Abstract
The homozygous NQO1*2 polymorphism results in a null NQO1 phenotype and is a susceptibility factor for occupational benzene poisoning. NQO1 plays an important role in detoxification of benzene-derived quinones but plays a role in numerous other non-metabolic cellular functions. NQO1 is expressed in endothelial cells of bone marrow which form the vascular stem cell niche important in stem cell homing and mobilization. We therefore employed a transformed human bone marrow endothelial cell (HBMEC) line to define the effects of compromising NQO1 on endothelial function. Either inhibition or knockdown of NQO1 led to decreased expression of the adhesion molecules E-selectin, VCAM-1 and ICAM-1 and decreased functional adhesion of CD34+ progenitor cells after TNFα stimulation. Suicide inhibition or knockdown of NQO1 decreased NFκB p105 precursor and NFκB p50 subunit levels as well as leading to decreased nuclear levels of NFκB phospho-p65. An additional function of endothelial cells is tube formation and angiogenesis which was inhibited by the benzene metabolite hydroquinone suggesting that endothelial function may be affected at multiple levels after exposure of NQO1*2 polymorphic individuals to benzene. These data demonstrate that NQO1 plays an upstream role in NFκB signaling and adhesion molecule expression in HBMEC and that NQO1 has important regulatory effects in its own right in addition to being a marker for Nrf-2 activation. Metabolic susceptibility factors such as NQO1 have roles in addition to detoxification of reactive intermediates and interrogation of these novel roles can inform both mechanisms of toxicity and human risk assessment.
Keywords: Benzene, NAD(P)H:quinone oxidoreductase 1 (NQO1), adhesion molecule, NFκB, bone marrow endothelium, vascular stem cell niche
1. NAD(P)H:Quinone oxidoreductase 1 (NQO1) and Benzene Toxicity
A null polymorphism in NQO1, the NQO1*2 polymorphism, has been associated with toxicity after occupational exposure of workers to benzene [1–4]. This effect has been considered a result of a lack of metabolic detoxification of reactive benzene-derived quinones in individuals deficient in NQO1. This interpretation is consistent with in-vitro data demonstrating that NQO1 is one of the metabolic determinants of the cell-specific toxicity of benzene metabolites in bone marrow cell populations [5–8]. However, it has become increasingly evident that NQO1 has multiple biological functions and the consequences of the NQO1*2 polymorphism may not be restricted to metabolism [9–11]. NQO1 has been associated with control of reduced/oxidized pyridine nucleotide ratios [11], superoxide scavenging [12], interaction with and modulation of proteasomal processing of p53 [13–15] and other proteins [16,17] and microtubule stabilization [18]. The concept that proteins associated with metabolism play diverse roles in cellular function is not new and examples include regulation of JNK signaling and cell proliferation by glutathione-S-transferases [19] and regulation of hematopoietic stem cell cycling and the balance between stem cell quiescence/proliferation by the Ah receptor [20–22]. The latter effect may be particularly relevant to mechanisms underlying benzene hematopoietic toxicity.
1.1 Additional roles of NQO1 influencing hematopoiesis ?
In addition to the association of increased benzene toxicity with compromised NQO1 status, the NQO1*2 polymorphism has also been found to be a susceptibility factor for de-novo leukemias [23–25] and for therapy-induced leukemias [26–28]. Further, unchallenged NQO1 knockout animals demonstrate myeloid hyperplasia [29] and disruption of the NQO1 gene in mice leads to radiation induced myeloproliferative disease [30]. These findings have led us to examine whether NQO1 might be influencing hematopoiesis directly in addition to its metabolic detoxification role.
