity, 30% to 70%) containing standard irradiated bedding and were provided irradiated rodent chow (Lab Diet 5053, PMI Nutrition International, St Louis, MO) and water ad libitum. Animals were weighed and observed for joint swelling and mobility once monthly. Mice were euthanized by CO2 asphyxiation at 20 or 40 wk of age (STR/ort, n = 8 or 9; CBA, n = 5 or 6). Blood was collected under isoflurane anesthesia from the retroorbital sinus, allowed to clot, and spun at 1000 × g for 10 min; serum was frozen at −80 °C until analysis. Stifle joints were collected for histologic analysis. The study was approved by the Genzyme Institutional Animal Care and Use Committee and conducted in accordance with humane guidelines for animal care. The animals underwent regular surveillance to ensure that they remained uninfected with a standard panel of mouse pathogens.
Histology.
Stifle joints were fixed in 10% normal buffered formalin, followed by decalcification in formic acid. Joints were embedded in paraffin, and 5-µm sections were stained with hematoxylin and eosin or toluidine blue. Joint morphology was scored by a board-certified veterinary pathologist according to a published system.10
Immunohistochemisty.
Sections were digested with 0.4 U/mL chondroitinase and 0.4 U/mL keratinase (Sigma Aldrich, St Louis, MO) at 37 °C for 1 h, followed by treating with pepsin (Dako, Carpinteria, CA) in 0.2 N HCl to block endogenous alkaline phosphatase activity (for detection by alkaline phosphatase activity) or by treating with 3% hydrogen peroxide (Sigma Aldrich) in methanol (JT Baker, Phillipsburg, NJ) to block the endogenous hydrogen peroxide activity (for detection by peroxidase activity). Nonspecific binding was blocked by using serum from the same host species as for the secondary antibodies, followed by incubation at 4 °C overnight with primary antibodies: RAGE (2.5 mg/mL; Santa Cruz Biotechnology, Santa Cruz, CA), S100A4 (2 mg/mL; MBL/Cyclex, Woburn, MA; confirmed to not detect other S100 proteins), AGE (2 mg/mL; TransGenic, Kobe, Japan; detects N′-carboxymethyllysine–protein adduct), HMGB1 (2.5 mg/mL; Sigma Aldrich), cathepsin K (Santa Cruz Biotechnology), and CD68 (AbD Serotec, Raleigh, NC). Matching isotype antibodies were used as negative controls. Sections were incubated with biotinylated secondary antibodies for 30 min at 25 °C, followed by incubation with alkaline phosphatase- or horseradish peroxidase-conjugated biotin antibody (Vector Laboratories, Burlingame, CA) for 30 min at 25 °C. Color was revealed by using Vector Red (Vector Laboratories) for alkaline-phosphatase conjugates (that is, detection of AGE, S100A4, and HMGB1) and diaminobenzimide substrate (Dako) for horseradish peroxidase conjugates (RAGE detection), followed by staining with hematoxylin (Dako). Parallel samples were run simultaneously for each antigen to avoid experimental variation. Numbers of positive cells and intensity of staining were scored by a board-certified veterinary pathologist in a blinded manner. For numbers of positive cells, the scoring scheme was: 0, no stained cells per 40× high-power field; 1, 1 to 5 stained cells; 2, 6 to 10 stained cells; 3, 11 to 20 stained cells; and 4, 21 or more stained cells. The intensity of staining was scored as: 0, no staining; 1, slight staining; 2, moderate staining; 3, strong staining; and 4, very bright staining.
Mouse serum cytokine analysis.
Cytokine levels (IL1α, IL1β, IL2, IL3, IL4, IL5, IL6, IL9, IL10, IL13, IL17, IL12p70, INFγ, GM–CSF, KC [murine IL8 homolog], MIP1β, RANTES, and TNFα) were measured in duplicate by using a mouse 21plex bead assay (Beadlyte, Upstate, Temecula, CA) according to the manufacturer's instructions.
Statistical analyses.
Immunohistochemical scores and osteoclast quantitation were compared by using the 2-tailed, unequal variance Student t test (Microsoft Excel, Redmond, WA). The significance of R2 values was analyzed by using the Spearman nonparametric test (SAS 9.1, SAS Institute, Cary, NC). ANOVA and Wilcoxon 2-sample tests (SAS Software) were used to compare cytokine levels. P values less than 0.05 were considered to be statistically significant in all analyses.
Results
Pathology of mouse stifle joints.
