Abstract
Background
Use of the Model for End-Stage Liver Disease (MELD) score has improved the efficiency of allocating deceased donor organs for liver transplant. However, its use may reduce access to deceased donor livers for patients with primary sclerosing cholangitis (PSC) due to the weighting of the MELD score variables. To overcome such barriers in the post-MELD era, clinicians might refer patients with PSC, relative to patients without PSC, for living donor transplants more frequently.
Methods
To test this hypothesis, we examined patients in the UNOS database from December 1st, 1994-May 31st, 2009.
Results
In multivariable models conditioned on transplant center, patients with PSC were significantly more likely to receive a living donor transplant in both the pre-MELD (OR=2.75; 95% CI 2.20–3.44) and post-MELD eras (OR=4.08; 95% CI 3.45–4.82). There was a significant interaction between PSC and post-MELD era of transplantation (OR=1.48; 95% CI 1.11–1.97), indicating that patients with PSC were more likely to receive living donor transplants at baseline relative to patients without PSC, and that this effect was magnified following the introduction of the MELD score.
Conclusions
These findings raise the possibility that allocating livers on the basis of MELD score may have yielded the unintended consequence of increasing rates for living donor transplants for patients with PSC relative to patients with other forms of end-stage liver disease. Future research is needed to determine whether the practice of selectively transplanting patients with PSC with living donor transplants is associated with differences in clinical outcomes.
Keywords: MELD score, bilirubin, cholangiocarcinoma, interaction, deceased donor transplantation
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unclear etiology with an estimated incidence of approximately 1 per 100,000 person-years.(1) Liver transplantation is the only known beneficial therapy for this progressive and potentially fatal disease, and survival rates post-transplantation are favorable, exceeding 80% at 5 years.(2) However, determining the optimal timing of transplantation is complicated, because in addition to complications occurring in all forms of end-stage liver disease due to portal hypertension, patients with PSC are at risk for ascending cholangitis due to biliary strictures and cholangiocarcinoma. The risk for cholangiocarcinoma is of particular concern because it develops in 6%–36% of patients with PSC and is associated with poor outcomes post-transplantation.(3–15) Thus some have speculated that “preemptive” transplantation may reduce the risks of developing cholangiocarcinoma.(16)
Prior to 2002, the allocation of livers was based on a combination of waitlist time plus the Child-Turcotte-Pugh (CTP) score. In response to concerns about the potential bias associated with measuring the CTP score, and its impact on the equity and efficiency of the allocation system, the Model for End Stage Liver Disease (MELD) score replaced the CTP score on February 27th, 2002. However, because the sample of patients on whom the MELD score was derived largely excluded those with PSC, it is possible that it does not capture such patients’ severities of illness.(17) Further, because patients with PSC manifest their disease by greater elevations in bilirubin than creatinine or INR, these patients may have lower MELD scores than patients with other forms of end-stage liver disease, despite being equally sick. This disproportionate elevation in bilirubin also may cause patients with PSC to have slower rises in their MELD score, extending their waiting time and generating increased risks for adverse outcomes while also prolonging their impaired quality of life.
In light of such concerns, patients with PSC may seek – and their transplant physician may recommend – a living donor liver transplant (LDLT). Because living donor organs are not allocated via the MELD score, patients and physicians may pursue this option so as to maximize the chance of getting transplanted before one deteriorates clinically, or to minimize suffering while on the waitlist.
We designed the present study to address two questions: are patients with PSC preferentially referred for LDLT, and if so, was this effect generated or magnified in the post-MELD era? In light of increasing concerns regarding the safety of procuring partial liver allografts from healthy living donors, and given recent data suggesting increased morbidity for recipients of LDLTs, answering these questions could help guide future liver allocation policy, particularly as it relates to patients with PSC.(18)
Results
The age, race, and gender distributions of the two groups (PSC vs. non-PSC) were different. Table 1 lists the demographic and laboratory values of all transplant recipients, classified by diagnosis and type of transplant. Within each classification, patients with PSC were younger, more likely to be white, and had higher values of bilirubin. Deceased donor recipients had higher values of bilirubin and creatinine at transplantation compared with living donor recipients.
