Abstract
Reproductive experience in female rats modifies acquired behaviors, induces long-lasting functional neuroadaptations and can also modify spatial learning and memory. The present study supports and expands this knowledge base by employing the Morris water maze, which measures spatial memory. Age-matched young adult (YNG) nulliparous (NULL; nonmated) and primiparous (PRIM; one pregnancy and lactation) female rats were tested 15 d after the litter's weaning. In addition, corresponding middle-aged (AGD) PRIM (mated in young adulthood so that pregnancy, parturition, and lactation occurred at the same age as in YNG PRIM) and NULL female rats were tested at 18 mo of age. Behavioral evaluation included: 1) acquisition of reference memory (platform location was fixed for 14 to 19 d of testing); 2) retrieval of this information associated with extinction of the acquired response (probe test involving removal of the platform 24 h after the last training session); and 3) performance in a working memory version of the task (platform presented in a novel location every day for 13 d, and maintained in a fixed location within each day). YNG PRIM outperformed NULL rats and showed different behavioral strategies. These results may be related to changes in locomotor, mnemonic, and cognitive processes. In addition, YNG PRIM exhibited less anxiety-like behavior. Compared with YNG rats, AGD rats showed less behavioral flexibility but stronger memory consolidation. These data, which were obtained by using a well-documented spatial task, demonstrate long lasting modifications of behavioral strategies in both YNG and AGD rats associated with a single reproductive experience.
Abbreviations: AGD, aged female rats; MWM, Morris water maze; NULL, nulliparous female rats; PRIM, primiparous female rats; YNG, group of young female rats; ITI, intertrial interval
Reproductive experience in female rats modulates cognitive processes and behavioral, neuroendocrine, and neurochemical function.6,7,9,11,14,16,23-26,30,35,36,39,40,52,56 The maternal brain undergoes functional changes during pregnancy and lactation,15,28,42,45,46,52,56 which are associated with enhanced behavioral repertoire. For instance, sensitivity to opiates is reduced and maternal aggression is increased in pregnant and lactating animals, respectively.29,39,40,42 In addition, both the intensity of striatal dopaminergic responses and basal serum prolactin levels are reduced in maternally-experienced female rats.5,7,8,11,23,24,45,46 Some acquired behaviors and functional neuroadaptations are long-lasting and suggest a powerful effect associated with reproduction and care of young.
Over the past decade, reproductive experience has been reported to modify spatial learning and memory processes20,30,49,50,51 and hippocampal and olfactory bulb neurogenesis.17,48,50,55 These changes are likely due to a combination of hormonal exposure during pregnancy, coupled to maternal–offspring interactions during lactation; the modifications to brain enhance the requisite tasks performed during motherhood, such as those related to providing food and protecting the offspring.16,30,33,49-51 Multiparous and primiparous (PRIM) female rats exhibit better performance in the radial-arm maze compared with nulliparous (NULL) female rats.48,49 Other evidence indicates that the initial reproductive experience may stimulate greater memory-related changes than subsequent maternal experiences,49-51 and the effects are long-lasting.21,39
The parity-induced modifications in learning and memory are tied to pregnancy and lactational hormonal profiles, especially estrogen and progesterone, oxytocin, and possibly prolactin, which can trigger modulations related to nervous system plasticity.15,17,49-51,59 The combination of pregnancy and lactation appears to be more effective at inducing changes in learning and memory processes than pregnancy alone.30,28,33,49-51 Acquired maternal behaviors are retained for future mothering, referred to as maternal memory, such that one-time mothers can be induced more rapidly. In addition, hippocampal modifications, including neurogenesis17 and synaptogenesis,37 and increased oxytocin-induced long-term potentiation59 have been shown to affect spatial learning and memory in parous female rats.
Sex hormones also have been implicated in reference and working memory processes. For example, female rats exhibit better performance in a nonspatial cue version of the Morris water maze (MWM) when tested during proestrus compared with estrus; in contrast, when tested in a spatial version of the task, female rats showed better performance when tested during estrus compared with proestrus.61 However, the stage of the estrous cycle did not affect the results obtained in working and reference memory tests using a dry-land maze.49-51 High circulating levels of estradiol may impair working memory, and low circulating levels may facilitate it.22 There have been reports that female rats in proestrus exhibit more spatial errors;61 however, there are also reports showing that estrus cycle does not interfere with learning and memory processes.49,51 Corticosterone and stress also can influence working memory processes,13,18,19,34,58 and parity affects stress and stress hormones.4 In addition, when it occurs during pregnancy, stress can abolish memory improvements attributed to motherhood.34 In addition, oxytocin plays a role in maternal experience and can facilitate long-lasting spatial learning during motherhood by increasing oxytocin synthesis and receptor expression in the hippocampus.43,59
Motor and motivational processes influence learning and memory acquisition as a function of reproductive experience in rats.24,30,49-51 There have been demonstrations that learning and memory processes in female rats exposed to reproductive experience are facilitated30,34,51 and that these changes may be related to hippocampal plasticity,17,31,48-51,55 monoaminergic activity,11,23,24,26,35,36,56 anxiety and stress.10,34,62 These modifications are reported to be long-lasting after lactation, including reduced open field activity.11,24
The present study investigated the effects of parity on learning and memory by using the MWM, a paradigm that involves different stress and motivational stimuli compared with the dry-land mazes applied in prior studies (for example, references 30 and 49 through 51). The MWM assesses long-term spatial reference memory by keeping the hidden platform location constant throughout all training sessions; using a probe test, with the platform removed, evaluates the spatial bias toward the platform's prior location and extinction of the memory for the missing platform.12,44 Spatial working memory is evaluated by moving the hidden platform to a different location on each day of training. This modification requires that novel spatial information is acquired each day and remains valid only for the trials of a single session (for details, see reference 63). Age-matched adult NULL and PRIM female rats were tested on day 15 after weaning (group YNG); middle-aged (AGD) PRIM dams (which experienced motherhood at the same age as the YNG PRIM) and NULL female rats were tested when then they were 18 mo old. The hypothesis under evaluation was that a single reproductive experience induces long-lasting changes in both mnemonic processes and behavioral strategies of female rats and that these changes might be revealed by testing these subjects in both reference and working-memory versions of the MWM task.
Materials and Methods
Animals housing and breeding.
