Abstract
Reported here is the novel finding of neuropathology in a patient with succinic semialdehyde dehydrogenase deficiency, an inherited disorder of γ-aminobutyric acid metabolism characterized by intellectual deficiency, hypotonia, and epilepsy, with 4-hydroxybutyric aciduria and abnormalities of the globus pallidus on neuroimaging. A 19-year-old woman of European origin with a neurodevelopmental disorder and epilepsy died unexpectedly in 1998. A postmortem examination was performed, with a final diagnosis of sudden unexpected death in epilepsy patients. Eight years later, her sister with a neurodevelopmental disorder presented at 13 years of age with seizures and was diagnosed with succinic semialdehyde dehydrogenase deficiency. In the decedent, succinic semialdehyde dehydrogenase deficiency was established at the molecular level, 10 years after her death, using genomic DNA from brain tissue specimens. The neuropathologic findings revealed striking discoloration of the globi pallidi, leptomeningeal congestion, and a scar in the frontal cortex. After detection of the pathogenic homozygous mutation c.1226G>A, p.Gly409Asp in the living sister, it was confirmed in the decedent. An underlying metabolic disease may be an additional risk factor for sudden unexpected death in epilepsy patients.
Introduction
Succinic semialdehyde dehydrogenase deficiency is an autosomal recessive disorder of γ-aminobutyric acid metabolism [1,2]. Its biochemical hallmark is the accumulation and excretion of γ-hydroxybutyric acid in biologic fluids [3]. Patients with succinic semialdehyde dehydrogenase deficiency do not typically present with metabolic disturbances such as acidosis, hyperammonemia or hypoglycemia, which would suggest an inborn error of metabolism, and instead have a temporal course that appears relatively static and nonprogressive. The clinical presentation is developmental delay in infancy with associated hypotonia and hyporeflexia. Approximately half of patients have generalized epilepsy, which is frequently refractory to medical therapy [4]. Magnetic resonance imaging reveals T2-weighted hyperintensities of the globus pallidus, with less common involvement of the cerebellar dentate nuclei. Confirmation of diagnosis is made by enzyme assay in lymphocytes or molecular analysis of the ALDH5A1 gene [5,6].
Reported here is the case of a family with two children having a similar phenotype in whom the presenting sibling was diagnosed with succinic semialdehyde dehydrogenase deficiency and her deceased older sister was then confirmed to have had the same mutation.
Case Report
The proband is a 13-year-old girl who presented with recent onset of generalized seizures. She initially had staring spells, and then manifest generalized tonic-clonic seizures. She had difficulties with inattention, hyperactivity, and anxiety and had received intervention with atomoxetine followed by methylphenidate. The latter was discontinued with the onset of the staring spells. She had a history of nonprogressive encephalopathy characterized by developmental delay and hypotonia noted from infancy and residual intellectual deficiency with marked impairment in expressive language. She received special education services in school and had a full-scale intelligence quotient measurement of 70. Developmental milestones were delayed; specifically, she began crawling at 12 months, walked at 20 months, and spoke her first words at 3.5 years. She was the product of an uncomplicated pregnancy and perinatal course to consanguineous parents of Greek ancestry who were third cousins.
The proband’s sister, who died 6 years earlier at 19 years of age, had a similar phenotype of a developmental disorder with epilepsy. This sibling had developmental delay from infancy and long-standing intellectual deficiency with virtually absent speech. She had seizure onset at age 13 years of age, with accelerating seizures in the last 6 months of her life despite on-going antiepileptic drug therapy. She died unexpectedly in her sleep and was diagnosed with sudden unexpected death in epilepsy patients. Postmortem analysis had been obtained.
Neurologic examination of the proband demonstrated an alert, visually interactive adolescent with more impairment in expressive than receptive language. The limited speech output was dysfluent and dyspractic. Cranial nerve functions were otherwise satisfactory. There was mild appendicular hypotonia with satisfactory strength and absence of tremor or dyskinesias. There was diffuse hyporeflexia, despite reinforcement maneuvers. Gait was mildly wide based; response to the Romberg test was normal.