Table 1.
Demographic Characteristics of Transplant Recipients*
| Living Donor Recipients | Deceased Donor Recipients | |||
|---|---|---|---|---|
| Variable | PSC | Non-PSC | PSC | Non-PSC |
| Race/Ethnicity, N (%) | White: 310 (88.6) | White: 1,602 (79.2) | White: 2,677 (82.7) | White: 41,326 (75.0) |
| Black: 21 (6.0) | Black: 73 (3.6) | Black: 386 (11.9) | Black: 4,106 (7.5) | |
| Hispanic: 16 (4.6) | Hispanic: 259 (12.8) | Hispanic: 120 (3.7) | Hispanic: 6,785 (12.3) | |
| Asian: 3 (0.9) | Asian: 71 (3.5) | Asian: 38 (1.2) | Asian: 2,299 (4.2) | |
| Male, N (%) | 229 (65.4) | 1131 (55.9) | 2,202 (68.0) | 36,589 (66.4) |
| Bilirubin at transplant, mg/dL | 5.3 ± 6.6 | 3.6 ± 4.5 | 9.3 ± 10.4 | 6.4 ± 9.2 |
| Creatinine at transplant, mg/dL | 0.84 ± 0.25 | 1.01 ± 0.62 | 1.2 ± 1.0 | 1.4 ± 1.3 |
| Age at transplant, years | 44.4 ± 13.0 | 51.8 ± 10.5 | 47.6 ± 12.4 | 52.5 ± 9.5 |
Summaries presented as mean ± standard deviation unless otherwise noted as N (%).
Trends in LDLT
Table 2 displays the percentage of transplants from living vs. deceased donors for patients with and without PSC. Since 1999, when the number of LDLTs increased, the percentage of LDLTs among patients with PSC was stable. There was a 30% decrease in LDLTs among patients without PSC in the post-MELD era.
Table 2.
Transplantation from Living Donors, by MELD era
| Transplants from living donors, N (%) | ||||
|---|---|---|---|---|
| Pre-MELD (1994–2002) | Pre-MELD (1999–2002) | Post-MELD (2002–2009) | ||
| PSC | 101 (5.9)* | 99 (13.7) † | 249 (14.0) | P<.0001* P=0.835† |
| Non-PSC | 681 (3.0) | 660 (5.9) | 1,344 (4.0) | P<.0001* P<.0001† |
Wilcoxon rank sum comparing post-MELD to 1994–2002 pre-MELD cohort
Wilcoxon rank sum comparing post-MELD to 1999–2002 pre-MELD cohort
Factors associated with LDLT
Table 3 lists the hypothesized risk factors of clinical importance. All of the variables assessed reached statistical significance and were included in the final model, except for insurance. UNOS region was not included in the final model because the model included fixed effects for transplant center. Pre-MELD, the odds that patients with PSC would receive a LDLT were 2.8 times that for patients without PSC (95% CI 2.20–3.44). Post-MELD, the odds that patients with PSC would receive a LDLT were four times that for non-PSC patients (95% CI 3.45–4.81). Importantly, there was a significant interaction between PSC and era of transplant (OR ratio=1.48; 95% CI 1.11–1.96, P=0.007), indicating that patients with PSC, despite having stable rates of LDLTs pre- and post-MELD, had significantly greater odds, relative to patients without PSC, of receiving a LDLT following introduction of the MELD allocation policy.
Table 3.