Wistar rats (n = 34; 80 to 90 d old at the beginning of the experiment) were individually housed in polypropylene cages (45 cm × 25 cm × 20 cm) with a controlled light:dark cycle (lights on, 0600 to 1800). Rats were provided by the Animal Facilities of the School of Veterinary Medicine of the Universidade de São Paulo. Water and food were available ad libitum. Rats were assigned randomly to 1 of 2 groups. Initially, one group of rats was mated. After mating, pregnant females were again individually housed and allowed to give birth. Their neonates were culled to 6 (3 male, 3 female) pups on the day after parturition, and these dams (PRIM) raised their litters until weaning on postpartum day 21. The second group of female rats (NULL) remained unmated for the same period of time. Approximately 2 to 3 wk after weaning, both the YNG PRIM (n = 8) and NULL (n = 9) female rats underwent daily behavioral testing. Corresponding middle-aged (AGD) NULL (n = 9) and PRIM (n = 8) female rats were included. AGD PRIM rats were mated at the same time as were YNG rats; however, behavioral testing of AGD PRIM rats did not begin until they were approximately 18 mo old. These rats received the same treatment as that given to the YNG NULL and PRIM.
The animals used in this study were maintained in accordance with the guidelines of the Committee on the Use of Laboratory Animals of the College of Veterinary Medicine of the University of São Paulo and the Guide for the Care and Use of Laboratory Animals.27
Estrous cycle.
Daily vaginal smears allowed identification of the estrus cycle phase, thereby enabling us to identify normal cyclicity and take it into account as a possible source of variation; this manipulation was performed at least 4 h before experimental procedures to account for its possible interaction with behavioral testing. Female rats that were 18 mo old with irregular cyclicity were considered to be AGD instead of old, because reproductive senescence occurs later in Wistar rats compared with other rat strains.53 Cells were collected through vaginal lavage with 20 μL saline, by using an automated pipette with disposable tips. A drop of vaginal lavage solution containing cells was placed on a glass slide and immediately examined under a microscope (10×). The estrus cycle phase was determined according to cell characteristics: epithelial, round nucleated, in proestrus; epithelial keratinized in estrus; and polymorphonuclear cells in metestrus.41
Apparatus.
A black fiberglass swimming pool (diameter, 200 cm; height, 50 cm; water depth, 25 cm; 26 ± 1 °C), with water rendered opaque by the addition of 200 mL milk, was used as the water maze apparatus. A movable, transparent, 9-cm diameter platform was placed in the pool about 2 cm below the water surface; the platform location depended on the behavioral procedure. A video camera connected to a microcomputer by an image interface device (VP112, HVS Image, Hampton, UK) allowed collection and analysis of the rat's swim path. The experimenter running the session placed the rats individually into the swimming pool, started and ended the data collection recording system at the beginning and end of each trial, respectively, and then transferred the subjects back to their cages. The swimming pool water was changed daily.
For descriptive data analyses, the pool was divided into 4 equal quadrants and 3 concentric 33-cm wide rings (inner, intermediate, and outer rings). In addition, four 27-cm-diameter counter areas, each of them located in the center of each quadrant, therefore concentric with each possible platform locations, were defined. The time spent within these areas, named critical counters when the platform was located within them, provided specific indices of spatial location. The MWM was located in a 3.13-m × 4.5-m room with several salient cues hanging on the walls (for example, a 40-cm red square).
Behavioral procedure.
A trial in both the reference and working memory versions of the MWM began by introducing the rat to the pool in a location close to the maze wall, facing the wall, and allowing the rat to swim until it found the platform. If the rat did not find the platform within 120 s, the experimenter manually guided it onto the platform, where the rat remained for 10 s. The rat then was transferred to its home cage until the next trial.
For the MWM reference memory task, the platform was positioned in a single, fixed location in the center of one of the quadrants. Each rat received 2 trials per session and one session per day. The training proceeded until the subjects achieved an asymptotic level of performance (14 d for YNG rats and 19 d for AGD). Each trial lasted 2 min; the intertrial interval (ITI) was 10 min. This approach renders the acquisition of the task more difficult relative to procedures that include more trials per day but with shorter ITIs;12,42,61 we thereby maximized the chances of distinguishing groups’ performances. Training occurred between 1400 and 1700 h. The starting point varied randomly from trial to trial to minimize the adoption, by the subjects, of strategies other than spatial. If a rat did not find the platform within 2 min, it was placed onto the platform where it stayed for 10 s. The rat then was transferred to and maintained in its home cage until the next trial. Acquisition was assessed by the latency to find the platform, path length, percentage of time in the quadrant where the platform was located, and percentage of time spent in the inner, intermediate (which contained the platform), and outer rings. The heading angle (a measure of initial divergence from the direct path to the platform) provided another measure of spatial bias. Swimming speeds were calculated by dividing the path length by the corresponding latency.
A 180-s probe test, with the platform removed, was conducted 24 h after the reference memory training phase. During this test, the rats were allowed to swim freely in the pool. The number of entries and percentage of time spent, in time bins of 60 s, in the critical counters allowed assessment of both long-term memory for the platform location and the rate of extinction of searching behavior along the 3 consecutive time bins. In addition, path length; percentage of time spent in the quadrant of the platform; percentages of time spent in inner, intermediate, and outer rings; and swim speed were recorded also.
Training in the MWM working memory task began 24 h after the probe test. On days 1 through 8, subjects were exposed to 4 trials daily, with an ITI of 10 min. On days 9 to 13, subjects were exposed to 3 trials daily, with an ITI of 60 min, thereby increasing the difficulty of the test. Different starting points were used for each trial. During the ITI, rats remained in their home cages. The platform location changed every day; therefore, in the first trial, the rats reached it by chance and scanning (so that latencies and path lengths reflect a lack of knowledge of the platform location). At the end of the first trial, however, the rats received information about the platform location on that specific day; therefore, they should have benefitted from this information during the second and remaining trials of that particular day, because the task requires matching-to-position for that day. In addition, rats tended to search for the platform within the area (quadrant and counter) where it was located on the previous day, indicating that they retained information about the platform location for at least 24 h.63
Swim speed was determined by dividing swim path length by latency, thus providing an index of motivation. The analyzed parameters were the same as those assessed in the reference memory and extinction tests.
Data analysis.
The parameters analyzed for reference memory included latency to find the platform; path length; percentage of time spent within the platform quadrant; percentage of time within the inner, intermediate, and outer rings; heading angle; swim speed; and time spent in the counter that contained the platform. For presentation purposes the scores recorded in the trials of each session were averaged thus facilitating visualization of the results; however, statistical analysis included trial scores separately.