2. NQO1 and bone marrow endothelium
In archived human bone marrow biopsies, NQO1 was found to be expressed at high levels in bone marrow endothelium [31] which is of potential functional significance. A single layer of bone marrow sinusoidal endothelial cells forms the stem cell vascular niche which is important in the trafficking of stem cells between bone marrow and peripheral compartments [32–34]. In addition, bone marrow endothelial cells have recently been shown to be essential for the self renewal and repopulation of stem cells [35]. Important functions of endothelial cells include adhesion molecule expression which is critical in stem cell homing to and mobilization from bone marrow [32–34] and capillary tube formation which is essential for angiogenesis [36,37]. Recently, recovery after myelosuppression, which occurs after benzene exposure, has been shown to depend on endothelial cell angiogenesis [38]. If a lack of NQO1 as a consequence of the NQO1*2 polymorphism resulted in compromised bone marrow endothelial cell function, it is conceivable given the key role of endothelium in both adhesion and angiogenesis that this may result in impaired hematopoiesis. To explore the question of whether a lack of NQO1 alters bone marrow endothelial cell function we have employed a transformed human bone marrow endothelial cell line which has been used to investigate mechanisms underlying adhesion of bone marrow progenitor cells to endothelium [39]. Multiple adhesion molecules and chemokines are likely important players in the adhesion process but in the transformed human bone marrow endothelial cell line employed in our studies, E-selectin, VCAM-1 and ICAM-1 have been demonstrated to play key roles. E-Selectin is important in the initial rolling behavior of attaching cells while VCAM-1 and ICAM-1 contribute to firm adhesion [39].
2.1 Does compromising NQO1 in human bone marrow endothelial cells result in modulation of adhesion molecule expression and functional adhesion of CD34+ progenitor cells ?
Using the transformed human bone marrow endothelial cell line (HBMEC), we first examined modulation of gene expression as a result of inhibition of NQO1 using mechanism-based (suicide) inhibitors of NQO1. Interestingly, data indicated that VCAM-1 was one of the genes transcriptionally down-regulated as a result of NQO1 inhibition and we then confirmed that either pharmacological inhibition or siRNA knock down of NQO1 led to decreased expression of VCAM-1 at the protein level in HBMEC [40]. TNF-α has been shown to markedly induce VCAM-1, E-selectin and ICAM-1 expression in HBMEC [39] and has been found to be critical to hematopoietic stem cell function and homing [41]. We therefore examined the effects of compromising NQO1 either pharmacologically or genetically on adhesion molecule expression in HBMEC after TNF-α stimulation. TNF-α led to increased expression of E-selectin, VCAM-1 and ICAM-1 in HBMEC and either pre-treatment with inhibitors of NQO1 or anti-NQO1 siRNA resulted in a marked reduction of adhesion molecule expression in HBMEC [42].
The functional correlate of decreased adhesion molecule expression is decreased adhesion of stem and progenitor cells to HBMEC. To explore this question we employed a flow chamber system to examine altered attachment of CD34+ KG1a cells to HBMEC under conditions of constant shear stress. Our data demonstrated approximately 60–70% decreased adhesion of CD34+ progenitor cells to HBMEC after inhibition or knockdown of NQO1 [42].
3. Signaling events modulated by compromising NQO1 in HBMEC. The potential importance of the NFκB system
The NFκB system is an important regulatory pathway involved in control of TNF-α stimulated adhesion molecule expression. IKBα is an inhibitor of NFκB activation and since previous work has demonstrated that NQO1 deficiency can regulate TNF-α signaling by inhibiting IKBα phosphorylation [43], we examined levels of IKBα and its phosphorylated form after treatment of HBMEC with anti-NQO1 siRNA or suicide inhibitors of NQO1. We found no differences in the kinetics of formation and degradation of IKBα and its phosphorylated form after TNF-α stimulation of HBMEC as a result of compromising NQO1 [44]. Similarly, no differences were observed in levels of nuclear NFκB p65 in HBMEC as a function of inhibition or knockdown of NQO1. However, we did observe decreased nuclear levels of NFκB phospho-p65, phospho-c-Jun and ATF2 in HBMEC after compromising NQO1 [42] and all of these signaling systems have been shown to play a role in TNF-α induced gene expression [43,45–47]. These observations provide a potential mechanistic link between altered adhesion in NQO1-compromised endothelial cells and nuclear levels of key transcription factors.
