Abstract
Background
Over the past several years, increased attention has been devoted to understanding regionally selective brain changes that occur in Huntington’s disease and their relationships to phenotypic variability. Clinical progression is also heterogeneous, and while the CAG repeat length influences age of onset, its role, if any, in progression has been less clear. We evaluated progression in HD using a novel longitudinal MRI analysis; our hypothesis was that the rate of brain atrophy is influenced by the age of onset of HD.
Methods
We scanned 22 patients with HD at approximately one-year intervals; individuals were divided into one of three groups, determined by the relative age of onset.
Results
We found significant differences in the rates of atrophy of cortex, white matter and subcortical structures; patients who developed symptoms earlier demonstrated the most rapid rates of atrophy as compared to those who developed symptoms during middle-age or more advanced age. Rates of cortical atrophy were topologically variable, with the most rapid changes occurring in sensori-motor, posterior frontal and portions of parietal cortex. There were no significant differences in the rates of atrophy in basal ganglia structures. While both CAG repeat length and age influenced the rate of change in some regions, there was no significant correlation in many regions.
Conclusion
Rates of regional brain atrophy are influenced the age of onset of HD symptoms and are only partially explained by the CAG repeat length. These findings suggest that other genetic, epigenetic and environmental factors play important roles in neurodegeneration in HD.
Keywords: MRI longitudinal atrophy, Phenotypic variability, Huntington’s disease, neurodegeneration, longitudinal MRI
Introduction
Ages of onset, clinical manifestations, and rates of progression in Huntington’s disease (HD) vary dramatically between patients. While the CAG repeat length (CAGn) is an important influence, it accounts for less than 50% of the variability in the age of onset and may have only a modest relationship to the rate of progression1–3. Clinical heterogeneity poses many challenges for designing clinical trials that are aimed at slowing the progression of HD. The primary instrument currently used to measure progression in clinical trials is the Total Functional Capacity (TFC) scale, part of the United Huntington’s Disease Rating Scale (UHDRS)4. The TFC measures progressive functional losses that accumulate in HD; however, because of clinical and measurement variability, hundreds of patients must be followed for several years to provide sufficient power for Phase III interventional studies. The TFC is also not useful in typical phase II studies for providing preliminary evidence of disease modification. More efficient and objective measures of clinical progression are greatly needed. Novel longitudinal neuro-imaging approaches provide sensitive, reliable and objective measures that could enhance the efficiency of clinical trials in HD; they also provide a unique opportunity to more fully characterize the neurobiology of clinical heterogeneity and to understand whether there may be definable subtypes that progress and respond to therapies differently.
A major source of clinical heterogeneity and of variability in the TFC is the marked difference in the rate of progression between patients. Our hypothesis, based on clinical experience, was that a more rapid progression, irrespective of CAGn, was associated with earlier age of onset, and, by extension, slower progression was associated with later age of onset. We used serial MRI to study rates of atrophy in individuals who were at approximately the same stage of disease, but who had developed symptoms at different ages. We took advantage of a novel within subject analytical algorithm to evaluate the rates of change in cortical thickness, and the volumes of gray and white matter and of subcortical structures. We limited our study to those individuals with CAGn ranging from 40 to 55, to avoid the extremes often encountered in juvenile HD, and to subjects in early stages. We found that individuals who had developed motor symptoms younger than 40 had more rapid rates of atrophy as compared to individuals who developed HD in mid-life; those who developed symptoms after age 55 progressed even more slowly. Both the rate and topological distribution of changes were distinct across groups. While CAGn was independently associated with the rate of change in a small number of regions, it did not entirely explain observed differences. Clinical progression was also more rapid in younger subjects, paralleling rates measured by MRI. Our study supports an important role of the cortex in explaining clinical variability, and demonstrates the potential utility of neuro-imaging biomarkers to monitor progression in the setting of marked phenotypic variability. This is the first study to systematically evaluate the relationship between age of onset and the rates of regional progressive changes in the brain in early HD and to demonstrate variable progression related to age.
