Abstract
Historically, Jamaica Bay was a site of extensive oyster beds and shellfish culture leases that supported a significant oyster fishery in the New York area. The industrial and urban expansion of the early 1900’s led to over-harvesting and a deterioration in water and bay sediment quality that coincided with shellfish decline and the ultimate disappearance of oysters from the bay. Over the past 50 years, efforts to arrest and reverse the pollution problems of Jamaica Bay have been undertaken but the area still contains metals and other pollutants at levels higher than NYS Water Quality Standards. Previous we showed that Crassostrea virginica seed transplanted to the bay had excellent growth and survival despite the bay’s pollution problems. In this study we measured the one-year bioaccumulation and tissue distribution of four metals in C. virginica seed that were transplanted to the bay at two different depths: one foot from the surface and one foot above the sediment. Tissues of C. virginica were dissected, dried and digested in nitric acid. Arsenic, cadmium, copper and zinc levels were measured using electrothermal vaporization with deuterium lamp background correction in an atomic absorption spectrophotometer fitted with a THGA graphite furnace. Metals were distributed in the various tissues in μg/g dry weight amounts, which correlate well with published values for whole oysters grown in other polluted areas. Metal distributions were not homogeneous throughout the animals and in most of the tissues tested, oysters grown near the surface accumulated more metal than those positioned near bay sediment.
Introduction
Jamaica Bay, a 26 square mile estuarial embayment situated between southern Brooklyn and Queens, NY and a major inlet opening to the Atlantic Ocean, lies just east of the entrance to NY Harbor and the mouth of the Hudson River. It is home to the Jamaica Bay Wildlife Refuge, which encompasses 9,155 acres of diverse habitats including salt marsh, upland field and woods, fresh, and brackish water ponds, and an open expanse of bay and islands. The bay is also a critical component of a larger watershed that drains naturally or via storm sewers, on the seaward-sloping outwash plain south of the harbor hill terminal moraine1.
At one time, wild stocks of the Eastern Oyster, Crassostrea virginica, also known as the American Oyster, were found all along the Atlantic and Gulf coasts of North America and for centuries, supported subsistence fishing by Native Americans and early European colonists2. Historically, C. virginica, flourished in Jamaica Bay and the NY/NJ Harbor area as either self-sustaining or farmed populations3. Jamaica Bay’s oyster industry observed a steady decline in production after its peak in the early 1900’s4. Lack of adequate supply of seed oysters, over-harvesting by commercial fishermen, increased pressure from natural predators, parasitic invasion, changing hydrographic patterns, siltation, and a decline in water quality are all cited as possible causes for the decline. The rapid urbanization and local industrialization of the area at the turn of the century was followed by years of unregulated industrial, urban and residential dumping which contaminated bay sediment, causing adverse effects on benthic organisms and bioaccumulation further up the food chain. Discharges of inadequately treated sewage were poisoning oysters, clams and ultimately people, and by 1921 the U.S. Department of Agriculture had closed shellfish lands in Jamaica Bay altogether. The shellfish problem was not unique to Jamaica Bay for around the same time declines in estuarine shellfish populations had occurred throughout the entire east coast of the United States and other important oyster fisheries, like that of Chesapeake Bay and Virginia’s James River, had also started to collapsed5,6,7. Today, very few if any wild oysters are found in Jamaica Bay, and the dramatic loss of this historic oyster bed has permanently altered the structure and function of the bay’s benthic ecosystem.
Over the last 50 years, major efforts have been undertaken by federal, state and local authorities to arrest and reverse the pollution problem and while natural stocks of C. virginica have not returned to the bay, there has been a resurgence of many other marine organisms. Despite improvements, Jamaica Bay still exhibits poor water quality8 and studies indicate the presence of various metal pollutants, including arsenic, cadmium, chromium, copper, iron, lead, mercury, nickel and zinc, in bay sediment9,10,11,12,13,14,15. Heavy metals have been found to inhibit growth in a variety of mollusc species including oysters16,17,18. In 2002, our lab did a study to determine the growth and survival of C. virginica seed transplanted to Jamaica Bay in protected containers at two ecologically different locations, at two different depths: one foot below the surface and one foot off the bottom. Despite bay conditions, the oysters at both locations and both depths had excellent growth and survival rates19. The results of this project generated many new questions such as: to what extent are oysters acquiring bay pollutants in their tissues, how are they able to adapt to this stress, what are the physiological effects on their various organ systems, and what are the consequences of the polluted environment on the long term survival and reproductive success in Jamaica Bay.
Bivalves are particularly good accumulators of heavy metals20,21,22,23 and being sessile, tend to reflect local contaminant concentrations more accurately than crustaceans and free-swimming finfish. The EPA considers C. virginica, as one of six target bivalve species recommended for contaminant monitoring in Mid-Atlantic coastal waters including Jamaica Bay24. Target metal analytes on the EPA Fish Contaminant Workgroup list include arsenic, cadmium, mercury, selenium and tributyltin25. The Mussel Watch Project of the National Oceanic and Atmospheric Administration (NOAA) monitors copper, lead, nickel, and zinc, in addition to the analytes on the EPA’s target list26. Numerous other reports have been made on the bioaccumulation of these and other heavy metals in oyster species of in the US and around the globe27,28,29,30,31,32.
In this study we measured the one year bioaccumulation and tissue distribution of four metal pollutants (arsenic, cadmium, copper and zinc) in C. virginica seed, that had been transplanted to the bay in 2002 at two different depths: in floats, one foot below the surface, and in hanging nets, one foot off the sediment. Since Jamaica Bay sediment is a major reservoir of metal pollutants, it was hypothesized that metal accumulations might be greater in the tissues of bottom positioned oysters, since they were grown nearer to a more concentrated source of metal pollutants.
Materials and Methods
In June 2002, four modified Taylor Floats33 of approximately 3′ × 4′ were constructed using PVC tubing and 1/4″ mesh nylon screening. Each float was designed to hold up to three 1/8″ mesh nylon boxes in which oyster seed were to be placed. Each float had 1/4″ nylon mesh lids to keep out predators. Oyster seed of approximately 20 mm anterio-posterial (height) shell lengths and 5 mm shell hinge width were obtained from Frank M. Flower & Sons, Inc. Oyster Nursery in Oyster Bay, NY. 150 oyster seeds were distributed among the 3 nylon boxes in each float. Two floats were positioned 1 foot below the water surface in Jamaica Bay at Fort Tilden at Gateway National Park Marine Station (GNPMS), and at Kingsborough Community College Marina (KBCCM) in Brooklyn’s Sheepshead Bay, a large cove of Jamaica Bay (NY/NJ Baykeeper license #LG P00 583). The two other floats that were designed to sink and position the oyster seed 1 foot off the bay bottom, were placed at the same two sites. Both surface and bottom floats were inspected and cleaned of any fouling, biweekly in the summer and monthly in the winter. After 2 months, the bottom floats were deemed clumsy/difficult to work with and were replaced with commercially constructed hanging nets, suspended one foot off the bottom, for the rest of the experiment.