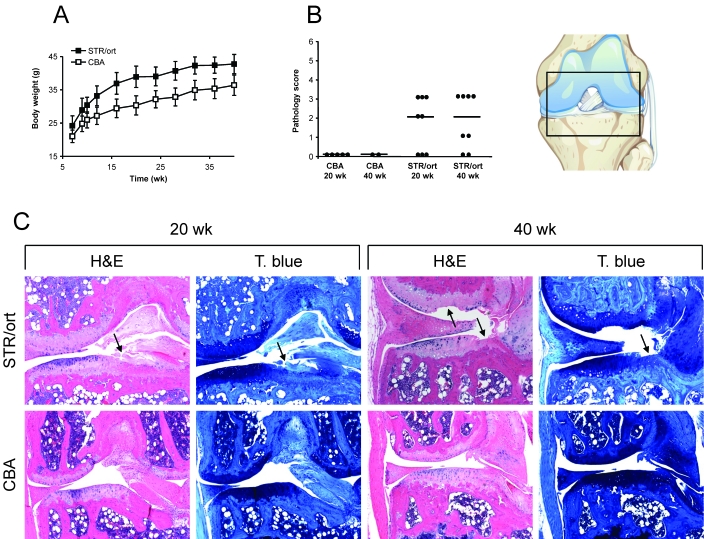
None of the mice exhibited any visual joint swelling, reduced mobility, or pain during the study. However, STR/ort mice had increased bodyweight compared with that of CBA mice (Figure 1 A). Histologic analysis and scoring of the stifle joints showed that at 20 wk, 62% of male STR/ort mice had signs of osteoarthritis; the incidence further increased to 75% by 40 wk (Figure 1 B and C). Histologic changes were detected in articular cartilage of medial tibial plateau and varied from mild focal superficial fibrillations (score, 1) to erosions that extended to subchondral bone (score, 4). Fibrillations frequently were accompanied by loss of chondrocytes and decreased cartilage proteoglycan staining. In addition, joints had mild chronic synovitis with predominantly lymphocyte and macrophage infiltration as well as moderate synovial hyperplasia. More severely affected joints exhibited degeneration of ligaments, focal degeneration of the medial meniscus, and osteophyte formation. No histologic signs of osteoarthritis were present in the joints of age-matched CBA mice, similar to previous reports;9,15,33,35,46 therefore, these mice were used as osteoarthritis-negative controls for subsequent analyses.
Figure 1.
Analysis of STR/ort and CBA mice. (A) Body weights during the study. Values represent group mean ± 1 SD (STR/ort, n = 8 or 9; CBA, n = 5 or 6) (B) Histologic scoring of cartilage surface at 20 and 40 wk based on sections stained with hematoxylin and eosin (H & E). The scatter plot shows scores for each mouse (filled circles) and for the group median (black line). A similar pattern was seen for sections stained with toluidine blue (T. blue; not shown; STR/ort, n = 8; CBA, n = 2 to 5). (C) Representative images from 20- and 40-wk mice of the location shown in the diagram. Arrows indicate articular cartilage degeneration, which mostly was present on the medial tibial plateau. Magnification, 100×.
Immunohistochemical analysis of inflammatory markers in mouse stifle joints.
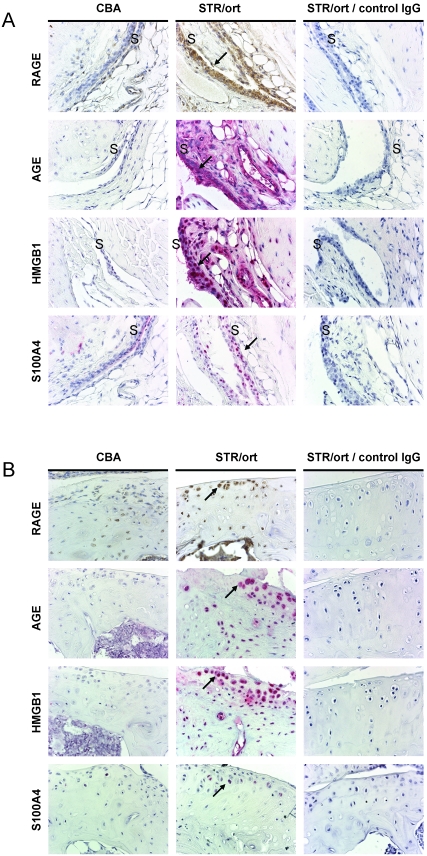
Joints from STR/ort and CBA mice were analyzed at 20 wk (Figure 2). Synovial lining cells around the cruciate ligament and medial and lateral sides were positive for RAGE and AGE, whereas HMGB1 staining was present mainly in mononuclear cells (Figure 2 A; similar to CD68-positive cells, not shown). In articular cartilage, AGE and RAGE showed intracellular staining, whereas HMGB1 exhibited intracellular and occasionally extracellular staining within chondrocyte lacunae and surrounding matrix (Figure 2 B). Little S100A4 staining was detected in STR/ort mice. In CBA mice, there was little detection of any of these markers.
Figure 2.
Immunolocalization of inflammatory markers in the joint synovium and cartilage at 20 wk of age. (A) Synovial and (B) tibial articular cartilage staining with RAGE, AGE, S100A4, and HMGB1 in CBA mice (left panel) and STR/ort mice (middle panel). Immunohistochemistry of STR/ort joints with appropriate isotype control antibodies also is shown (right panel). See Methods for details. Arrows point to positive cells. S, synovium. Magnification, 600×.