Risk Factors for Living Donor Transplantation
| Variable* | Univariable | Multivariable | P-Value† |
|---|---|---|---|
| Odds Ratio (95% Confidence Interval)
|
|||
| PSC | 3.85 (3.68–4.03) | 2.76 (2.20–3.44) | <0.0001 |
| Post-MELD | 1.10 (1.00–1.20) | 0.56 (0.51–0.62) | <0.0001 |
| PSC* Post-MELD | 1.55 (1.36–1.76) | 1.48 (1.11–1.96) | 0.007 |
| Blood type | |||
| O | 1.0 | 1.0 | |
| A | 1.11 (1.07–1.15) | 0.97 (0.89–1.07) | 0.56 |
| B | 0.83 (0.79–0.88) | 0.76 (0.66–0.88) | <0.0001 |
| AB | 0.54 (0.47–0.62) | 0.42 (0.30–0.57) | <0.0001 |
| Male gender | 0.77 (0.74–0.79) | 0.73 (0.67–0.79) | <0.0001 |
| Race/Ethnicity | |||
| White | 1.0 | 1.0 | |
| Black | 0.41 (0.38–0.45) | 0.46 (0.37–0.57) | <0.0001 |
| Hispanic | 0.63 (0.60–0.66) | 0.76 (0.66–0.88) | <0.0001 |
| Age at listing† | 0.98 (0.98–0.98) | 0.98 (0.98–0.99) | |
Variable of pre vs. post-1/1/1999 was statistically significant in both models, but not shown, and UNOS region and insurance type (private vs. public) were significant only in the univariable model.
Reported P-values are from the multivariable model.
Age defined as 1-year increment in age at the time of listing
Secondary analyses
Similar results were obtained for three of the secondary analyses (Table 4). The interaction between diagnostic category and MELD era remained statistically significant when the sample was restricted to (1) patients removed from the waitlist after 1999; (2) patients who received either a living or deceased donor transplant, adjusting for severity of illness at transplantation; and (3) comparing cholestatic patients (PSC and PBC) versus all others. Excluding patients from UNOS region 7 to prevent the results from being overly sensitive to the unique practices of a single institution, yielded an odds ratio for the interaction term of 1.30 (P=0.11).
Table 4.
Odds Ratios for Interaction Term in Secondary Analyses
| Model | No. of patients | OR (95% CI) |
|---|---|---|
| Main Model | 89,321* | 1.48 (1.11–1.96) |
| Post-1999 Removals† | 71,960 | 1.44 (1.08–1.92) |
| Transplantation only‡ | 46,713 | 1.62 (1.18–2.22) |
| PSC/PBC vs. others | 89,321 | 1.72 (1.36–2.17) |
| Region 7 Exclusion | 79,886 | 1.30 (0.94–1.78) |
When conditioned on transplant center, 73 groups (22,588) observations dropped because of all positive or negative outcomes.
Restricted model to only patients removed from waitlist starting January 1, 1999.
Restricted model to patients who received a transplant, with outcome being living vs. deceased donor transplantation. Final model adjusted for creatinine and bilirubin at time of transplantation.
Waiting time prior to transplantation
Wait times prior to transplantation decreased in the post-MELD era. Median wait times for a deceased donor transplant for patients with PSC decreased from 253 days (inter-quartile range: 103–540) for those listed pre-MELD, to 105 days (32–302) for those listed post-MELD. For patients without PSC, the respective wait times decreased from 211 days (73–469) to 70 days (19–217). Figure 1 displays the distribution of the wait times prior to deceased donor transplantation by year of listing. Wait times are greater for patients with PSC across the study period, but the magnitude of the difference in median wait times increases substantially starting at year 4 post-MELD. The logrank test comparing the distribution of wait times between PSC and non-PSC patients was significant across the study period (P <0.0001).
Figure 1.