Cranial magnetic resonance imaging revealed symmetric homogeneous T2-weighted hyperintensity of the globus pallidi. Her waking and sleeping electroencephalograms were unremarkable.
Laboratory testing included urine organic acids, which demonstrated 4-hydroxybutyric aciduria. Succinic semialdehyde dehydrogenase enzymatic activity in lymphocytes was profoundly deficient, confirming the diagnosis of succinic semialdehyde dehydrogenase deficiency. DNA sequence analysis demonstrated a homozygous mutation in the ALDH5A1 gene (c.1226G>A; p.Gly409Asp), a known pathogenic mutation resulting in less than 1% of normal succinic semialdehyde dehydrogenase activity in mammalian cells [6].
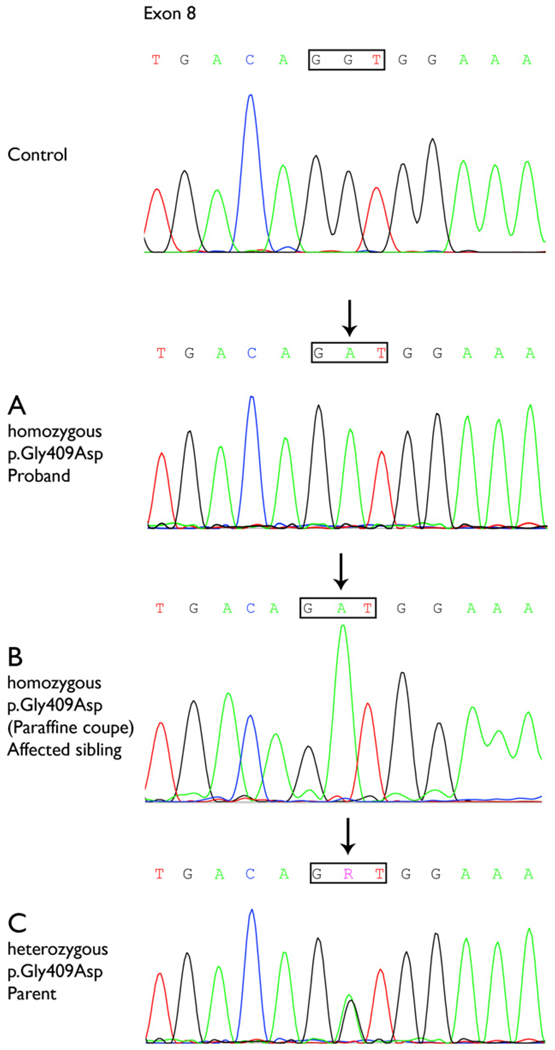
Both parents were tested and confirmed as heterozygous for this mutation. Subsequently, homozygosity for this mutation was detected in the decedent, confirming the postmortem diagnosis of succinic semialdehyde dehydrogenase deficiency (Fig 1).
Figure 1.
Sequence analysis of part of exon 8 of the ALDH5A1 gene of the proband (A), the affected sibling using genomic DNA extracted from brain tissue slides (B), and a parent (C). The decedent (B) was homozygous for the pathogenic mutation (c.1226G>A; p.Gly409Asp), as was her younger sibling (A). Both parents were heterozygous for this mutation, confirming homozygosity in both children. The mutation is indicated by an arrow. R indicates either adenine or guanine (A or G).