Methods
Subjects
Twenty-two individuals with early symptomatic HD (Stage I or II, as defined by the UHDRS TFC) were recruited. We used the median age of onset of 45 in our cohort to define groups. Individuals were classified as “Mid” who developed motor symptoms between 40 and 55, (N=9, 6F/3M, mean age 49.3 ± 4.2, CAGn 43.8±1.6), as “Young”, those who developed symptoms when younger than 40 (N=5, 2F/3M; mean age 31.4±3.4; CAGn 52.2±2.4) and as “Old” those who developed symptoms older than 55 (N=8, 4F/4M, mean age 62.2±6.3; CAGn 42.1±1.5). Subjects had comparable TFC’s at the time of the first scan (no significant difference between groups, p=0.13). While CAGn was larger in Young, there was no significant difference between Mid and Old (p=0.3). All subjects were recruited through the HD Center at Massachusetts General Hospital (MGH) and assessed by a neurologist with HD expertise (HDR). Subjects returned approximately one year later for follow-up. Procedures were explained and consent obtained according to the Declaration of Helsinski. Protocols were approved by the MGH Internal Review Board (IRB). Baseline group characteristics are provided in Table 1.
Table 1.
Group Demographics
| HD Young (N=6) |
HD Mid (N=9) |
HD Old (N=8) |
|
|---|---|---|---|
| Age | 33.1 ± 3.4 | 49.3 ± 3.6 | 62.2 ± 6.3 |
| Sex | 3F/3M | 7F/3M | 4F/4M |
| CAG Repeat | 52.2 ± 2.1 | 43.8 ± 1.6 | 42.1 ± 1.5 |
| TFC | 9.3 ± 1.2 | 10.6 ± 1.4 | 10.0 ± 2 |
| Verbal Fluency | 9 ± 2 | 14 ± 7 | 11 ± 3 |
| Stroop | 25 ± 6 | 31 ± 10 | 22 ± 8 |
| Symbol Digit | 21 ± 6 | 34 ± 13 | 23 ± 10 |
Image acquisition and processing
Scan Acquisition
Two T1-weighted images (TE=3.31ms, TR = 2730ms, flip angle 7°, FOV 256mm, matrix 256 × 171, 1.33 mm sagittal acquisition, Siemens 1.5T Avanto System, (Erlangen, Germany)) with 1.3×1x1.3 mm resolution were acquired. The image processing methods, using FreeSurfer 4.5, have been previously described in detail. 5,6. Thickness values were calculated in the native MRI scanner space of an individual subject’s brain and computed as the shortest distance between the pial and the gray/white surfaces. These methods have been previously shown to be reliable7 and comparable to manual measurements8.
We developed a novel, improved, inverse consistent rigid registration, allowing for more accurate within-subject registrations, to determine the rate of within subject change9. Briefly, an unbiased template image was created from two time points for each subject, used as an initiation point for anatomical segmentations and surface reconstructions, and then submitted to nonlinear iterative optimizations including topology correction, nonlinear atlas registration and nonlinear spherical surface registrations. Within subject measurements have been shown to reduce variability9–11. Cortical regions of interested were obtained as previously described 12.
To compute the rate of change per year, we normalized the average thickness or volume across the two time points. If M1 and M2 represent the cortical thickness measures, and T1 and T2 represent the respective time points, we obtain the “symmetrized” percent change (SPC) as: SPC = 2(M2−M1)/[(M1+M2)(T2−T1)]13. An automated segmentation scheme was used to obtain whole brain, subcortical volumes, adjusted for intracranial volume.
Statistical Analysis
We evaluated differences in the slopes of change from T1 to T2 amongst groups in regional thickness and volume measures using one-way ANOVA models, as well as t-tests. Statistical analyses were performed using R14. To more precisely determine the relationship, if any, between CAGn and regional rates of cortical thinning, CAGn was regressed on a vertex-by-vertex basis. T-statistics at each vertex were used to test the hypothesis that the slope coefficient was equal to zero. Results are presented uncorrected.
Results
Group Comparisons: Rate of Cortical Atrophy/Thinning
Whole brain volumes were reduced at a rate of 3.6% per year in Young (p < 0.05, as compared to Mid and Old); in Mid, the rate of whole brain atrophy was 1.55% per year; and in Old, 1.77% per year (not significantly different, p=0.7). Rates of gray and white matter loss over one year, however, were significantly different amongst the groups. In Young, white matter volume was lost at a rate of approximately 4.6% per year and at approximately 1.8% per year in both Mid and Old (p =0.02 for the groups combined; p = 0.02 Young vs Mid, Young vs Old). Similarly, total grey matter volume was lost at approximately five percent per year in Young, approximately 2.1% in Mid and 1.7% in Old (p=0.02 across groups, p=0.01 Young vs Old; p=0.04 Young vs Mid).