Seed survival and growth, as well as various parameters of bay water quality including temperature, pH, dissolved O2, chlorophyll a, salinity, and turbidity were monitored throughout the one year experimental period. In July 2003, samplings of one-year old oysters were removed from each site and depth to determine the bioaccumulations of arsenic, cadmium, copper and zinc in various tissues.
Prior to tissue isolation, all glassware was acid-washed in dilute (5%, wt/wt) nitric acid in deionized water to remove any bound metals. Washing was followed by a thorough rinse with deionized water to remove any remaining acid. All acids used were Fisher trace-metal grade. Representative oysters were cleaned, shucked, and their tissues dissected to determine metal bioaccumulations. The mantle, gill, palps, posterior adductor muscle and stomach were removed with stainless steel instruments. The tissues were blotted and placed in pre-weighed Pyrex flasks to determine wet weights. A 1–2 g sampling of each animal’s shell was also removed, weighed, and processed similarly for metal analysis. All tissues were dried in an oven at 120°C and reweighed to determine dry weights. Dried tissue samples were digested in concentrated nitric acid on hotplates. Digested samples were adjusted to a final volume of 10 ml in dilute (0.2 %, wt/wt) nitric acid. Aliquots of each sample were analyzed for arsenic, cadmium, copper and zinc determinations by electrothermal vaporization with deuterium lamp background correction in a Perkin Elmer AAnalyst 800 Atomic Absorption Spectrophotometer with a THGA Graphite Furnace. Metal levels were recorded as μg/g dry weight. Statistical analysis was determined by a Wilcoxon Matched-Pairs Signed Ranks Test using GraphPad InStat version 3.00.
Results
C. virginica at both locations and depths were deemed to have excellent growth and survival. After one year, oyster seed at both sites and depths had over 75% survival, and growth, as measured by shell height, had increased over 300% with average growth rates for bottom maintained oysters exceeding those maintained near the surface by approximately 20%19.
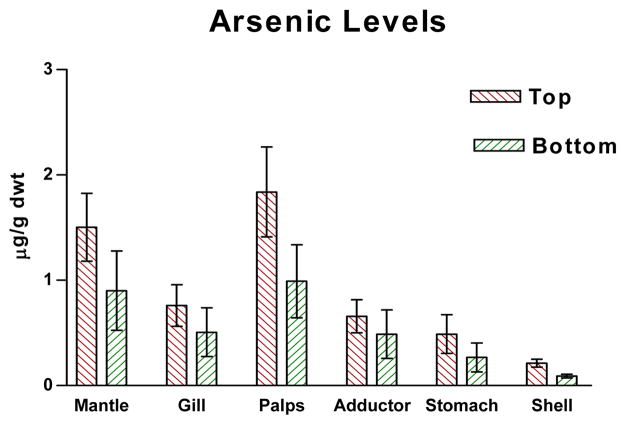
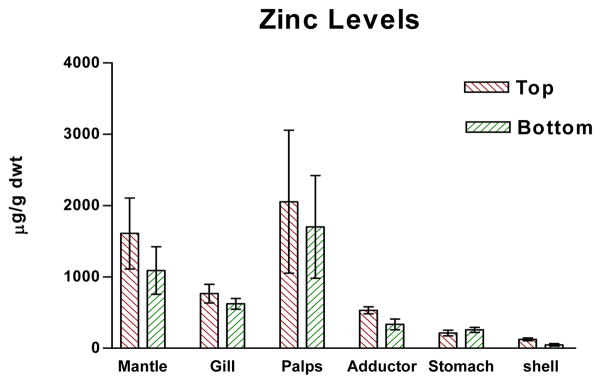
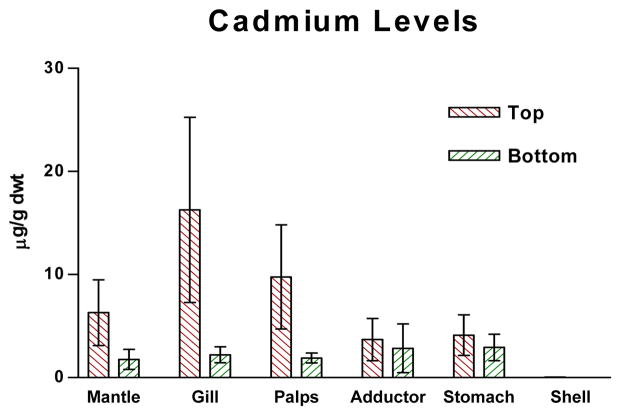
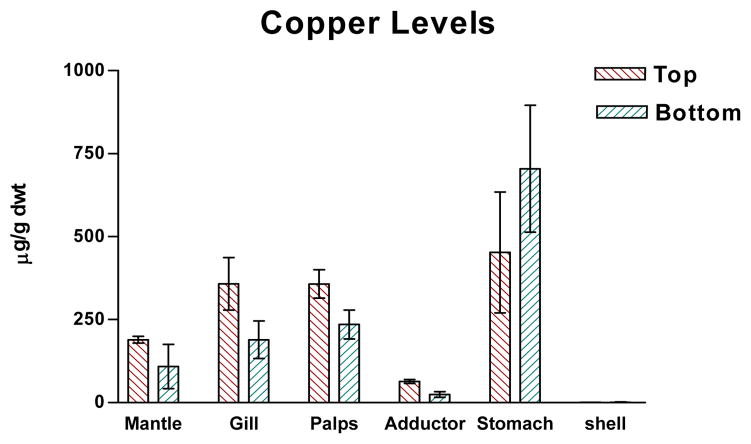
Figures 1–4 show the one-year bioaccumulations of arsenic, cadmium, copper and zinc in the mantle, gill, palps, adductor muscle, stomach and shell of top positioned oysters compared to bottom positioned oysters. For each metal, top oysters from both sites (GNPMS and KBCCM) were combined, and bottom oysters from both sites were combined, to generate top/bottom comparisons in each chart of the figure. No significant difference in tissue metal accumulation could be discerned between top position oysters at GNPMS and top positioned oysters at KBCCM, or between bottom position oysters at GNPMS and bottom positioned oysters at KBCCM (data not shown). The various tissues of the one-year old Jamaica Bay oysters readily accumulated arsenic, cadmium, copper and zinc. The metal content of the tissues were not homogeneously distributed and were different for the oysters maintained at the top as compared to the bottom. Soft tissues accumulated metals in the μg/g dwt range, which is comparable to other published reports for C. virginica growing in other areas34,35. Of the four metals tested, zinc showed the most bioaccumulation in all oyster tissues including shell.