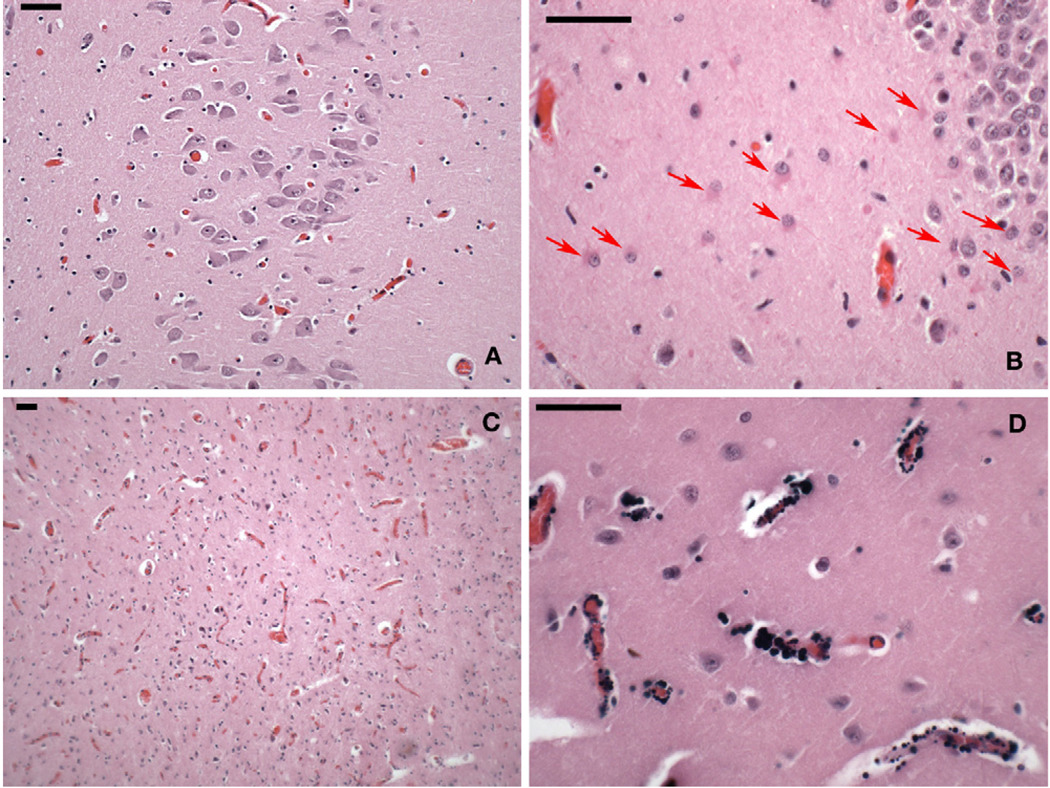
Autopsy of the older sibling was reviewed. There were no detected systemic or toxicologic abnormalities. The brain weight was 1420 g, and there were no gross neuropathologic findings, except for leptomeningeal congestion. Multiple coronal sections through the cerebral hemispheres showed an intact cortical gray ribbon and well-myelinated subcortical white matter. Striking bilateral, sharply circumscribed, dark tan discoloration in the deep portion of both globus pallidi was noted. The other histologic findings were congestion of small blood vessels in the leptomeninges, globus pallidi, and superior colliculus (Fig 2). The small blood vessels of the basal ganglia were mineralized. A small linear zone of architectural disruption, partial neuronal loss, and astrocytic proliferation was found in the depth of a frontal sulcus. No other remarkable changes were noted.
Figure 2.
Photomicrographs of postmortem brain tissue of the 19-year-old decedent (hematoxylin and eosin stain). Each scale bar = 50 µm. (A) CA1 sector of hippocampus, the area of the brain most vulnerable to systemic hypoxia-ischemia, with no indication of loss of neurons or gliosis. (B) CA4-subplate region of the hippocampus with a band of reactive astrocytes below the dentate layer of the hippocampus (arrows). (C) Hyperemia of the superior colliculus, also seen in the globus pallidus. (D) Granular perivascular calcification in putamen, uncommon in this age group and location.
No other changes were evident in the other deep cerebral nuclei, including the caudate, putamen, thalamus, and hypothalamus, as well as amygdala, cerebellum, brainstem, and cervico-medullary junction. The CA1 sector of hippocampus, the area most vulnerable to systemic hypoxia and ischemia, showed no neuronal loss or gliosis. In the CA4-subplate region of the hippocampus, there was a band of reactive astrocytes below the dentate gyrus, as occasionally found in patients with seizures. Granular perivascular calcifications were found in the globus pallidus, cerebellar dentate nucleus, and hippocampus. The ventricular system was normal in size and configuration.
Materials and Methods
The postmortem examination included external and internal examinations with histologic analysis on the general autopsy and gross and microscopic neuropathologic analysis. Multiple coronal sections were taken through the cerebral hemispheres, and multiple horizontal sections were taken through the brain stem and cerebellum. Histologic sections were examined from representative portions of cerebral cortex, basal ganglia, hippocampus, diencephalon, cerebellum, midbrain, pons, medulla, cervico-medullary junction, and amygdala.