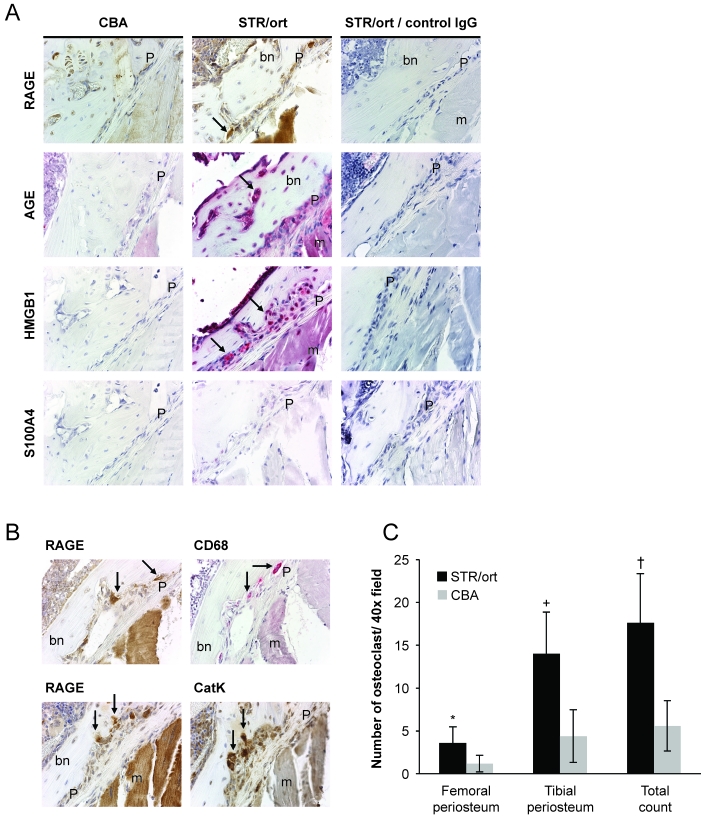
In the periosteum, RAGE was present in osteoclasts of STR/ort joints (confirmed by colocalization with cathepsin K and CD68 staining, both common osteoclast markers; Figure 3 A and B). AGE and HMGB1 showed strong signals that localized to multiple cell types; no staining for S100A4 was observed. There was a striking increase in the number of CD68-positive osteoclasts (particularly in the lateral tibial periosteum) in the periosteum of STR/ort mice compared with CBA mice (Figure 3 C).
Figure 3.
Immunohistochemical analysis of inflammatory markers in the joint periosteum at 20 wk of age. (A) RAGE, AGE, S100A4, and HMGB1 staining at the lateral tibial side of CBA (left panel) and STR/ort (middle panel) joints and STR/ort joints with isotype control antibodies (right panel). Magnification, 600×. (B) Colocalization of RAGE staining with CD68 and cathepsin K in periosteal osteoclasts. Arrows, osteoclasts. Magnification, 600×. bn, bone; m, muscle; P, periosteum. (C) Quantitation of osteoclasts in the joint periosteum. CD68-positive cells at the femoral and tibial periosteum were counted. Data points represent group mean ± 1 SD (STR/ort, n = 8; CBA, n = 5). Significance was assessed by using a 2-tailed Student t test (*, P < 0.05; +, P < 0.01; †, P < 0.001).
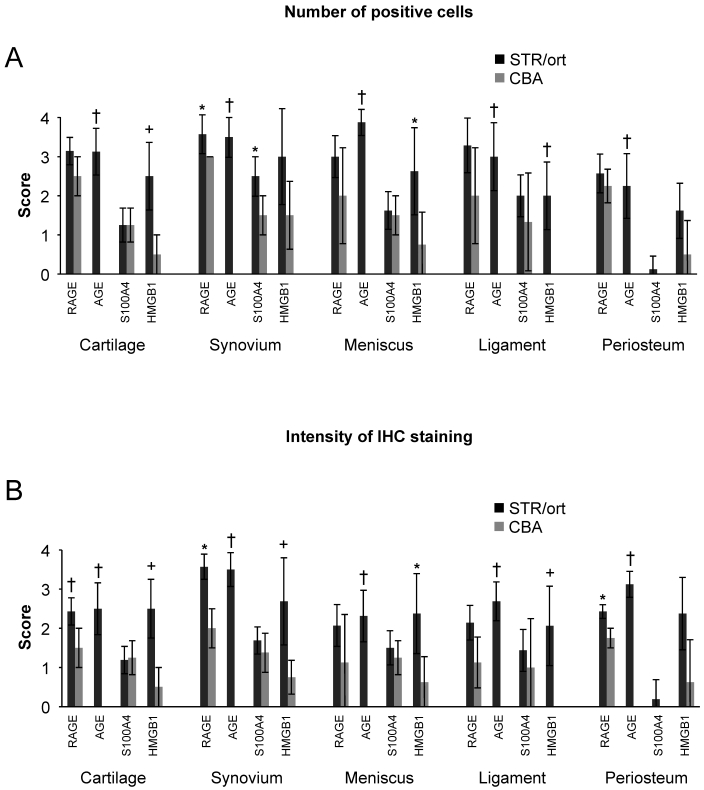
Staining intensity and number of positive cells for all markers except S100A4 showed consistent and significant increases in STR/ort mice (Figure 4). In particular, HMGB1 levels in the cartilage, synovium, ligaments, and meniscus exhibited strong correlation with cartilage histopathologic scores (Table 1). These scores also correlated with AGE levels detected in synovium, ligaments, and meniscus, whereas only RAGE levels in the synovium were associated with cartilage damage.