For the 180-s probe test, the parameters analyzed included the numbers of entries and percentages of time spent by the subjects in the critical counters; path length; percentage of time spent in the quadrant of the platform; percentages of time spent in inner, intermediate, and outer rings; and swim speed. For presentation purposes, the scores recorded in the trials of each session were averaged of time bins of 60 s; statistical analysis also included trial scores separately.
For working memory scores, the means of latency; path length; percentage of time spent in the critical quadrant on the preceding day; percentages of time spent within the inner, intermediate, and outer rings; numbers of entries and percentage of time spent within the previous day's critical platform counter; and swim speeds scores for the 4 trials across the 8 d with a 10-min ITI (days 1 to 8) and for the 3 trials across the 5 d with a 60-min ITI (days 9 to 13) were calculated.
A 3-way ANOVA was performed for the MWM reference memory data, with group (NULL versus PRIM) as the between-subjects factor and sessions (either 14 sessions for YNG subjects or 19 sessions for AGD subjects) and trials (first and second) as within-subjects factors. A 2-way ANOVA was used to analyze probe test results, with group (NULL versus PRIM) as the between-subjects factor and time bin (first, second, and third minute) as the within-subjects factor. Finally, a 3-way ANOVA was performed for the MWM working memory data, with group (NULL versus PRIM) as the between-subjects factor and ITI (10 versus 60 min) and trial (first, second, and third) as within-subjects factors. In every case, separate ANOVA were used for each measure.
We also conducted a Bartlett test for homogeneity of variance on these variables. To determine the effects of estrous cycle, interactions between variables and estrous cycle phase were evaluated as covariates. Significant effects were analyzed with Fisher least significant difference (LSD) and Dunnett posthoc tests. All analyses were done by using SAS software (SAS Institute, Cary, NC). Statistical significance was defined as a P value of less than 0.05.
Results
The results show differences between NULL and PRIM rats in both mnemonic processes and behavioral strategies. These differences were not influenced by estrous cycle (P > 0.05), a finding that is consistent with earlier studies.50,51
YNG female rats.
Reference memory.
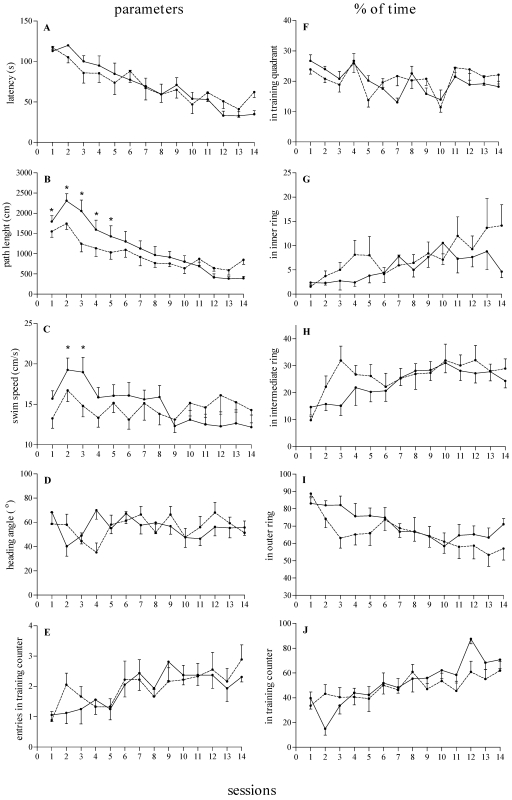
With regard to latency, path length, and percentage of time within the training quadrant (Figure 1), ANOVA revealed significant effects of day (F13,195 = 4.29 to 23.02, P < 0.0001 [note that the minimal and maximal F values for the referred parameters were presented in association with the P value of the minimal F value and thus are conservative]) and trial (F1,15 = 21.50 to 51.97, P < 0.0003) and a significant day × trial interaction (F13,195 = 1.79 to 9.47, P < 0.04) but no effect of Group (F1,15 = 0.01 to 1.47), indicating that both NULL and PRIM adult female rats learned the MWM reference memory task. ANOVA also revealed a significant day × group interaction for path length (F13,195 = 3.05, P = 0.0009) and swim speed (F13,195 = 4.38, P = 0.0005) and a trial × group interaction for swim speed (F1,15 = 4.71, P = 0.046). The posthoc contrast analysis revealed that these effects were related to shorter path lengths and slower swim speeds by the NULL adult female rats compared with PRIM adult female rats during early reference memory training (Figure 1), particularly during the first trial (data not shown). As training proceeded and the subjects learned the task, the apparent differences disappeared. ANOVA did not reveal any significant differences in percentages of time in the inner, intermediate, and outer rings; heading angle; entries in the training counter; or percentage of time in the training counter.
Figure 1.
Effects of reproductive experience on the acquisition of reference memory in adult nulliparous (n = 9, dashed lines) and primiparous (n = 8, continuous lines) female rats along 14 training sessions. (A) Latency to find the platform (s). (B) Path length (cm). (C) Swim speed (cm/s). (D) Heading angle relative to platform location (degrees). (E) Number of entries within the training counter. (F) Percentage of time spent within the training quadrant. (G) Percentage of time spent in the inner ring. (H) Percentage of time spent in the intermediate ring. (I) Percentage of time spent in the outer ring. (J) Percentage of time spent in the training counter. Data are expressed as mean ± SEM of 4 trials daily. *, Significant (P < 0.05) difference between groups.
Probe test.
As mentioned earlier, the probe test assesses long-term memory of platform location and the rate of extinction of search behavior during 3 consecutive time bins. The ANOVA revealed no effect of group (F1,15 = 0.0 to 1.55, P > 0.23) or time bin (F2,30 = 0.12 to 1.57, P > 0.22) or group × time bin interaction (F2,30 = 0.05 to 1.80, P > 0.18) for path length; percentage of time within the training quadrant; percentages of time within the inner, intermediate, and outer rings; entries in the training counter; percentage of time in the critical counter; or swim speed. The probe test, therefore, did not reveal any significant differences between the performances of PRIM and NULL adult rats.
Working memory.