3.1 NQO1 as an upstream regulator of the NFκB signaling system
Ahn et al.[43] used keratinocytes from NQO1 knockout animals to demonstrate that NQO1 modulated IκBα kinase activation, IκBα phosphorylation and IκBα degradation implicating NQO1 as playing a role in NFκB signaling. Importantly, NQO1 has very recently been shown to be an upstream regulator of the NFκB precursor p105 and NFκB subunit p50 in melanoma [48]. These authors found that NQO1 stabilized BCL3 leading to upregulation of NFκB p50 which in turn correlated with cell cycle changes mediated by altered cyclin expression and increased proliferation of melanoma cells. NQO1 is mainly a cytosolic enzyme but, consistent with previous work in lung cancer cell lines, Garate et al [48] found significant NQO1 levels in the nucleus of melanoma cells. This suggests that in addition to its cytosolic functions, NQO1 may play significant roles in the nuclear compartment which may include effects on key transcriptional modulators.
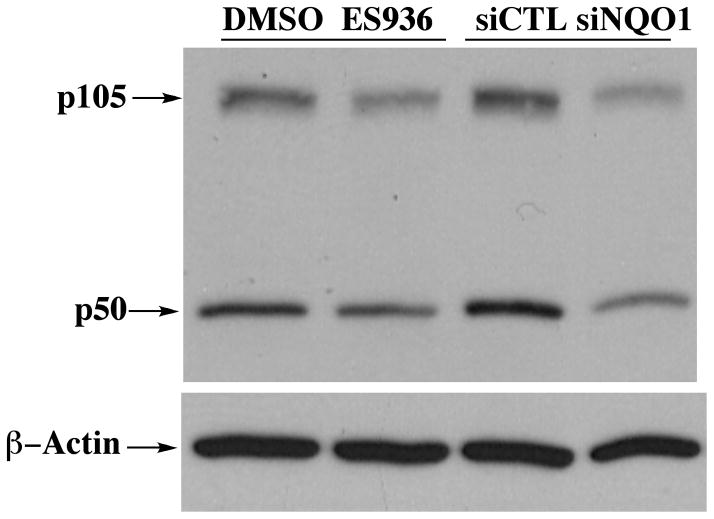
We therefore examined whether inhibition or knockdown of NQO1 might result in a similar effect in HBMEC. As demonstrated in Figure 1, our data is consistent with that of Garate et al [48] and shows decreased p105 and p50 levels as a result of NQO1 inhibition or knockdown in HBMEC implicating NQO1 as an upstream regulator of the NFκB system in HBMEC. We also found significant levels of NQO1 in the nuclear compartment of HBMEC [42] suggesting that nuclear roles for NQO1, potentially at the level of transcription factor stability, need to be explored in more detail.
Figure 1. The effect of compromising NQO1 on the levels of NFk-B p105 and p50 in HBMEC.
Treatment of HBMEC with anti-NQO1 siRNA or the mechanism-based inhibitor ES936 results in decreased levels of p105 and p50. Cells were harvested after treatment with 100nM ES936 for 2h or siRNA for 72h. (siCTL = scrambled siRNA control).
3.2 NQO1 and adhesion molecule expression in HBMEC
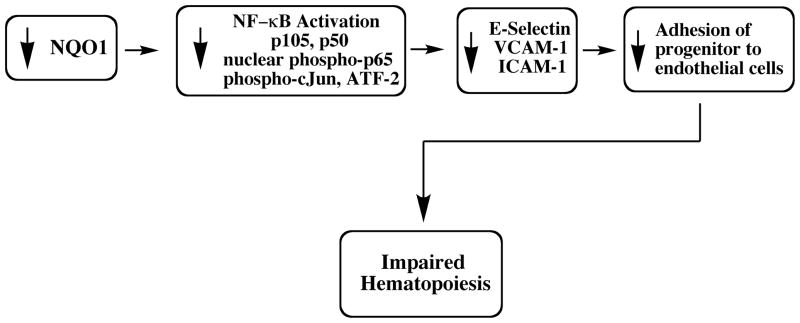
Our current understanding of the role of compromising NQO1 status in impaired endothelial adhesion is shown in Figure 2. Our current working hypothesis is that the impaired adhesive function of the endothelial layer compromises the function of the vascular stem cell niche leading to impaired hematopoiesis. This may provide a common mechanism to explain both the increased incidence of leukemias and the increased myeloid toxicity of benzene exposure in individuals lacking NQO1 as a result of the NQO1*2 polymorphism.
