Abstract
Intranasal application of zinc gluconate has commonly been used to treat the common cold. The safety of this treatment, however, has come into question recently. In addition to a United States recall of a homeopathic product that contains zinc gluconate, abundant literature reports cytotoxic effects of zinc on the olfactory epithelium. Additional research suggests that divalent cations (such as zinc) can block ion channels that facilitate the transduction of odors into electrical signals on the olfactory epithelium. The purpose of the current study was 2-fold: to confirm whether zinc gluconate causes anosmia and to reveal whether any other divalent cationic compounds produce a similar effect. Groups of mice underwent a buried food-pellet test to gauge olfactory function and then were nasally irrigated with 1 of 3 divalent cationic compounds. When tested after treatment, mice irrigated with zinc gluconate and copper gluconate experienced a marked increase in food-finding time, indicating that they had lost their ability to smell a hidden food source. Control mice irrigated with saline had a significantly lower increase in times. These results confirm that zinc gluconate can cause anosmia and reveal that multiple divalent cations can negatively affect olfaction.
Abbreviations: BFP, buried food pellet
An over-the-counter medicine that supposedly reduces the severity and duration of the common cold is applied intranasally and is composed primarily of a diluted form of zinc gluconate. This particular remedy has enjoyed moderate popularity, selling over 35 million units since its introduction in 1999.10 Despite its widespread use, the product has recently been linked with the loss of the sense of smell. After receiving more than 130 consumer reports of product-induced anosmia, the US Food and Drug Administration warned the public against using these nasal gels and swabs, and these products were removed from the market.17 The manufacturer maintains that its products are completely innocuous and dismisses the cases of anosmia as a result of the common cold.10
However, the hazards associated with the intranasal application of zinc have been well documented. During the testing of a potential polio treatment in the 1930s, zinc sulfate was found to disrupt the sense of smell in children.16 Numerous subsequent studies have produced olfactory nerve damage and anosmia in the mouse through nasal irrigation of ZnSO4.5,11,14 In a 1982 study on the effects of various salts on the olfactory epithelium of catfish,2 both zinc chloride and zinc sulfate damaged olfactory cells. The same study also demonstrated that anions were inactive on the olfactory epithelium since sodium sulfate and sodium chloride produced no effect. Furthermore, medical evaluations of patients exhibiting anosmia after use of the product mentioned earlier have isolated zinc application as the cause of sensory loss in several subjects.1,7
The process by which zinc causes olfactory dysfunction is not fully understood, but it most likely occurs during the transduction of odors into electrical signals on the olfactory epithelium. When odorants enter the nose, they are believed to attach to olfactory receptors, stimulating the release of cAMP and the formation of inositol-l,4,5-trisphosphate. These compounds open various ‘cation channels,’ which allow Ca2+ ions to enter the olfactory neurons. Ca2+ entry eventually results in depolarization of the cells, which consequently release action potentials that carry information to the olfactory bulb.12 Ba2+ was shown to block the aforementioned inositol-l,4,5-trisphosphate- and cAMP-gated channels in animals12 and in humans.7 If these channels are nonspecific in fact, they could be blocked by divalent cations other than Ca2+, thereby explaining why Zn2+ causes anosmia.
The manufacturer of the aforementioned product asserts that results from studies showing the harmful effects of zinc sulfate cannot be applied to zinc gluconate.9 Zinc gluconate, however, possesses the same divalent cation present in ZnSO4 and therefore could negatively affect olfactory function in a similar manner. Before 2009, this hypothesis was empirically tested only once in a 2007 study involving the direct effects of intranasal zinc gluconate on olfaction. The findings of this study failed to support claims that the product causes anosmia, but it should be noted that this study was funded in part by the manufacturers,15 and a similar experiment in 2009 yielded somewhat conflicting results.8 Although one study assessed the effects of several salts on the olfactory epithelium,2 no previous studies have evaluated the effects of divalent cations other than zinc on olfactory function.