DNA was isolated from blood of the proband and her parents. All 10 exons and the adjacent splice sites of the ALDH5A1 gene of the index patient were analyzed by DNA sequence analysis. Paraffin-embedded brain tissue slides were used to extract DNA from the decedent. Given degradation of the decedent’s DNA over 10 years, a 120-bp region of the gene that includes amino acid 409, the site of the pathogenic mutation, was amplified and sequenced.
Discussion
The diagnosis of succinic semialdehyde dehydrogenase deficiency in the older patient was made posthumously and retrospectively after the diagnosis in a younger sibling, with subsequent molecular confirmation in the living proband and, consequently, the decedent.
The main morphologic feature in the decedent was discoloration of the globus pallidus, corresponding to the site of the most constant abnormality evident on neuroimaging. Granular perivascular calcifications found in the globus pallidus, cerebellar dentate, and hippocampus are atypical in young patients and likely attributable to chronic progressive excitotoxic injury.
The prominent pallidal findings in this present case of succinic semialdehyde dehydrogenase deficiency are distinct from other childhood neurodegenerative disorders, including the neuronal loss, gliosis, and iron deposition in the globus pallidus found in pantothenate kinase deficiency [7]. Focal hyperemia in superior colliculus and globus pallidus, as described in the present case, is seen in response to energy insufficiency and with comparable anatomic distribution of similar but more severe lesions in Leigh syndrome, thiamine deficiency, and cyanide poisoning. Sparing of CA1 indicates that systemic hypoxic or ischemia related to seizures was not a likely pathogenetic factor.
Basal ganglia calcification occurs in other metabolic disorders, including mitochondrial cytopathies, cerebral hypoxia, N-methyl-d-aspartate excitotoxicity, hypoparathyroidism, and premature aging conditions with increased oxidative stress such as Cockayne syndrome [8]. In a study of 215 patients with mitochondrial disorders ages 33–93 years, basal ganglia calcifications were found in 36 cases (17%) and were associated with additional imaging findings including cerebral atrophy and subcortical white matter lesions (leukoaraiosis) [8]. In succinic semialdehyde dehydrogenase deficiency, mitochondrial dysfunction may occur as γ-aminobutyric acid degradation is coupled to the Krebs cycle and hence, to the respiratory chain through succinate and α-ketoglutarate [9]. In Aldh5a1−/− mutant mice, decreased activities of respiratory chain complexes I–IV were found in the hippocampus together with decreased glutathione, suggesting both mitochondrial dysfunction and increased oxidative stress.
The decedent reported here had a final diagnosis of sudden unexpected death in epilepsy patients when she died in 1998. This syndrome is defined as a sudden unexpected nontraumatic and nondrowning death, witnessed or unwitnessed, in a patient with epilepsy with or without evidence of a seizure. Status epilepticus is excluded from sudden unexpected death in epilepsy patients, and autopsy does not reveal a toxicologic or anatomic cause for death [10]. Risk factors for sudden unexpected death in epilepsy patients have been identified as young age, early seizure onset, presence of generalized tonic-clonic seizures, male sex, and body position [11]. The pathophysiologic mechanisms implicated in sudden unexpected death in epilepsy patients include central apnea, pulmonary edema, and cardiac arrhythmia [11,12].
In conclusion, the predominant neuropathologic changes in succinic semialdehyde dehydrogenase deficiency involve the basal ganglia and resemble the imaging findings. There is evidence of chronic excitotoxic neuronal injury. An underlying metabolic disease may be an additional risk factor for sudden unexpected death in epilepsy patients.
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grant NS 40270/HD58553 (K.M.G., P.L.P.) and by the Pediatric Neurotransmitter Disease Association (K.M.G., P.L.P.), the Delman Family Fund for Pediatric Neurology Research (P.L.P., S.C.), and SHS International (I.K.).