Figure 4.
Summary of immunohistochemical analysis (IHC) for inflammatory markers in knee joints. The staining in each mouse for RAGE, AGE, S100A4, and HMGB1 was scored for the (A) number of positive cells and (B) intensity of IHC staining in STR/ort and CBA joints. See Methods for details. Data points are shown as group mean ± 1 SD (n = 8 for STR/ort and n = 4 for CBA mice). Significance by 2-tailed Student t test (*, P < 0.05; +, P < 0.01; †, P < 0.001).
Table 1.
P values of comparison of immunohistochemical staining (IHC) intensity with cartilage histopathology scores
| Compared with score for |
|||
| IHC score | Hematoxylin and eosin | Toluidine blue | |
| Cartilage | RAGE | 0.0834 | 0.0473 |
| AGE | 0.2287 | 0.0770 | |
| S100A4 | 0.7707 | 0.7098 | |
| HMGB1 | 0.0146 | 0.0006 | |
| Synovium | RAGE | 0.0266 | 0.0732 |
| AGE | 0.0327 | 0.0019 | |
| S100A4 | 0.2732 | 0.4158 | |
| HMGB1 | 0.0032 | 0.0003 | |
| Ligament | RAGE | 0.5503 | 0.2447 |
| AGE | 0.0474 | 0.0024 | |
| S100A4 | 0.6836 | 0.3612 | |
| HMGB1 | 0.0018 | 0.0010 | |
| Meniscus | RAGE | 0.1240 | 0.0635 |
| AGE | 0.0162 | 0.0031 | |
| S100A4 | 0.8647 | 0.8067 | |
| HMGB1 | 0.0070 | 0.0006 | |
r2 values were determined from scoring cartilage histology (hematoxylin and eosin, Figure 1 B, or toluidine blue) at 20 wk compared with immunohistochemical staining intensity scores for RAGE, AGE, S100A4, and HMGB1 in the cartilage, synovium, ligaments, or meniscus (Figure 4 B). P values were determined by Spearman nonparametric test; significant (P < 0.05) differences are shown in bold. In the periosteum, histologic and immunohistochemical scorings showed no correlation (data not shown).
Serum cytokine analysis.
Eight (IL1β, IL3, IL4, IL5, IL10, IL12p70, IFNγ, and MIP1β) of the 21 serum cytokines analyzed were elevated approximately 2-fold in STR/ort compared with age-matched CBA mice at 20 wk (Table 2). These 8 cytokines remained elevated at 40 wk, and additional cytokines showing increased levels at the 40-wk mark included RANTES, IL1α, IL2, IL9, IL17, and IL12p40. Compared with their levels in 20-wk-old STR/ort mice, RANTES, IL1α, IL1β, IL9, IL17, GM–CSF, and KC were significantly higher in 40-wk-old STR/ort mice (P < 0.05). The use of age-matched CBA mice proved appropriate, given that the majority of the cytokines analyzed increased with age in CBA mice even though their levels were always lower than those in STR/ort mice.
Table 2.
Serum cytokine and chemokine levels in STR/ort and osteoarthritis-resistant CBA mice at 20 and 40 wk of age
| 20 wk |
40 wk |
|||||
| STR/ort | CBA | P | STR/ort | CBA | P | |
| IL5 | 37 ± 6 | 9 ± 2 | 0.000 | 45 ± 8 | 15 ± 12 | 0.000 |
| RANTES | 84 ± 11 | 66 ± 14 | 0.154 | 139 ± 25 | 80 ± 22 | 0.000 |
| IL1α | 28 ± 6 | 5 ± 3 | 0.252 | 126 ± 62 | 14 ± 9 | 0.000 |
| MIP1β | 449 ± 51 | 300 ± 66 | 0.000 | 477 ± 27 | 362 ± 73 | 0.002 |
| IL1β | 242 ± 99 | 64 ± 17 | 0.007 | 317 ± 125 | 154 ± 89 | 0.008 |
| IL12p70 | 361 ± 61 | 115 ± 123 | 0.003 | 419 ± 100 | 224 ± 182 | 0.008 |
| IFNγ | 46 ± 8 | 31 ± 9 | 0.007 | 55 ± 7 | 39 ± 15 | 0.009 |
| IL10 | 179 ± 55 | 73 ± 32 | 0.008 | 232 ± 67 | 134 ± 71 | 0.008 |
| IL3 | 52 ± 13 | 22 ± 11 | 0.002 | 60 ± 13 | 39 ± 16 | 0.011 |
| IL4 | 17 ± 4 | 8 ± 4 | 0.001 | 19 ± 3 | 14 ± 5 | 0.023 |
| IL9† | 59 ± 18 | 105 ± 39 | 0.019 | 115 ± 27 | 67 ± 31 | 0.001 |
| IL17† | 39 ± 4 | 64 ± 14 | 0.001 | 55 ± 11 | 41 ± 14 | 0.012 |