We evaluated rates of thinning across the cortical parcellations and found significant differences between in Young versus Old in R precentral (p=0.053), R posterior frontal (pars opercularis) (p =0.026), and a trend in R inferior parietal (p=0.052), L cuneus (p=0.053) and right caudal middle frontal (p=0.059). There were significant differences between Young and Mid in L posterior cingulate (p=0.031) and a trend toward significance in portions of L anterior cingulate (p=0.057). Table 2 outlines the rates for several cortical parcellations, for the entire sample as well as for the distinct groups. In most instances, the rates of thinning were faster in Young.
Table 2.
Rates of Thinning: Select Cortical Parcellation
| Parcellation | Overall Rate | Young | Mid | Old |
|---|---|---|---|---|
| L / R superior frontal | 1.6% / 2.6% | 1.4%/0.6% | 2.3%/ 3.8% | 1.0%/ 2.5% |
| L / R Anterior middle frontal | 2.0%/ 5.2% | 0.4% / 4.2% | 3.9%/7.0% | 0.9% / 3.7% |
| L / R Posterior Middle Frontal | 3.1% / 5.5 % | 2.9% / 8.9% | 4.7% / 6.1% | 1.4% / 2.8% |
| L / R Posterior Inferior Frontal* | 4.5% / 3.4% | 6.3% / 5.7% | 5.0% / 4.7% | 2.9% / 4.6% |
| L / R Anterior Cingulate | 1.1%/ 3.7% | 1.9% / 3.6% | 1.6% / 7.5% | 0.3% / 0.5% |
| L / R Precentral* | 4.0% / 5.0% | 5.5% / 9.0% | 4.2% / 3.0% | 3.1%/ 4% |
| L / R Postcentral | 1.7% / 3.7% | 0.4% / 7.2% | 1.5% / 1.5% | 2.8% / 4.0% |
| L / R supramarginal | 3.0%/ 4.9% | 2.3%/ 8.1% | 2.7%/ 4.7% | 3.6%/ 3.1% |
| L / R superior parietal | 1.7% /3.7% | 3.5% /7.5% | 1.4%/2.8% | 0.9%/ 2.3% |
| L / R paracentral | 1.2% / 2.2% | 0.5% / 0.2% | 2.3% / 3.4% | 0.5% / 2.2% |
| L / R Inferior Parietal* | 2.8% / 5.3% | 6.8% / 1.1% | 2.3% / 4.2% | 0.9% / 3.3% |
| L /R Precuneus* | 2.5% / 4.0% | 5.7% / 5.4% | 2.5% / 5.0% | 0.7% / 2.0% |
| L / R Isthmus Cingulate | 3.5% / 5.7% | 8.6% / 1.0% | 1.1% / 5.4% | 3.0% / 4.7% |
| L / R superior temporal | 2.6% / 4.0% | 1.8% / 5.2% | 3.6%/ 2.8% | 1.9% / 4.7% |
| L / R Middle Temporal | 3.0% / 4.0% | 2.7% / 6.8% | 3.3% / 3.5% | 2.7% / 3.0% |
| L / R Inferior Temporal | 3.1%/ 5.4% | 5.0% / 6.0% | 4.0% / 7.2% | 1.2% / 3.0% |
| L / R Parahippocampal | 2.6%/ 4.6% | 2.7% / 5.3% | 4.3% / 7.0% | 0.4% / 1.4% |
| L / R Cuneus* | 1.4% / 1.9% | 3.2% / 1.0% | 0.7% / 3.5% | 1.0% / 1.8% |
| L / R pericalcarine | 1.6% / 0.4% | 0.3% / 0.6% | 1.7%/ 0.1% | 2.5% / 0.1 % |
| L / R Lingual | 2.2% / 2.4% | 0.3% / 0.9% | 2.7% / 3.2% | 3.2% / 2.3% |
| L / R Fusiform | 3.2% / 4.8% | 4.3% / 5.0% | 4.0% / 5.3% | 2.0% / 4.2% |
| L / R Lateral Occipital | 2.1% / 2.6% | 2.8% / 3.0% | 1.8% / 2.2% | 1.8% / 2.7% |
Cortical parcellations in which the rate of thinning was significantly faster in the younger patients, p<0.05, uncorrected.