Figure 2.
A proposed mechanism for the role of NQO1 deficiency in the impairment of endothelial progenitor cell adhesion and hematopoiesis.
4. Do benzene metabolites affect endothelial cell tube formation in HBMEC?
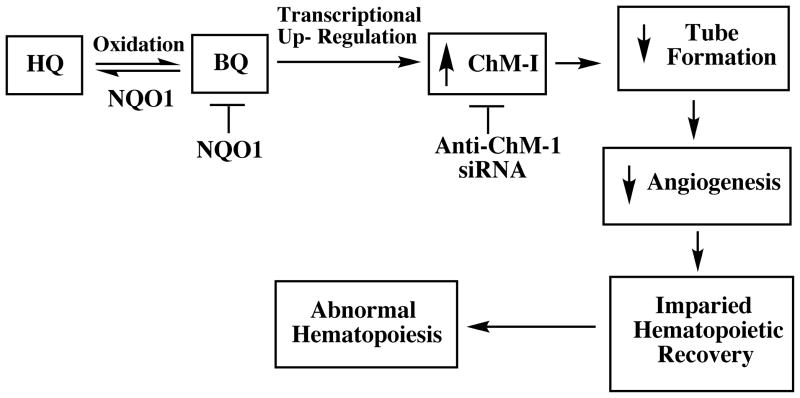
Another key function of HBMEC is tube formation leading to angiogenesis. Recovery after myelosuppression, which occurs after benzene exposure, has been shown to depend on endothelial cell angiogenesis [38] so we examined HBMEC tube formation after treatment with the benzene metabolite hydroquinone. Hydroquinone treatment of HBMEC led to inhibition of tube formation [49]. Experiments employing gene array analysis indicated that hydroquinone-induced upregulation of chondromodulin 1, an anti angiogenic gene expressed by endothelial cells, was partly responsible for inhibition of tube formation by hydroquinone. Purified chondromodulin 1 reproduced the inhibitory effects of hydroquinone on tube formation and anti-ChM1 siRNA protected against the effects of hydroquinone [49]. The effects of hydroquinone at the level of tube formation in HBMEC are summarized in Fig 3.
Figure 3.
The role of ChM-1 in hydroquinone-induced inhibition of tube formation by HBMEC and the protective effect of NQO1.
5. Summary
Our data indicates the potential importance of NQO1 in bone marrow with respect to both endothelial signaling and endothelial function. Compromising NQO1 in HBMEC led to modulated NFκB transcription, decreased adhesion molecule expression and decreased functional adhesion to stem/progenitor cells [42]. Since adhesion molecules have documented roles in hematopoietic cell homing and mobilization, impairment of the endothelial/vascular stem cell niche may play a role in both the hematopoietic pathology associated with a lack of NQO1 [23–28] and the increased susceptibility of individuals lacking NQO1 to benzene toxicity [1–4].
Another important endothelial function is tube formation and angiogenesis and the benzene metabolite hydroquinone is capable of inhibiting endothelial tube formation [49]. A summary of these two distinct effects, a lack of NQO1 impairing endothelial adhesion and benzene metabolites impairing endothelial tube formation, is summarized in Figure 4. It follows that exposure of NQO1*2 polymorphic individuals to benzene could result in impairment of endothelial function at multiple levels and in future work, whether the bone marrow endothelial cell is a critical target of benzene should be investigated in-vivo employing endothelial-specific biomarkers.
Figure 4.
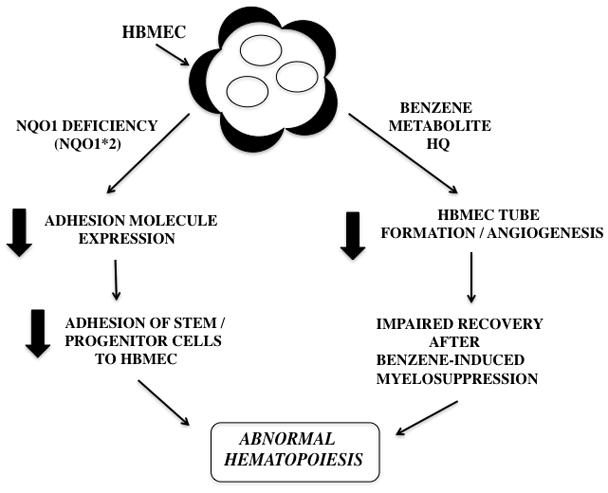
The proposed roles of NQO1 deficiency and hydroquinone on bone marrow endothelial cell adhesion molecule expression and angiogenesis.