| IL2 | 13 ± 6 | 6 ± 3 | 0.093 | 20 ± 7 | 12 ± 8 | 0.016 |
| IL12p40 | 98 ± 17 | 75 ± 14 | 0.143 | 130 ± 14 | 104 ± 37 | 0.011 |
| VEGF | 32 ± 11 | 21 ± 3 | 0.071 | 33 ± 5 | 29 ± 17 | 0.484 |
| IL6 | 40 ± 25 | 18 ± 4 | 0.298 | 69 ± 53 | 29 ± 23 | 0.056 |
| IL13 | 321 ± 135 | 195 ± 111 | 0.199 | 474 ± 113 | 465 ± 240 | 0.925 |
| GM-CSF | 68 ± 21 | 42 ± 10 | 0.201 | 103 ± 14 | 73 ± 60 | 0.116 |
| KC | 38 ± 13 | 30 ± 9 | 0.483 | 67 ± 10 | 68 ± 28 | 0.414 |
| MCP1 | 110 ± 75 | 58 ± 11 | 0.429 | 166 ± 117 | 78 ± 60 | 0.073 |
| TNFα | 22 ± 25 | 10 ± 2 | 0.546 | 46 ± 54 | 17 ± 11 | 0.136 |
Cytokine and chemokine levels (pg/mL) were obtained by serum multiplex analysis and are shown as group mean ± 1 SD (STR/ort mice, n = 8 or 9; CBA mice, n = 5 or 6).
P values were obtained by nonparametric ANOVA; bolded values indicate factors that were significantly (P < 0.05) higher in STR/ort mice.
IL5, MIP1β, IL1β, IL12p70, IFNγ, IL10, IL3, and IL4 were all significantly (P < 0.05) elevated in STR/ort mice at both 20 and 40 wk, whereas IL9 and IL17 were significantly (P < 0.05) decreased in 20-wk-old but significantly (P < 0.05) increased in 40-wk-old STR/ort compared with CBA mice.
At 20 wk, histopathology scores of osteoarthritic cartilage at 20 wk were correlated most closely (P < 0.01) with IL12p70, IL3, IL4, MIP1b, IL5, and IL10 levels, indicating that increases in these cytokines coincided with the appearance of cartilage changes (Table 3). All of these cytokines were elevated at both 20 and 40 wk in STR/ort mice compared with CBA mice.
Table 3.
Correlation between systemic cytokine levels and cartilage histology scores in STR/ort mice at 20 wk of age.
| Cytokine | R2 | P |
| IL12p70 | 0.702 | 0.000 |
| IL3 | 0.675 | 0.001 |
| IL4 | 0.624 | 0.001 |
| MIP1β | 0.575 | 0.003 |
| IL5 | 0.507 | 0.006 |
| IL10 | 0.484 | 0.008 |
| IL17 | 0.441 | 0.441 |
| IL2 | 0.400 | 0.020 |
| IFNγ | 0.400 | 0.020 |
| VEGF | 0.380 | 0.025 |
| IL1β | 0.361 | 0.030 |
| IL13 | 0.306 | 0.050 |
| RANTES | 0.282 | 0.062 |
| IL1α | 0.273 | 0.067 |
| IL6 | 0.241 | 0.089 |
| KC | 0.196 | 0.130 |
| GM–CSF | 0.176 | 0.154 |
| IL9 | 0.188 | 0.160 |
| IL12p40 | 0.100 | 0.293 |
| TNFα | 0.056 | 0.436 |
| MCP1 | 0.049 | 0.468 |
Data were analyzed by nonparametric Wilcoxon 2-sample tests; P values lower than 0.05 are bolded.
Levels of IL12p70, IL3, IL4, MIP1β, IL5, IL10, IL2, IFNγ, VEGF, and IL1β were all significantly (P < 0.05) correlated with cartilage changes in STR/ort compared with CBA mice.
Discussion
Multiple factors are involved in the development of osteoarthritis, but the early initiating factors are particularly poorly understood. We assessed joints from the mouse spontaneous osteoarthritis model, STR/ort, for the presence of oxidative and inflammatory markers at early stages of osteoarthritis, when about 62% of animals showed histologic changes in the joints. Our results showed that the inflammation marker HMGB1 and oxidative stress marker AGE both were increased strongly in STR/ort joints but were absent in joints from the osteoarthritis-resistant CBA mouse strain. Systemic inflammation was also evident in STR/ort mice in that multiple inflammatory cytokines were upregulated, and their levels correlated with changes in knee joints. Therefore, these data support the presence of both local and systemic inflammation in STR/ort mice, similar to findings during human osteoarthritis.
