Abstract
Δ9-Tetrahydrocannabinol (THC) discrimination in rodents is a behavioral assay that has been used to probe differences among classes of cannabinoids in rats. The purpose of this study was to determine whether traditional and anandamide-like cannabinoids were distinguishable in cannabinoid discrimination procedures in mice. Male mice were trained to discriminate 30 mg/kg THC or 70 mg/kg methanandamide from vehicle in a two-lever milk-reinforced drug discrimination procedure. After acquisition, agonist tests with THC, methanandamide, CP55,940, and anandamide were conducted, as were antagonism tests with rimonabant. Substitution (agonism) and antagonism tests were also performed in female mice trained to discriminate THC. THC and CP55,940 fully substituted in THC-trained mice of both sexes. Further, THC substitution was rimonabant reversible. In contrast, mice injected with methanandamide or anandamide failed to respond substantially on the THC lever, even up to doses that decreased overall responding. In methanandamide-trained mice, methanandamide fully generalized to the methanandamide training dose. Rimonabant did not reverse this generalization. Although THC, CP55,940 and anandamide also increased responding on the methanandamide lever, the magnitude of substitution was less than for methanandamide. These results suggest incomplete overlap in the underlying mechanisms mediating endocannabinoid pharmacology and marijuana intoxication. Further, they suggest that methanandamide discrimination may involve a non-CB1 receptor mechanism that is particularly prominent at higher doses.
Keywords: anandamide, cannabinoids, discriminative stimulus, methanandamide, mouse, rimonabant, sex differences, Δ9-tetrahydrocannabinol
Introduction
Plant-derived cannabinoids, including Δ9-tetrahydrocannabinol (THC), the principal psychoactive constituent of marijuana, produce their psychoactive effects primarily through activation of cannabinoid CB1 receptors in the brain (Compton et al., 1996). Physiologically, these receptors are components of an endocannabinoid system that is activated by endogenous lipid ligands, with anandamide being the best characterized example. While THC and exogenously administered anandamide produce similar effects in many pharmacological assays (Fride and Mechoulam, 1993; Smith et al., 1994), several lines of research suggest that differences also exist. For example, anandamide and its analogs are only half as efficacious as THC at producing hypothermia (Smith et al., 1994; Ryan et al., 1997). Further, correlations between CB1 receptor affinities of anandamide analogs and their in vivo potencies are not as strong as for traditional cannabinoids (Adams et al., 1995a,b). In mice, the cannabimimetic effects of anandamide itself (i.e., decreased locomotion, hypothermia, antinociception, and catalepsy) were not reversed by the CB1 antagonist, rimonabant, although it did block those of a more stable anandamide analog, 2-methyl-2'-fluoroethylanandamide (Adams et al., 1998). Differences in the mechanism through which anandamide produces spinal antinociception in mice also have been noted (Smith et al., 1994; Welch et al., 1998; Welch and Eads, 1999; Houser et al., 2000). Finally, CB1 knockout and wildtype mice showed similar sensitivity to the pharmacological effects of anandamide in contrast with the decreased sensitivity of CB1 knockout mice to THC's effects (Di Marzo et al., 2000).
Determination of the mechanisms responsible for these differences has been complicated by challenges in working with anandamide. Under physiological conditions, anandamide is synthesized on demand and then rapidly metabolized by fatty acid amide hydrolase (FAAH; Cravatt et al., 1996). Exogenously administered anandamide also undergoes rapid FAAH-induced degradation and inactivation, which contrasts with the comparably slow oxidative metabolism of THC by cytochrome P450 enzymes to active and inactive metabolites (Klausner and Dingell, 1971). To evaluate the pharmacological effects of anandamide, various strategies to compensate for anandamide’s quick metabolism have been employed, including co-administration of compounds that inhibit FAAH, such as URB-597 (Mor et al., 2004) and PF-3845 (Ahn et al., 2009), investigation of anandamide effects in genetically modified mice lacking FAAH (Cravatt et al., 2001), and examination of the effects of metabolically stable analogs of anandamide (Adams et al., 1995b).