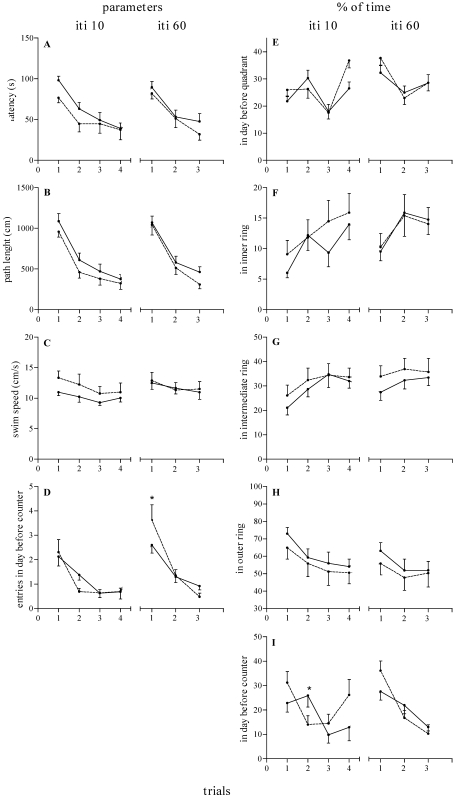
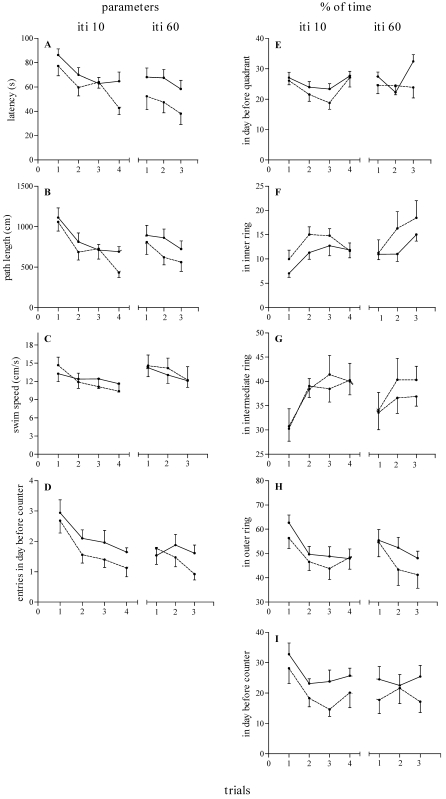
In the working memory task, rats were required to learn a new platform location each day, in which they were exposed to a matching-to-place procedure. In this way the critical location of the platform on each day was acquired during the first trial, allowing the rat to make use of this information to reach the platform more quickly when the ITI was 10 min (Figure 2, left panels) or 60 min (Figure 2, right panels).
Figure 2.
Effect of reproductive experience on working memory in adult nulliparous (n = 9, dashed lines) and primiparous (n = 8, continuous lines) female rats when the ITI was 10 min (ITI 10) and 60 min (ITI 60). (A) Latency to find platform (s). (B) Path length (cm). (C) Swim speed (cm/s). (D) Number of entries within the day before critical counter. (E) Percentage of time spent within the quadrant in which the platform was located on the previous day. (F) Percentage of time spent in the inner ring. (G) Percentage of time spent within the intermediate ring. (H) Percentage of time spent in the outer ring. (I) Percentage of time spent in the previous day's critical counter. Data are expressed as mean ± SEM for trials across days 1 through 8 (ITI 10) and days 9 through 13 (ITI 60). *, Significant (P < 0.05) difference between groups.
ANOVA of latency, path length, and swim speed scores when the ITI was 10 min revealed significant effects of trial (F3,45 = 12.22 to 44.98, P < 0.0001), a nonsignificant trend for effect of group (F1,15 = 1.39 to 3.23, P > 0.09), and no group × trial interaction (F3,45 = 0.20 to 0.56, P > 0.64). Similarly, ANOVA for latency, path length, and swim speed scores when the ITI was 60 min revealed significant effects of trial (F2,30 = 4.86 to 35.98, P < 0.02), no effect of group (F1,15 = 0.02 to 1.30, P > 0.27), and no group × trial interaction (F2,30 = 0.30 to 0.55, P > 0.58). Together, these data indicate that both PRIM and NULL adult female rats acquire the spatial working memory version of the MWM task at similar rates and maintained similar levels of performance independent of the requirement to retain critical information for either 10 or 60 min.
With regard to the number of entries (Figure 2 D) and percentage of time spent in the prior day's critical counter (Figure 2 I), indexes reflecting the subject's memory of the platform location used on the previous day and the extinction of their search within this location over trials, ANOVA revealed a significant effect of group (F1,15 = 4.96, P = 0.0417) when the ITI was 10 min (Figure 2 I, left curves) and a group × trial interaction effect when the ITI was 60 min (Figure 2 D, right curves; F2,30 = 3.48, P = 0.0436). Figure 2 D shows that during the first trial, whereas NULL female rats promptly redirected their behavior to look for the platform in other locations as soon as they could not find it in the prior day's location, PRIM female rats persisted in entering the counter where the platform had been placed on the day before, particularly during trials 1 (Figure 2 D, right curves) and 2 (Figure 2 I, left curves). However, when the ITI was 60 min, NULL female rats entered the quadrant for the previous day's location of the platform (Figure 2 D) more often, especially on the first trial, than did PRIM rats. ANOVA did not reveal any significant differences (P > 0.05) in percentages of time spent in the previous day's critical quadrant or in the inner, intermediate, and outer rings.
AGD female rats.
Reference memory.