The role of inflammation in the development of osteoarthritis is controversial. Compared with rheumatoid arthritis, osteoarthritis shows only mild synovial inflammation. However, mononuclear cell infiltrates and inflammatory cytokines are detected in the synovium of human patients with early osteoarthritis,5 a finding supported by a recent study in human patients, in which synovitis was a predictive factor for increased cartilage damage.3 Furthermore, a marker of low-grade inflammation and innate immunity activation, RAGE, has been detected in arthritic joints including human osteoarthritis cartilage, rheumatoid arthritis synovium, and synovial fluids.8,17,25,40,41 Consistent with the human data, our results showed widespread localization and expression of RAGE in STR/ort mice compared with CBA mice. Although the number of RAGE-positive cells sometimes was not increased in STR/ort joints, RAGE staining intensity was always increased in the cartilage and synovium of these samples. This finding likely reflects increased RAGE expression in response to ligand stimulation, which has been shown to provide a positive transcriptional feedback in environments with increased levels of RAGE ligands.37
RAGE ligands and the inflammation markers AGE, HMGB1, and S100A4 also have been reported in cartilage and synovium from arthritic patients.20,21,30,39,41,47 We detected AGE and HMGB1 in multiple locations (cartilage, synovium, meniscus, ligaments, and periosteum) in joints of STR/ort mice, whereas S100A4 (detected with an antibody free of crossreactivity to other related S100 proteins) was present at low levels only, contradictory to results reported for human osteoarthritis cartilage.47 The colocalization of RAGE and its ligands AGE and HMGB1 in hyperplastic synovium suggests the role of synovial RAGE activation in enhanced inflammation and production of matrix metalloproteases, leading to cartilage degradation. This notion was supported by the correlation of increased synovial levels of RAGE, AGE, and HMGB1 levels with changes in cartilage histopathology.
The role of increased levels of AGE and HMGB1 in STR/ort joints is unknown. Oxidative stress, potentially caused by mechanical stress (that is, by patellar displacement in these mice45), is increased in the joints of STR/ort mice, as shown by their elevated levels of 3-nitrotyrosine, a marker of oxidative stress,33 and may initiate intracellular accumulation of AGE.12,32 AGE generation was clearly an early event, in that all STR/ort mice had significantly increased AGE levels, but this glycation product could not be detected in the joints of CBA mice. AGE has been shown to cause permanent modification of intracellular proteins, cell death, and amplification of inflammatory pathways.12,16,39 We also noted that STR/ort mice had increased concentrations of HMGB1, as has been reported in human osteoarthritis.17,40 HMGB1 is a nonhistone nuclear protein with inflammatory cytokine-like properties when secreted by macrophages or released from dying cells.30,31,41 Intraarticular administration of HMGB1 induces synovial inflammation in mice.31 We detected HMGB1 in macrophage-like synoviocytes in STR/ort mice, and our preliminary in vitro experiments confirmed the increased proinflammatory phenotype of HMGB1-stimulated human monocytic cells (data not shown). Furthermore, HMGB1 levels in STR/ort cartilage positively correlated with cartilage histopathology.
Bone remodeling is part of osteoarthritis pathology, and our results showed that STR/ort mice have an increased number of periosteal osteoclasts. Additional studies are required to determine whether the increased levels of RAGE, AGE, and HMGB1 in STR/ort periosteum led to the increased osteoclast number and whether they stimulated bone resorption in these mice. However, a role of RAGE in bone resorption is supported by the reduced number of osteoclasts and increased bone mass in mice lacking RAGE.13,48 Similarly, HMGB1 knockout mice have fewer osteoclasts, and this protein chemoattracts osteoclasts.30,42 Furthermore, AGE can increase osteoclastic activity in diabetic mice.34
Our serum cytokine analyses showed that STR/ort mice exhibited low-grade systemic inflammation, unlike the CBA mouse strain which lacked any histologic sign of osteoarthritis.9,15,33,35,46 Several proinflammatory serum cytokines were elevated in STR/ort mice, including the macrophage-specific cytokines IL12p70, MIP1β, and IL1β, which likely enhance the susceptibility of this strain to osteoarthritis.7,22,23 This notion was supported further by the correlation between the concentrations of these cytokines and the cartilage histopathology scores in our study. Elevated serum cytokines (including IL17, IL12p70, and IL5) during spontaneous osteoarthritis have been documented in Hartley strain guinea pigs.18 The cytokine most increased in both models, IL5, has been linked to enhanced innate immunity by stress-induced modifications of autologous proteins and lipids11 For example, stimulation with oxidation-induced modification of LDL led to increased systemic IL5 levels in a mouse atherosclerosis model.6 Although more research is required to understand the role of IL5 in osteoarthritis models, changes associated with oxidative stress, such as increases in AGE, may act similarly to oxidation-induced modification of LDL to activate stress-induced signaling.