Two of these metabolically stable anandamide analogs, R-(+)-methanandamide [(R)-(+)-arachidonyl-1’-hydroxy-2’-propylamide] and O-1812 [(R)-(20-cyano-16,16-dimethyl docosa-cis-5,8,11,14-tetraenoyl)-1'-hydroxy-2'-propylamine], have been used in several previous studies to compare the discriminative stimulus effects of THC and anandamide-like cannabinoids in rats (Burkey and Nation, 1997; Järbe et al., 2001; Wiley et al., 2004). THC discrimination is a highly selective and pharmacologically specific assay (for review, see Wiley, 1999) and has been proposed as an animal model of marijuana intoxication in humans (Balster and Prescott, 1992). Although the majority of these studies have been conducted in rats and non-human primates, recent studies have established THC discrimination in mice (McMahon et al., 2008; Vann et al., 2009). The primary goals of the present study were to establish discrimination of a methylated anandamide analog, methanandamide [2-methylarachidonyl-(2'-hydroxyethyl)amide], in mice and to compare substitution patterns to those obtained in mice trained to discriminate THC from vehicle. Among the advantages of developing a mouse model of discrimination is that it is a first step towards utilization of genetically modified mice as an additional tool for mechanistic investigation in this area. Given the scarce supply of some knockout mice, their use in this type of procedure may require use of available mice without regard to sex. Hence, a secondary goal of this study was to compare THC discrimination in male and female mice.
Methods
Subjects
Experimentally naive male and female C57BL/6 mice (20–25g), bred at Virginia Commonwealth University, were housed individually in clear plastic cages (18 × 29 × 13 cm) with steel wire fitted tops and wood-chip bedding. Mice were kept in a light- (12-h light:dark cycle; lights on at 06.00h) and temperature- (20–22°C) controlled vivarium, except during experimental sessions. Mice were maintained at 85% of free-feeding body weight during the initial phase of the study. When stable rates of responding were established upon both levers, mice were allowed to gain weight gradually as discrimination training progressed, culminating in free feed status if responding remained at greater than 80% correct on the appropriate levers. Water was freely available in the home cage. Animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Virginia Commonwealth University and the ‘Guidelines For The Care And Use Of Mammals In Neuroscience And Behavioral Research’ (National Research Council, 2003).
Apparatus
Six computer-interfaced operant chambers (Med-Associates, St. Albans, VT), housed within light- and sound-attenuating cubicles, were used for behavioral training and testing. Each inner chamber contained two response levers (8 cm apart). A recessed well centered between the two levers contained a liquid dipper that delivered 0.02 ml of sweetened-condensed milk (by volume: one part condensed milk, one part sugar, and two parts water) as reinforcement. A house light was centered on the rear wall opposite the levers and was illuminated during experimental sessions.
Procedure
Training in the discrimination procedure was similar to that described previously (Vann et al., 2009). Briefly, each mouse was placed in a standard two-lever operant conditioning chamber and trained to press one lever following administration of THC or methanandamide and to press the opposite lever following vehicle injection according to a fixed ratio 10 (FR10) schedule of milk reinforcement, under which 10 consecutive presses on the correct (drugappropriate) lever resulted in delivery of a small dipper of milk. The training dose of THC was 30 mg/kg. The training dose of methanandamide was 70 mg/kg. (Lower training doses of 56 and 65 mg/kg methanandamide were used in previous groups of mice, but failed to maintain accurate drug-appropriate responding.) Responses on the incorrect lever reset the ratio requirement on the correct lever. Daily injections were administered on a double alternation sequence of THC or methanandamide and vehicle (e.g., drug, drug, vehicle, vehicle). Daily 15 min training sessions were held Monday-Friday until the mice had met two criteria during 8 of 10 consecutive sessions: (1) the first completed FR10 was on the correct lever and (2) ≥ 80% of the total responding occurred on the correct lever. When the two criteria were met, acquisition of the discrimination was established and substitution testing began. Male mice were trained in both discriminations. Female mice were only trained in the THC discrimination procedure.