Figure 1.
Graph of arsenic levels in oyster tissues. p<0.01 for comparison of tissue arsenic levels for top verses bottom, Wilcoxon Matched-Pairs Signed Ranks test.
Figure 4.
Graph of zinc levels in oyster tissues. p<0.01 for comparison of tissue zinc levels for top verses bottom, Wilcoxon Matched-Pairs Signed Ranks test.
In soft tissues, zinc accumulations were greater than copper, which were greater than cadmium. Arsenic showed the lowest soft tissue accumulations. For arsenic, cadmium and copper, the gills, mantle and palps accumulated the most metals; while for copper, the stomach accumulated considerable more than the other tissues. With all four metals, the soft tissues of oysters positioned one foot below the surface accumulated more metal than their bottom positioned counterpart, with the exception of stomach which not only showed very high copper accumulations but demonstrated a greater copper accumulation in bottom dwelling oysters.
Shell tissue also accumulated metals but to a lesser extent. With the exception of zinc, shell metal accumulations were 10–100× less than metal accumulations seen in any one type of oyster tissue. Zinc and copper accumulation were in the μg/g dwt range while cadmium and arsenic accumulations were in the ng/g dwt range. Average shell zinc levels (μg/g) were higher in top positioned oysters (top: 123 ± 20, bottom: 47 ± 16); average shell cadmium levels (ng/g) were higher in top positioned oysters (top: 28 ± 9, bottom: 12 ± 4); and average shell arsenic levels (ng/g) were higher in top positioned oysters (top: 213 ± 37, bottom: 92 ± 18). Only copper showed slightly higher average accumulations (μg/g) in bottom positioned oysters (top: 1.033 ± 0.145, bottom: 1.450 ± 0.352.
Discussion
Marine bivalves are filter feeders that take up and accumulate metals and other pollutants from the water column or via ingestion of contaminants adsorbed to phytoplankton, detritus and sediment particles. Because they are sessile, they reflect local contaminant concentrations more accurately than crustaceans and free-swimming finfish. Marine bivalves such as oysters and mussels have been extensively used as model organisms in environmental studies of water quality36,37. Trace metals are taken up and accumulated by oysters and many other marine invertebrates to tissue and body concentrations usually much higher on a wet weight basis than concentrations in the surrounding seawater38.
In this study we measured the one year bioaccumulation and tissue distribution of four metal pollutants (arsenic, cadmium, copper and zinc) in C. virginica seed, that had been transplanted to the bay in 2002 at two different depths: one foot below the surface and one foot above the sediment. Past years of unregulated industrial, rural and residential dumping, and previously inadequate wastewater treatment has caused Jamaica Bay sediment to be a major reservoir of metal pollutants. While the content and degree of metal contamination in the sediment can vary greatly depending upon site tested, the presence of many metal pollutants throughout Jamaica Bay sediment is widespread. In a 2002 report to the New York State Department of Environmental Conservation and the NY/NJ Port Authority13, sediment sampled at 3 different sites in Jamaica Bay indicated that the content of many metals including arsenic, cadmium, copper and zinc were present at levels above the “Effects Range Low Level” (ERL) guideline and at some sites were above the “Probable Effects Level” (PEL) guideline. Arsenic levels ranged from 8.6–11 mg/kg dwt. (ERL and PEL, 8.2 and 41.6, respectively). Cadmium levels ranged from 2.2–5.7 mg/kg dwt. (ERL and PEL, 1.2 and 4.2, respectively). Copper levels ranged from 85–160 mg/kg dwt. (ERL and PEL, 34 and 108.2, respectively). Zinc levels ranged from 45–307 mg/kg dwt. (ERL and PEL, 150 and 271, respectively). A 1991 report on the annual input of heavy metals to Jamaica Bay revealed that sewage effluent carries the largest quantities of zinc, copper and cadmium to the bay39.
Our results indicate that over a one-year period of growth in Jamaica Bay, considerable amounts of arsenic, cadmium, copper and zinc can accumulate in the tissues of oyster seed. Arsenic and cadmium are believed to serve no essential role in animal or plant metabolism40,41. Copper and zinc function as micronutrients, but when in excess even these essential elements can cause ecotoxicological effects. Therefore, organisms that are exposed to and accumulate metal pollutants require a means of physiological detoxification, typically by binding to high affinity sites in inorganic granules, or to various proteins like apoferritin or cysteine-rich metallothionein42,43.
Arsenic is the twentieth most abundant element in the earth’s crust and sources of aquatic arsenic are both natural as well as anthropogenic. It is released naturally to the atmosphere from volcanic eruptions and forest fires44 and to water via natural weathering processes45. Anthopogenic sources include pollution due to fossil fuel combustion, mining/smelting, effluents from sewage treatment facilities, leaching from hazardous waste disposal sites, and production and use of arsenic compounds as pesticides and as a wood preservative46. The toxicity of arsenicals is highly dependent upon the nature of the compound with trivalent arsenic compounds considered more toxic than pentavalent arsenic compounds, and inorganic forms more toxic than organic forms47,48. Arsenic and arsenic-containing organic compounds have not been shown to accumulate to any great extent in aquatic organisms49, with perhaps the exception of C. virginica, which in a bioaccumulation test was able to generate a very high bioconcentration factor (BCF) of 350 after 112 days of exposure to phytoplankton containing arsenic50. In addition C. virginica may be particularly resistant to the toxic effects of arsenic for data comparing the acute toxicity of inorganic arsenic (III) indicated a very high acute value for C. virginica compared to C. gigas, a related species (7500 ug/l; 326 ug/l, respectively)51.
Cadmium, a trace metal widely distributed in soil, air, water, and living things40, is naturally present in zinc, lead, and copper deposits. It is also released into the environment from several anthropogenic sources including smelting and mining, electroplating, application of phosphate fertilizers, waste disposal operations, and the manufacturing and disposal of paints, alloys, batteries, and plastics52,53,54,55,56. Cadmium is a cumulative human toxicant that can cause a variety of adverse health problems including renal dysfunction and degenerative bone disease57,58. Cadmium has been found to bioaccumulate in fish and shellfish tissues in estuarine/marine waters59,60 nationwide and New York has issued advisories for this metal in all of its marine coastal waters.