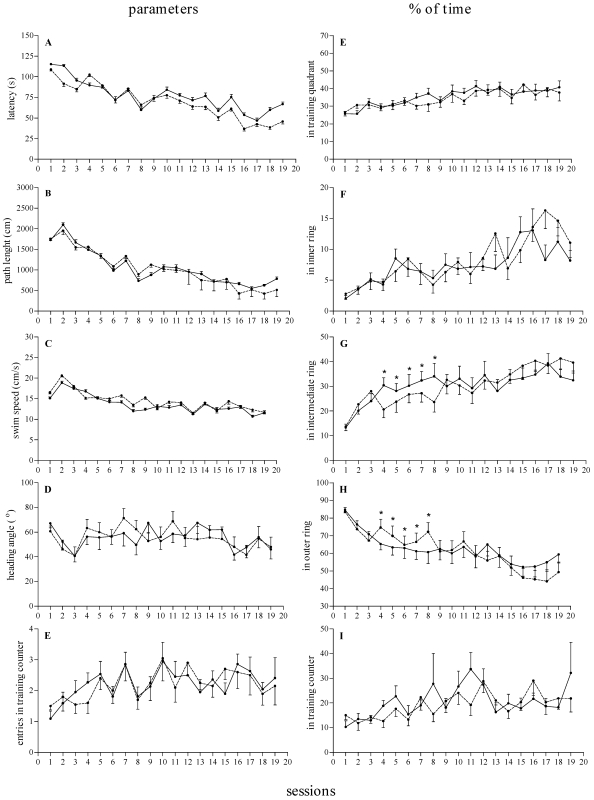
Middle-aged PRIM and NULL rats were exposed to the MWM reference memory test for 19 consecutive days. ANOVA revealed a significant effect of day for latency, path length, swim speed, and percentage of time within the training quadrant (F18,342 = 5.52 to 20.54, P < 0.0001; Figure 3 A through C and E) and a significant effect of trial for latency, path length, and swim speed (F1,19 = 9.60 to 18.57, P < 0.005). The data indicate that both AGD PRIM and NULL rats acquired the MWM reference memory version of the task and did not differ from each other. In addition, ANOVA revealed significant day × group interactions for percentage of time within the intermediate (F18,342 = 1.80, P = 0.0245) and outer (F18,342 = 1.68, P = 0.0407) rings of the pool (Figure 3 G and H). Posthoc analysis revealed that although PRIM rats spent significantly (P < 0.05) longer in the intermediate ring of the pool on days 4 through 8, NULL rats spent significantly (P < 0.05) longer within the outer ring of the pool on those same days. These results suggest that although AGD PRIM and NULL rats learned the reference memory version of the MWM task, they adopted different search strategies during the acquisition phase.
Figure 3.
Effects of reproductive experience on the acquisition of reference memory in middle-aged nulliparous (n = 9, dashed lines) and primiparous (n = 8, continuous lines) female rats along 19 training sessions. (A) Latency to find the platform (s). (B) Path length (cm). (C) Swim speed (cm/s). (D) Heading angle relative to platform location (degrees). (E) Number of entries within the training counter. (F) Percentage of time spent within the training quadrant (G) Percentage of time spent within the inner ring. (H) Percentage of time spent within the intermediate ring. (I) Percentage of time spent within the outer ring. (J) Percentage of time spent within the training counter. Data are expressed as mean ± SEM of 4 trials per day for each group. *, Significant (P < 0.05) difference between groups.
Probe test.
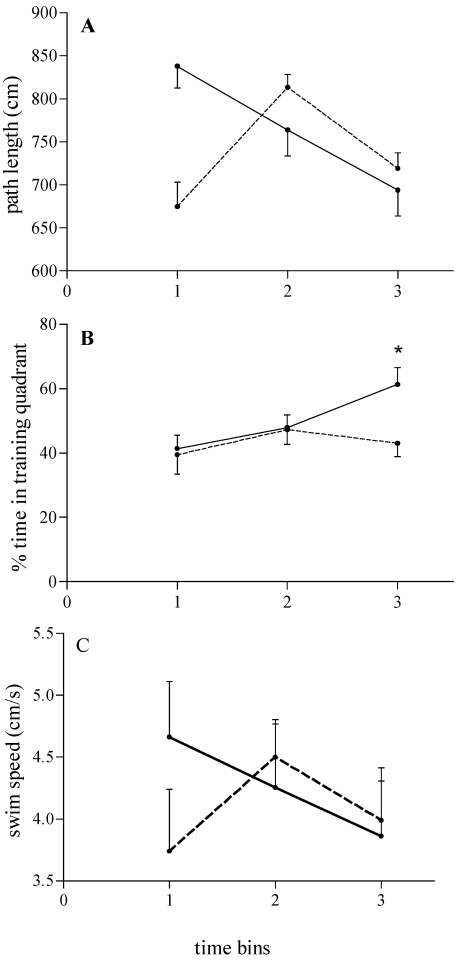
ANOVA revealed no effect of group for path length, percentage of time within the training quadrant, swim speed, or percentage of time spent within the training counter (F1,19 = 0.08 to 2.05, P > 0.16) but a significant effect of time bin for percentage of time within the training quadrant (F2,38 = 4.69, P = 0.0151; Figure 4 B). In addition, ANOVA revealed significant group × time bin interactions for path length, percentage of time within the training quadrant, and swim speed, (F2,38 = 3.21 to 3.66, P < 0.05; Figure 4 A through C), suggesting that AGD PRIM and NULL female rats adopted different strategies in the MWM: AGD PRIM rats exhibited a more consistent search strategy when attempting to find the missing platform early in the probe test (Figure 4 A and C) and persisted longer over time bins in the search (Figure 4 B).
Figure 4.
Effects of reproductive experience on retrieval of the information about the platform location and extinction of reference memory in middle-aged nulliparous (n = 9, dashed lines) and primiparous (n = 8, continuous lines) female rats along 3 consecutive time bins of 60 min each, during the probe test. (A) Path length (cm). (B) Percentage of time spent within the training quadrant. (C) Swim speed (cm/s). Data are expressed as mean ± SEM along 3 consecutive time bins of 60 s each. *, Significant (P < 0.05) difference between groups.
Working memory.
When the ITI was 10 min, ANOVA revealed a significant effect of trial for latency, path length, and swim speed (F3,57 = 11.24 to 19.52, P < 0.0001) but no effect of group (F1,19 = 0.05 to 2.61, P > 0.12; Figure 5). Moreover, ANOVA revealed no group × trial interaction for latency or path length (F3,57 = 1.38 to 1.85, P > 0.15) but a significant group × trial interaction for swim speed (F3,57 = 3.79, P < 0.0176). These findings indicate that both AGD PRIM and NULL rats learned the working memory version of the MWM task at similar rates when the ITI was 10 min but differed in their search strategies.
Figure 5.
Effects of reproductive experience on working memory in middle-aged nulliparous (n = 9, dashed lines) and primiparous (n = 8, continuous lines) female rats when the ITI was 10 min (ITI 10) or 60 min (ITI 60). (A) Latency to find platform (s). (B) Path length (cm). (C) Swim speed (cm/s). (D) Number of entries into the previous day's critical counter. (E) Percentage of time spent within the quadrant in which the platform was located on the previous day. (F) Percentage of time spent within the inner ring. (G) Percentage of time spent within the intermediate ring. (H) Percentage of time spent within the outer ring. (I) Percentage of time spent within the previous day's critical counter. Data are expressed as mean ± SEM for the trials across days 1 through 8 (ITI 10) and days 9 through 13 (ITI 60).