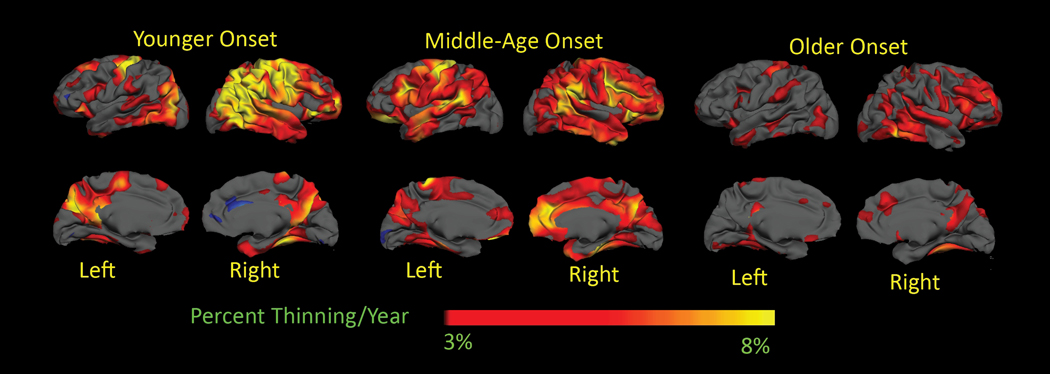
We also evaluated more generated surface-based maps on a voxel by voxel basis, shown in Figure 1. In Young, the rate of thinning approximated 8% per year in sensori-motor (pre- and post-central, corresponding with BA 4, 3,2,1 p<0.05), inferior parietal (corresponding with BA 5), and precuneus, bilaterally, superior and middle frontal, superior parietal and middle frontal on the right hemisphere, and approximated 5% per year over right superior, middle and inferior temporal right hemisphere regions. The rate of thinning in Mid followed the same general distribution as Young, but was less, between 3% and 5% yearly, over those regions. In contrast, the rate of thinning in Old approached 3% only in superior temporal, lingual gyrus, portions of the superior and inferior frontal right hemisphere and over right precuneus. When thresholds were reduced, rates of cortical thinning in Mid were generally greater than 1.5% per year throughout most cortical regions restricted to sensori-motor, portions of superior, middle and inferior temporal regions, superior and inferior frontal, portions of inferior parietal, and parahippocampal gyrus, in Old. In comparison, rates of thinning in healthy older adults have been reported as approximately 0.5% per year 15.
Figure 1.
Surface –based maps of the rate of cortical thinning. A. Young B. Middle Age C. Old. Younger patients have a much more rapid rate of thinning, especially in sensori-motor and parietal cortical regions. The general distribution of thinning was similar, however, in the young and middle age groups. In older patients, the rate of thinning was much slower than either group. Maps are presented on a semi-inflated cortical surface of an average brain. The color scale at the bottome represents the yearly rate of thinning, transitioning from red (3% or greater) to yellow (8% or greater).
The rate of decline in the TFC in Young was on the average of 1.7% per year; in contrast, the change in TFC was closer to 0.8% per year in the other two groups. While the differences were not significant (p=0.13), a test for linear trend approached significance (p=0.06).
