Abstract
Many changes produced by chronic stress are similar to those seen in cannabinoid CB1 receptor–deficient mice. In the current study, we examined both anxiety-like behavior and dendritic complexity within the prefrontal cortex and basolateral amygdala (BLA) in wild-type and CB1 receptor–deficient mice, under basal conditions and following exposure to 21 days of protracted restraint stress. CB1 receptor–deficient mice exhibited increased indices of anxiety in the elevated plus maze under basal conditions that were similar in magnitude to changes seen in wild-type mice exposed to chronic stress. Chronic stress or deletion of the CB1 receptor also produced a reduction in both apical dendritic length and branch points of neurons within layer II/III of the prelimbic region of the prefrontal cortex. Pyramidal neurons in the (BLA) of CB1 receptor–deficient mice were found to have increased dendritic length compared with wild type. Chronic stress increased dendritic length of these amygdalar neurons in both wild-type and CB1 receptor–deficient mice. Collectively, these data demonstrate that loss of cannabinoid CB1 receptor signaling produces a chronic stress-like phenotype under basal conditions and provide a putative neural substrate that may subserve the changes in emotional behavior seen following disruption of CB1 receptor signaling.
Keywords: anxiety, basolateral amygdala, endocannabinoid, prefrontal cortex, stress
Introduction
Corticolimbic neural circuits are integral to the central processing of aversive stimuli and the subsequent generation of neuroendocrine, autonomic, and behavioral responses (Price and Drevets 2010). Two important nodes in this circuitry are the prefrontal cortex and the amygdala. The amygdala acts as an interface between sensory inputs and cortical processing, and activation of this structure is directly linked to the generation of fear and anxiety (Sah et al. 2003; Shin and Liberzon 2010). The medial prefrontal cortex (mPFC), on the other hand, is involved in higher order processing and is explicitly involved in the recognition of aversive stimuli and in drawing conclusions about the controllability of the stimuli (Amat et al. 2005; Maier et al. 2006). Since mPFC projections to the amygdala are largely excitatory afferents that synapse onto inhibitory neuronal networks within the amygdala, mPFC activation is inversely related to amygdalar activity (McDonald et al. 1996; Sotres-Bayon et al. 2004). The functional coupling of mPFC and amygdala is widely believed to be critical for cognitive and emotional flexibility to aversive and stressful stimuli and for stress adaptation and stress resilience (Feder et al. 2009; Price and Drevets 2010). In fact, dysregulation of prefrontal cortical–amygdalar communication is associated with many pathological states. For example, neuroimaging studies have revealed that patients with major depression and posttraumatic stress disorder exhibit both volumetric reductions in distinct subregions of the mPFC and increased amygdalar activity (Price and Drevets 2010; Shin and Liberzon 2010). This shift in the functional coupling of the mPFC and amygdala is believed to produce an amygdalocentric form of information processing, which favors aversive responses to environmental stimuli, and is believed to underlie the generation of pathological states of anxiety and depression (Price and Drevets 2010).
Protracted exposure to stress is one of the most reproducible precipitating factors in the development of episodes of both mood and anxiety disorders. Preclinical studies have revealed that exposure of rodents to chronic stress results in neuronal remodeling within the mPFC and amygdala which parallels the structural/functional changes associated with mood and anxiety disorders. Exposure to chronic stress of various intensities and durations consistently results in simplification and retraction of the apical dendritic tree in pyramidal neurons of the mPFC (Cook and Wellman 2004; Radley et al. 2004, 2005; Brown et al. 2005; Liston et al. 2006; Cerqueira et al. 2007; Liu and Aghajanian 2008; Dias-Ferreira et al. 2009; Goldwater et al. 2009; Shansky et al. 2009; Bloss et al. 2010). Conversely, chronic stress results in an expansion and arborization of the dendritic tree of pyramidal neurons in the basolateral nucleus of the amygdala (basolateral amygdala (BLA); Vyas et al. 2002, 2004, 2006; Johnson et al. 2009). Similar to what is seen in mood and anxiety disorders, this bidirectional regulation of dendritic complexity within the mPFC and BLA is thought to result in a preferential shift to amygdalar processing of environmental stimuli, in part through a loss of prefrontal cortical negative regulation of amygdalar activation, which favors the expression of fearful and anxiety-like behaviors (McLaughlin et al. 2009).