Footnotes
Gerard Catanese, MD, of the Nassau County, New York, coroner’s office, supplied the autopsy report and tissue blocks. Majella S. Boutmy-De Lange, BS, and Ana Pop, BS, of Vrije University in Amsterdam, assisted with DNA sequencing. Ofir T. Betsalel, BSc, Metabolic Unit, Amsterdam, assisted with Figure 1. Katherine Forester, BA, assisted with data and manuscript preparation.
References
- 1.Gibson KM, Gupta M, Pearl PL, et al. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (γ-hydroxybutyric aciduria) Biol Psychiatry. 2003;54:763–768. doi: 10.1016/s0006-3223(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 2.Knerr I, Pearl PL, Bottiglieri T, Snead OC, Jakobs C, Gibson KM. Therapeutic concepts in succinate semialdehyde dehydrogenase (SSADH; ALDH5a1) deficiency (γ-hydroxybutyric aciduria): hypotheses evolved from 25 years of patient evaluation, studies in Aldh5al−/− mice and characterization of γ-hydroxybutyric acid pharmacology. J Inherit Metab Dis. 2007;30:279–294. doi: 10.1007/s10545-007-0574-2. [DOI] [PubMed] [Google Scholar]
- 3.Gibson KM, Goodman SI, Frerman FE, Glasgow AM. Succinic semialdehyde dehydrogenase deficiency associated with combined 4-hydroxybutyric and dicarboxylic acidurias: potential for clinical misdiagnosis based on urinary organic acid profiling. J Pediatr. 1989;114:607–610. doi: 10.1016/s0022-3476(89)80706-1. [DOI] [PubMed] [Google Scholar]
- 4.Pearl PL, Gibson KM, Acosta MT, et al. Clinical spectrum of succinic semialdehyde dehydrogenase deficiency. Neurology. 2003;60:1413–1417. doi: 10.1212/01.wnl.0000059549.70717.80. [DOI] [PubMed] [Google Scholar]
- 5.Gibson KM, Lee CF, Chambliss KL, et al. Hydroxybutyric aciduria: application of a fluorometric assay to the determination of succinic semialdehyde dehydrogenase activity in extracts of cultured human lymphoblasts. Clin Chim Acta. 1991;196:219–221. doi: 10.1016/0009-8981(91)90076-o. [DOI] [PubMed] [Google Scholar]
- 6.Akaboshi S, Hogema BM, Novelletto A, et al. Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease-causing mutations in patients with SSADH deficiency. Hum Mutat. 2003;6:442–450. doi: 10.1002/humu.10288. [DOI] [PubMed] [Google Scholar]
- 7.Galvin JE, Giasson B, Hurtig HI, Lee VM, Trojanowski JQ. Neurodegeneration with brain iron accumulation, type 1 is characterized by α-, β-, and γ-synuclein neuropathology. Am J Pathol. 2000;157:361–368. doi: 10.1016/s0002-9440(10)64548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsterer J, Kopsa W. Basal ganglia calcification in mitochondrial disorders. Metab Brain Dis. 2005;20:219–226. doi: 10.1007/s11011-005-7209-9. [DOI] [PubMed] [Google Scholar]
- 9.Sauer SW, Kölker S, Hoffmann GF, et al. Enzymatic and metabolic evidence for a region specific mitochondrial dysfunction in brains of murine succinic semialdehyde dehydrogenase deficiency (Aldh5a1−/− mice) Neurochem Int. 2007;50:653–659. doi: 10.1016/j.neuint.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia. 1997;38 Suppl. 11:S6–S8. doi: 10.1111/j.1528-1157.1997.tb06130.x. [DOI] [PubMed] [Google Scholar]
- 11.Monté CP, Arends JB, Tan IY, Aldenkamp AP, Limburg M, de Krom MC. Sudden unexpected death in epilepsy patients: risk factors: a systematic review. Seizure. 2007;16:1–7. doi: 10.1016/j.seizure.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Davis GG, McGwin G., Jr Comparison of heart mass in seizure patients dying of sudden unexplained death in epilepsy to sudden death due to some other cause. Am J Forensic Med Pathol. 2004;25:23–28. doi: 10.1097/01.paf.0000113930.53578.f8. [DOI] [PubMed] [Google Scholar]