Given the apparent void in research on this subject, the current study was performed to confirm whether zinc gluconate causes anosmia and to reveal whether other divalent cationic compounds produce a similar effect.
Materials and Methods
Animals.
Female mice (Mus musculus; CD1; viral-antigen–free;n= 42; age, 76 or 77 d) were provided by Charles River Laboratories (Wilmington, MA). Mice were housed in groups of 3 or 4 in heavy-duty polysulfone cages (18.4 × 29.2 × 12.7 cm; Alternative Design, Siloam Springs, AR). Water was provided ad libitum, and mice were allowed free access to irradiated laboratory rodent food (Harlan Laboratories, Indianapolis, IN) at all times except 17 h before behavioral tests. All experimental procedures were approved by the University of Central Florida Institutional Animal Care and Use Committee. Furthermore, all animals were treated humanely during this research, in accordance with the Guide for the Care and Use of Laboratory Animals.6
Compounds.
The compounds used for nasal irrigation (zinc gluconate, copper gluconate, and magnesium gluconate) were manufactured by MP Biomedicals (Solon, OH) and purchased from Fisher Scientific (Pittsburg, PA). Each salt was put into solution with deionized water in a concentration of 33 mM, the same concentration at which zinc gluconate is found in aforementioned commercial product. In addition, PBS was purchased from Fisher Scientific.
Pretreatment behavioral tests.
To assess olfactory function, all members of the zinc gluconate control and experimental groups were evaluated by using a modification of the buried food-pellet (BFP) test, similar to a method used previously.13 This behavioral test, which has been used numerous times to gauge olfactory function,3-5,14,18 tasks the subjects with relying on the sense of smell to locate food.
After the animals fasted for 17 h, one mouse was placed in a 25.9 × 47.6 × 20.9 cm rat cage (Allentown, Allentown, NJ) containing an approximately 4-g pellet of rodent food buried under approximately 3 cm of Sani-Chips bedding (Harlan Laboratories, Indianapolis, IN). The time (in seconds) that the mouse required to uncover the food and grab it in its forepaws or teeth was recorded. The mouse was allowed to consume a portion of the pellet and then was returned to its home cage. Each control (n = 7) and experimental (n = 7) mouse was tasked with finding the food in the same manner. The bedding was changed for each mouse, with the food being buried in the same location. Each subject then underwent the same test 2 more times, with the food hidden in a new position chosen for each round of trials. By the end of these pretreatment trials, each mouse had located the pellet in the same 3 locations, with these scores setting a baseline for olfactory function.
Zinc gluconate nasal irrigation.
The zinc gluconate treatment began an hour after completion of pretreatment testing. The first control mouse was placed in a DecapiCone animal restraint (Braintree Scientific). While the subject was held in place by an experienced laboratory animal technologist, an 8-mm 30-gauge needle (blunted at the tip) was inserted approximately 2 to 3 mm into the mouse's left nostril. PBS (100 µL; Fisher Scientific) then forcefully was expelled into the nostril from a syringe attached to the needle. Animal feeding needles were considered but were too large for insertion into a mouse's nostril. After irrigation, the mouse was returned to its home cage. This process was repeated for each control mouse, and each experimental mouse was irrigated with 100 µL 33 mM zinc gluconate solution. Because several mice experienced tremors and impaired respiration after being flushed with 100 µL solution, the volume injected subsequently was reduced to 50 µL when each animal's right nostril was irrigated with the same solution 45 min later. The mice responded more favorably to the smaller volume, so 50 µL was used in future irrigations. Despite the observed difficulties, the survival rate of the treatment was in excess of 92%.
Posttreatment behavioral tests.
At 30 min after treatment, each control (n = 7) and remaining experimental (n =6) mouse underwent the BFP test only once. Mice that could not find the pellet in 6 min were returned to the home cage, and latency was recorded as 360 s. The BFP test was repeated at 48 and 96 h after the first pretreatment time trial. All mice were fasted 17 h prior to each test. Food-finding time once again was recorded as 360 s for any mouse that could not find the food in 6 min.
Copper gluconate tests and treatments.