Copper is plentiful in the environment and is an essential micronutrient for the normal growth and metabolism of all living organisms61,62,63. The United States is the major world producer and consumer of copper and its compounds. Copper releases to the global biosphere—which may approach 1.8 million metric tons per year64 - come mostly from anthropogenic activities65. Copper is among the most toxic of the heavy metals in freshwater and marine biota61,66. Excess copper can cause cellular damage by generating oxygen free radicals and inactivating biological thiols into disulfides62. Inputs of copper into aquatic ecosystems have increased sharply during the past century due to a number of reasons including atmospheric fallout from industrial activities, waste and industrial discharges, and leaching of antifouling marine paints and wood preservatives67,68. Copper is elevated in sediments of many marinas, probably as a result of copper containing antifouling paints used on boats housed in these marinas69. Bioavailability and toxicity of copper to aquatic organisms depends on the total concentration of copper and its speciation70. In marine ecosystems, the high copper levels measured in heavily contaminated coastal areas sometimes approach the incipient lethal concentrations for some organisms71. Among marine organisms, the highest accumulations are generally found in molluscan tissues and soft parts, especially those of cephalopods and oysters65. Bioconcentration factors for copper are highest for American oysters after exposure for 140 days (20,700–28,200), and lowest for bay scallops (Argopecten irradians) after exposure for 112 days (3,310) and for softshell clams after exposure for 35 days (3,300)72.
Zinc is ubiquitous in the tissues of plants and animals73 and functions as a cofactor for many enzymes in both aquatic and terrestrial organisms74. Like many other trace metals, zinc can be toxic if present at high concentrations in aquatic ecosystems75,76,77. Most of the zinc introduced into aquatic environments eventually is partitioned into the sediments. Anthropogenic sources include domestic and industrial sewage, combustion of fossil fuels and solid wastes, corrosion of zinc alloys and galvanized surfaces, road surface runoff, smelting and mining operations, and erosion of agricultural soils78,79,80,81,82. In seawater zinc exists in a dissolved state, as a solid precipitate, or adsorbed to particle surfaces. Zinc in molluscs is usually associated with high molecular weight proteins, with diet (as opposed to ambient water zinc concentrations), from collection locales with elevated sediment zinc burdens, and with particulate matter from dredging and storm perturbations83,84. Molluscan life processes do not seem to be affected by excess zinc accumulations and zinc is frequently accumulated far in excess of the organism’s immediate needs83.
In this study C. virginica seed, transplant to Jamaica Bay, readily accumulated arsenic, cadmium, copper and zinc and the distribution of these metals were not homogeneous throughout oyster tissues. The unequal distribution of all 4 metals among the tested oyster tissue is not likely a random event. Copper values were particularly high in stomach tissue and are more likely a reflection of the copper-laden microalgea and detritus the animal ingested rather than actual tissue accumulations. For the other tissues, the cellular concentration of metallothionein and other metal binding organic or inorganic compounds probably varies depending upon physiological parameters and each tissue’s role in metal detoxification. In the case of copper and zinc, which are micronutrients, the unequal distribution and known extensive accumulations possible in the American oyster may correlate with some physiological function for these two metals. Both of these nutrients are exclusively sequestered in oyster amebocytes, a cell type credited with many indispensable responsibilities for oyster survival, including antimicrobial activities for defense and nutrition85. The fact that copper and zinc accumulations, which were most abundant in the gills, mantal and palps, were not homogeneous throughout oyster tissue may be more associated with amebocyte function and distribution than a detoxified storage of these metals.
A number of studies have reported on metal accumulations in the shell of C. virginica from various sites86,87,88,89. Our results are in accordance with previous findings with shell tissue accumulating metals but to a lesser extent. Copper and zinc concentrations were in the ug/g dwt range, while cadmium and arsenic concentrations were in the ng/g range. Even though metal accumulations in shell were much lower than that found in oyster soft tissue, if one considers that the shell of the animal typically makes up over 75% of the animal’s weight, then the overall amount of metal present in the shell of the whole animal could be significant87. Therefore measurements of metal accumulations in oyster shell are important for they can represent a terrestrial reservoir of aquatic pollutants that can be adsorbed or released into the environment depending upon the nature of the metal and ambient concentrations. In addition, metals and other aquatic pollutants may influence the normal mineralization and metal composition of oyster shells, possibly decreasing shell strength and stability. Frazier90 believed that shell thinning was linked to effects of copper and cadmium on shell calcification enzymes. If competent shell structure depends on metal composition, then deviations in metal composition may adversely affect shell quality, compromising the long-term health and survival of the animal.
Comparing metal accumulations in C. virginica seed grown at different depths in the bay, our results indicate that for most of the tissues studied, top-maintained oysters accumulated more metal pollutants than oysters maintained near the sediment. Differences in general water quality parameters such as pH, temperature, dissolved O2, salinity, and turbidity could not account for our findings because these parameters, which were monitored throughout the experimental period, indicated no statistical variation at either site or at either depth19. Our results also indicate that wide variations in metal accumulations were possible even among individuals positioned at the same site and same depth. This is commonly reported by monitoring agencies for bioindicator organisms and can be due to many causes including age, seasonal factors and individual variability within the population34. While our oysters were all of the same age and reproductive status (subadult), differences in metal accumulations could still exist due to feeding patterns, extent of glycogen stores, adaptability to acute stresses, or overall health of the organism91,92,93. Even sex can be a factor for variations in metal accumulations for in the bivalve Donax trunculus L., females were shown to accumulate higher concentrations of zinc than males94. Differences in feeding patterns or overall health may also be an explanation for the disparity in metal accumulations between surface and bottom dwelling oysters. Bivalves accumulate pollutants not only from the water column and metal-laden detritus, but also via ingestion of metal contaminated phytoplankton. While our measurements of chlorophyll-a in surface/bottom water showed no consistent variation19, the extent of feeding and the species and metal content of the microalgae available to the oysters near the surface might have been different from that available to oysters growing near the sediment. It also is possible that the increase in metal accumulations seen in top maintained oysters may have been due to a difference in top/bottom overall health or early infection rates with known oyster pathogens such as Haplosporidium nelsoni (MSX disease) or Perkinsus marinus (Dermo disease). While neither organism is harmful to humans, nor a health threat to humans who ingest infected shellfish, these parasites can chronically weaken and eventually kill C. virginica over a period of years95,96. Other studies have shown a positive correlation between tissue burden of certain metals, especially copper and zinc, and immuno-defense related characteristic of oysters85,97,98. Also to consider is the fact that top but not bottom dwelling oysters may have been exposed to additional bay contaminants, including floatables and organic pollutants, that either enhanced metal accumulations or compromised oyster health and therefore soft tissue growth. Differences in soft tissue weights can significantly affect trace metal concentrations by simply diluting or concentrating the animal’s total metal body burden99,100,101,102,103,104,105. It was a surprise that oysters positioned near the sediment not only had lower overall metal accumulations, but also had better growth rates when compared to top grown oysters19. It is currently unknown whether this pattern of greater growth and lesser metal accumulations in Jamaica Bay is site specific or if metal accumulation would have been greater and growth worse in bottom dwelling oysters if a more polluted site were available for our study. The fact that the bottom placed oysters accumulated less tissue arsenic, cadmium, copper and zinc than their top placed counterpart is interesting and may itself be an explanation for the faster bottom growth rate. However, only 5 tissues and these 4 metal pollutants were studied in this report. More work needs to be done here and at additional sites in Jamaica Bay to determine if other metal pollutants and other oyster tissues show similar patterns of metal accumulations.