For all mice that successfully acquired the discrimination, stimulus substitution tests were conducted on Tuesdays and Fridays during 15 min test sessions, with maintenance of training continuing on intervening days. During test sessions, responses on either lever delivered reinforcement according to an FR10 schedule. To be tested, mice must have completed the first FR10 on the correct lever and ≥ 80% of the total responding must have occurred on the correct lever during the prior day’s training session. In addition, the mouse must have met these same criteria during previous training sessions with the alternate training compound (training drug or vehicle). If these criteria were not met, the mouse received a regular training session and was not tested. A dose-effect determination with the training drugs, THC or methanandamide, was conducted in both groups of male mice. THC and methanandamide dose-effect curves were also determined in the group of female mice. After these tests, substitution tests were conducted with CP 55,940, anandamide, and PCP in the male mice only. Mice were injected with rimonabant 10 min before administration of either training drug or vehicle. Prior to conducting each dose-effect curve, control tests were determined with vehicle and the training drug (i.e., 30 mg/kg THC or 70 mg/kg methanandamide).
Drugs
THC [National Institute on Drug Abuse (NIDA), Rockville, MD], methanandamide [2-methylarachidonyl-(2'-hydroxyethyl)amide; O-680; Organix, Inc., Woburn, MA], anandamide (Organix), rimonabant (Pfizer Inc., Groton, CT) and CP55,940 (Pfizer) were mixed in a vehicle of ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ) and saline in a 1:1:18 ratio. Phencyclidine HCl (PCP; NIDA) was dissolved in 0.9% saline. Doses of each drug were administered in ascending order via i.p. injections at a volume of 10 ml/kg. THC and CP 55,940 were administered 30 min prior to the test session. Methanandamide, anandamide, and PCP were administered 10 min prior to test sessions. Rimonabant was injected 10 min before administration of vehicle or the training drug (THC or methanandamide). Pre-session injection intervals were based upon our extensive prior work with these cannabinoids (Wiley et al., 1995a,b, 1997). Methanandamide, used as a training drug in this manuscript and in previous studies in our lab (Wiley et al., 1998; Vann et al., 2009), is structurally distinct from R-(+)-methanandamide that has been used in previous studies in other labs (Järbe et al., 2001; McMahon et al., 2008), in that the methyl substituent for methanandamide is contained within the arachidonoyl portion of the anandamide template (Adams et al., 1995b) whereas the methyl group of R-(+)-methanandamide is located on the ethanolamide part of the anandamide molecule (Abadji et al., 1994).
Data Analysis
For each operant session, the percentage of responses on the drug lever and response rate (responses/sec) were calculated. When appropriate, effective dose 50 (ED50) values were calculated for each drug using least squares linear regression analysis, followed by calculation of 95% confidence intervals (CI). Since mice that responded less than 10 times during a test session did not press either lever a sufficient number of times to earn a reinforcer, their data were excluded from analysis of drug lever selection, but response rate data were included. Response-rate data were analyzed using a repeated-measures analysis of variance (ANOVA). Significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means. Sex differences in mean number of sessions to acquisition of the THC discrimination were evaluated through use of a two-tailed t-test (α = 0.05).
Results
Male mice (n=7) acquired the 30 mg/kg THC vs. vehicle discrimination in a mean of 66 sessions (range: 11–140). Female mice (n=4) acquired the 30 mg/kg THC vs. vehicle discrimination in a mean of 45 sessions (range: 29–60). The mean number of sessions to acquisition of the THC discrimination was not significantly different between the sexes. Mice (all males, n=8) acquired the final 70 mg/kg methanandamide vs. vehicle discrimination in a mean of 42 sessions (range: 13–100).