Copper gluconate control (n = 7) and experimental (n = 7) mice were fasted and underwent BFP tests. An hour after completion of the time trials, each mouse was nasally irrigated in the same manner as described earlier. The only modification implemented was the replacement of zinc gluconate solution with copper gluconate solution, 50 µL of which was expelled into each nostril of each mouse. The survival rate for these irrigations was 100%. Posttreatment behavioral tests were performed at 30 min, 48 h, and 144 h after the first pretreatment test. The last posttreatment tests were performed 144 h after the first test, because the animal husbandry staff omitted to fast the mice in time for the 96-h tests; they were performed at the next possible time that would avoid any circadian variation in food-finding times. Animals were fasted 17 h before each of the last 2 trials.
Magnesium gluconate tests and treatments.
One final compound—magnesium gluconate—was tested. Experimental (n = 7) and control (n = 7) mice were fasted and evaluated in BFP tests as previously outlined; mice then were irrigated with either 50 µL saline or magnesium gluconate solution. No fatalities occurred during these irrigations, although one mouse died several hours after treatment. Posttreatment time trials were performed 30 min, 48 h, and 96 h after treatment, with the subjects being fasted 17 h prior to the last 2 tests.
Follow-up behavioral tests.
After the last BFP test, the mice given copper gluconate and zinc gluconate were allowed to reacclimate for about 3 wk. At 32 d after pretreatment testing, each mouse was evaluated in the BFP test once more to check for any change in olfactory function. After termination of the study, mice were donated to the University of Central Florida Wild Animal Facility to be used for teaching purposes or were transferred to other approved projects.
Statistical analysis.
The mean latency of each experimental group was compared with that of its corresponding control group by using 2-way ANOVA with an alpha level of 0.05. In addition, a one-way ANOVA was used to compare the mean food-finding times of the 3 control groups. All statistical analysis was performed by using PASW Statistics 18 for Windows (SPSS, Chicago, IL).
Results
Pretreatment food-finding ability.
During the pretreatment behavioral tests, every mouse in every group was able to find the buried pellet in less than 6 min. As illustrated in Figures 1 through 3, the mean food-finding times were relatively low in the pretreatment trials (trials 1 to 3), and there was no significant difference between the means of the experimental and control groups.
Performance after zinc gluconate treatment.
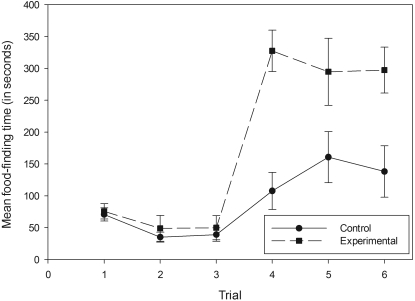
In the posttreatment behavioral tests, both the zinc gluconate control and experimental groups experienced an increase in mean food-finding times (Figure 1). The mean latency of the experimental group was significantly higher than that of the control group in posttreatment tests; treatment had a significant effect (F[1,36] = 34.967; P < 0.001), whereas trial had no significant effect (F[2,36] = 0.015; P = 0.985). There was no significant interaction between treatment and trial (F[2,36] =0.846; P = 0.437). In most of these trials, subjects in the experimental group were unable to find the food at all, whereas all control mice found the pellet every time, with one exception.
Figure 1.
Mean food-finding times for the zinc gluconate control and experimental groups. Trials 1, 2, and 3 were performed the morning before treatment. Trial 4 was performed 30 min after treatment, and trials 5 and 6 occurred 48 and 96 h after trial 1, respectively. All data points represent mean ± SEM.
Behavioral differences between the groups were observed in addition to the differences in food-finding times. When placed in the test cage, control mice appeared to constantly sniff the bedding on which they walked and dug into the bedding very little until they had located the food. Treated mice were more inclined to dig and burrow in all areas of the cage, rarely sniffing the bedding. These tendencies were observed with very few exceptions. The constant digging occasionally resulted in an experimental mouse finding the pellet by chance.