The endocannabinoid system is a neuromodulatory system that regulates processing of aversive stimuli and the subsequent generation of neuroendocrine and behavioral responses (Hill and McEwen 2010). The role of this system is highlighted by findings that pharmacological or genetic disruption of endocannabinoid signaling produces a phenotypic state which is strikingly reminiscent of changes that are evoked by exposure to chronic stress, such as increased emotionality (Haller et al. 2002; Martin et al. 2002; Rodgers et al. 2005; Patel and Hillard 2006; Aso et al. 2008; Thiemann et al. 2009; Beyer et al. 2010), exaggerated stress responses (Barna et al. 2004; Patel et al. 2004; Steiner, Marsicano, Wotjak, and Lutz 2008; Steiner, Marsicano, Nestler, et al. 2008), and reductions in hippocampal plasticity and cellular resilience (Jin et al. 2004; Aguado et al. 2005; Kim et al. 2006; Lee et al. 2009; Beyer et al. 2010). Furthermore, reduced levels of circulating endocannabinoids have been documented in individuals diagnosed with major depression (Hill, Miller, et al. 2008; Hill et al. 2009), and the widespread use in humans of a CB1 receptor antagonist revealed that blockade of endocannabinoid signaling increased indices of anxiety and depression in a subset of individuals with no history of mental illness (Christensen et al. 2007; Nissen et al. 2008; Hill and Gorzalka 2009; Moreira et al. 2009).
The neural circuit subserving regulation of emotional behavior by endocannabinoid signaling has yet to be determined. Both CB1 receptors and the endocannabinoids anandamide and 2-arachidonoylglycerol are present within the corticolimbic circuit, which is implicated in mood and anxiety disorders (Gorzalka et al. 2008). More interestingly, however, is the finding that corticolimbic endocannabinoid signaling is compromised by exposure to chronic stress (Hill et al. 2005; Hill, Carrier, et al. 2008; Rossi et al. 2008; Reich et al. 2009). Coupled with the aforementioned clinical findings, these data have led to the hypothesis that deficient endocannabinoid signaling increases susceptibility to mood and anxiety disorders in the face of stressful environmental conditions (Hill and Gorzalka 2009); however, the neural mechanisms by which deficient endocannabinoid signaling relates to changes in emotional behavior and increased sensitivity to stress is not well understood. In the present study, we have examined the effects of chronic stress on anxiety-like behavior and dendritic arborization and complexity of pyramidal neurons within the mPFC and BLA of wild-type and CB1 receptor–null mice.
Materials and Methods
Subjects
Male ICR mice, aged 10–12 weeks and housed in cages of 3–5 mice per cage, were used in these studies. Mice were maintained on a 12 h light:dark cycle with lights on at 06:00. Food and water were available ad libitum. CB1 receptor–null mice were bred in house from a founder line generously provided by Roche Laboratories and backcrossed for 9 generations onto the ICR strain (Pan et al. 2008). CB1 receptor null mice were generated through heterozygous breeding pairs. Wild-type mice derived from the same backcrossing were used as controls in those studies. Genotypes were determined by polymerase chain reaction using DNA isolated from ear tissue obtained at weaning. All procedures carried out with mice were approved by the Institutional Animal Use and Care Committee of the Medical College of Wisconsin.
Procedure
Both wild-type (n = 12) and CB1 receptor–deficient mice (n = 11) were divided into 2 conditions, nonstress or stress. Mice in the stress condition were subjected to 6 h of restraint stress per day for 21 days. Restraint stress took place each day approximately between 10:00 and 16:00 h. Mice were placed into wire mesh restrainers (5″ × 4.5″) that were sealed with binder clips, and the restrainers were placed in the home cage for the duration of the stressor. Nonstress mice were left undisturbed for the duration of the experiment, except for cage changing, which occurred twice weekly.
Behavioral Testing
Twenty-four hours following the final stressor, all subjects were examined for anxiety-like behavior within the elevated plus maze. Testing occurred within the colony room, so no habituation to the testing room was required. The elevated plus maze (San Diego Instruments) was constructed from beige plastic and consisted of 2 open arms (30 × 5 cm) and 2 enclosed arms (30 × 5 × 15 cm) that extended from a central platform (5 × 5 cm). The maze was elevated 40 cm above the floor. Lighting conditions in the testing room were optimized to reveal an anxiogenic phenotype of CB1 receptor–deficient mice as previously reported (Haller et al. 2004). Specifically, testing occurred in a dark room with only a single light that was placed in close proximity to the open arm and produced a bright field of light directly onto the open arm (ca. 200 lx). Each animal was placed in the central quadrant of the elevated plus maze facing an open arm and entries into open and closed arms, and time spent in the open arm were scored over a 5-min trial by a blind observer. Entries into the open arm were calculated as a full body entry to the open arm such that the entire body up to the base of the tail was on the open arm. At the conclusion of each test, the elevated plus maze was wiped clean with a 10% ethanol solution to remove urine and scent.
