Abstract
Characterization of laryngeal flow velocity fields is important to understanding vocal fold vibration and voice production. One common method for acquiring flow field data is particle image velocimetry (PIV). However, because using PIV with models that have curved surfaces is problematic due to optical distortion, experimental investigations of laryngeal airflow are typically performed using models with idealized geometries. In this paper a method for acquiring PIV data using models with realistic geometries is presented. Sample subglottal, intraglottal, and supraglottal PIV data are shown. Capabilities and limitations are discussed, and suggestions for future implementation are provided.
Introduction
Because airflow through the larynx affects vocal fold vibration and speech quality, it is important to be able to accurately characterize laryngeal flow structures. Many experimental studies have focused on supraglottal aerodynamics; however, optical access has limited the investigation of intraglottal and subglottal aerodynamics. Further, most studies on supraglottal aerodynamics have used idealized supraglottal geometries1 or have focused on a free jet without supraglottal structures.2 Subglottal, glottal, and supraglottal airflow data obtained using models with realistic geometries is necessary to accurately characterize laryngeal airflow and ascertain its effects on voice production.
Several types of models have been used to study laryngeal airflow. These include static,3 driven,1 and self-oscillating4 vocal fold models. In such studies, subglottal and supraglottal geometries are typically approximated using simplified, constant area cross-sections. Excised larynx models2, 5, 6 incorporate realistic subglottal and glottal geometries, but are limited in terms of parameterization, can only be used for a short period of time, and supraglottal geometry, if included, is typically idealized. Finally, computational models provide another method for studying laryngeal flow.7, 8 Because computational models are not limited by optical access, they can yield detailed flow data throughout the larynx. However, accurate simulations are complex, computationally expensive, and require thorough validation using experimental data.
Particle image velocimetry (PIV) is a common method of choice for acquiring spatially-resolved flow field data. However, using PIV with models that have curved surfaces is problematic due to optical distortion caused by light refraction at the model-fluid interface. In this paper a method for acquiring detailed flow field data using PIV with synthetic models that have realistic geometries is presented. The refraction difficulty is overcome by creating a model with flat, smooth outer surfaces, and by matching the index of refraction of the working fluid with that of the surrounding material. This method, based on that reported by Hopkins et al.9 and used elsewhere10, 11, 12 for nasal cavity airflow measurements, incorporates the use of a clear silicone model with a cavity the shape of the laryngeal airway and a glycerol-water mixture for the working fluid. Sample subglottal, intraglottal, and supraglottal PIV data sets are shown. Capabilities and limitations of the method are discussed, and suggestions for future implementation are provided.
Methods
A model consisting of a transparent silicone rectangular prism with a larynx airway-shaped cavity was created as follows. A 3D computer model of the airway was first created via MRI image extraction. A corresponding prototype was then built on a three-dimensional printer (Z Corporation) using soluble plastic powder. The prototype was coated with several thin layers of polyvinyl acetate, and suspended in a rectangular acrylic box. Clear silicone (Sylgard 184, Dow Corning) was carefully poured around the prototype, filling the acrylic box, and allowed to cure. The acrylic box was then removed and inlet and outlet holes were cut into the silicone to allow for fluid flow. The prototype was dissolved in cold running water, leaving a silicone model with a cavity of the shape of the laryngeal airway.
A mixture of glycerol and water (55% glycerol, 45% water by volume; kinematic viscosity11 = 6.55 × 10−6 m2∕s) was used as the working fluid to match the index of refraction of the silicone. To find the optimal glycerol-water mixture, the silicone model was connected to a flow loop with a grid placed behind the model. An initial glycerol-water mixture with high glycerol concentration was pumped through the model and then slowly titrated until gridline distortions were minimized. Figure 1 shows the effect of refractive index matching when the model is filled with air, water, and the optimal glycerol-water mixture. When the model is filled with air the grid is obscured and there is no optical access to the cavity interior. With water the grid is visible, but distorted, so that PIV measurements are not possible. When filled with the 55∕45 glycerol-water mixture, the index of refraction of the silicone is matched, the grid is visible with minimal distortion, and PIV can be performed on the flow in the cavity interior.
Figure 1.
Photographs depicting the effects of index of refraction matching between the fluid and the model. The silicone model with a larynx airway-shaped cavity is placed in front of a grid. Shown are views of the cavity filled with (a) air, (b) water, and (c) 55∕45 glycerol-water mixture.