Given the dramatic increase in latency and obvious behavioral differences, 5 of the 6 surviving treated mice were declared anosmic. The remaining treated mouse found the pellet in each posttreatment test and did not exhibit any of the behaviors characteristic of the other experimental subjects. This mouse appeared to retain its olfactory function, perhaps due to poor nasal irrigation technique.
Performance after copper gluconate treatment.
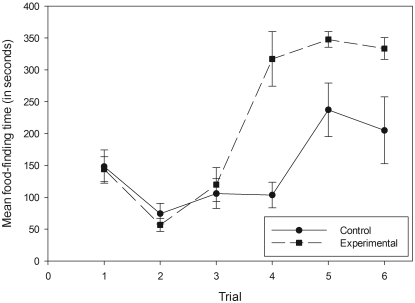
As for zinc gluconate, copper gluconate treatment produced a marked effect (Figure 2). In the posttreatment tests, the mean food-finding time of the experimental group again was significantly higher than that of the control group, with treatment having a significant effect (F[1,36] = 28.312; P < 0.001). Trial had no significant effect (F[2,36] = 2.978; P = 0.064), and there was no significant interaction between treatment and trial (F[2,36] = 1.262; P = 0.265).
Figure 2.
Mean food-finding times for the copper gluconate control and experimental groups. Trials 1, 2 and 3 were performed the morning before treatment. Trial 4 was performed 30 min after treatment, and trials 5 and 6 occurred 48 and 144 h after trial 1, respectively. All data points represent mean ± SEM.
The same behavioral differences observed during the zinc gluconate posttreatment trials were apparent during the copper gluconate trials. All 7 of the treated mice were declared anosmic: 3 of them never found the food pellet after treatment, and the remaining 4 mice located the pellet only once.
Performance after magnesium gluconate treatment.
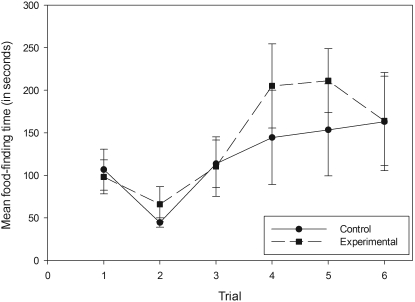
Magnesium gluconate treatment produced little to no effect (Figure 3), with no significant difference between the mean food-finding times of the control and experimental groups in the posttreatment BFP tests (F[1,34] = 1.917; P = 0.175). No behavioral differences between the groups were observed, and none of the mice were declared anosmic.
Figure 3.
Mean food-finding times for the magnesium gluconate control and experimental groups. Trials 1, 2 and 3 were performed the morning before treatment. Trial 4 was performed 30 min after treatment, and trials 5 and 6 occurred 48 and 96 h after Trial 1, respectively. All data points represent mean ± SEM.
Control group comparison.
One-way ANOVA comparing the posttreatment mean food-finding times of the 3 control groups revealed no significant difference (F[2,58] = 0.867; P = 0.426).
Follow-up behavioral tests.
In BFP tests performed 32 d after treatment, the effects of the zinc gluconate and copper gluconate treatments had diminished. Significant differences were no longer detected between the mean food-finding times of the experimental and control groups.