Morphological Analysis
Immediately following testing in the elevated plus maze, mice were rapidly decapitated. Brains were quickly removed, washed in distilled water, and were processed for staining of individual neurons following the manufacturer's instructions for the rapid Golgi kit (FD Neurotech). Golgi-stained tissue containing the mPFC and BLA was sliced (100 μm), mounted with coverslip, and used for morphological analysis. Neurons were 3D reconstructed using a Nikon Eclipse microscope (40×) and the Neurolucida software package (MicroBrightField). For each region, 6 neurons were reconstructed per animal by a trained experimenter blind to the conditions, and an average for each animal was generated and used for analysis. Sholl analysis was also performed, in which the center of the soma was used as a reference point and dendritic length and branch points were quantified both as a total measure per cell as well as a function of radial distance from the soma in 30 μm increments.
Within the mPFC, pyramidal neurons within layer II/III of the prelimbic region were examined for dendritic architecture as these neurons have been repeatedly characterized as those which exhibit remodeling in response to chronic stress (Cook and Wellman 2004; Radley et al. 2004, 2005). Pyramidal neurons within this region are defined as having a cell body which is immediately lateral to layer I, which is relatively absent of cells. These neurons are also defined by the presence of a basilar dendritic tree and a clearly defined single apical dendrite that projects toward the pial surface. Neurons within the prelimbic region of the mPFC used for analysis were chosen from slices that were between 1.5 and 2.3 mm anterior to bregma. For analysis, cells were chosen that met the following criteria: 1) cell bodies within the midsection of the tissue to minimize dendritic truncation; 2) relative isolation of the cell body from neighboring impregnated neurons; 3) cell bodies exist between 150 and 250 μm from the pial surface to prevent artifacts due to unrepresentative sampling from neurons of varying distance from the midline; and 4) the presence of an intact primary, secondary, and tertiary dendrites (as evidenced by well-defined endings) to at least 50 μm from the cell body. As all the previous studies that have examined the effects of stress on this neuronal class within the mPFC have found selective effects on the apical dendrite (Cook and Wellman 2004; Radley et al. 2004, 2005; Brown et al. 2005; Liston et al. 2006; Goldwater et al. 2009; Shansky et al. 2009; Bloss et al. 2010), as opposed to the basilar dendritic tree, analysis was restricted to the apical dendritic tree.
Within the BLA, pyramidal neurons were examined for dendritic architecture as this class of neurons has been repeatedly characterized as those that exhibit remodeling in response to chronic stress. Pyramidal neurons within this region were identified based on previously established criteria (Vyas et al. 2002; Wellman et al. 2007), which requires the presence of a single clearly defined apical dendrite and at least 2 intact basilar dendrites extending from the base of the cell body. Further, as previous studies have identified that it is the spiny pyramidal neurons in the BLA which exhibit architectural changes in response to stress (Vyas et al. 2002, 2004, 2006), our analysis was also restricted to the spiny class of pyramidal neurons, which was defined by the obvious presence of spines along the apical and basilar dendritic trees. Neurons within the BLA used for analysis were chosen from slices that were between 1.0 and 2.2 mm posterior to bregma, and the boundaries of the BLA were clearly defined within Golgi-impregnated material by the fiber tracts from the external capsule that defined the dorsal, medial, and lateral boundaries of the BLA. For analysis, cells were chosen that met the following criteria: 1) cell bodies within the midsection of the tissue to minimize dendritic truncation; 2) relative isolation of the cell body from neighboring impregnated neurons; 3) cell bodies exist within the defined boundaries of the BLA; and 4) the presence of an intact primary, secondary, and tertiary dendrites (as evidenced by well-defined endings) to at least 50 μm from the cell body. Based on the analysis performed in previous studies, we analyzed all dendritic material in both the apical and basilar trees collapsed together as one variable. As such, neurons required the presence of at least 2, and no more than 5 basilar dendritic trees to be quantified, and each animal was balanced so that an equal number of basilar dendritic trees was represented across the 6 neurons quantified for each animal.
Statistics
Anxiety-like behavior, dendritic length, and branch points were all analyzed using a 2-way analysis of variance (ANOVA), with stress and genotype as fixed factors. Bonferroni post hoc tests were used to determine specific differences between experimental groups. Data generated from Sholl analysis were analyzed using a repeated measure ANOVA, with distance from soma acting as a within-subject factor and stress and genotype acting as between-subject factors. Corrected Bonferroni post hoc tests were used to determine specific differences between experimental groups at different segments from the soma. Significance for all tests was established at a P < 0.05. All data presented in the figures are listed as mean values ± standard error.