Corpus Callosum
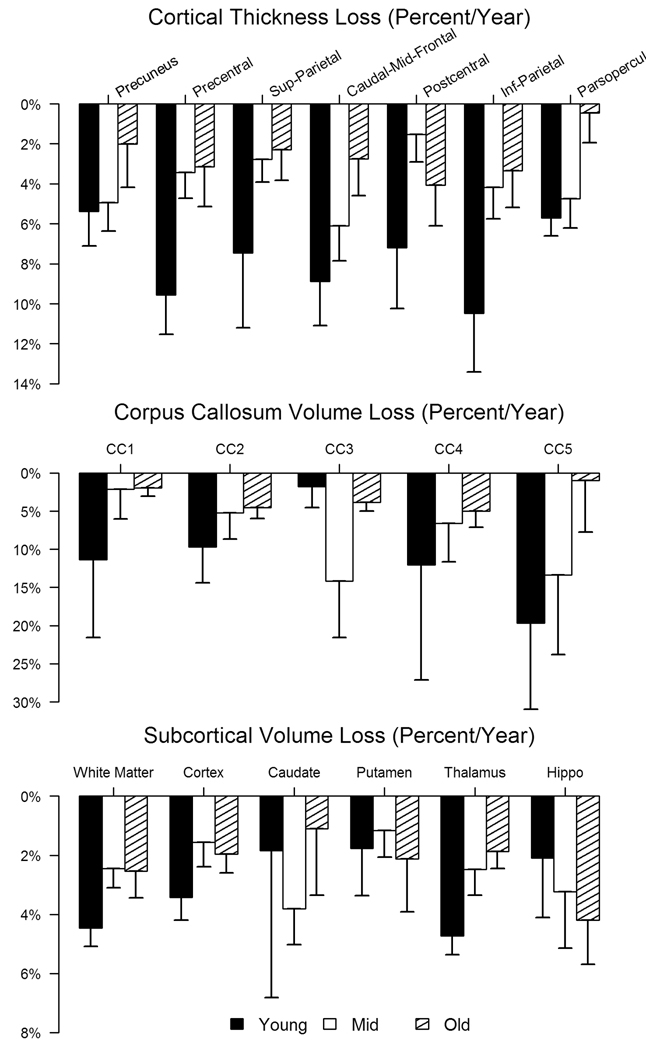
We evaluated rates of thinning of the corpus callosum (CC). With the exception of the mid-portion of the CC, the rate of thinning was in general faster in Young, across all other regions of the CC, with differences reaching significance in the most posterior segment (CC5, p=0.037) and a trend in the most anterior segment (CC1, p=0.063). Results are shown in Figure 2.
Figure 2.
Rate of change of thickness of select parcellations. Results from the cortical parcellations recapitulate the heterogeneity in the rate and topological distribution of progressive cortical thinning in the three groups.
Subcortical Structures
Also shown in Figure 2 are rates of volume change of subcortical structures in the three groups. There was no significant difference in the rate of atrophy in any basal ganglia structure. In contrast to what was expected, the rate of caudate atrophy was slower in the Young as compared to Old, suggesting a possible floor effect. Consistent with this, baseline volumes for Young were significantly smaller (Table 3), adjusted caudate volumes in Young were, on the average, 40% smaller than those of either the Mid or Old; putamen and pallidum volumes were approximately 25% and 20% smaller, respectively.
Table 3.
Baseline Subcortical Volumes: proportion of ICV
| LCaud | RCau | L Put | R Put | L Pall | R Pall | LHipp | RHipp | Lamyd | Ramyd | |
|---|---|---|---|---|---|---|---|---|---|---|
| Young | 0.09* | 0.10* | 0.19* | 0.18* | 0.061* | 0.060* | 0.21 | 0.21 | 0.06 | 0.078 |
| Middle | 0.14 | 0.15 | 0.26 | 0.24 | 0.077 | 0.066 | 0.25 | 0.25 | 0.08 | 0.09 |
| Old | 0.15 | 0.16 | 0.24 | 0.22 | 0.077 | 0.070 | 0.21 | 0.21 | 0.07 | 0.08 |
ICV adjusted volumes for the caudate, putamen and pallidum were significantly smaller in the younger onset group, as compared to the Middle and old groups.
p< 0.05, uncorrected.