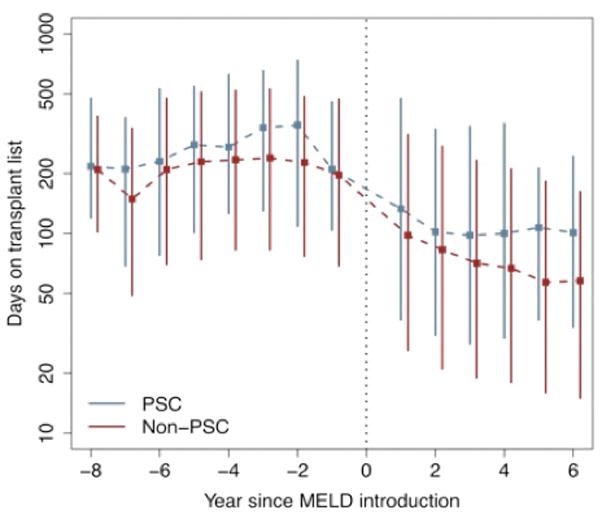
Wait time prior 10 transplantation for liver-transplant recipients, based on year of listing, for PSC and non-PSC patients. The symbol ‘■’ indicates the median wait time; solid lines indicate the inter-quartile range.
Discussion
This study of all adult patients listed for liver transplantation in the United States during a 16-year period shows that patients with PSC are significantly more likely to receive a LDLT than are patients with other liver diseases, and the magnitude of this discrepancy increased following the introduction of the MELD allocation system. While the data does not allow us to determine whether the MELD-based allocation system caused these changes, the results show that these changes were temporally linked with this transition in allocation policy.
Following the increase in the frequency of LDLTs in 1999, approximately 13% of liver transplants in patients with PSC were from living donors, compared to approximately 4.5% in all other patients. While the overall decline in the total number of LDLTs (according to OPTN data as of January 14, 2011) may reflect a response to the well-publicized death of a living donor on January 16th, 2002, the fact that the proportion of LDLTs among patients with PSC was unchanged suggests that introduction of MELD-based allocation at approximately the same time may have specifically influenced transplantation patterns for patients without PSC, especially relative to patients with PSC. (19)
While the implementation of MELD-based allocation appears to have achieved its overall goal of decreasing the waiting time for the very sick prior to transplantation, thereby reducing mortality on the waitlist, this increased overall efficiency may carry unintended consequences for patients with PSC. Because the MELD score gives equal weight (relative to the variables’ scales(20)) to bilirubin, creatinine, and INR, patients with PSC, whose liver dysfunction is most prominently manifested by elevations in bilirubin, are not only more likely to have lower MELD scores than patients with equally advanced, hepatocellular forms of liver disease, but are also more likely to have slower rises in MELD scores. Indeed, we demonstrate that post-MELD, median wait times for deceased donor transplantation are longer for patients with PSC.
Increased time on the transplant waitlist has several implications for patients with PSC. First, increased wait time exposes patients to greater risks for developing cholangiocarcinoma or other complications of end-stage liver disease that could result in patients being too sick to be transplanted. Second, extended relative wait times on the transplant list increases the time that these patients must deal with symptoms of their disease that may negatively impact their quality of life. In light of these considerations, clinicians may try to enable earlier transplantation via LDLT. Although patients with PSC may be more likely to receive a LDLT because of increased waiting time, this does not imply that increased waiting time in and of itself is associated with a bad outcome.
Other than differences in waiting time, there are other possible explanations for the changes in the risk of LDLTs in the post-MELD era for patients with PSC. Differential rates of LDLTs could be driven by non-hepatic complications (i.e. patients with IBD and dysplasia needing colectomies, but liver disease precluding surgery). However, among patients with PSC listed for transplantation, and those successfully transplanted, the proportions with IBD are similar in the pre- and post-MELD eras. Finally there is the potential impact of the hepatocellular carcinoma (HCC) exception point protocol implemented in the post-MELD era. However this does not seem to be an important explanation because after excluding patients with HCC, a significant association remained between PSC and era of transplant (OR ratio=1.41; 95% CI 1.06–1.87, P=0.019).
Despite the hypothesized reasons as to why LDLT is more prevalent in patients with PSC, these data are insufficient to draw firm conclusions. Many programs do not offer LDLTs as an option, and there may be unmeasured factors at play that contribute to the phenomenon we have encountered.