Results
Anxiety-Like Behavior
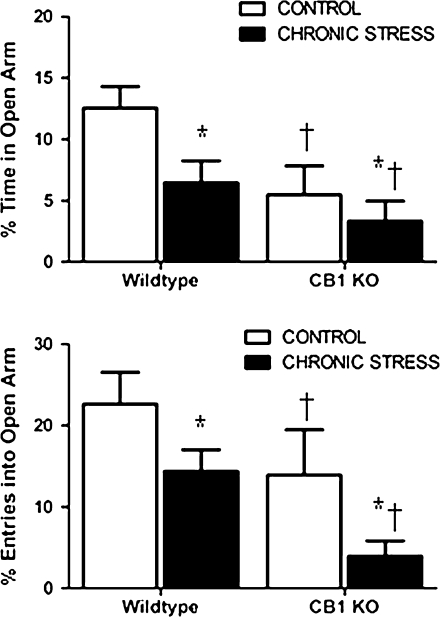
Analysis of percent time spent in the open arms revealed that there was not a significant interaction between stress and genotype (F1,19 = 3.43, P > 0.05; Fig. 1). There was, however, a main effect of both stress exposure (F1,19 = 15.01, P < 0.05) and genotype (F1,19 = 22.94, P < 0.02). That is, both exposure to chronic stress and deletion of the CB1 receptor reduce the percent of time spent in the open arm.
Figure 1.
Behavioral analysis of cannabinoid CB1 KO mice (CB1 KO) or their wild-type counterparts (WT), under conditions of no stress (CONTROL) or following 21 days of 6 h/day restraint stress (CHRONIC STRESS), in the elevated plus maze. Data are displayed as mean frequency ± standard error of the mean and for all groups n = 5–7 per condition. *, denotes significant differences (P < 0.05) between control and stressed animals and †, denotes significant differences (P < 0.05) between wild-type and CB1 receptor–deficient animals.
A similar phenomenon was seen for percentage of entries into the open arms, with analysis revealing that there was no significant interaction between genotype and stress (F1,19 = 0.81, P > 0.05; Fig. 1). There was, however, a main effect of both stress exposure (F1,19 = 20.27, P < 0.02) and genotype (F1,19 = 22.51, P < 0.02). That is, both exposure to chronic stress and deletion of the CB1 receptor reduce the percent of entries in the open arm.
With respect to closed arm entries, there was not a significant interaction between stress and genotype on closed arm entries (F1,19 = 0.33, P > 0.05; data not shown) nor a main effect of genotype on closed arm entries (F1,19 = 2.14, P > 0.05). There was, however, a main effect of stress on closed arm entries (F1,19 = 13.71, P < 0.005) such that regardless of genotype all animals that were stressed exhibited more entries into the open arms.
Morphological Analysis of Pyramidal Neurons within the mPFC
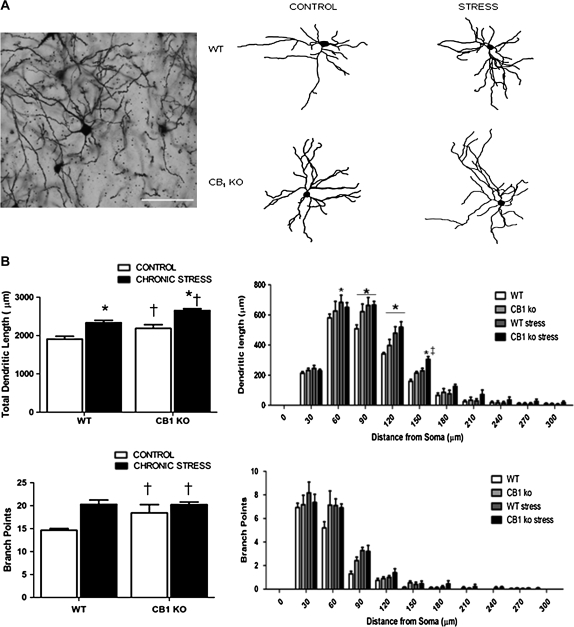
Apical dendrites of neurons in the mPFC of mice exposed to chronic restraint stress (CRS) were shorter and less branched than those of control mice. Apical dendrites of neurons in mPFC of nonstressed CB1 KO mice had shorter and less branched dendrites than wild-type mice and showed no significant effects of CRS on dendritic length or branching. Statistical analyses demonstrate a significant interaction between genotype and stress on the length of the apical dendritic tree on pyramidal neurons within layer II/III of the prelimbic region of the mPFC (F1,16 = 5.12, P < 0.04; Fig. 2). Post hoc analysis revealed that within wild-type mice, exposure to chronic stress resulted in approximately a 24% reduction of length of the apical dendritic tree (P < 0.01). CB1 KO mice exhibited a reduction in the length of the apical dendritic tree relative to nonstressed wild-type mice (P < 0.01). There was no significant difference in the length of the apical dendrite between nonstressed and CRS-exposed CB1 KO mice (P > 0.05) nor was there a significant difference in apical dendrite length between wild-type mice subjected to chronic stress and CB1 KO mice exposed to chronic stress (P > 0.05).