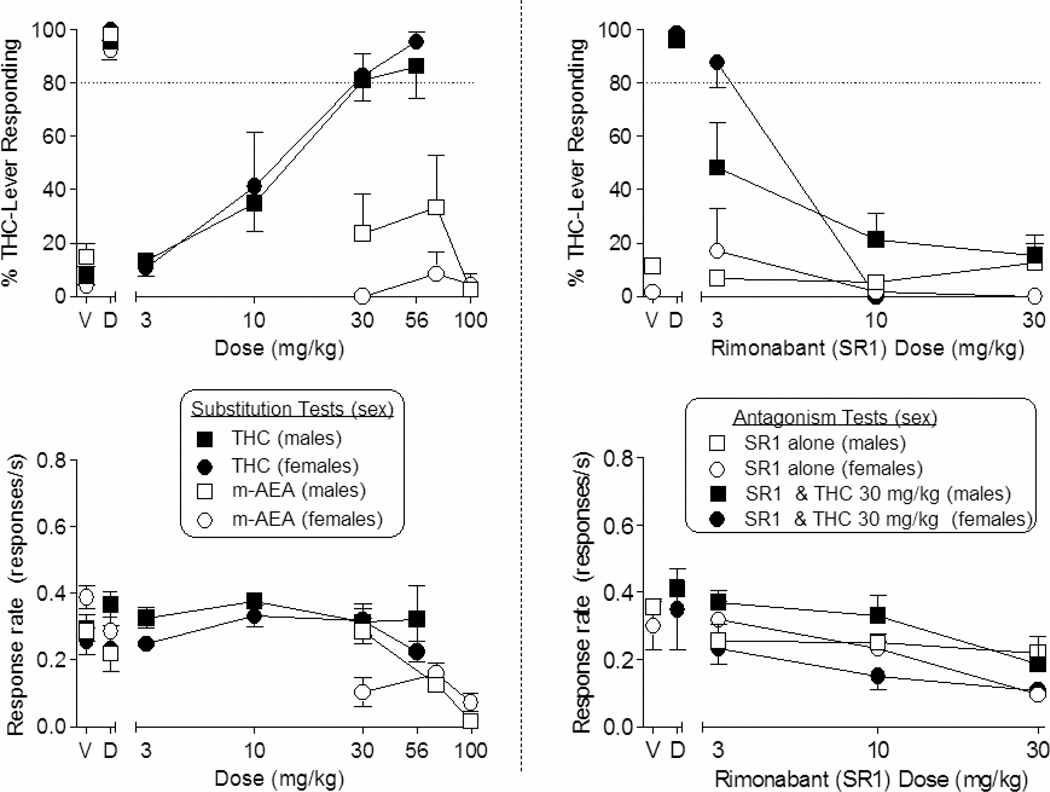
Figure 1 (left panels) shows the effects of THC and methanandamide substitution tests in male and female mice trained to discriminate 30 mg/kg THC from vehicle. Dose-dependent generalization was observed with THC at similar potency in both sexes, with ED50 values of 12 mg/kg (95% CI: 7 – 21 mg/kg) for males and 12 mg/kg (95% CI: 8–17 mg/kg) for females. In contrast, methanandamide failed to generalize to THC in either sex. When administered alone, rimonabant also did not generalize to THC (Fig. 1, right panels); however, it dose-dependently antagonized generalization by 30 mg/kg THC in both sexes, with attenuation occurring at a lower dose in males than in females (3 vs 10 mg/kg, respectively).
Fig. 1.
Effects of THC (filled symbols) and methanandamide (open symbols) on % THC-lever responding (top left panel) and response rate (bottom left panel) in male (squares) and female (circles) mice trained to discriminate 30 mg/kg THC vs. vehicle. Right panels show the effects of rimonabant alone (unfilled symbols, both panels) and with the training dose of 30 mg/kg THC (filled symbols, left panels) in mice trained to discriminate THC from vehicle. Points above V and D represent the results of control tests with vehicle and the training drug, respectively. Values represent the mean (±SEM) of data from 5–7 male mice or 4 female mice, with the exceptions that n=2 male mice for % drug-lever responding at 100 mg/kg methanandamide and n=2 female mice for both dependent measures at 10 and 30 mg/kg rimonabant. *p < 0.05 compared to mean rates of responding during the control test with vehicle (V).