Finally, these results could have broader implications for the role of NQO1 in biological systems. NQO1 has been examined in detail but it is often used only as a sentinel marker for activation of Nrf-2 in biological systems. Our observations and previous data of Ahn et al.[43] and Garate et al. [48] suggest that NQO1 has important regulatory effects in its own right in addition to being a marker for Nrf-2 activation. It may be useful to consider these regulatory effects when examining the consequences of modulation of NQO1 in biological systems.
Footnotes
Summary of a paper presented at Biological Reactive Intermediates VIII, Barcelona, Spain, July 15th–18th, 2010 in a session entitled Benzene, 1,3-Butadiene and Biological Reactive Intermediates. Lessons learned and the way ahead.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Rothman N, Smith MT, Hayes RB, Traver RD, Hoener BA, Campleman S, Li GL, Dosemeci M, Linet M, Zhang LP, Xi LQ, Wacholder S, Lu W, Meyer KB, Titenko-Holland N, Stewart JT, Yin SN, Ross D. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQ01609C-->T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57:2839–2842. [PubMed] [Google Scholar]
- 2.Wan J, Shi J, Hui L, Wu D, Jin X, Zhao N, Huang W, Xia Z, Hu G. Association of genetic polymorphisms in CYP2E1, MPO, NQO1, GSTM1, and GSTT1 genes with benzene poisoning. Environ Health Perspect. 2002;110:1213–1218. doi: 10.1289/ehp.021101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan J, Shi J, Guan J, Ye R, Gao X, Liu W, Hui L, Cao D, Jin X, Hu G, Xia Z. Relation of genetic polymorphism of NQO1 and GSTT1 with risks of chronic benzene poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2002;20:340–343. [PubMed] [Google Scholar]
- 4.Chen Y, Li G, Yin S, Xu J, Ji Z, Xiu X, Liu L, Ma D. Genetic polymorphisms involved in toxicant-metabolizing enzymes and the risk of chronic benzene poisoning in Chinese occupationally exposed populations. Xenobiotica. 2007;37:103–112. doi: 10.1080/00498250601001662. [DOI] [PubMed] [Google Scholar]
- 5.Smart RC, Zannoni VG. DT-Diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984;26:105–111. [PubMed] [Google Scholar]
- 6.Thomas DJ, Sadler A, Subrahmanyam VV, Siegel D, Reasor MJ, Wierda D, Ross D. Bone marrow stromal cell bioactivation and detoxification of the benzene metabolite hydroquinone: comparison of macrophages and fibroblastoid cells. Mol Pharmacol. 1990;37:255–262. [PubMed] [Google Scholar]
- 7.Ross D, Siegel D, Schattenberg DG, Sun XMM, Moran JL. Cell-specific activation and detoxification of benzene metabolites in mouse and human bone marrow: Identification of target cells and a potential role for modulation of apoptosis in benzene toxicity. Environ Health Perspect. 1996;104 (Suppl 6):1177–1182. doi: 10.1289/ehp.961041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trush MA, Twerdok LE, Rembish SJ, Zhu H, Li YB. Analysis of target cell susceptibility as a basis for the development of a chemoprotective strategy against benzene-induced hematotoxicities. Environ Health Perspect. 1996;104 (Suppl 6):1227–1234. doi: 10.1289/ehp.961041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross D. Quinone reductases. Multitasking in the metabolic world. Drug Metabolism Reviews. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 10.Ross D, Zhou H. Relationships between metabolic and non-metabolic susceptibility factors in benzene toxicity. Chemico-Biological Interactions. 2010;184:222–228. doi: 10.1016/j.cbi.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Archives of Biochemistry and Biophysics. 2010 Mar 31; doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Molecular Pharmacology. 2004;65:1–10. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 13.Asher G, Lotem J, Cohen B, Sachs L, Shaul Y. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci USA. 2001;98:1188–1193. doi: 10.1073/pnas.021558898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong X, Kole L, Iskander K, Jaiswal AK. NRH:quinone oxidoreductase 2 and NAD(P)H:quinone oxidoreductase 1 protect tumor suppressor p53 against 20s proteasomal degradation leading to stabilization and activation of p53. Cancer Res. 2007;67:5380–5388. doi: 10.1158/0008-5472.CAN-07-0323. [DOI] [PubMed] [Google Scholar]