Figure 2.
(A) Representative photomicrograph of a pyramidal neuron in layer II/III of the prelimbic region of the mPFC and representative tracings of the apical dendritic tree of pyramidal neurons from cannabinoid CB1 KO mice (CB1 KO) or their wild-type counterparts (WT), under conditions of no stress (CONTROL) or following 21 days of 6 h/day restraint stress (CHRONIC STRESS). Scale bars (100 μm) are seen in white in the photomicrograph. (B) Morphological analysis of pyramidal neurons in layer II/III of the prelimbic region of the mPFC in cannabinoid CB1 KO mice (CB1 KO) or their wild-type counterparts (WT), under conditions of no stress (CONTROL) or following 21 days of 6 h/day restraint stress (CHRONIC STRESS). Analysis encompassed dendritic length (upper panel) and branch points (bottom panel) of pyramidal neurons and was performed on the total apical dendritic tree (left panel) and through Sholl analysis of 30 μm concentric rings from the soma (right panel). Data are displayed as mean frequency ± standard error of the mean and for all groups n = 5 per condition. Significant differences from WT-CONTROL group denoted by *P < 0.05.
Sholl analysis was employed to determine the region of the apical dendritic tree at which the reduction in length was seen. This analysis revealed a significant interaction between stress, genotype, and distance from soma (F11,176 = 1.54, P < 0.05; Fig. 2). Post hoc analysis revealed that relative to nonstressed wild-type mice, exposure to CRS reduced dendritic material at segments which were 180–270 μm from the soma (P < 0.05 for 180–210, 210–240, and 240–270 μm segments). Similarly, nonstressed CB1 KO mice exhibited reductions in dendritic material relative to nonstressed wild-type mice at segments between 180 and 300 μm from the soma (P < 0.05 for 180–210, 210–240, 240–270, and 270–300 μm segments). There were no differences in dendritic length at any segment between stressed wild-type, nonstressed CB1 KO mice, and stressed CB1 KO mice at any segment of the apical dendritic tree.
Branch points were analyzed as a measure of dendritic complexity. Analysis revealed a significant interaction between genotype and stress on total branch points within the apical dendrite (F1,16 = 5.05, P < 0.04; Fig. 2). Post hoc analysis revealed that, relative to nonstressed wild-type mice, stressed wild-type mice and nonstressed CB1 KO mice exhibited significant reductions in the total number of branch points within the apical dendrite (both P < 0.05). There were no differences in branch points between CRS-exposed CB1 KO mice and either CRS-exposed, wild-type mice, or nonstressed CB1 KO mice.
Sholl analysis of the branch points was subjected to statistical analysis, which did not reveal a significant interaction between distance, genotype, and stress (F11,176 = 1.25, P > 0.05; Fig. 2) nor interactions between distance and stress (F11, 176 = 0.95, P > 0.05) or between distance and genotype (F11,176 = 1.11, P > 0.05).
Morphological Analysis of Pyramidal Neurons within the BLA
CRS increased dendritic material in pyramidal neurons in the BLA of both wild-type and CB1 KO mice, and nonstressed CB1 KO mice displayed greater dendritic material than nonstressed wild-type mice. With respect to the length of all dendritic material within pyramidal neurons in the BLA, there was no interaction between stress and genotype (F1,16 = 0.09, P > 0.05; Fig. 3). There was, however, a significant main effect of stress to increase the length of total dendritic material (F1,19 = 38.58, P < 0.001) and a main effect of CB1 receptor deficiency to increase the length of total dendritic material (F1,19 = 17.07, P < 0.01). That is, CB1 KO mice exhibited more dendritic material on BLA pyramidal neurons than wild-type mice, but unlike what was seen in the mPFC, CB1 KO status did not abrogate the effects of stress to increase in dendritic length in CB1 KO mice.
Figure 3.
(A) Representative photomicrograph of a pyramidal neuron in the BLA and representative tracings of the total dendritic tree of pyramidal neurons from cannabinoid CB1 KO mice (CB1 KO) or their wild-type counterparts (WT), under conditions of no stress (CONTROL) or following 21 days of 6 h/day restraint stress (CHRONIC STRESS). Scale bars (100 μm) are seen in white in the photomicrograph. (B) Morphological analysis of pyramidal neurons in the BLA in cannabinoid CB1 KO mice (CB1 KO) or their wild-type counterparts (WT), under conditions of no stress (CONTROL) or following 21 days of 6 h/day restraint stress (CHRONIC STRESS). Analysis encompassed dendritic length (upper panel) and branch points (bottom panel) of pyramidal neurons and was performed on the total dendritic tree (left panel) and through Sholl analysis of 30 μm concentric rings from the soma (right panel). Data are displayed as mean frequency ± standard error of the mean and for all groups n = 5 per condition. *, denotes significant differences (P < 0.05) between control and stressed animals and †, denotes significant differences (P < 0.05) between wild-type and CB1 receptor–deficient animals.