The effect of the expanded CAG Repeat Length and Rate of progression
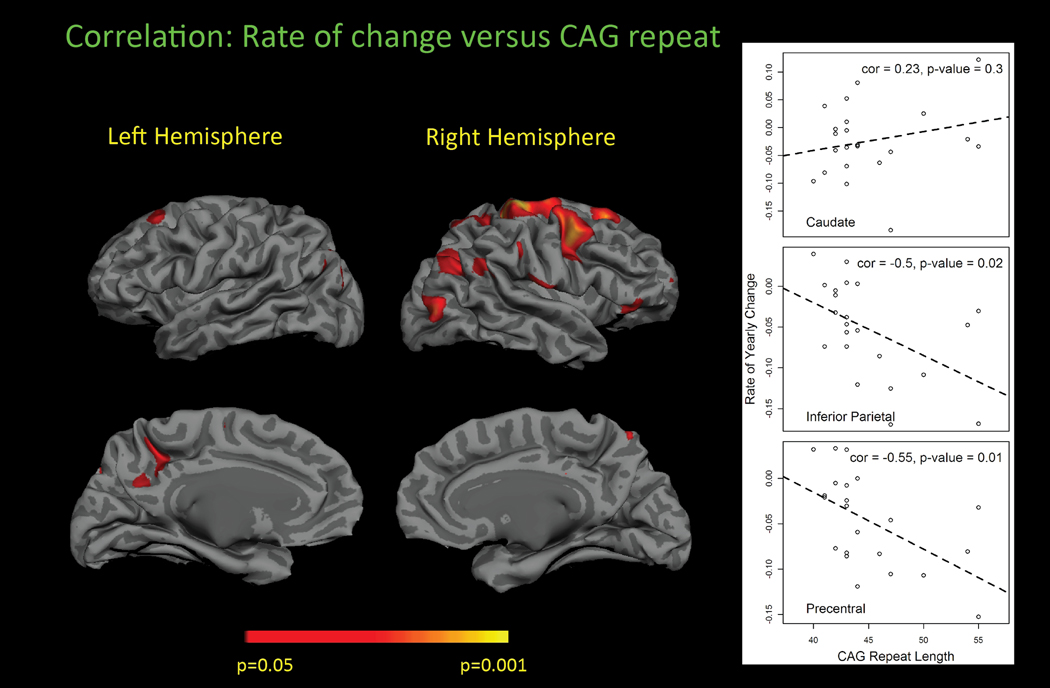
Surface based maps suggested a correlation between CAGn and regional thinning in restricted cortical regions. In the cortical parcellations, we found significant correlations with CAGn and R precentral (p=0.009), R caudal middle frontal (p=0.017), R inferior parietal (p=0.018), L precuneus (p=0.028), L pars triangularis (p=0.043), and R post central (p=0.043). On average, for those areas that were correlated, each unit increase in CAGn was associated with a 0.3% faster rate of thinning. The surface based correlations are displayed in Figure 3. We found no significant relationship between the expanded CAGn and rate of volume loss for any subcortical structure, including caudate, putamen, and thalamus. In contrast, we found a very significant relationship between cross-sectional caudate volumes at baseline and CAGn (p=0.0002).
Figure 3.
Relationship between cortical thinning and CAG repeat. A. Surface based maps. B. Scatterplots showing the relationship of the caudate and examples of the cortex. Higher CAG repeats were associated with faster rates in distinct cortical regions, but not globally. This suggests that factors, other than the CAG repeat length, may be important in the variable rate of progression.
Discussion
This is the first study to systematically evaluate variable rates of progression in HD using structural neuro-imaging. The most striking finding was that both the rate of progression and the topological distribution of cortical thinning were highly influenced by the age of onset as defined clinically, rather than by the length of the CAG repeat. The rates of change for more than two-thirds of cortical regions demonstrated more rapid thinning in Young than in either Mid or Old.
In Young, we found regional rates of cortical thinning greater than twice those of either Mid or Old in several regions. This was true for sensori-motor cortex, portions of interior frontal, superior parietal, inferior parietal and the cuneus, where the rates of thinning were greater than eight percent. However, even within Young, the rate of cortical thinning was highly heterogenous, with much slower rates of thinning in superior temporal, peri-calcarine, and lingual regions. In contrast, the rates of cortical thinning were considerably slower in Old, where the most rapid changes were on the order of at most, three percent per year, primarily in portions of precentral, superior, middle and inferior temporal and the precuneus; thinning of most other brain regions was much slower. Mid, in general, demonstrated rates of thinning intermediate between Young and Old. It is noteworthy that this pattern was recapitulated in the corpus callosum, the major conduit for information transfer between the cortical hemispheres11. Total white matter and gray matter volume reductions were also significantly more rapid in Young. Reductions in whole brain volumes, while not significant, were also more rapid in Young subjects than in either Mid or Old subjects. The general implication is that phenotypic variability in Huntington’s disease is, indeed, complex16 and extends to include highly variable rates of progression.
While the numbers of subjects studied was relatively small, the findings in the cortex were recapitulated in the more rapid volume loss in whole brain volumes and in the corpus callosum in Young; rates in Old were significantly slower.
In contrast to cortex, and perhaps surprisingly, the rates of change in subcortical structures were not significantly different amongst the groups. Atrophy rates for the basal ganglia structures were consistent with what has been published previously17. Of note, the initial volumes measured in Young were significantly smaller than those of the other two groups, suggesting a potential “floor” effect. These findings also independently support an important role of the cortex in clinical progression.