- 15.Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, Pietenpol JA, Ross D. Interaction of Human NAD(P)H:Quinone Oxidoreductase 1 (NQO1) with the Tumor Suppressor Protein p53 in Cells and Cell-free Systems. Journal of Biological Chemistry. 2003;278:10368–10373. doi: 10.1074/jbc.M211981200. [DOI] [PubMed] [Google Scholar]
- 16.Asher G, Bercovich Z, Tsvetkov P, Shaul Y, Kahana C. 20S proteasomal degradation of ornithine decarboxylase is regulated by NQO1. Mol Cell. 2005;17:645–655. doi: 10.1016/j.molcel.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Garate M, Wong RP, Campos EI, Wang Y, Li G. NAD(P)H quinone oxidoreductase 1 inhibits the proteasomal degradation of the tumour suppressor p33(ING1b) EMBO Rep. 2008;9:576–581. doi: 10.1038/embor.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wignall SM, Gray NS, Chang YT, Juarez L, Jacob R, Burlingame A, Schultz PG, Heald R. Identification of a novel protein regulating microtubule stability through a chemical approach. Chem Biol. 2004;11:135–146. [PubMed] [Google Scholar]
- 19.Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339–345. [PubMed] [Google Scholar]
- 20.Yoon BI, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, Kanno J, Kim DY, Fujii-Kuriyama Y, Inoue T. Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol Sci. 2002;70:150–156. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 21.Hirabayashi Y, Yoon BI, Li GX, Fujii-Kuriyama Y, Kaneko T, Kanno J, Inoue T. Benzene-induced hematopoietic toxicity transmitted by AhR in wild-type mouse and nullified by repopulation with AhR-deficient bone marrow cells: time after benzene treatment and recovery. Chemosphere. 2008;73:S290–S294. doi: 10.1016/j.chemosphere.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30:11–19. doi: 10.1093/carcin/bgn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MT, Wang Y, Kane E, Rollinson S, Wiemels JL, Roman E, Roddam P, Cartwright R, Morgan G. Low NAD(P)H:quinone oxidoreductase 1 activity is associated with increased risk of acute leukemia in adults. Blood. 2001;97:1422–1426. doi: 10.1182/blood.v97.5.1422. [DOI] [PubMed] [Google Scholar]
- 24.Smith MT, Wang Y, Skibola CF, Slater DJ, Nigro LL, Nowell PC, Lange BJ, Felix CA. Low NAD(P)H:quinone oxidoreductase activity is associated with increased risk of leukemia with MLL translocations in infants and children. Blood. 2002;100:4590–4593. doi: 10.1182/blood-2001-12-0264. [DOI] [PubMed] [Google Scholar]
- 25.Wiemels JL, Pagnamenta A, Taylor GM, Eden OB, Alexander FE, Greaves MF. A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. United Kingdom Childhood Cancer Study Investigators. Cancer Res. 1999;59:4095–4099. [PubMed] [Google Scholar]
- 26.Larson RA, Wang Y, Banerjee M, Wiemels J, Hartford C, Beau MM, Smith MT. Prevalence of the inactivating 609C-->T polymorphism in the NAD(P)H:Quinone oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia [In Process Citation] Blood. 1999;94:803–807. [PubMed] [Google Scholar]
- 27.Naoe T, Takeyama K, Yokozawa T, Kiyoi H, Seto M, Uike N, Ino T, Utsunomiya A, Maruta A, Jin-nai I, Kamada N, Kubota Y, Nakamura H, Shimazaki C, Horiike S, Kodera Y, Saito H, Ueda R, Wiemels J, Ohno R. Analysis of genetic polymorphism in NQO1, GST-M1, GST-T1, and CYP3A4 in 469 Japanese patients with therapy-related leukemia/myelodysplastic syndrome and de novo acute myeloid leukemia. Clinical Cancer Research. 2000;6:4091–4095. [PubMed] [Google Scholar]