Sholl analysis of radial distance from the soma revealed a significant interaction between distance, genotype, and stress (F10,160 = 1.80, P < 0.05; Fig. 3). Post hoc analysis revealed that relative to nonstressed wild-type mice, nonstressed CB1 KO mice exhibited an increase in dendritic material only at the 90–120 μm segment of the dendritic tree (P < 0.05). Wild-type mice exposed to CRS demonstrated increases in dendritic material at segments between 60 and 150 μm from the cell body relative to nonstressed wild-type mice (P < 0.05 for all of 60–90, 90–120, and 120–150 μm segments). Relative to nonstressed CB1 KO mice, CB1 KO mice exposed to CRS exhibited significant increases in dendritic material at the 150–180 μm segment of the dendritic tree (P < 0.01).
Analysis of branch points within the entire dendritic tree of BLA pyramidal neurons revealed that there was neither an interaction between stress and genotype (F1,16 = 3.24, P > 0.05; Fig. 3) nor a main effect of CB1 receptor deficiency (F1,16 = 3.02, P > 0.05]. There was, however, a main effect of stress to increase branch points within BLA pyramidal neurons (F1,16 = 12.08, P < 0.005).
Similar to what was seen in the mPFC, analysis of Sholl data revealed that there was no interaction between radial distance of branch points from the soma, genotype, and stress (F10,160 = 1.23, P > 0.05; Fig. 3) nor was there a significant interaction between genotype and distance (F10,160 = 0.8, P > 0.05). However, there was a significant interaction between stress and distance (F10,160 = 1.78, P < 0.05). Post hoc analysis revealed that mice exposed to CRS, regardless of genotype, exhibited increases in branch points along the dendritic tree at the segments between 60 and 120 μm from the cell body (P < 0.05 for both 60–90 and 90–120 μm segments).
Discussion
Exposure of wild-type mice to CRS produced an increase in anxiety-like behavior in the elevated plus maze, together with reductions in the dendritic complexity of the apical dendritic tree within pyramidal neurons in layer II/III of the prelimbic region of the mPFC and expansion of dendritic arborization and complexity within pyramidal neurons in the BLA, which is consistent with previous reports (reviewed in McLaughlin et al. 2009). Interestingly, nonstressed CB1 receptor KO mice exhibited a phenotype like that of wild-type mice exposed to CRS, including increased anxiety-like behavior and similar changes in prefrontal cortical and amygdalar dendritic arborization and complexity.
Chronic stress produced a reduction in the arborization and complexity of pyramidal neurons in layer II/III of the prelimbic region of the mPFC. Sholl analysis revealed that this reduction in dendritic complexity was driven by a loss of dendritic material and branching within distal regions of the apical dendritic tree (i.e., regions of the apical dendrite that were beyond 180 μm from the cell body). A selective effect of chronic stress on the distal portions of the apical dendritic tree is consistent with the previous reports of chronic stress effects on dendritic complexity within the mPFC (Cook and Wellman 2004; Radley et al. 2004). The distal regions of the apical dendritic tree, which are found in layer I of the cortical laminae and are within close proximity to the midline, largely receive input from subcortical structures, such as the hippocampus and thalamus (Swanson and Cowan 1977; Groenewegen 1988), and are not the primary target of intracortical projections (Scheibel ME and Scheibel AB 1970). The mechanism by which chronic stress produces dendritic retraction is not fully understood but is believed to involve the actions of glucocorticoids and possibly excitatory amino acids (Brown et al. 2005; Liu and Aghajanian 2008). Similar to the dendritic retraction which has been documented within the CA3 region of the hippocampus following chronic stress, the effects of chronic stress on PFC neurons is blocked by glucocorticoid receptor antagonism (Liu and Aghajanian 2008). Within the hippocampus, this effect of glucocorticoids on dendritic remodeling is driven by an enhancement of excitatory amino acid release (Magarinos and McEwen 1995). The dendritic retraction that occurs in response to the increase in excitatory amino acid release, produced by stress and glucocorticoids, is thought to represent a compensatory response to limit excessive excitation and excitotoxic damage (McEwen 1999). As such, a similar phenomenon is likely involved in the dendritic retraction that occurs in the mPFC following exposure to chronic stress.