Relationship to CAG repeat length
CAG repeat length appeared to have some influence in the rates of progressive thinning, but in only fewer than eleven percent of cortical regions was the correlation significant. In these regions, each increase in repeat length was associated with a 0.1% faster rate of change; a similar association has been reported with the TFC 2, 18. CAG repeats were, as expected, larger in Young as compared to Old, but there was considerable overlap with Mid. There was no significant difference in CAGn between Mid and Old; in spite of this, there was still a difference in the rates of thinning for many cortical regions, suggesting that cortical thinning is not strongly dependent on the CAGn. It is also important to note that patients with repeat lengths in the ranges that have been associated with juvenile onset, typically larger than 55, were not included in these analyses, as juvenile HD is difficult to equate clinically with adult HD. We suspect that rates of progression in juvenile HD are even faster than our Young group.
We did not find any relationship between CAGn and the rate of volume loss in any subcortical structure, but did find a relationship between the cross-sectional volume at the baseline scan and CAGn, as has been reported previously19. Other studies have put into question the role of the CAGn in clinical heterogeneity 16. Our findings suggest that the CAGn does not appear to play a significant role in the rate of atrophy for any structure or region, but rather, that once the genetic influence set off the pathogenic cascade, subsequent factors dominate the pathology and influence disease progression much more than CAGn. Our findings also illustrate the importance of carefully evaluating models developed from cross-sectional data in longitudinal studies.
Relationship to Clinical Progression
It is important to note that the groups were in similar stages of disease at the time of the first assessment, thereby reducing a potential confound due to disease severity; clinical rates of progression may vary according to stage 20. Nevertheless, the change in the TFC in Young was more than double the change in either Mid or Old. Typically, the TFC has been reported to change by approximately 0.72 units yearly. In our study, Young had a mean reduction of approximately two points over one year, suggesting that Young were also clinically progressing more quickly than expected; change scores in Mid and Old, were closer to expected values. Age, which has been shown to affect cortical changes independently, did not significantly influence regional rates of progression.
Conclusion
Selective vulnerability in the brain in Huntington’s disease remains poorly understood. This selectivity appears to extend to regional cortical rates of progression. One possible explanation is differential transcriptional dysregulation, which is believed to be heterogeneous throughout the brain and may render certain regions more vulnerable21. Oxidative stress may independently contribute to vulnerability 22–24. Differentially altered metabolism and cerebral perfusion may also be important; these too have been demonstrated to differ regionally25. If neither CAG n. nor age can fully account for the variability in progression, there must be additional environmental, genetic, or epigenetic factors that modulate progression26. Much remains to be understood about clinical heterogeneity in a disorder defined by a single genetic mutation.
Our findings also have important implications for clinical trials. At present, the high clinical variability in HD necessitates enrolling hundreds of patients who must be followed for years in neuroprotective Phase III studies in which the TFC is the primary outcome measure. The TFC also has little power to provide preliminary evidence of efficacy in early phase studies so it is difficult to either systematically build preliminary data for promising therapies or to terminate them. Our study demonstrates that progression, defined structurally by MRI, is indeed highly variable. However, it also demonstrates that neuro-imaging approaches can objectively and sensitively measure the variability and thus help contain it. Thus, there is great potential for neuro-imaging to enhance our understanding of important phenotypic variability and to provide biomarkers to enhance the efficiency of clinical trials.
Caveats
Total numbers of subjects included in this study are small; findings need to be replicated. Young tended to have a longer CAGn; while not unexpected, one cannot entirely rule out the potential influence of CAGn on progression. While adjustment for multiple comparisons was not done, the consistency of trends (higher rates in Young versus Mid and in Mid versus Old) observed in a majority of regions and the similarity of results, using different analytical algorithms, give us confidence that our conclusions would be replicated and supported in larger studies.
Acknowledgements
Financial Support
Support for this research was provided in part by that National Institutes of Health, National Institute for Neurological Disorders and Stroke (R01 NS042861, NS058793, NS05792, NS052585, R21NS072652), National Institute of Nursing Research (NR010827), the National Center for Research Resources (P41-RR14075, and the NCRR BIRN Morphometric Project BIRN002, U24 -01). Additional support was RR021382), the National Institute for Biomedical Imaging and Bioengineering (R01EB006758), the National Institute on Aging (AG022381), Additional support was provided by CHDI and The Autism & Dyslexia Project funded by the Ellison Medical Foundation.