For the 60-min ITI data, ANOVA revealed no significant effect of trial for latency, path length, or swim speed (F2,38 = 1.35 to 2.49, P > 0.09), no significant effect of group for path length or swim speed, (F1,19 = 0.49 to 1.60, P > 0.22) but a trend toward a significant effect of group for latency (F1,19 = 3.95, P = 0.0615). There was no group × trial interaction for latency or path length (F2,38 = 0.09 to 0.35, P > 0.70) but a significant group × trial interaction for swim speed (F2,57 = 3.76, P = 0.0324). Therefore, AGD PRIM and NULL rats did not differ in the expression of the acquisition and maintenance of working memory when the ITI was 60 min.
Both AGD PRIM and NULL rats improved their performance over trials in the MWM when the ITI was 10 min but not when the ITI was 60 min. In YNG rats, improvements in performance in the working memory task were detected independent of the ITI, indicating the sensitivity of this task for detecting difficulties in spatial working memory in older rats with longer ITI. With regard to the percentage of time spent in the prior day's critical counter, ANOVA revealed a significant effect of trial (F3,57 = 8.59, P = 0.0002) but no effect of group (F1,19 = 1.92, P = 0.18) and no group × trial interaction (F3,57 = 0.40, P = 0.72) when the ITI was 10 min (Figure 5 I, left panel). Moreover, ANOVA revealed no effect of trial (F2,38 = 0.83, P = 0.44) or group (F1,19 = 2.22, P = 0.15), and no group × trial interaction (F2,38 = 1.24, P = 0.29) when the ITI was 60 min (Figure 5 I, right panel). Together, these results indicate that both AGD PRIM and NULL female rats exhibited more consistent searching for the prior day's critical counter when the ITI was shorter.
Discussion
The present data revealed differences in learning and memory processes and behavioral strategies as a function of reproductive experience. Although both groups of rats could learn and remember the task, YNG PRIM rats exhibited a proactive behavior pattern, whereas YNG NULL female rats tended toward caution. YNG PRIM rats adopted a different search strategy from YNG NULL after the 10th experimental day during the reference memory test. Compared with YNG NULL rats, YNG PRIM rats swam slower yet found the platform with shorter latency and shorter path length. These data suggest that YNG PRIM rats continued learning after the 10th day, resulting in stronger memory consolidation. In addition, although YNG PRIM rats could find the platform after the 10th experimental day, they did not rush to reach it, revealing behavioral modification.
Reproductive experience was associated with increased performance by rats in learning and memory tasks and in a dry-land maze and the MWM.30,34,50,51 Our results consistently show the effects of reproductive experience on MWM in spatial reference and working memory and suggest behavioral strategy modifications associated with aging20. The main difference between the dry-land maze and MWM is that stress and the motivational stimuli are quite different, and this difference may have influenced the results.21 Stress can modify performance that depends on cognitive function, particularly during early training in a water-maze task (when subjects are still learning that a safe refuge, the platform, exists; the effect of stress on cognitive function exhibits a U-shaped curve, depending on corticosterone levels.2,54 The deleterious effects of stress affect learning and memory throughout life.34 Chronic stress is associated with increased corticosterone and facilitates working memory.4 Therefore, the MWM can be adapted to selective visual and spatial factors in learning and working memory but may be less suitable for repeated measures or for assessing long-term memory deficits. In contrast, a dry-land maze, such as the radial arm maze, detects steady-state reference and working memory deficits and is suitable for repeated measures, leading to better analysis of involved processes.21
The effects of reproductive experience on modulation of both learning and memory processes20,30,31,49,51 and stress sensitivity may be considered an important adaptation because they are likely to contribute to offspring survival, protection, and nutrition. In addition, as mentioned earlier, the interaction of stress and motherhood may explain long-lasting effects on memory.4,10,34,54,62 Decreased swim speed suggests habituation to the maze and perhaps reflects less anxiety-like behavior expressed by the YNG PRIM females.60 Similarly, YNG PRIM female rats have been reported to express less anxiety-like behavior by staying longer in the open arms of an elevated plus maze.10,38 In addition, a prior study from our laboratory revealed motor activity differences between YNG PRIM and YNG NULL female rats exposed to the open field test.25 In the current study, reproductive experience had no effect on extinction of reference memory in adult female rats. In contrast, during the working memory test, YNG NULL rats spent more time searching for the platform in the quadrant in which it was located the day before, especially during the first trial, when the ITI was 60 min, suggesting that YNG PRIM and YNG NULL rats adopted different strategies for the requirements of the working memory version of the MWM. These findings suggest, therefore, that both groups could learn the task, although perhaps YNG PRIM rats could also change their behavioral strategy to increase task success.
The results in AGD rats revealed behavioral differences that were distinct from those in adult female rats. However, the observed behavior might also be related to changes in adaptive strategies attributable to reproductive experience. Acquisition of reference memory in AGD female rats revealed no significant differences between groups; the prolonged reference memory consolidation that might have occurred in experienced adult female rats did not occur in AGD rats. Similar to the results from experienced YNG females, AGD rats exhibited decreased time spent in the outer area, an observation that may suggest decreased stress and anxiety. Alternatively, these results may suggest increased memory retention and behavioral precision,10 because the platform was placed in the intermediate area, not in the outer area, of the water maze. Moreover, the results can be interpreted as less behavioral flexibility in middle-aged female rats or as difficulties in redirecting the behavior and searching for the platform in other locations. Because behavior is regulated at many different levels, there are likewise many levels at which the effects we report here may be regulated.
Working memory relies on a different neurocircuitry than does reference memory.47 For example, for a correct answer in a reference memory test, recalling the last time the stimulus was presented is not necessary, unlike for working memory.47 Rats, however, may take advantage of the reference learning process to perform working memory tests.10,44 In the MWM working memory test, we noted a slower extinction rate and behavioral redirection in PRIM rats, suggesting that decreased behavioral flexibility may have occurred as a consequence of aging. YNG rats exhibited improved performance in the working memory task independent of ITI, emphasizing the sensitivity of this task for detecting difficulties in spatial working memory at longer ITI with increasing age. The MWM was sensitive to this difference: when the ITI was increased to 60 min, the performance of PRIM female rats differed from that of NULL rats, exhibiting increased latency, path length, and time spent near the prior day's platform location. PRIM rats, therefore, were more persistent in their search for the platform in the prior day's quadrant, taking more time to redirect their preference to search for the platform in other locations during each trial. Difficulties in extinction of memory and redirecting behavior may be a consequence of stronger memory consolidation or different behavioral strategies. We noted these differences only when the ITI was 60 min, perhaps reflecting an effect of age. Aging-related reductions in cognition similar to those noted in the current study have been well reported.3,60
Because behavioral testing was performed in intact YNG and AGD female rats, estrous-cycle–related gonadal hormones may have influenced the results. However, estrous phase does not appear to influence radial-arm (dry-land) maze performance in female rats,30,50,51 although other reports have suggested that gonadal hormones may positively influence radial maze performance.1,12,31,57 Although we examined the data closely statistically, we noted no interactions between estrous cycle and the results obtained in the water maze in the present study.