Global genetic deletion of the CB1 receptor also produced a decrease in the arborization and complexity of pyramidal neurons in layer II/III of the prelimbic region of the mPFC that was, like CRS, due to loss of dendritic material and branching within distal regions of the apical dendritic tree. Our findings are in accord with previous studies demonstrating that CB1 KO mice exhibit reductions in dendritic complexity of pyramidal neurons within the motor cortex (Ballesteros-Yanez et al. 2007). Furthermore, chronic administration of a CB1 receptor agonist has been reported to increase the dendritic complexity and arborization of neurons within the mPFC (Kolb et al. 2006). Cannabinoid CB1 receptors are known to gate excitatory transmission within cortical structures (Auclair et al. 2000; Barbara et al. 2003), and a recent report has demonstrated that CB1 receptor–deficient mice exhibit an impairment in synaptic glutamatergic clearance and a reduction in the expression of the astrocytic glutamate transporter within the prefrontal cortex (Zoppi et al. 2010). These data indicate that a loss of CB1 receptors within the prefrontal cortex increases excitatory transmission, which in turn could be the driving force for dendritic retraction in these mice. Interestingly, this paper also demonstrated that chronic stress exposure produced a comparable shift in excitatory neurotransmission within the prefrontal cortex, and more so that activation of CB1 receptors prevented the excitotoxic effects of chronic stress (Zoppi et al. 2010). Accordingly, it appears quite reasonable to assume that heightened glutamatergic signaling within the prefrontal cortex may mediate both the effects of chronic stress and a CB1 receptor deficiency on the dendritic morphology of frontocortical pyramidal neurons. Alternately, it has been demonstrated that there is a critical role for CB1 receptors in the development and maturation of developing cortical neurons and their establishment of synaptic contacts (Mulder et al. 2008). As such, the reductions in dendritic arborization and complexity of cortical pyramidal neurons seen in CB1 receptor–deficient mice could be due to developmental alterations in the synaptic architecture of these neurons which compromises their mature phenotype.
The functional relevance of the dendritic retraction of neurons within the mPFC has been highlighted by behavioral studies which have documented that exposure to chronic stress produces deficits in PFC-mediated higher order cognitive processes, such as cognitive flexibility, fear extinction, and working memory (Hill et al. 2005; Bondi et al. 2008; Baran et al. 2009; Hains et al. 2009; Mitra and Sapolsky 2009), to a comparable degree to that which is seen following lesions of the mPFC (Birrell and Brown 2000; Quirk et al. 2000; Lacroix et al. 2002; Gisquet-Verrier and Delatour 2006). Interestingly, CB1 KO mice also exhibit deficits in many of these same PFC-mediated tasks (Marsicano et al. 2002; Varvel and Lichtman 2002; Varvel et al. 2005; Kamprath et al. 2006). Our current findings of reduced dendritic complexity within the mPFC of CB1 KO mice are consistent with these behavioral findings and could contribute to the deficits in PFC-mediated tasks seen in these mice. Given the parallels between chronic stress and the genetic deletion of the CB1 receptor, we hypothesize that these phenomenon are functionally related such that a stress-induced loss of prefrontal cortical endocannabinoid signaling is responsible for the structural and functional changes within the mPFC. In support of this hypothesis, previous studies have shown that administration of a CB1 receptor agonist to rodents following chronic stress reduces stress-induced impairments in cognitive flexibility (Hill et al. 2005).
In line with previous reports (Vyas et al. 2002, 2004, 2006; Johnson et al. 2009), chronic stress produced an expansion of dendritic arborization and an increase in dendritic complexity of BLA pyramidal neurons. Also, unlike the mPFC, in which the effects of stress on the dendritic tree was restricted to the distal region, the effects of stress on amygdalar neurons were restricted to regions of the dendritic tree which were more proximal to the soma (between 60 and 150 μm from the soma), consistent with previous reports (Vyas et al. 2002). This pattern indicates that CRS exposure increases proximal dendritic material and branch points rather than causes extension of the existing dendritic tree. Similar to what was seen in the mPFC, CB1 KO mice exhibited a similar phenotype to chronic stress under steady state conditions, although the effects here were of a much smaller magnitude than what was seen in the mPFC. Also, in opposition to what was seen in the mPFC, CB1 KO mice exhibited a significant increase in dendritic length following chronic stress, indicating that endocannabinoid signaling is not required for the effects of CRS to alter dendritic morphology in the amygdala.
At the behavioral level, both chronic stress and deletion of the CB1 receptor produced an increase in anxiety-like behavior as assessed by the elevated plus maze. The facilitation in emotionality following chronic stress is thought to be related to structural changes within the corticolimbic circuit we have examined herein (McLaughlin et al. 2009; Roozendaal et al. 2009; Price and Drevets 2010); a similar mechanism could underlie the heightened emotionality in CB1 KO mice. The reduction in dendritic complexity of pyramidal neurons within the mPFC, coupled to the expansion of dendritic arborization within the BLA, could result in a shift in information processing that compromises the inhibitory influence of the mPFC and facilitates excitability and activation of the amygdala. If so, then this shift would then favor amygdala-based processing of environmental information and may result in an enhancement of aversive salience to environmental cues, which would then manifest as increased anxiety, vigilance, and fear-like behavior.