In the current study, we did not note consistent increases in IL6 and TNFα levels, 2 cytokines commonly increased in chronic inflammatory diseases and associated with adiposity,22,24,27 despite the 22% higher weight of STR/ort mice compared with CBA strain. This outcome may be due to lack of positive correlation between STR/ort body weight and the severity of osteoarthritis in knee joints, indicating that increased body weight is not a cause for osteoarthritis in these mice.19,26,35,38 Furthermore, although they weigh more than CBA mice, STR/ort mice weigh less than ICR and db/db mice and do not have abnormally large fat deposits.43 However, STR/ort mice have altered lipid metabolism, with increased serum cholesterol and triglyceride levels and low adiponectin levels.43 Learning whether and how these factors contribute to osteoarthritis development will require additional studies. Furthermore, although the reason for the increased cytokines in STR/ort mice is currently unclear, they may explain the increased incidence of periodontal disease and hepatomas reported in these mice, given that both conditions can be augmented by inflammatory conditions.28,29
In summary, our current study has demonstrated widespread and strong increases in the inflammatory markers AGE and HMGB1 in the joints of STR/ort mice. These changes are similar to those in human osteoarthritic joints and may contribute to joint destabilization and subsequent development of osteoarthritis. The colocalization of AGE and HMGB1with RAGE in hyperplastic synovium points to their roles as early amplifiers of synovial inflammation. In addition, STR/ort mice had increased serum cytokines, indicating a potential systemic mechanism for disease development. Because these mice share several characteristics with many human osteoarthritis patients, who exhibit low-grade inflammation both locally and systemically, the STR/ort strain represents a relevant animal model for testing new agents for osteoarthritis treatment.
Acknowledgments
We thank Xian-Jie Yu for statistics, Brian DelGuidice for illustrations, and David Lee-Parritz for critical review of the manuscript. We also appreciate support from Fen Chen, Peter DiBenedetto, Queendy Yu, Genzyme Animal Facility and Pathology Departments. This work was funded by Genzyme Corporation.
References
- 1.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayer F, Zimmer R, Bartnik E. 2006. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54:3533–3544 [DOI] [PubMed] [Google Scholar]
- 2.Ameye LG, Young MF. 2006. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol 18:537–547 [DOI] [PubMed] [Google Scholar]
- 3.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. 2005. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1-year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13:361–367 [DOI] [PubMed] [Google Scholar]
- 4.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, DeCaterina R. 2002. Advanced glycation end-products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 105:816–822 [DOI] [PubMed] [Google Scholar]
- 5.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. 2005. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64:1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. 2004. IL5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 114:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blom AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooijen N, van der Berg WB. 2007. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis. Arthritis Rheum 56:147–157 [DOI] [PubMed] [Google Scholar]
- 8.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. 2005. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol 175:8296–8302 [DOI] [PubMed] [Google Scholar]
- 9.Chambers MG, Bayliss MT, Mason RM. 1997. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis Cartilage 5:301–308 [DOI] [PubMed] [Google Scholar]
- 10.Chambers MG, Kuffner T, Cowan SK, Cheah KS, Mason RM. 2002. Expression of collagen and aggrecan genes in normal and osteoarthritic murine knee joints. Osteoarthritis Cartilage 10:51–61 [DOI] [PubMed] [Google Scholar]
- 11.Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. 2008. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med 263:479–488 [DOI] [PubMed] [Google Scholar]
- 12.DeGroot J, Verzijl N, Wenting-van Wijk MJ, Jacobs KM, Van El B, Van Roermund PM, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP. 2004. Accumulation of advanced glycation end-products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum 50:1207–1215 [DOI] [PubMed] [Google Scholar]
- 13.Ding KH, Wang ZZ, Hamrick MW, Deng ZB, Zhou L, Kang B, Yan SL, She JX, Stern DM, Isales CM, Mi QS. 2006. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes-related bone loss. Biochem Biophys Res Commun 340:1091–1097 [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE. 2007. Activation of RAGE induces elevated O2– generation by mononuclear phagocytes in diabetes. J Leukoc Biol 81:520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannelly J, Chambers MG, Dudhia J, Hembry RM, Murphy G, Mason RM, Bayliss MT. 2002. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage 10:722–733 [DOI] [PubMed] [Google Scholar]
- 16.Franke S, Sommer M, Ruster C, Bondeva T, Marticke J, Hofmann G, Hein G, Wolf G. 2009. Advanced glycation end-products induce cell-cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res Ther 11:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Arnandis I, Guillen M, Gomar F, Pelletier J, Martel-Pelletier J, Alcaraz M. 2010. High-mobility group box 1 potentiates the proinflammatory effects of interleukin 1β in osteoarthritic synoviocytes. Arthritis Res Ther 12:R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huebner JL, Kraus VB. 2006. Assessment of the utility of biomarkers of osteoarthritis in the guinea pig. Osteoarthritis Cartilage 14:923–930 [DOI] [PubMed] [Google Scholar]