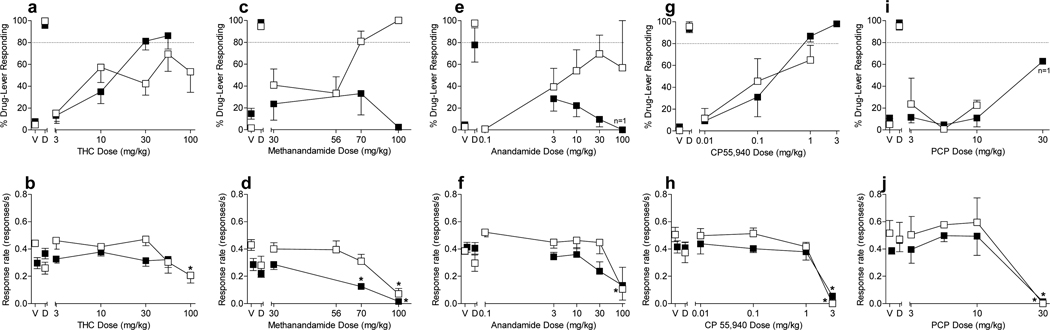
Figure 2 shows the effects of substitution tests with THC, methanandamide, CP55,940 and PCP on drug-lever responding and response rates in male mice trained to discriminate 30 mg/kg THC or 70 mg/kg methanandamide. THC fully and dose-dependently generalized to the 30 mg/kg training dose in the THC-trained mice (Fig. 2, panel a), yielding an ED50 of 12 mg/kg (95% CI: 7 – 21 mg/kg). Response rates were not significantly decreased at doses up to 56 mg/kg (Fig. 2, panel b). In mice trained to discriminate methanandamide, the maximum mean percentage responding on the drug lever after THC injection was 69% at 56 mg/kg THC, with 4 of the 7 mice exhibiting ≥ 80% responding on the methanandamide lever at this dose (Fig. 2, panel a). Because responding on the THC-lever exceeded 50%, an ED50 was calculated in the methanandamide-trained group (27 mg/kg with 95% CI: 8 – 85 mg/kg) for the purpose of comparison to the value obtained in THC-trained mice. Hence, THC was approximately 2-fold more potent in producing discriminative stimulus effects in THC-trained mice versus methanandamide-trained mice, with the caveat that efficacies and dose-effect curve slopes were not equal between the two groups. Relative to vehicle, response rates were significantly decreased by 100 mg/kg THC in methanandamide-trained mice (Fig. 2, panel b).
Fig. 2.
Effects of THC (panels a and b), methanandamide (panels c and d), anandamide (panels e and f), CP 55,940 (panels g and h), and PCP (panels i and j) on % drug-lever responding (top panels) and response rate (bottom panels) in mice trained to discriminate either 30 mg/kg THC (filled symbols) or 70 mg/kg methanandamide (unfilled symbols) vs. vehicle. Points above V and D represent the results of control tests with vehicle and the training drug, respectively. Values represent the mean (±SEM) of data from 6–7 THC-trained mice for substitution tests with THC, methanandamide, and anandamide, with the exceptions that n=2 mice for % drug-lever responding at 100 mg/kg methanandamide and n=1 for this measure and n=3 for response rate at 100 mg/kg anandamide. For CP55,940 and PCP substitution tests in THC-trained mice, n=4–5 mice, except n=1 for % drug-lever responding at 30 mg/kg PCP. N=6–8 methanandamide-trained mice tested with methanandamide and THC except for n=4 for % drug-lever responding at 100 mg/kg methanandamide; n = 4–6 methanandamide-trained mice tested with anandamide except for n=2 for % drug-lever responding at 100 mg/kg anandamide; and n=2–3 methanandamide-trained mice tested with CP55,940 and PCP. *p < 0.05 compared to mean rates of responding during the control test with vehicle (V).
In contrast to the effects of THC in THC-trained mice, methanandamide failed to generalize to THC in THC-trained mice (Fig. 2, panel c), even up to doses that significantly reduced overall response rates (Fig. 2, panel d). In the methanandamide-trained mice, however, methanandamide dose-dependently generalized to the training dose, with an ED50 of 45 mg/kg (95% CI: 33 – 62 mg/kg) [Fig. 2, panel c]. Compared to vehicle response rates, only the 100 mg/kg methanandamide dose produced significant decreases in rates of responding (Fig. 2, panel d). Hence, generalization occurred at doses that did not affect response rates.