Deficits in endocannabinoid signaling are associated with an increased propensity for mood and anxiety disorders (Hill and Gorzalka 2009) as circulating levels of endocannabinoids are reduced in individuals with major depression (Hill, Miller, et al. 2008; Hill et al. 2009), and administration of an antagonist to the CB1 receptor in humans increases indices of both anxiety and depression (Christensen et al. 2007; Nissen et al. 2008). Furthermore, genetic variants in the CB1 receptor are associated with an increased susceptibility to depression following stressful life events (Juhasz et al. 2009). The current data demonstrate that a loss of CB1 receptor signaling produces structural alterations within the mPFC and amygdala, which parallel volumetric changes that have been documented in populations afflicted by mood and anxiety disorders (Price and Drevets 2010) and are similar to the changes produced by exposure to chronic stress (McLaughlin et al. 2009). These structural alterations could result in an amygdalocentric form of information processing which favors an increased salience to aversive environmental cues, which may in turn be a critical step in the formation of pathological mood and anxiety states. In fact, combined preclinical/clinical studies have highlighted that mood disorders are associated with molecular changes within the amygdala that favor hyperexcitability of this structure (Sibille et al. 2009) and likely relate to increased levels of anxiety, emotionality, and vigilance. Collectively, these data provide evidence that a loss of CB1 receptor signaling produces effects on dendritic morphology in both the mPFC and amygdala similar to those produced by chronic stress and support the hypothesis that reduced cannabinoid signaling and chronic stress have convergent effects on corticolimbic circuits.
Funding
This research was supported by National Institutes of Health grants (MH41256 to B.S.M., DA026996 and DA09155 to C.J.H.) and a postdoctoral fellowship from the Canadian Institutes of Health Research to MNH.
Acknowledgments
The authors would like to thank Katharine McCarthy, Kara Stuhr, Ilia Karatsoreos, and Melinda Miller for their technical assistance on the execution of these studies. Conflict of Interest: None declared.
References
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Yanez I, Valverde O, Ledent C, Maldonado R, DeFelipe J. Chronic cocaine treatment alters dendritic arborization in the adult motor cortex through a CB1 cannabinoid receptor-dependent mechanism. Neuroscience. 2007;146:1536–1545. doi: 10.1016/j.neuroscience.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, Caboche J, Soubrie P, Crepel F. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signalling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–990. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, Chan K, Manners MT, Samad TA, Kennedy JD, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Delatour B. The role of the rat prelimbic/infralimbic cortex in working memory: not involved in the short-term maintenance but in monitoring and processing functions. Neuroscience. 2006;141:585–596. doi: 10.1016/j.neuroscience.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behavior in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Impairments in endocannabinoid signaling and depressive illness. JAMA. 2009;301:1165–1166. doi: 10.1001/jama.2009.369. [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioral effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163:34–39. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Chase D, Pegg E, Downey D, Toth ZG, Stones K, Platt H, Mekli K, Payton A, Elliott R, et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology. 2009;34:2019–2027. doi: 10.1038/npp.2009.19. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Ledent C, Jin K, Greenberg DA. Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther. 2006;319:150–154. doi: 10.1124/jpet.106.107698. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim DH, Yoon SH, Ryu JH. Sub-chronic administration of rimonabant causes loss of antidepressive activity and decreases doublecortin immunoreactivity in the mouse hippocampus. Neurosci Lett. 2009;467:111–116. doi: 10.1016/j.neulet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behavior. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress. 2009;12:305–312. doi: 10.1080/10253890802379955. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, Despres JP, Kastelein JJ, Steinhubl SR, Kapadia S, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, Usiello A, Centonze D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Elementary processes in selected thalamic and cortical subsystems—the structural substrates. In: Schmitt FO, editor. The neurosciences: 2nd study program. New York: Rockefeller University Press; 1970. pp. 443–457. [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33:1165–1170. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Thiemann G, Watt CA, Ledent C, Molleman A, Hasenohrl RU. Modulation of anxiety by acute blockade and genetic deletion of the CB (1) cannabinoid receptor in mice together with biogenic amine changes in the forebrain. Behav Brain Res. 2009;200:60–67. doi: 10.1016/j.bbr.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi S, Perez Nievas BG, Madrigal JL, Manzanares J, Leza JC, Garci-Bueno B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.214. doi: 10.1038/npp.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]