We are very grateful to the New England Center HDSA Center of Excellence and its patients who so generously contributed to this work.
Footnotes
Financial Disclosures : There are no financial disclosures or conflicts of interest for any author.
Author Roles
1) Research Project: A. Conception, B. Organization, C. Execution
2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique
3) Manuscript: A. Writing of the first draft, B. Review and Critique H. Diana Rosas -1A.1B.1C.2C.3A.B. Martin Reuter: 1C.2B.2C.3B. Gheorghe Doros: 2A.B.C.3A. Stephanie Y. Lee; 1A.1B.3C. Tyler Triggs:1B.C.3B Keith Malarick: 1B.3B Bruce Fischl: 2B.3B. David H. Salat: 1C.3C. Steven M. Hersch:1B.2C.3B
All authors listed report no financial disclosures.
References
- 1.Kieburtz K, MacDonald M, Shih C, et al. Trinucleotide repeat length and progression of illness in Huntington's disease. J Med Genet. 1994;31(11):872–874. doi: 10.1136/jmg.31.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravina B, Romer M, Constantinescu R, et al. The relationship between CAG repeat length and clinical progression in Huntington's disease. Mov Disord. 2008;23(9):1223–1227. doi: 10.1002/mds.21988. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt A, Liang KY, Zhou H, et al. The association of CAG repeat length with clinical progression in Huntington disease. Neurology. 2006;66(7):1016–1020. doi: 10.1212/01.wnl.0000204230.16619.d9. [DOI] [PubMed] [Google Scholar]
- 4.Group HS. Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 5.Rosas HD, Salat DH, Lee SY, et al. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 7.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 9.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 11.Rosas HD, Lee SY, Bender AC, et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection". Neuroimage. 49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Berry DA, Ayers GD. Symmetrized Percent Change for Treatment Comparisons. The American Statistician. 2006;60:27–31. [Google Scholar]
- 14.Team RDC. R: A language and environment for statistical computing. 2010 In. [Google Scholar]
- 15.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thu DC, Oorschot DE, Tippett LJ, et al. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 133(Pt 4):1094–1110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- 17.Aylward EH, Li Q, Stine OC, et al. Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology. 1997;48(2):394–399. doi: 10.1212/wnl.48.2.394. [DOI] [PubMed] [Google Scholar]
- 18.Henley SM, Wild EJ, Hobbs NZ, et al. Relationship between CAG repeat length and brain volume in premanifest and early Huntington's disease. J Neurol. 2009;256(2):203–212. doi: 10.1007/s00415-009-0052-x. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington's disease: correlations with CAG repeat numbers. Neurology. 1998;50(5):1357–1365. doi: 10.1212/wnl.50.5.1357. [DOI] [PubMed] [Google Scholar]
- 20.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington's disease. Huntington Study Group. Neurology. 2000;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 21.Cha JH. Transcriptional signatures in Huntington's disease. Prog Neurobiol. 2007;83(4):228–248. doi: 10.1016/j.pneurobio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polidori MC, Mecocci P, Browne SE, Senin U, Beal MF. Oxidative damage to mitochondrial DNA in Huntington's disease parietal cortex. Neurosci Lett. 1999;272(1):53–56. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- 23.Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447(7143):447–452. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton TM, Graham BH, Corral-Debrinski M, et al. Marked increase in mitochondrial DNA deletion levels in the cerebral cortex of Huntington's disease patients. Neurology. 1995;45(10):1879–1883. doi: 10.1212/wnl.45.10.1879. [DOI] [PubMed] [Google Scholar]
- 25.Feigin A, Leenders KL, Moeller JR, et al. Metabolic network abnormalities in early Huntington's disease: an [(18)F]FDG PET study. J Nucl Med. 2001;42(11):1591–1595. [PubMed] [Google Scholar]
- 26.van Dellen A, Hannan AJ. Genetic and environmental factors in the pathogenesis of Huntington's disease. Neurogenetics. 2004;5(1):9–17. doi: 10.1007/s10048-003-0169-5. [DOI] [PubMed] [Google Scholar]