The behavioral effects could have been affected by motor activity as a consequence of parity,24 because parous female rats exhibit less locomotor activity.10,24 This change could be related to the lower swim speed of both YNG and AGD experienced rats in the MWM reference memory task. PRIM rats may have shown different behavioral strategies than NULL rats because of subtle differences in locomotor function.24,30 More likely, these differences are a consequence of mnemonic and cognitive processes, but the interaction cannot be ruled out. Slower swim speed and heading angle related to the platform may suggest that once the task is learned and memorized, the experienced animals take the best (fastest) route. In addition, as suggested by the present results and earlier studies,60 experienced female rats may present less anxiety-like behavior; they therefore may ‘use’ this alteration to their advantage by enhancing their behavioral strategies. Therefore, because performance in PRIM female rats is more precise and direct, their strategies might be more successful, efficient, or economical. Improvements in mothers compared with nonmothers have been viewed in neuroeconomic terms: efficiencies translated into advantages.32 This possibility appears to apply to both reference and working memory.
In conclusion, the present study differentiated mnemonic and cognitive improvements; suggested modifications in anxiety-like behavior; found effects of age in parous rats; and demonstrated long-lasting differences in behavioral strategies as a function of a single reproductive experience both in young adult and middle-aged rats.
Acknowledgments
This research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, no. 07/04224-0) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, no. 351107/92-4) awarded to LFF.
References
- 1.Aguiar RB, Dickel OE, Cunha RW, Monserrat JM, Barros DM, Martinez PE. 2006. Estradiol valerate and tibolone: effects on memory. Pharmacol Biochem Behav 85:689–696 [DOI] [PubMed] [Google Scholar]
- 2.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. 2004. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem 11:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. 2009. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging 30:646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman RE, Zrull MC, Luine VN. 2001. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res 904:279–289 [DOI] [PubMed] [Google Scholar]
- 5.Bridges RS, Dibiase R, Loundes DD, Doherty P. 1985. Prolactin stimulation of maternal behavior in female rats. Science 227:782–784 [DOI] [PubMed] [Google Scholar]
- 6.Bridges RS, Felicio LF, Pellerin LJ, Stuer AM, Mann PE. 1993. Prior parity reduces postcoital diurnal and nocturnal prolactin surges in rats. Life Sci 53:439–445 [DOI] [PubMed] [Google Scholar]
- 7.Bridges RS, Henriquez BM, Sturgis JD, Mann PE. 1997. Reproductive experience reduces haloperidol-induced prolactin secretion in female rats. Neuroendocrinology 66:321–326 [DOI] [PubMed] [Google Scholar]
- 8.Bridges RS, Mann PE. 1994. Prolactin–brain interactions in the induction of maternal behavior in rats. Psychoneuroendocrinology 19:611–622 [DOI] [PubMed] [Google Scholar]
- 9.Byrnes EM, Bridges RS. 2000. Endogenous opioid facilitation of maternal memory in rats. Behav Neurosci 114:797–804 [DOI] [PubMed] [Google Scholar]
- 10.Byrnes EM, Bridges RS. 2006. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav 50:70–76 [DOI] [PubMed] [Google Scholar]
- 11.Byrnes EM, Byrnes JJ, Bridges RS. 2001. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav 68:481–489 [DOI] [PubMed] [Google Scholar]
- 12.Daniel JM, Roberts SL, Dohanich GP. 1999. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav 66:11–20 [DOI] [PubMed] [Google Scholar]
- 13.Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. 2003. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology 144:5268–5276 [DOI] [PubMed] [Google Scholar]
- 14.Felicio LF, Florio JC, Sider LH, Cruz-Casallas PE, Bridges RS. 1996. Reproductive experience increases striatal and hypothalamic dopamine levels in pregnant rats. Brain Res Bull 40:253–256 [DOI] [PubMed] [Google Scholar]
- 15.Fleming AS, Korsmit M. 1996. Plasticity in the maternal circuit: effects of maternal experience on Fos–Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci 110:567–582 [DOI] [PubMed] [Google Scholar]
- 16.Fleming AS, O'Day DH, Kraemer GW. 1999. Neurobiology of mother–infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev 23:673–685 [DOI] [PubMed] [Google Scholar]
- 17.Furuta M, Bridges RS. 2009. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull 80:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. 2008. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol 62:247–260 [DOI] [PubMed] [Google Scholar]
- 19.Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM. 1987. Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone, and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113:435–444 [DOI] [PubMed] [Google Scholar]
- 20.Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. 2005. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull 66: 91–98 [DOI] [PubMed] [Google Scholar]
- 21.Hodges H. 1996. Maze procedures: the radial arm and water maze compared. Brain Res Cogn Brain Res 3:167–181 [DOI] [PubMed] [Google Scholar]
- 22.Holmes MM, Wide JK, Galea LA. 2002. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci 116:928–934 [DOI] [PubMed] [Google Scholar]
- 23.Hucke EE, Cruz-Casallas PE, Florio JC, Felicio LF. 1998. Reproductive experience reduces striatal dopaminergic responses in freely moving female rats. Neuroreport 9:3589–3593 [DOI] [PubMed] [Google Scholar]
- 24.Hucke EE, Cruz-Casallas PE, Sider LH, Felicio LF. 2001. Reproductive experience modulates dopamine-related behavioral responses. Pharmacol Biochem Behav 68:575–582 [DOI] [PubMed] [Google Scholar]
- 25.Hucke EETS, Felicio LF. 2002. Respostas neuroquímicas estriatais e hipotalâmicas in vivo a injeções de anfetamina (AMPH) ou apomorfina (APO). Rev Bras Reprod An 26:101–103 [Google Scholar]
- 26.Hucke EETS, Felicio LF. 2008. Effects of prolactin on in vivo striatal monoaminergic activity are modulated by a previous reproductive experience. Braz J Vet Res Anim Sci 44:71–80 [Google Scholar]
- 27.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 28.Kinsley CH. 2008. The neuroplastic maternal brain. Horm Behav 54:1–4 [DOI] [PubMed] [Google Scholar]