The increased relative rates of LDLTs of patients with PSC (vs. non-PSC) might be viewed not as a disparity, but as an efficient solution to an allocation dilemma. While LDLTs do not have inferior survival outcomes compared to deceased donor transplants, there is increased morbidity associated with LDLTs (e.g., increased hospital admissions and days hospitalized for biliary complications), along with potential risks to the donor. (18, 21) This can be seen as resulting in inequity if one segment of the donor pool disproportionately receives such transplants, and thus is placed at risk of these potential increased complications. Further, the decline in the total number of LDLTs (according to OPTN data as of January 14, 2011), may disproportionately affect access to such organs for patients with PSC.
Our study has several limitations. The UNOS database limited the data elements available for covariate adjustment, preventing us from adjusting for severity of illness in our primary analysis. However, the secondary analysis focusing on transplanted patients, allowing for a severity of illness adjustment, yielded similar results. Second, although the relative rates of LDLTs for patients with vs. without PSC have changed since the introduction of the MELD score, we cannot be certain that this is due to the use of the MELD score. Because MELD-based allocation was implemented for all patients at once, it is not possible to fully disentangle the effects of MELD-based allocation from those of other temporal trends. We cannot exclude the possibility that the death of a living donor in 2002, rather than MELD per se, preferentially altered allocation practices for certain types of patients. Lastly, as mentioned, we could only perform a secondary analysis excluding region 7 instead of solely the Mayo Clinic Rochester. However, we don’t believe that the exclusion of other centers in the region significantly altered the results for this secondary analysis, and it did not alter the conclusions of our study.
In summary, this study highlights a previously unexplored discrepancy in rates of LDLTs among patients with different diseases that seems to have been magnified in the post-MELD era. Patients with PSC have received LDLTs more than four times more commonly than patients with other diseases in the post-MELD era, and among those who did receive deceased donor transplants, patients with PSC waited substantially longer for such organs. There exist no data that the MELD is unfair to patients with PSC, either through increased pre-transplant mortality of reduced quality of life. However, if patients with PSC are preferentially given LDLTs under the MELD system, and the supply of this resource continues to decline, these trends likely will disadvantage patients with PSC. Furthermore, the present data suggest that if overall rates of LDLTs continue to decline, modifications to the allocation system may be needed to avoid substantially adverse consequences for patients with PSC who currently rely more heavily on LDLTs than other patients.
Materials and Methods
Patients
This was conducted using the United Network for Organ Sharing (UNOS) Organ Procurement and Transplant Network (OPTN) database. We identified all patients listed for a liver transplant from December 1st, 1994 through May 31st, 2009. These dates were chosen to provide equivalent time before and after implementation of the MELD-based allocation system on February 27th, 2002. However, because few LDLTs were performed prior to January 1st, 1999, we performed secondary analyses restricting the pre-MELD sample to patients removed from the waitlist after January 1st, 1999.
We excluded patients under age 18 for two reasons. Patients under age 12 are allocated livers by a distinct model, the Pediatric End-Stage Liver Disease score; second, patients ages 12–18 exhibit a substantially different spectrum of primary diagnoses than do those older than 18. We excluded patients listed for retransplantation to ensure that all observations represent unique individuals. We excluded patients listed as status 1 (fulminant hepatic failure) because the MELD score is not considered in allocating organs to such patients, and status-1 patients rarely have chronic liver diseases. Of the remaining 113,593 patients, we excluded 1,529 patients (1.3%) with a missing primary diagnosis.
Statistical Analysis
We used Fisher’s exact tests and chi-square tests to compare dichotomous variables. Student t tests or Wilcoxon rank-sum tests were used for the comparison of continuous variables, depending on their distribution.
We used a logistic regression model to assess the relationship between diagnosis (PSC vs. non-PSC) and receipt of a LDLT (LDLT vs. all other outcomes). We chose this binary outcome, rather than time-to-transplant, because the clinical outcome of primary interest is whether patients with PSC ultimately were more likely to receive a LDLT, irrespective of waiting time. Waiting time was evaluated as a secondary outcome, as we hypothesized that differences in waiting time prior to transplantation could serve as a mechanism by which relative rates of LDLTs changed after the implementation of the MELD system.