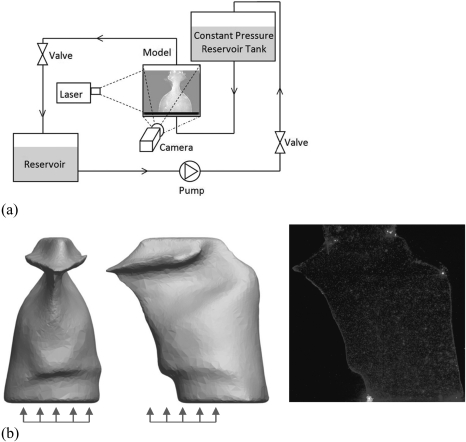
Figure 2a illustrates the experimental setup. The silicone model was connected to a flow loop. A constant pressure reservoir tank placed 0.67 m above the model supplied steady flow. Because this model was intended for demonstration purposes, the larynx inlet and outlet region did not include flow conditioning features (e.g., smooth transitions for reduction of turbulence and secondary flow patterns). After exiting the model the fluid flowed into a lower reservoir where a pump (Teel, 1P579F) returned it to the constant pressure reservoir tank. The flow was seeded with hollow glass spheres (approximately 50–70 μm diameter). PIV measurements were acquired along five sagittal and five frontal cross-sections, as shown in Fig. 2b, using a double-pulsed laser (New Wave Laser, Solo II-15 Nd:YAG, Fremont, CA) and a synchronized camera (LaVision Imager Intense, Goettingen, Germany). At each cross section 500 image pairs were recorded at 4.95 frames∕s with a resolution of 1376 × 1024 pixels and a 300 μs inter-frame delay.
Figure 2.
(Color online) (a) Schematic of the experimental setup. (b) (left) and (middle) Arrows denoting approximate locations of sagittal and frontal image planes, respectively. Distance between adjacent planes was approximately 5 mm. (right) Raw PIV image.
The velocity fields for each cross section were analyzed using an FFT-based cross correlation algorithm to determine particle displacement and velocity vectors. Multiple passes with successively smaller interrogation regions were used, beginning with 64 × 64 pixel windows and a 50% overlap, and ending with 16 × 16 windows (also with a 50% overlap). Velocity fields at each cross section were then averaged.
Results and discussion
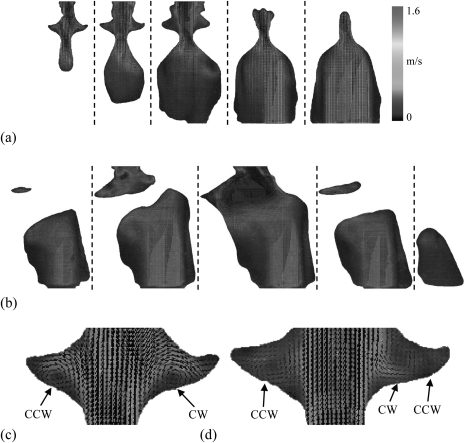
Figure 3 shows time-averaged frontal and sagittal velocity distributions. The Reynolds number based on the average inlet velocity was approximately 1570. The velocity magnitude was fairly uniform over large regions of the subglottis. Velocities quickly accelerated from around 0.4 m∕s in the subglottis to around 1.2 m∕s in the glottis, corresponding to approximately 0.97 m∕s and 2.91 m∕s, respectively, in air (the vocal folds were abducted in the MRI data set, resulting in lower glottal velocities than what is typical during phonation). Velocity magnitudes decreased close to the subglottal walls due to wall friction and the flow inlet condition. Some vortical structures can be seen in the subglottis, inferior to the vocal folds, although again, these may be related to the inlet flow properties, and further investigation is necessary with a well-conditioned inlet to determine whether they may be present in the human case. In the present case it appears that these subglottal vortices were likely formed when the incoming jet collided with the static vocal folds, causing recirculation to occur along the subglottal walls. Exploration of velocity patterns within the laryngeal ventricles, viewed in the frontal planes, reveals recirculation zones consisting of single vortices [Fig. 3c] and dual counter-rotating vortices [Fig. 3d]. Visual inspection of the velocity vector fields obtained from individual image pairs (before time-averaging) suggested that the vortices in this flow field were relatively stationary.
Figure 3.
(Color online) Average velocity distributions in (a) five frontal planes, and (b) five sagittal planes. (c) and (d) Velocity vectors in laryngeal ventricles in two frontal planes (the plane on the right was located approximately 5 mm dorsally to the plane on the left). Counterclockwise (CCW) and clockwise (CW) vortices are seen in the left and right ventricles, respectively, in both planes. An additional counter-rotating vortex is seen in the lateral extent of the right ventricle in the plane shown at right.