- 28.Krajinovic M, Sinnett H, Richer C, Labuda D, Sinnett D. Role of NQO1, MPO and CYP2E1 genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Int J Cancer. 2002;97:230–236. doi: 10.1002/ijc.1589. [DOI] [PubMed] [Google Scholar]
- 29.Long DJ, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, Jaiswal AK. Disruption of the NAD(P)H:Quinone Oxidoreductase 1 (NQO1) Gene in Mice Causes Myelogenous Hyperplasia. Cancer Res. 2002;62:3030–3036. [PubMed] [Google Scholar]
- 30.Iskander K, Barrios RJ, Jaiswal AK. Disruption of NAD(P)H:quinone oxidoreductase 1 gene in mice leads to radiation-induced myeloproliferative disease. Cancer Res. 2008;68:7915–7922. doi: 10.1158/0008-5472.CAN-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel D, Ryder J, Ross D. NAD(P)H: quinone oxidoreductase 1 expression in human bone marrow endothelial cells. Toxicology Letters. 2001;125:93–98. doi: 10.1016/s0378-4274(01)00426-x. [DOI] [PubMed] [Google Scholar]
- 32.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology(Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 33.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997;158:3408–3416. [PubMed] [Google Scholar]
- 37.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 38.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweitzer KM, Vicart P, Delouis C, Paulin D, Drager AM, Langenhuijsen MM, Weksler BB. Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest. 1997;76:25–36. [PubMed] [Google Scholar]
- 40.Zhou H, Dehn D, Kepa JK, Siegel D, Ross D. Downregulation of the cellular adhesion molecule VCAM-1 in human bone marrow endothelial cells after inhibition of NQO1 activity. Proc Amer Assoc Cancer Res. 2007;48:5371. [Google Scholar]
- 41.Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier IJ, Ildstad ST. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180:49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H, Dehn DL, Kepa JK, Siegel D, Scott D, Tan W, Ross D. NQO1-compromised human bone marrow endothelial cells exhibit decreased adhesion moolecule expression and CD34+ hematopoietic cell adhesion. J Pharmacol Exp Ther. 2010;334:1–9. doi: 10.1124/jpet.110.167841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem. 2006;281:19798–19808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 44.Ahn KS, Gong X, Sethi G, Chaturvedi MM, Jaiswal AK, Aggarwal BB. Deficiency of NRH:quinone oxidoreductase 2 differentially regulates TNF signaling in keratinocytes: up-regulation of apoptosis correlates with down-regulation of cell survival kinases. Cancer Res. 2007;67:10004–10011. doi: 10.1158/0008-5472.CAN-07-2213. [DOI] [PubMed] [Google Scholar]
- 45.Min W, Pober JS. TNF initiates E-selectin transcription in human endothelial cells through parallel TRAF-NF-kappa B and TRAF-RAC/CDC42-JNK-c-Jun/ATF2 pathways. J Immunol. 1997;159:3508–3518. [PubMed] [Google Scholar]
- 46.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. The FASEB Journal. 1995;9:899–909. [PubMed] [Google Scholar]
- 47.Kordula T, Bugno M, Rydel RE, Travis J. Mechanism of interleukin-1- and tumor necrosis factor alpha-dependent regulation of the alpha 1-antichymotrypsin gene in human astrocytes. J Neurosci. 2000;20:7510–7516. doi: 10.1523/JNEUROSCI.20-20-07510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garate M, Wani AA, Li G. The NAD(P)H:Quinone Oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Radic Biol Med. 2010;48:1601–1609. doi: 10.1016/j.freeradbiomed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Zhou H, Kepa JK, Siegel D, Miura S, Hiraki Y, Ross D. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol Pharmacol. 2009;76:579–587. doi: 10.1124/mol.109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]