Discussion
Mice nasally irrigated with a zinc gluconate solution lost their ability to find a hidden food pellet. Whereas control mice irrigated with saline were able to locate the pellet, treated mice could not detect the volatile odors emitting from the familiar food source. These results support the notion that zinc gluconate can impair olfactory function, consistent with previous findings.8
The results of a 2007 study on the effects of zinc gluconate on olfaction did not support the claims that a commercial solution containing zinc gluconate causes anosmia.15 In mice irrigated with volumes of 2 and 8 µL, the authors reported only minimal effects on olfaction and the olfactory epithelium. Mice irrigated with 50 µL zinc gluconate were noted to be “hyposmic, but clearly not anosmic.”15 The authors also argued that the application of 50 µL was not relevant in proving that the product causes anosmia, because that volume would equal about 94 times the human dose.15
Although the volume of zinc gluconate used in the current study is many times the recommended human dose, using a smaller volume may have yielded misleading results. It is extremely difficult to ensure that small volumes of liquid actually reach the olfactory epithelium in unanaesthetized mice.8 Using quantities as small as 2 and 8 µL, it is entirely possible that the authors of the previous study15 treated only a portion of the olfactory epithelium or missed the epithelium altogether. Furthermore, the increased volume of zinc gluconate cannot be assumed to fully account for the observed anosmia. Large volumes of other intranasal medications have been shown not to cause olfactory dysfunction.8
The volume used in the current study was determined by referencing a previously published table,11 which displayed volumes administered in behavioral studies using nasal irrigation of zinc sulfate to disrupt olfaction in mice. The 100-µL aliquot we chose initially was a commonly used volume that was deemed large enough to reasonably ensure contact with the olfactory epithelium. However, when one mouse died during the first round of the zinc gluconate treatment, the volume was reduced to 50 µL, the next smallest volume listed in the aforementioned article.11
When used as directed, the commercial product for humans enters only the nostrils and does not get near the olfactory epithelium. This situation may lead some to believe that nasal irrigation is not an appropriate method for testing the toxicity of zinc gluconate. However, many patients who experienced anosmia after use report sniffing the product in deeply.1 In this way, these subjects could have forced the solution onto the olfactory epithelium. In addition, the gel form of the product is forcefully expelled from a nasal pump. Those users who happen to have a ‘straight shot’ anatomically to the olfactory cleft could potentially squirt the product directly onto the olfactory receptors.
Another difference between administration of the commercial product and nasal irrigation is the fact that that the gel form remains in the nasal cavity. The current study involved nasal irrigation of an aqueous solution instead of application of a gel because applying large amounts of gel into the nasal cavity likely would make it difficult for the mice to breathe. Furthermore, the objective was merely contact with the olfactory epithelium; the adverse reaction to zinc gluconate treatment reportedly is immediate,1 so there was no benefit to having the solution remain in the nasal cavity of the mice in the current study.
The anosmia we observed was only temporary; the anosmic mice clearly had regained olfactory function when they were tested 1 mo after the first BFP test. However, the fact that anosmia was transient does not negate the fact that the anosmia occurred. Sensory loss of any duration is detrimental to the health of an organism.
At first glance, some readers may perceive substantial variability in the mean food-finding times of the control groups displayed in the figures. Handling-associated stress was the most likely cause of the increases control food-finding times, along with the fact that simply expelling liquid into the nasal cavity may cause slight sensory loss. However, the increases in food-finding times of the zinc and copper experimental groups were significantly higher than those observed in their corresponding control groups. In addition, the statistical analysis showed no significant effect of trial on food-finding time, and no significant interaction between the effects of treatment and trial. Furthermore, a one-way ANOVA comparing all 3 control groups found no statistically significant difference between groups.
Even more important than the confirmation that zinc gluconate can cause anosmia are the results revealing that other divalent cationic compounds can produce a similar effect. Mice treated with copper gluconate also experienced olfactory dysfunction, suggesting that the zinc-induced anosmia phenomenon is not isolated to zinc. Although another author observed that certain other divalent cationic compounds were “moderately active” on olfactory receptor cells,2 ours is the first study in which such a compound has been shown to negatively affect olfaction.
Our discovery that Cu2+ causes anosmia supports the theory that divalent cations can block channels that facilitate the sense of smell. However, magnesium gluconate did not produce anosmia in any of the subjects. This result can probably be explained by the small molecular mass of magnesium. The ions that have been shown to cause anosmia or block cation channels on the olfactory epithelium (zinc, barium, and copper) all have molecular masses larger than that of calcium. These larger ions are more likely to physically block Ca2+ channels than are magnesium ions, which has a molecular mass considerably less than that of calcium. Therefore, a reasonable conclusion is that divalent cations larger than Ca2+ can negatively affect olfaction.