Anandamide and CP 55,940 produced different profiles in THC-trained mice (Fig. 2). Whereas anandamide failed to generalize to THC (Fig. 2, panel e), CP 55,940 produced dose-dependent generalization (Fig. 2, panel g), with an ED50 of 0.15 mg/kg (95% CI: 0.08 – 0.31 mg/kg). In contrast, anandamide and CP 55,940 produced similar patterns of responding in the methanandamide-trained mice (Fig. 2, panels e and g, respectively). Each drug dose-dependently increased responding on the methanandamide-associated lever up to a maximum of just under 80% at 30 mg/kg (anandamide) and 1 mg/kg (CP 55,940). Associated ED50 values in the methanandamide-trained mice were 7 mg/kg (95% CI: 2 – 28 mg/kg) and 0.2 mg/kg (95% CI: 0.05 – 1.1 mg/kg) for anandamide and CP55,940, respectively. Hence, CP55,940 was 1.3-fold more potent at producing drug-lever responding in the THC-trained mice as compared to in the methanandamide-trained mice. Higher doses of each drug did not increase responding on the methanandamide-associated lever, but rather significantly decreased overall responding on either lever (Fig. 2, panels f and h).
In contrast to the cannabinoids, PCP failed to generalize to either training drug (Fig. 2, panel i). Although PCP produced a moderate amount of THC-appropriate responding (>60%) at 30 mg/kg, only 1 animal responded at this dose. Further, the 30 mg/kg dose of PCP significantly reduced response rates in both training groups (Fig. 2, panel j).
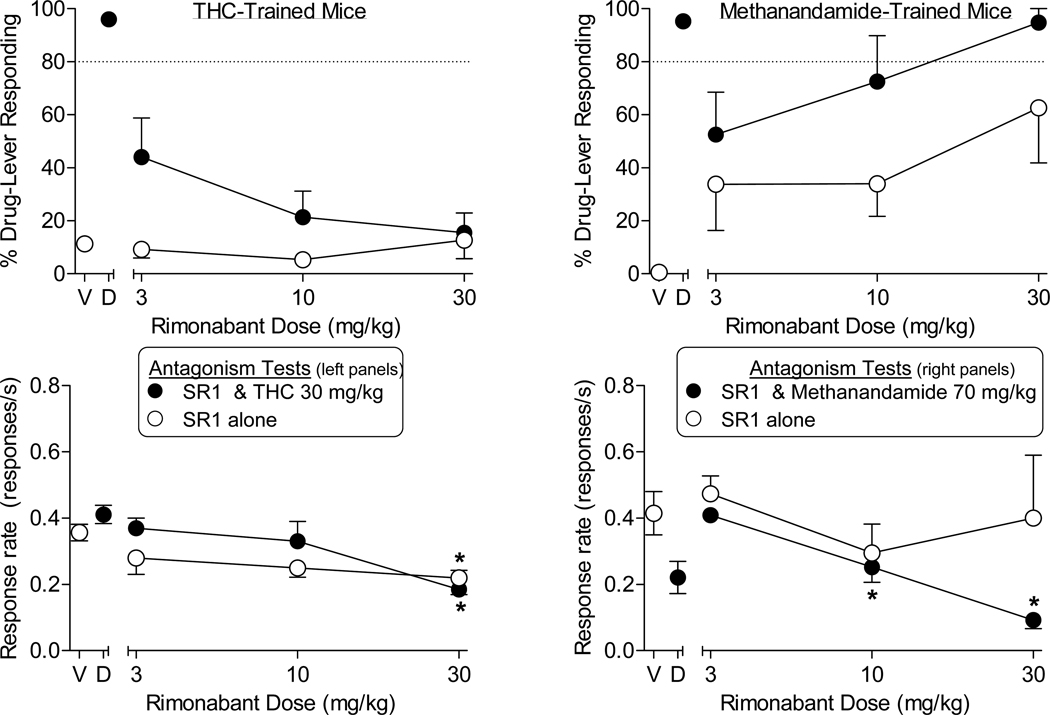
Figure 3 shows the effects of rimonabant with vehicle and in combination with the 30 mg/kg training dose of THC (Fig. 3, left panels). Mice responded predominantly on the vehicleassociated lever when administered rimonabant alone. Further, when rimonabant was injected prior to THC, it dose-dependently decreased responding on the THC-associated lever. Response rates were significantly decreased at the 30 mg/kg dose of rimonabant, regardless of whether it was administered alone or with THC.
Fig. 3.