Figure 2.
Graph of cadmium levels in oyster tissues. p<0.01 for comparison of tissue cadmium levels for top verses bottom, Wilcoxon Matched-Pairs Signed Ranks test.
Figure 3.
Graph of copper levels in oyster tissues. p<0.01 for comparison of tissue copper levels for top verses bottom, Wilcoxon Matched-Pairs Signed Ranks test.
Acknowledgments
We wish to acknowledge Frank M. Flower and Sons Oyster Farm, Oyser Bay, NY for supplying oysters. This work was supported in part by grants 0420359 of the MRI Program of NSF, 0622197 of the DUE Program of NSF, 2R25GM06003-05 of the Bridge Program of NIGMS, 0516041071 of NYSDOE and 67876-0036 of PSC-CUNY.
References
- 1.United States Fish and Wildlife Service (USFWS) Jamaica Bay and Breezy Point, Complex. 1997. Nov 16, Significant Habitats and Habitat Complexes of the New York Bight Watershed, Southern New England–New York Bight Coastal Ecosystems Program. [Google Scholar]
- 2.MacKenzie CL Jr, Burrell VG Jr, Rosenfield A, Hobart WL, editors. Atlantic and Gulf Coasts. US Dept. Commerce, NOAA Tech. Rep. NMFS 127. Vol. 1. Seattle, WA: 1997. The history, present condition, and future of the molluscan fisheries of North and Central America and Europe; p. 234. [Google Scholar]
- 3.New York/New Jersey Harbor Estuary Program (NYNJHEP) Health of the Harbor - The first Comprehensive Look at the State of the NY/NJ Harbor Estuary. Hudson River Foundation; NY: 2004. p. 82. [Google Scholar]
- 4.Franz DR. An historical perspective on mollusks in lower New York Harbor, with emphasis on oysters. In: Mayer GF, editor. Ecological Stress and the New York Bight: Science and Management. Estuarine Research Federation; Columbia, South Carolina: 1982. pp. 181–197. 715 p. [Google Scholar]
- 5.Rothschild BJ, Ault JS, Goulletquer P, Heral M. Decline of the Chesapeake Bay oyster population: a century of habitat destruction and overfishing. Mar Ecol Prog Ser. 1994;111:29–39. [Google Scholar]
- 6.Woods H, Hargis WJ, Jr, Hershner CH, Mason P. Disappearance of the natural emergent 3-dimensional oyster reef system of the James River, Virginia, 1871–1948. J Shellfish Res. 2005;24:139–142. [Google Scholar]
- 7.MacKenzie CL. History of oystering in the United States and Canada, featuring the eight greatest oyster estuaries. Mar Fish Rev. 1996a;58:1–78. [Google Scholar]
- 8.United States Army Corps of Engineers (USACE) Hudson-Raritan Estuary Environmental Restoration Study, Section 905(b) WRDA 86 Preliminary Analysis. New York: 2000. Jun, Reconnaissance Study; p. 38. [Google Scholar]
- 9.Ramondetta PJ, Harris WH. Heavy metals distribution in Jamaica Bay sediments. Environmental Geology Volume. 1978;2(3):145–149. [Google Scholar]
- 10.Franz DR, Harris WH. Final Report. Jamaica Bay Wildlife Refuge, Gateway National Recreation Area; Brooklyn, New York: 1985. Benthos Study. Contract #CX 1600-l-0031. [Google Scholar]
- 11.Staubitz WW, Wolcott SW. Resources Investigations Report 85-4085. New York: U.S. Geological Survey Water; 1985. Hydraulic and sediment characteristics at the North Channel Bridge, Jamaica Bay; p. 43. [Google Scholar]
- 12.Adams DA, O’Connor JS, Weisberg SB. Final Report: Sediment Quality of the NY/NJ Harbor System- An Investigation under the Regional Environmental Monitoring and Assessment Program (REMAP). EPA/902-R-98-001. USEPA-Region 2. Division of Environmental Science and Assessment; Edison, NJ: 1998. www.epa.gov/emap/remap/html/docs/nynjharbor.html. [Google Scholar]
- 13.Barry A, editor. New York State Department of Environmental Conservation (NYSDEC) and the NY/NJ Port Authority (NYNJPA) Report on Bioassay analyses conducted on sediments collected from Jamaica Bay, Far Rockaway, New York June 2002. Vittor & Associates, Inc; Mobile, AL: 2002. Sep, [Google Scholar]
- 14.Adams DA, Benyi S. An Investigation under the Regional Environmental Monitoring and Assessment Program (REMAP). EPA/902-R-03-002. USEPA-Region 2. Division of Environmental Science and Assessment; Edison, NJ: 2003. Final Report: Sediment Quality of the NY/NJ Harbor System: A 5 year revisit 1993/4 –1998. http://www.epa.gov/emap/remap/html/docs/NY_NJHarbor98.pdf. [Google Scholar]
- 15.Ortiz MT, Stavroulakis AM, Love A, Lanzetta P, Pilchman P. Characterization of Potential Transplantation Sites for Eelgrass (Zostera marina L.) in Jamaica Bay, New York and Eelgrass Growth in a Laboratory Microcosm Mimicking Field Conditions. In Vivo. 2005;26(3):4–18. [Google Scholar]
- 16.Wikfors GH, Twarog J, Joseph W, Ferris GE, Smith BC, Ukeles R. Survival and growth of post-set oysters and clams on diets of cadmium-contaminated microalgal cultures. Mar Environ Res. 1994;37(3):257–281. [Google Scholar]
- 17.Keppler C, Ringwood AH. Expression of P-glycoprotein in the gills of oysters, Crassostrea virginica: seasonal and pollutant related effects. Aquat Toxicol. 2001;54(3–4):195–204. doi: 10.1016/s0166-445x(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 18.Gifford SP, MacFarlane GR, O’Connor WA, Dunstan RH. Effect of the pollutants lead, zinc, hexadecane and octocosane on total growth and shell growth in the akoya pearl oyster, Pinctada imbricate. Journal of Shellfish Research. 2006;25(1):159–165. [Google Scholar]
- 19.Sarinsky G, Carroll MA, Nduka E, Catapane EJ. Growth and Survival of the American Oyster Crassostrea virginica in Jamaica Bay, New York. In Vivo. 2005;27(1):15–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham PA. The use of bivalve molluscs in heavy metal pollution research. In: Vernberg WB, Thurberg FP, Calabrese A, Vernberg FJ, editors. Marine Pollution: Functional Responses. Academic Press; NY: 1979. [Google Scholar]