In conclusion, this study has demonstrated the harmful effects of certain compounds on olfaction. Using a well-established test of olfactory function, we found that mice irrigated intranasally with zinc gluconate and copper gluconate became anosmic. Considering the importance of the sense of smell in both human survival and quality of life, further research must be performed to discover how and why these divalent cationic compounds cause olfactory dysfunction. Until the risks associated with these compounds are fully understood, they may not be safe for use in the nasal passage.
Acknowledgments
We thank Stacey Cohen (Charles River Laboratories) for arranging a gratis order that provided the mice used in this study and Richard Carter (Harlan Laboratories) for donating the Sani-Chips bedding needed for the food-finding tests. We also thank Dr Joseph Bielitzki, Jennifer Reed, Jenna Richards, and Dr Annette Khaled (University of Central Florida) for their continued advice and support during development and execution of the research.
References
- 1.Alexander TH, Davidson TM. 2006. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope 116:217–220 [DOI] [PubMed] [Google Scholar]
- 2.Cancalon P. 1982. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell 14:717–733 [DOI] [PubMed] [Google Scholar]
- 3.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. 2002. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419:70–74 [DOI] [PubMed] [Google Scholar]
- 4.Getchell TV, Kwong K, Saunders CP, Stromberg AJ, Getchell ML. 2006. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol Behav 87:848–856 [DOI] [PubMed] [Google Scholar]
- 5.Harding JW, Getchell TV, Margolis FL. 1978. Denervation of the primary olfactory pathway in mice. V. Long-term effects of intranasal ZnSO4 irrigation on behavior, biochemistry, and morphology. Brain Res 140:271–285 [DOI] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 7.Jafek BW, Linschoten MR, Murrow BW. 2004. Anosmia after intranasal zinc gluconate use. Am J Rhinol 18:137–141 [PubMed] [Google Scholar]
- 8.Lim JH, Davis GE, Wang Z, Li V, Wu Y, Rue TC, Storm DR. 2009. Zicam-induced damage to mouse and human nasal tissue. PLoS One 4: e7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matrixx Iniatives [Internet]. 2009. FDA questions. [Cited 11 November 2009]. Available at: http://www.zicam.com/products/faqs/fda
- 10.Matrixx Iniatiaves [Internet]. 2009. Matrixx initiatives voluntarily withdraws Zicam Cold remedy swabs, Zicam cold remedy nasal gel. [Cited 10 November 2009]. Available at: http://www.matrixxinc.com/releasedetail.cfm?ReleaseID=390200
- 11.McBride K, Slotnick B, Margolis FL. 2003. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses 28:659–670 [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto T, Restrepo D, Teeter JH. 1992. Voltage-dependent and odorant-regulated currents in isolated olfactory receptor neurons of the channel catfish. J Gen Physiol 99:505–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. 2004. Olfactory function in apoE knockout mice. Behav Brain Res 150:1–7 [DOI] [PubMed] [Google Scholar]
- 14.Sipos ML, Wysocki CJ, Nyby JG, Wysocki L, Nemura TA. 1995. An ephemeral pheromone of female house mice: perception via the main and accessory olfactory systems. Physiol Behav 58:529–534 [DOI] [PubMed] [Google Scholar]
- 15.Slotnick B, Sanguino A, Husband S, Marquino G, Silberberg A. 2007. Olfaction and olfactory epithelium in mice treated with zinc gluconate. Laryngoscope 117:743–749 [DOI] [PubMed] [Google Scholar]
- 16.Tisdall FF, Brown A, Defries DR. 1938. Persistent anosmia following zinc sulfate nasal spraying. J Pediatr 18:60–62 [Google Scholar]
- 17.United States Food and Drug Administration [Internet]. 2009. FDA advises consumers not to use certain Zicam cold remedies. [Cited 08 November 2009]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm167065.htm
- 18.Yee KK, Rawson NE. 2000. Retinoic acid enhances the rate of olfactory recovery after olfactory nerve transection. Brain Res Dev Brain Res 124:129–132 [DOI] [PubMed] [Google Scholar]