Conclusions and future work
A method for acquiring detailed glottal flow measurements using PIV in a geometrically realistic model of the laryngeal airway has been presented and demonstrated. The method is based on the work of Hopkins et al.9 for nasal cavity airflow studies. This method allows for the acquisition of whole-field velocity data, even in complex model geometries. Velocity fields in sagittal and frontal planes are shown here, but PIV data acquisition in the transverse plane is also possible. This method is anticipated to find use in subsequent studies characterizing the three-dimensional velocity field, including vortical patterns and turbulence levels, found in the human larynx.
A few observations regarding limitations and future work are worth noting. First, while Reynolds number similarity is maintained, because a liquid is used as the working fluid, Mach number similarity is not. This is an inherent limitation of all liquid-based larynx model experiments. The same model could be used for limited acoustic analysis in air. Second, use of a static model does not yield information about the effect of vibrating vocal folds on the flow field. However, the development and use of a similar model with dynamically driven vocal folds is conceivable. Third, the preliminary PIV data presented in this study are two-dimensional, but the results show that the flow is clearly three-dimensional. This experimental technique is not restricted to two-dimensional PIV, and future studies with three-dimensional PIV using this method are possible. Fourth, future studies of this type should include conditioned inlet and outlet sections to reduce turbulence and other potential flow-loop-produced flow artifacts, ideally including the upper portion of the trachea, the pharynx, and the oral and nasal cavities to more closely model the physiological inlet and outlets. Fifth, since steady flow conditions were used in this experiment, the use of a low-speed PIV system without triggering was adequate. However, in future studies involving unsteady flow conditions or vibrating vocal folds, high-speed imaging or a combination of phase-locking and ensemble averaging would be required for temporal resolution of the flow field. Finally, the use of pressure taps (also with a matched index of refraction working fluid) in conjunction with velocity field data could be implemented.
Acknowledgments
This work was supported by Grant R01 DC009616 from the National Institute on Deafness and Other Communication Disorders. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health (NIH).
References and links
- Triep M. and Bru¨cker C., “Three-dimensional nature of the glottal jet,” J. Acoust. Soc. Am. 127, 1537–1547 (2008). [DOI] [PubMed] [Google Scholar]
- Khosla S., Murugappan S., Gutmark E., and Scherer R., “Vortical flow during phonation in an excised canine larynx model,” Ann. Otol. Rhinol. Laryngol. 116, 217–228 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour F. and Scherer R. C., “Pressure and velocity profiles in a static mechanical hemilarynx model,” J. Acoust. Soc. Am. 112, 2996–3003 (2002). [DOI] [PubMed] [Google Scholar]
- Drechsel J. S. and Thomson S. L., “Influence of supraglottal structures on the glottal jet exiting a two-layer synthetic, self-oscillating vocal fold model,” J. Acoust. Soc. Am. 123, 4434–4445 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour F. and Scherer R. C., “Characterizing glottal jet turbulence,” J. Acoust. Soc. Am. 119, 1063–1073 (2006). [DOI] [PubMed] [Google Scholar]
- Oren L., Khosla S., Murugappan S., King R., and Gutmark E., “Role of subglottal shape in turbulence reduction,” Ann. Otol. Rhinol. Laryngol. 118, 232–240 (2009). [DOI] [PubMed] [Google Scholar]
- Mihaescu M., Khosla S., Murugappan S., and Gutmark E., “Unsteady laryngeal airflow simulations of the intra-glottal vortical structures,” J. Acoust. Soc. Am. 127, 435–444 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Bielamowicz S., Luo H., and Mittal R., “A computational study of the effect of false folds on glottal flow and vocal fold vibration during phonation,” Ann. Biomed. Eng. 37, 625–642 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins L. M., Kelly J. T., Wexler A. S., and Prasad A. K., “Particle image velocimetry measurements in complex geometries,” Exp. Fluids 29, 91–95 (2000). [Google Scholar]
- Kim J., Yoon J., Kim C., Nam T., Shim D., and Shin H., “Particle image velocimetry measurements for the study of nasal airflow,” Acta Otolaryngol. 126, 282–287 (2006). [DOI] [PubMed] [Google Scholar]
- Kim S. K. and Chung S. K., “An investigation on airflow in disordered nasal cavity and its corrected models by tomographic PIV,” Meas. Sci. Technol. 15, 1090–1096 (2004). [Google Scholar]
- Doory D., Taylor D. J., Franke P., and Schroter R. C., “Experimental investigation of nasal airflow,” J. Eng. Med. 222, 439–453 (2008). [DOI] [PubMed] [Google Scholar]