- 29.Kinsley CH, Bridges RS. 1988. Parity-associated reductions in behavioral sensitivity to opiates. Biol Reprod 39:270–278 [DOI] [PubMed] [Google Scholar]
- 30.Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. 1999. Motherhood improves learning and memory. Nature 402:137–138 [DOI] [PubMed] [Google Scholar]
- 31.Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. 2006. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav 49:131–142 [DOI] [PubMed] [Google Scholar]
- 32.Lambert K, Kinsley CH. 2008. The neuroeconomics of motherhood: the costs and benefits of maternal investment, p 481–491 : Bridges RS. Neurobiology of the parental brain. New York (NY): Academic Press [Google Scholar]
- 33.Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. 2005. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav 84:799–806 [DOI] [PubMed] [Google Scholar]
- 34.Lemaire V, Billard JM, Dutar P, George O, Piazza PV, Epelbaum J, Le Moal M, Mayo W. 2006. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci 23:3368–3374 [DOI] [PubMed] [Google Scholar]
- 35.Lévy F, Guevara-Guzman R, Hinton MR, Kendrick KM, Keverne EB. 1993. Effects of parturition and maternal experience on noradrenaline and acetylcholine release in the olfactory bulb of sheep. Behav Neurosci 107:662–668 [DOI] [PubMed] [Google Scholar]
- 36.Lévy F, Kendrick KM, Goode JA, Guevara-Guzman R, Keverne EB. 1995. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: changes with maternal experience and effects on acetylcholine, γ-aminobutyric acid, glutamate, and noradrenaline release. Brain Res 669:197–206 [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. 2000. Maternal care, hippocampal synaptogenesis, and cognitive development in rats. Nat Neurosci 3:799–806 [DOI] [PubMed] [Google Scholar]
- 38.Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester N, Glasper E, DeVries C, Kinsley C, Lambert K. 2005. Maternal experience produces long-lasting behavior modifications in the rat. Behav Neurosci 119:1084–1096 [DOI] [PubMed] [Google Scholar]
- 39.Mann PE, Bridges RS. 1992. Neural and endocrine sensitivities to opioids decline as a function of multiparity in the rat. Brain Res 580:241–248 [DOI] [PubMed] [Google Scholar]
- 40.Mann PE, Kinsley CH, Ronsheim PM, Bridges RS. 1989. Long-term effects of parity on opioid and nonopioid behavioral and endocrine responses. Pharmacol Biochem Behav 34:83–88 [DOI] [PubMed] [Google Scholar]
- 41.Marcondes FK, Bianchi FJ, Tanno AP. 2001. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614 [DOI] [PubMed] [Google Scholar]
- 42.Moltz H, Robbins D. 1965. Maternal behavior of primiparous and multiparous rats. J Comp Physiol Psychol 60:417–421 [DOI] [PubMed] [Google Scholar]
- 43.Monks DA, Lonstein JS, Breedlove SM. 2003. Got milk? Oxytocin triggers hippocampal plasticity. Nat Neurosci 6:327–328 [DOI] [PubMed] [Google Scholar]
- 44.Morris R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60 [DOI] [PubMed] [Google Scholar]
- 45.Musey VC, Collins DC, Brogan DR, Santos VR, Musey PI, Martino-Saltzman D, Preedy JR. 1987. Long term effects of a first pregnancy on the hormonal environment: estrogens and androgens. J Clin Endocrinol Metab 64:111–118 [DOI] [PubMed] [Google Scholar]
- 46.Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JR. 1987. Long-term effect of a first pregnancy on the secretion of prolactin. N Engl J Med 316:229–234 [DOI] [PubMed] [Google Scholar]
- 47.Olton DS, Papas BC. 1979. Spatial memory and hippocampal function. Neuropsychologia 17:669–682 [DOI] [PubMed] [Google Scholar]
- 48.Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. 2009. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 30:343–357 [DOI] [PubMed] [Google Scholar]
- 49.Pawluski JL, Galea LA. 2006. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol 66:71–81 [DOI] [PubMed] [Google Scholar]
- 50.Pawluski JL, Galea LA. 2007. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 149:53–67 [DOI] [PubMed] [Google Scholar]
- 51.Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. 2006. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res 175:157–165 [DOI] [PubMed] [Google Scholar]
- 52.Rosenblatt JS, Olufowobi A, Siegel HI. 1998. Effects of pregnancy hormones on maternal responsiveness, responsiveness to estrogen stimulation of maternal behavior, and the lordosis response to estrogen stimulation. Horm Behav 33:104–114 [DOI] [PubMed] [Google Scholar]
- 53.Saal FS, Finch CE, Nelson JF. 1994. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates, 1213–1314 : Knobil E, Neill JD. The physiology of reproduction, 2nd ed. New York (NY): Raven Press [Google Scholar]
- 54.Sandi C, Loscertales M, Guaza C. 1997. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 9:637–642 [DOI] [PubMed] [Google Scholar]
- 55.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. 2003. Pregnancy-stimulated neurogenesis in the adult forebrain mediated by prolactin. Science 299:117–120 [DOI] [PubMed] [Google Scholar]
- 56.Sider LH, Hucke EE, Florio JC, Felicio LF. 2003. Influence of time of day on hypothalamic monoaminergic activity in early pregnancy: effect of a previous reproductive experience. Psychoneuroendocrinology 28:195–206 [DOI] [PubMed] [Google Scholar]
- 57.Spencer JL, Waters EM, Milner TA, McEwen BS. 2008. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience 155:1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stern JM, Goldman L, Levine S. 1973. Pituitary–adrenal responsiveness during lactation in rats. Neuroendocrinology 12:179–191 [DOI] [PubMed] [Google Scholar]
- 59.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. 2003. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6:384–390 [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Li S, Dong HP, Lv S, Tang YY. 2009. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 85:127–135 [DOI] [PubMed] [Google Scholar]
- 61.Warren SG, Juraska JM. 1997. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci 111:259–266 [DOI] [PubMed] [Google Scholar]
- 62.Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. 2003. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav 79:373–381 [DOI] [PubMed] [Google Scholar]
- 63.Xavier GF, Oliveira-Filho FJ, Santos AM. 1999. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in ‘place strategy’ because of a lack of flexibility in the use of environmental cues? Hippocampus 9:668–681 [DOI] [PubMed] [Google Scholar]