To quantify the association of post-MELD era with probability of receiving a LDLT, we needed to account for patients listed pre-MELD but still on the waitlist post-MELD. We created a continuous variable representing MELD era with values between 0 and 1 to represent the proportion of time each patient spent in the post-MELD era. Patients listed and removed pre-MELD were coded as 0, while those listed and removed (or still listed) post-MELD were coded as 1. Patients listed pre-MELD but removed (or still listed) post-MELD were coded as an intermediate value that represented the proportion of this interval spent in the post-MELD era. This approach enabled classification of patients according to their true time “at risk” for receiving a LDLT in each era.
In addition to the main effects of diagnosis and MELD era, we evaluated the statistical interaction between diagnosis and MELD era to determine whether the difference in odds of LDLTs between patients with and without PSC differed before and after introduction of the MELD score. This was the primary variable of interest.
To account for the significant increase in rates of LDLTs in 1999, we created a binary variable for two time periods, 12/1/1994–12/31/1998 and 1/1/1999–5/31/2009. This allowed us to account for differing rates of LDLTs during these two time periods, without affecting the relevant contrast of pre- vs. post-MELD.
We included fixed effects for transplant center to account for differences in the risk of receiving a LDLT across centers.(22) We selected other independent variables for inclusion in the final model if they were independently associated with the outcome (P<0.05) or if their removal changed the coefficient of the diagnosis-by-MELD interaction term by ≥10%. Variables tested included age, gender, race/ethnicity, blood type, UNOS region, and insurance type (private vs. public).
Clinical and laboratory data are limited in the UNOS dataset on patients in the pre-MELD era; thus we could not adjust for severity of illness in the form of MELD, CTP scores, or baseline laboratory values. However, values of creatinine and bilirubin at the time of transplantation are available for >97% patients pre-MELD, so we performed a secondary analysis adjusting for these variables using a restricted sample of patients who either received a living or decreased donor transplant.
To determine if changes in rates of LDLTs pertained only to patients with PSC, or to patients with other cholestatic liver diseases, we constructed a secondary logistic regression model, grouping patients with PSC and primary biliary cirrhosis (PBC) together. Also, due to increasing numbers of LDLTs as part of the Mayo Clinic’s (Rochester, MN) cholangiocarcinoma protocol, we performed a secondary analysis excluding patients from UNOS region 7 (due to UNOS’s data use agreement, the analysis could only exclude the entire region and not an individual center) to ensure that the results were not unduly influenced by rates of LDLTs for patients with PSC and cholangiocarcinoma at that center.(23) Finally, to assess differences in waiting times prior to transplantation, we compared medians and interquartile ranges of wait time prior to transplantation. Patients were grouped based on year of listing for transplantation (defined as years before vs. after MELD). We used the logrank test to compare wait times between the two groups of patients by year of listing, and for overall difference in wait times.
All statistical analyses were completed using STATA 10.(24)
Statistical Power
Our primary aim was to determine the odds ratio for the diagnosis-by-MELD period interaction term using a pre-existing dataset containing approximately 110,000 subjects. To calculate power to detect this interaction, we determined that 50% more observations would be needed for detection of a comparably sized main effect.(25)After dividing our actual sample size by 1.5, we had >85% power to detect an odds ratio of at least 1.40 at the conventional two-sided alpha level of 0.05, based on OPTN data indicating that 1.9% of patients listed for a liver transplant received a LDLT as of May 14, 2010.
Abbreviations Page
- CTP
Child-Turcotte-Pugh
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PBC
Primary biliary cirrhosis
- PSC
Primary sclerosing cholangitis
- UNOS
United Network for Organ Sharing
- HCC
Hepatocellular carcinoma
- LDLT
Living donor liver transplant
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the journal Transplantation.
Contributor Information
David Seth Goldberg, Department of Medicine, Division of Gastroenterology, University of Pennsylvania.