- 21.Bourgoin BP. Mytilus edulis shell as a bioindicator of lead pollution: Considerations on bioavailability and variability. Mar Ecol Prog Ser. 1990;61:253–262. [Google Scholar]
- 22.Phillips D, Rainbow P. Biomonitoring of trace aquatic contaminants. London, New York: Elsevier Applied Science; 1993. p. 371. [Google Scholar]
- 23.Boening DW. An evaluation of bivalves as biomonitors of heavy metal pollution in marine waters. Environ, Monit Assess. 1999;55:459–470. [Google Scholar]
- 24.U.S. EPA. Vol. 1 Fish Sampling and Analysis. 3. Vol. 3. 1993a. p. 16. [Google Scholar]
- 25.U.S. EPA. Vol 1 Fish Sampling and Analysis. 3. Vol. 4. 1993b. p. 3. [Google Scholar]
- 26.NOAA. “Chemical Contaminants in Oysters and Mussels” by Tom O’Connor. 1998. (on-line) [Google Scholar]
- 27.Capar SG, Yess NJ. US Food and Drug Administration survey of cadmium, lead and other elements in clams and oysters. Food Addit Contam. 1996;13(5):553–560. doi: 10.1080/02652039609374440. [DOI] [PubMed] [Google Scholar]
- 28.Bu-Olayan AH, Subrahmanyam MN. Accumulation of copper, nickel, lead and zinc by snail, Lunella coronatus and pearl oyster, Pinctada radiata from the Kuwait coast before and after the Gulf War oil spill. Sci Total Environ. 1997;197(1):161–165. doi: 10.1016/s0048-9697(97)05428-4. [DOI] [PubMed] [Google Scholar]
- 29.Spooner DR, Maher W, Otway N. Trace Metal Concentrations in Sediments and Oysters of Botany Bay, NSW, Australia. Archives of Environmental Contamination and Toxicology. 2003;45(1):0092–0101. doi: 10.1007/s00244-002-0111-0. [DOI] [PubMed] [Google Scholar]
- 30.Scanes PR, Roach AC. Determining natural background concentrations of trace metals in oysters from New South Wales. Australia Environ Poll. 1999;105(3):437–446. [Google Scholar]
- 31.Abbe GR, Riedel GF, Sanders JG. Factors that influence the accumulation of copper and cadmium by transplanted eastern oysters (Crassostrea virginica) in the Patuxent River, Maryland. Marine Environmental Research. 2000;49(4):377–396. doi: 10.1016/s0141-1136(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 32.Fang ZQ, Cheung RYH, Wong MH. Heavy Metal concentrations in edible bivalves and gastropods available in the major markets of the Pearl River Delta. J Environ Sci. 2001;13(2):210–217. [PubMed] [Google Scholar]
- 33.Luckenbach MW, O’Beirn FX, Taylor J. An Introduction to Culturing Oysters in Virginia. Virginia Institute of Marine Science Press; Gloucester Point, VA: 1999. p. 24. On-line at http://www.vims.edu/oystergarden/CulturingOysters.pdf. [Google Scholar]
- 34.Daskalakis KD. Variability of metal concentrations in oyster tissue and implications to biomonitoring. Marine Pollution Bulletin. 1996;32(11):794–801. [Google Scholar]
- 35.Karouna-Renier NK, Snyder RA, Allison JG, Wagner MG, Rao KR. Accumulation of organic and inorganic contaminants in shellfish collected in estuarine waters near Pensacola, Florida: Contamination profiles and risks to human consumers. Environmental Pollution. 2007;145(2):474–488. doi: 10.1016/j.envpol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Wang WX, Fisher NS, Luoma SN. Kinetic determinations of trace element bioaccumulation in the mussel Mytilus edulis. Mar Ecol Prog Ser. 1996;140:91–113. [Google Scholar]
- 37.Griscom SB, Fisher NS, Luoma SN. Geochemical Influences on Assimilation of Sediment-Bound Metals in Clams and Mussels. Environ Sci Technol. 2000;34:91–99. [Google Scholar]
- 38.Rainbow PS. The Significance of Trace Metal Concentrations in Marine Invertebrates, Ecotoxicology of Metals in Invertebrates.. Proceedings of a session at the First Society of Environmental Toxicology and Chemistry-Europe Conference; Sheffield, England. 7–10 April 1991; Boca Raton, Florida: Lewis Publishers; 1993. pp. 3–23. 8 tab, 58 ref. [Google Scholar]
- 39.Seidemann D. Metal pollution in sediments of Jamaica Bay, New York, USA - An urban estuary. Environmental Management. 1991;15(1):73–81. [Google Scholar]
- 40.Pinot F, Kreps SE, Bachelet M, Hainaut P, Bakonyi M, Polla BS. Cadmium in the environment: sources, mechanisms of biotoxicity, and biomarkers. Rev Environ Health. 2000;15:299–323. doi: 10.1515/reveh.2000.15.3.299. [DOI] [PubMed] [Google Scholar]
- 41.Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: role for cadmium, P450 and heme oxygenases? Tohoku J Exp Med. 2006;208:179–202. doi: 10.1620/tjem.208.179. [DOI] [PubMed] [Google Scholar]
- 42.Rainbow PS. Ecophysiology of Trace Metal Uptake in Crustaceans. Estuarine, Coastal and Shelf Science. 1997;44:169–175. [Google Scholar]
- 43.Roesijadi G. Metallothionein and its role in toxic metal regulation. Comp Biochem Physiol C. 1996;113:117–123. [Google Scholar]
- 44.Walsh PR, Duce RA, Fasching JL. Considerations of the enrichment, sources, and flux of arsenic in the troposphere. J Geophys Res. 1979;84(C4):1719–1726. [Google Scholar]
- 45.U.S. EPA (U.S. Environmental Protection Agency) Intermedia Priority Pollutant Guidance Documents. Office of Pesticides and Toxic Substances; Washington, DC: 1982. Arsenic. [Google Scholar]
- 46.Eisler R. Biological Report, 85(1.12), Contaminant Hazard Reviews, Report No. 12. Fish and Wildlife Service, U.S. Department of the Interior; Laurel, MD: 1988. Arsenic Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. [Google Scholar]
- 47.Vallee BL, Ulmer DD, Wacker WEC. Arsenic toxicology and biochemistry. Arch Ind Health. 1960;21:132–151. [Google Scholar]
- 48.Penrose WR. Arsenic in the marine and aquatic environments: Analysis, occurrence and significance. CRC Crit Rev in Environ Contam. 1974;4:465–482. [Google Scholar]
- 49.National Academy of Sciences (NAS) Arsenic; Committee on Medical and Biologic Effects of Environmental Pollutants; Washington, DC: National Research Council; 1977a. [Google Scholar]