Effects of rimonabant alone (unfilled symbols, both panels) and with the training dose of 30 mg/kg THC (filled symbols, left panels) or 70 mg/kg methanandamide (filled symbols, right panels) in mice trained to discriminate THC (left panels) or methanandamide (right panels) from vehicle. Points above V and D represent the results of control tests with vehicle and the training drug, respectively. Values represent the mean (±SEM) of data from 5–6 THC-trained mice and 4–6 methanandamide-trained mice. *p < 0.05 compared to mean rates of responding during the control test with vehicle (V).
The pattern of responding produced by rimonabant in methanandamide-trained mice differed from that seen in THC-trained mice (Fig. 3, right panels). When administered alone, rimonabant dose-dependently increased responding on the methanandamide-associated lever up to a maximum of 62% at the 30 mg/kg dose. Further, whereas 3 mg/kg rimonabant attenuated the percentage of methanandamide-lever responding after injection with the 70 mg/kg training dose of methanandamide [compared to control test with training drug (D)], higher doses of 10 and 30 mg/kg did not alter responding on the drug lever, despite significant decreases in overall response rates produced by the rimonabant / methanandamide combination.
Discussion
The discriminative stimulus effects of THC have been characterized in multiple species, including rats (Järbe and Mathis, 1992; Barrett et al., 1995), non-human primates (Gold et al., 1992; Wiley et al., 1995c), and most recently, mice (McMahon et al., 2008; Vann et al., 2009). Patterns of substitution for plant-derived (e.g., THC) and synthetic bicyclic (e.g., CP55,940) and indole-derived cannabinoids (e.g., WIN 55,212-2) are similar across these species, with potency for dose-dependent generalization in THC discrimination procedures and CB1 receptor affinity showing a strong correspondence (Compton et al., 1993). To the extent evaluated, symmetrical cross-generalization with THC has been observed in studies using CP55,940 or WIN 55,212-2 as a training drug in rats (Wiley et al., 1995b; Perio et al., 1996). The finding that THC and CP55,940 fully and dose-dependently generalized to the THC training dose in THC-trained mice in the present study is consistent with these previous reports. Further, the results here showed an absence of sex differences in ability to discriminate the cannabimimetic effects of THC, suggesting that future studies with genetically manipulated mice with low availability could feasibly use mice of both sexes. CB1 receptor mediation of the discriminative stimulus effects of THC in both sexes was indicated by rimonabant reversal, as has been shown in male rodents (Wiley et al., 1995d), but had not been previously evaluated in female rodents. Sex differences in the potency of rimonabant to reverse the discriminative stimulus effects of THC were noted.
In contrast to the generalization observed for THC and CP55,940 in the THC-trained mice, anandamide and methanandamide failed to generalize, even when tested up to doses that significantly decreased overall responding. These results are consistent with previous reports that, in the absence of FAAH inhibition, anandamide and R-(+)-methanandamide do not substitute for THC in mice trained to discriminate 10 mg/kg THC (McMahon et al., 2008; Vann et al., 2009). Substitution tests with anandamide and anandamide analogs have produced mixed results in rats trained to discriminate THC. While some, but not all, anandamide analogs have fully generalized to THC, this generalization is often accompanied by significant decreases in overall response rates (Wiley et al., 1997, 2004), suggesting differences in the discriminative stimulus effects of THC and anandamide-like compounds. This idea is further supported by findings that THC training dose affects efficacy in the discrimination procedure, with anandamide analogs being more likely to generalize to THC in rats trained with lower doses of THC. For example, in rats trained to discriminate 1.8 mg/kg THC versus vehicle, R-(+)-methanandamide generalized to the training drug, whereas R-(+)-methanandamide failed to generalize in rats discriminating 5.6 mg/kg THC versus vehicle (Järbe et al., 2000). In rats, higher training doses have been shown to be associated with greater specificity in drug discrimination procedures with cannabinoids (Järbe et al., 1998, 2000), as well as with other classes of drugs (Mansbach and Balster, 1991). Notably, the 30 mg/kg THC training dose used here is 3-fold higher than that used in previous mouse studies and 10-fold higher than the 3 mg/kg dose that we have typically used to train rats in THC discrimination (Wiley et al., 1998, 2004). Hence, maximization of the specificity of the THC discriminative stimulus would be expected in the present study. Further, demonstration of rimonabant reversal of the discriminative stimulus effects of THC confirms that they were CB1 receptor mediated. Interestingly, anandamide-like cannabinoids failed to generalize to THC in mice either in this study or when the training dose was 10 mg/kg (McMahon et al., 2008; Vann et al., 2009).