Benjamin French, Department of Biostatistics and Epidemiology, University of Pennsylvania; Leonard Davis Institute of Health Economics, University of Pennsylvania.
Arwin Thomasson, Department of Biostatistics and Epidemiology, University of Pennsylvania.
K Rajender Reddy, Department of Medicine, Division of Gastroenterology, University of Pennsylvania.
Scott D. Halpern, Department of Biostatistics and Epidemiology, University of Pennsylvania; Department of Medicine; Division of Pulmonary, Allergy, and Critical Care, University of Pennsylvania; Leonard Davis Institute of Health Economics, University of Pennsylvania.
References
- 1.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003 Nov;125(5):1364–9. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004 Jul;10(7):886–97. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 3.Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, et al. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987 Aug;22(6):655–64. doi: 10.3109/00365528709011139. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002 Mar;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 5.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995 Nov;22(5):1404–8. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 6.Farges O, Malassagne B, Sebagh M, Bismuth H. Primary sclerosing cholangitis: liver transplantation or biliary surgery. Surgery. 1995 Feb;117(2):146–55. doi: 10.1016/s0039-6060(05)80078-9. [DOI] [PubMed] [Google Scholar]
- 7.Helzberg JH, Petersen JM, Boyer JL. Improved survival with primary sclerosing cholangitis. A review of clinicopathologic features and comparison of symptomatic and asymptomatic patients. Gastroenterology. 1987 Jun;92(6):1869–75. doi: 10.1016/0016-5085(87)90618-4. [DOI] [PubMed] [Google Scholar]
- 8.Miros M, Kerlin P, Walker N, Harper J, Lynch S, Strong R. Predicting cholangiocarcinoma in patients with primary sclerosing cholangitis before transplantation. Gut. 1991 Nov;32(11):1369–73. doi: 10.1136/gut.32.11.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ, Jr, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991 Jan;213(1):21–5. doi: 10.1097/00000658-199101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knechtle SJ, D’Alessandro AM, Harms BA, Pirsch JD, Belzer FO, Kalayoglu M. Relationships between sclerosing cholangitis, inflammatory bowel disease, and cancer in patients undergoing liver transplantation. Surgery. 1995 Oct;118(4):615–9. doi: 10.1016/s0039-6060(05)80026-1. discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 11.Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand J Gastroenterol. 1997 Oct;32(10):1042–5. doi: 10.3109/00365529709011222. [DOI] [PubMed] [Google Scholar]
- 12.Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in primary sclerosing cholangitis. J Hepatol. 1994 Dec;21(6):1061–6. doi: 10.1016/s0168-8278(05)80618-x. [DOI] [PubMed] [Google Scholar]
- 13.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004 Mar;99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 14.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005 Sep;50(9):1734–40. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 15.Bjornsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999 Dec;19(6):501–8. doi: 10.1111/j.1478-3231.1999.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 16.Patt CH, Thuluvath PJ. Liver transplantation for primary sclerosing cholangitis: Screening for biliary malignancy and the role for biliary malignancy and role of preemptive transplantation. Curr Opinion Organ Transplant. 2002;2002(7):129–36. [Google Scholar]
- 17.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000 Apr;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 18.Merion RM, Shearon TH, Berg CL, Everhart JE, Abecassis MM, Shaked A, et al. Hospitalization rates before and after adult-to-adult living donor or deceased donor liver transplantation. Ann Surg. 2010 Mar;251(3):542–9. doi: 10.1097/SLA.0b013e3181ccb370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady D. Donor’s death at hospital halts some liver surgeries. New York Times; Jan 16th, 2002. [Google Scholar]
- 20.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008 Nov;135(5):1575–81. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007 Dec;133(6):1806–13. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French B, Heagerty PJ. Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008 Oct 30;27(24):5005–25. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010 Jul;23(7):692–7. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 25.Byar DP, Piantadosi S. Factorial designs for randomized clinical trials. Cancer Treat Rep. 1985 Oct;69(10):1055–63. [PubMed] [Google Scholar]