- 50.Zaroogian GE, Hoffman GL. Arsenic uptake and loss in the American oyster, Crassostrea virginica. Environ Monit Assessment. 1982;1:345–358. doi: 10.1007/BF00403835. [DOI] [PubMed] [Google Scholar]
- 51.EPA. Ambient Water Quality Criteria for Arsenic - 1984. 1985. Jan, EPA 440/5-84-033. [Google Scholar]
- 52.May TW, McKinney GL. Cadmium, lead, mercury, arsenic and selenium concentrations in freshwater fish, 1976–1977 - National Pesticide Monitoring Program. Pesticides Monitoring Journal. 1981;15(1):14–38. [PubMed] [Google Scholar]
- 53.Farag AM, Woodward DF, Goldstein JN, Brumbaugh W, Meyer JS. Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur D’Alene River Basin, Idaho. Arch Environ Contam Toxicol. 1998;34:119–127. doi: 10.1007/s002449900295. [DOI] [PubMed] [Google Scholar]
- 54.U.S. EPA (U.S. Environmental Protection Agency) Metal Bioaccumulation in Fish and Aquatic Invertebrates. Environmental Research Laboratory, Office of Research and Development; Springfield, VA: 1978. EPA-600/3-78-103. [Google Scholar]
- 55.U.S. EPA (U.S. Environmental Protection Agency) Health Assessment Document for Cadmium. Environmental Standards and Criteria, Office of Research and Development; Research Triangle Park, NC: 1979. EPA-600/8-79-003. [Google Scholar]
- 56.U.S. EPA (U.S. Environmental Protection Agency) Cadmium Health Advisory Draft. Office of Drinking Water; Washington, DC: 1987. [Google Scholar]
- 57.Goering PL, Waalkes MP, Klaassen CD. Toxicology of cadmium. In: Goyer RA, Cherian MG, editors. Toxicology of Metals. Springer-Verlag; Berlin, Germany: 1995. pp. 190–214. [Google Scholar]
- 58.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 59.NOAA (National Oceanic and Atmospheric Administration) NOAA Technical Memorandum NOS OMA 38. U.S. Department of Commerce; Rockville, MD: 1987. National Status and Trends Program for Marine Environmental Quality-Progress Report: A Summary of Selected Data on Chemical Contaminants in Tissues Collected During 1984, 1985 and 1986. [Google Scholar]
- 60.NOAA (National Oceanic and Atmospheric Administration) NOAA Technical Memorandum NOS OMA 49. U.S. Department of Commerce; Rockville, MD: 1989. National Status and Trends Program for Marine Environmental Quality - Progress Report: A Summary of Selected Data on Tissue Contamination from the First Three Years (1986–1988) of the Mussel Watch Project. [Google Scholar]
- 61.Schroeder HA, Nason AP, Tipton IH, Balassa JJ. Essential trace metals in man: copper. Journal of Chronic Diseases. 1966;19:1007–1034. doi: 10.1016/0021-9681(66)90033-6. [DOI] [PubMed] [Google Scholar]
- 62.Aaseth J, Norseth T. Copper. In: Friberg L, Nordberg GF, Vouk VB, editors. Handbook on the toxicology of metals. 2. II: specific metals. Elsevier; New York: 1986. pp. 233–254 . [Google Scholar]
- 63.Carbonell G, Tarazona JV. Toxicokinetics of copper in rainbow trout (Oncorhynchus mykiss) Aquatic Toxicology. 1994;29:213–221. [Google Scholar]
- 64.National Academy of Sciences (NAS) Committee on Medical and Biologic Effects of Environmental Pollutants. National Research Council, National Academy of Sciences; Washington, D.C.: 1977b. Copper; p. 115. [Google Scholar]
- 65.Eisler R. US Geological Survey, Biological Resources Division, Biological Science Report USGS/BRD/BSR--1998-0002. 1998. Copper hazards to fish, wildlife, and invertebrates: a synoptic review. [Google Scholar]
- 66.Betzer SB, Yevich PP. Copper toxicity in Busycon canaliculatum L. Biological Bulletin. 1975;148:16–25. doi: 10.2307/1540646. [DOI] [PubMed] [Google Scholar]
- 67.Nriagu JO. The global copper cycle. In: Nriagu JO, editor. Copper in the environment. Part 1: ecological cycling. John Wiley; N Y: 1979a. pp. 1–17 . [Google Scholar]
- 68.Nriagu JO. Copper in the atmosphere and precipitation. In: Nriagu JO, editor. Copper in the environment. Part 1: ecological cycling. John Wiley; NY: 1979b. pp. 45–75 . [Google Scholar]
- 69.Hall WS, Bushong SJ, Hall LW, Jr, Lenkevich MS, Pinkney AE. Monitoring dissolved copper concentrations in Chesapeake Bay, U.S.A. Environmental Monitoring and Assessment. 1988;11:33–42. doi: 10.1007/BF00394510. [DOI] [PubMed] [Google Scholar]
- 70.Hung TC, Chuang A, Wu SJ, Tsai CCH. Relationships among the species and forms of copper and biomass along the Erhjin Chi coastal water. Acta Oceanographic Taiwanica. 1990;25:65–76. [Google Scholar]
- 71.Neff JM, Anderson JW. The effects of copper (II) on molting and growth of juvenile lesser blue crabs Callinectes similis. In: Giam CS, editor. Pollutant effects on marine organisms. D.C. Heath and Company; Lexington, Massachusetts: 1977. pp. 155–165 . [Google Scholar]
- 72.U.S. Environmental Protection Agency (USEPA) US Environmental Protection Agency Report 440/5-80-036. 1980. Ambient water quality criteria for copper; p. 162. [Google Scholar]
- 73.Rosser BWC, George JC. Molt-induced muscle atrophy decreases the zinc content of the pectoralis of the giant Canada goose (Branta canadensis maxima) Experientia. 1986;42:549–550. [Google Scholar]
- 74.Coombs TL. The distribution of zinc in the oyster Ostrea edulis and its relation to enzymic activity and to other metals. Mar Biol. 1972;12:170–178. [Google Scholar]
- 75.Eisler R, Gardner GR. Acute toxicology to an estuarine teleost of mixtures of cadmium, copper and zinc salts. J Fish Biol. 1973;5:131. [Google Scholar]
- 76.Eisler R. US Fish and Wildlife Service Biological Report 10. 1993. Zinc hazards to fish, wildlife, and invertebrates: a synoptic review; p. 106. [Google Scholar]
- 77.Eisler R, Wapner M. Second annotated bibliography on biological effects of metals in aquatic environments. U.S. Environmental Protection Agency, Office of Research and Development, Environmental Research Laboratory; Narragansett, RI: 1975. EPA-600/3-75-008. [Google Scholar]