- 19.Jaeger K, Selent C, Jaehme W, Mahr S, Goebel U, Ibrahim S, Vollmar B, Mueller-Hilke B. 2008. The genetics of osteoarthritis in STR/ort mice. Osteoarthritis Cartilage 16:607–614 [DOI] [PubMed] [Google Scholar]
- 20.Klingelhöfer J, Senolt L, Baslund B, Nielsen GH, Skibhoj I, Pavelka K, Neidhart M, Gay S, Ambartsumian N, Hansen BS, Petersen J, Lukanidin E, Grigorian M. 2007. Upregulation of metastasis-promoting S100A4 (Mts1) in rheumatoid arthritis: putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 56:779–789 [DOI] [PubMed] [Google Scholar]
- 21.Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furle R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE. 2002. High-mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum 46:2598–2603 [DOI] [PubMed] [Google Scholar]
- 22.Lementowski PW, Zelicof SB. 2008. Obesity and osteoarthritis. Am J Orthop 37:148–151 [PubMed] [Google Scholar]
- 23.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. 2005. Innate immunity and inflammation in aging: a key for understanding age-related diseases. Immun Ageing 2:8–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, Spector TD. 2009. Interleukin 6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum 60:2037–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD. 2005. Articular chondrocytes express the receptor for advanced glycation end-products: potential role in osteoarthritis. Arthritis Rheum 52:2376–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. 2001. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage 9:85–91 [DOI] [PubMed] [Google Scholar]
- 27.Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, Sculco TP, Crow MK. 2007. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage 15:516–523 [DOI] [PubMed] [Google Scholar]
- 28.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. 2004. NFκB functions as a tumor promoter in inflammation-associated cancer. Nature 431:461–466 [DOI] [PubMed] [Google Scholar]
- 29.Pischon N, Heng N, Bernimoulin JP, Kieber BM, Willich SN, Pischon T. 2007. Obesity, inflammation, and periodontal disease. J Dent Res 86:400–409 [DOI] [PubMed] [Google Scholar]
- 30.Pisetsky DS, Erlandsson-Harris H, Andesson U. 2008. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther 10:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, Tarkowski A. 2003. High-mobility group box chromosomal protein 1, a DNA-binding cytokine, induces arthritis. Arthritis Rheum 48:1693–1700 [DOI] [PubMed] [Google Scholar]
- 32.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. 2005. Advanced glycation end-products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15:16R–28R [DOI] [PubMed] [Google Scholar]
- 33.Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, Glueck D, Heijnen H, Mason R, Crapo J. 2005. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum 52:3479–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. 2003. A role for advanced glycation end-products in diminished bone healing in type 1 diabetes. Diabetes 52:1502–1510 [DOI] [PubMed] [Google Scholar]
- 35.Sarukawa J, Takahashi M, Doi M, Suzuki D, Nagano A. 2010. A longitudinal analysis of urinary biochemical markers and bone mineral density in STR/ort mice as a model of spontaneous osteoarthritis. Arthritis Rheum 62:463–471 [DOI] [PubMed] [Google Scholar]
- 36.Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini A, Atzeni F, Canesi B. 2005. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum 35:1–10 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt AM, Yan SD, Yan SF, Stern DM. 2001. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108:949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokoloff L, Mickelsen O, Silverstein E, Jay GE, Jr, Yamamoto RS. 1960. Experimental obesity and osteoarthritis. Am J Physiol 198:765–770 [DOI] [PubMed] [Google Scholar]
- 39.Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, Lafeber FP, Toes RE, DeGroot J. 2006. Activation of receptor for advanced glycation end-products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum 54:253–263 [DOI] [PubMed] [Google Scholar]
- 40.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Makino H. 2006. Increased expression of receptor for advanced glycation end-products by synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum 54:97–104 [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyma I. 2003. High-mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum 48:971–981 [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi N, Yoshida K, Ito T, Tsuda M, Mishima Y, Furumatsu T, Ronfani L, Abeyama K, Kawahara K, Komiya S, Maruyama I, Lotz M, Bianchi ME, Asahara H. 2007. Stage-specific secretion of HMGB1 in cartilage regulates endochondrial ossification. Mol Cell Biol 27:5650–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchida K, Urabe K, Naruse K, Ogawa Z, Mabuchi K, Itoman M. 2009. Hyperlipidemia and hyperinsulinemia in the spontaneous osteoarthritis mouse model, STR/ort. Exp Anim 58:181–187 [DOI] [PubMed] [Google Scholar]
- 44.Walton M. 1978. A spontaneous ankle deformity in an inbred strain of mouse. J Pathol 124:189–194 [DOI] [PubMed] [Google Scholar]
- 45.Walton M. 1979. Patella displacement and osteoarthrosis of the knee joint in mice. J Pathol 127:165–172 [DOI] [PubMed] [Google Scholar]
- 46.Watters JW, Chen C, Pickarski M, Wesolowski GA, Zhuo Y, Hayami T, Wang W, Szumiloski J, Phillips RL, Duong LT. 2007. Inverse relationship between matrix remodeling and lipid metabolism during osteoarthritis progression in the STR/ort mice. Arthritis Rheum 56:2999–3009 [DOI] [PubMed] [Google Scholar]
- 47.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. 2006. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end-products. Arthritis Rheum 54:2901–2911 [DOI] [PubMed] [Google Scholar]
- 48.Zhou Z, Immel D, Xi CX, Bierhaus A, Feng X, Mei L, Nawroth P, Stern DM, Xiong WC. 2006. Regulation of osteoclast function and bone mass by RAGE. J Exp Med 203:1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]