- 78.Weatherley AH, Lake PS, Rogers SC. Zinc pollution and the ecology of the freshwater environment. In: Nriagu JO, editor. Zinc in the environment. Part I: ecological cycling. John Wiley; NY: 1980. pp. 337–417. [Google Scholar]
- 79.Spear PA. Zinc in the aquatic environment: chemistry, distribution, and toxicology. National Research Council of Canada; 1981. p. 145. Publication NRCC 17589. [Google Scholar]
- 80.Mirenda RJ. Acute toxicity and accumulation of zinc in the crayfish, Orconectes virilis (Hagen) Bulletin of Environmental Contamination and Toxicology. 1986;37:387–394. doi: 10.1007/BF01607778. [DOI] [PubMed] [Google Scholar]
- 81.Llobet JM, Domingo JL, Colomina MT, Mayayo E, Corbella J. Subchronic oral toxicity of zinc in rats. Bulletin of Environmental Contamination and Toxicology. 1988a;41:36–43. doi: 10.1007/BF01689056. [DOI] [PubMed] [Google Scholar]
- 82.Buhl KJ, Hamilton SJ. Comparative toxicity of inorganic contaminants related by placer mining to early life stages of salmonids. Ecotoxicology and Environmental Safety. 1990;20:325–342. doi: 10.1016/0147-6513(90)90010-3. [DOI] [PubMed] [Google Scholar]
- 83.Eisler R. Trace metal concentrations in marine organisms. Pergamon Press; NY: 1981. p. 687. [Google Scholar]
- 84.Eisler R. Trace metal changes associated with age of marine vertebrates. Biological Trace Element Research. 1984;6:165–180. doi: 10.1007/BF02916933. [DOI] [PubMed] [Google Scholar]
- 85.Fisher WS. Antimicrobial activity of copper and zinc accumulated in Eastern oyster amebocytes. Journal of Shellfish Research. 2004;23(2):321–351. [Google Scholar]
- 86.Smith RA, Wright ER. Elemental composition of oyster shell. Texas J Sci. 1962;14:222–224. [Google Scholar]
- 87.Wolfe DA. Levels of stable Zn and 65Zn in Crassostrea virginica from North Carolina. J Fish Res Bd Canada. 1970;27:47–57. [Google Scholar]
- 88.Windom HL, Smith RG. Distribution of iron, manganese, copper, zinc, and silver in oysters along the Georgia coast. J Fish Res Bd Canada. 1972;29:450–452. [Google Scholar]
- 89.Ferrell RE, Carville TE, Martinez JD. Trace metals in oyster shells. Environ Lett. 1973;4:311–316. doi: 10.1080/00139307309435502. [DOI] [PubMed] [Google Scholar]
- 90.Frazier JM. The dynamics of metals in the American oyster, Crassostrea virginicaII. Environmental effects. Chesapeake Sci. 1976;17:188–197. [Google Scholar]
- 91.Phillips DJH. The common mussel Mytilus edulis as an indicator of pollution by zinc, cadmium, lead and copper. 1. Effects of environmental variables on uptake of metals. Mar Biol. 1976;38:71–80. [Google Scholar]
- 92.Popham JD, D’Auria JM. Combined effect of body size, season, and location on trace element levels in mussels (Mytilus edulis). Archs environ. Contam Toxicol. 1983;12:1–14. doi: 10.1007/BF01054995. [DOI] [PubMed] [Google Scholar]
- 93.Martin N, Ichikawa G, Goetzl J, de Reyes M, Stephenson MD. Relationships between physiological stress and trace toxic substances in the bay mussel, Mytilus edulis from San Francisco Bay, California. Mar Environ Res. 1984;11:91–110. [Google Scholar]
- 94.Marina M, Enzo O. Variability of zinc and manganese concentrations in relation to sex and season in the bivalve Donax trunculus. Marine Pollution Bulletin. 1983;14(9):342–346. [Google Scholar]
- 95.Ford SE, Tripp MR. Diseases and Defense Mechanisms. In: Kennedy VS, Newell RIE, Eble AF, editors. The Eastern Oyster Crassostrea virginica. Maryland Sea Grant College; College Park, Maryland: 1996. pp. 581–660. [Google Scholar]
- 96.Lenihan HS, Micheli F, Shelton SH, Peterson CH. The influence of multiple environmental stressors on susceptibility to parasites: an experimental determination with oysters. Limnology and Oceanography. 1999;44(3):910–924. [Google Scholar]
- 97.Fisher WS, Oliver LM, Winstead JT, Long ER. A survey of oysters Crassostrea virginica from Tampa Bay, Florida: associations of internal defense measurements with contaminant burdens. Aquatic Toxicology. 2000;51(1):115–138. doi: 10.1016/s0166-445x(00)00082-5. [DOI] [PubMed] [Google Scholar]
- 98.Oliver LM, Fisher WS, Winstead JT, Hemmer BL, Long ER. Relationships between tissue contaminants and defense-related characteristics of oysters (Crassostrea virginica) from five Florida bays. Aquatic Toxicology. 2001;55(3,4):203–222. doi: 10.1016/s0166-445x(01)00161-8. [DOI] [PubMed] [Google Scholar]
- 99.Simpson RD. Uptake and loss of zinc and lead by mussels (Mytilus edulis) and relationships with body weight and reproductive cycle. Mar Pollut Bull. 1979;10:74–78. [Google Scholar]
- 100.Strong CR, Luoma SN. Variations in the correlation of body size with concentrations of Cu and Ag in the bivalve Macoma balthica. Can J Fish Aquat Sci. 1981;38:1059–1064. [Google Scholar]
- 101.Lobel PB, Wright DA. Relationship between body zinc concentration and allometric growth measurements in the mussel Mytilus edulis. Mar Biol. 1982a;66:145–150. [Google Scholar]
- 102.Lobel PB, Wright DA. Gonadal and nongonadal zinc concentrations in mussels. Mar Pollut Bull. 1982b;13:320–323. [Google Scholar]
- 103.Lowe DM, Moore MN. The cytochemical distributions of zinc (Zn 11) and iron (Fe 111) in the common mussel, Mytilus edulis, and their relationship with lysosomes. J mar biol Ass U K. 1979;59:851–858. [Google Scholar]
- 104.Cossa D, Bourget E, Piuze J. Sexual maturation as a source of variation in the relationship between cadmium concentration and body weight of Mytilus edulis. L Mar Pollut Bull. 1979;10:174–176. [Google Scholar]
- 105.Watling HR, Watling RJ. Trace metals in Chorornytilus mendionalis. Mar Pollut Bull. 1976;7:91–94. [Google Scholar]