At least two possible alternative explanations for the lack of substitution by anandamide-like compounds are plausible. First, anandamide may not have generalized to THC due to its rapid metabolism (Deutsch and Chin, 1993). This hypothesis receives support from the finding that co-administration of phenylmethylsulfonyl fluoride (PMSF), a nonspecific irreversible amidase inhibitor, and anandamide produced full generalization to THC in THC-trained mice (Vann et al., 2009). Co-administration of a selective FAAH inhibitor, URB-597, and anandamide also produced full generalization to THC in THC-trained rats (Solinas et al., 2007). However, these previous results do not offer a tenable explanation for failure of methanandamide generalization in THC-trained mice in the present study, as methanandamide is not subject to rapid degradation by endogenous enzymes. A second possibility for lack of generalization is that anandamide and its analogs may interact with other receptors, including novel cannabinoid receptors and/or non-cannabinoid receptors, in a way that influences their discriminative stimulus effects. Indeed, previous research has shown that anandamide activates transient receptor potential vanilloid1 (TRPV1) receptors at concentrations 10 to 20 times higher than those at which it activates CB1 receptors (Zygmunt et al., 1999; Smart et al., 2000). This action that has been shown to mediate some of the behavioral effects of anandamide (Panlilio et al., 2009), albeit other work has shown that some TRPV1 receptor ligands do not alter the effects of anandamide or its analogs in cannabinoid discrimination (Wiley et al., 2004; Solinas et al., 2007). Anandamide also has been shown to interact with potassium and calcium channels as well as 5-HT3 receptors (for a review, see Di Marzo et al., 2002) and R-(+)-methanandamide activates GPR55, an orphan G-protein coupled receptor that may represent a novel cannabinoid receptor (Ryberg et al., 2007; Lauckner et al., 2008). Findings from previous reports offer indirect support for contribution of non-CB1 receptor mechanism(s) to the pharmacological effects of anandamide-like cannabinoids. For example, Baskfield et al. (2004) reported that methanandamide decreased operant responding to a similar extent in both CB1 wild-type and knockout mice. In addition, the finding in the present study that rimonabant co-administration dose-dependently enhanced (rather than reversed) substitution of the training dose in methanandamide-trained mice suggests that negation of any putative CB1 receptor component of the methanandamide cue may actually be faciliatory for detection of the cue. Different metabolic and mechanistic pathways are not mutually exclusive explanations and a contribution of both factors to the observed differences in discriminative stimulus effects of THC versus anandamide-like cannabinoids is possible. It is also plausible that structural variations among anandamide analogs may alter not only their CB1 receptor affinity and activity, but also their actions at other receptors (e.g., Di Marzo et al., 2001), suggesting a factor that could also contribute to variable generalization patterns of anandamide analogs across studies.
To further examine the discriminative stimulus effects of anandamide-like cannabinoids, several groups have used anandamide analogs as training drugs, including R-(+)-methanandamide (Järbe et al., 2001), O-1812 [(R)-(20-cyano-16,16-dimethyl docosa-cis-5,8,11,14-tetraenoyl)-1'-hydroxy-2'-propylamine] (Wiley et al., 2004), and AM1346 [alkoxyacid amide of N-eicosa-(5Z,8Z,11Z,14Z)-tetraenylamide] (Järbe et al., 2006a). Results have shown that THC produces reliable, full dose-dependent generalization in rats trained to discriminate each of these anandamide analogs from vehicle (Järbe et al., 2001, 2006a; Wiley et al., 2004) and, to the extent tested, cross-generalization occurs with anandamide and other anandamide-like cannabinoids (Järbe et al., 2001, 2006a). While substitution test results are generally consistent with those of previous studies in rats, in that cannabinoids that were not the training drug (i.e., methanandamide) generalized in most mice, THC and CP55,940 were less efficacious in methanandamide-trained mice than in THC-trained mice because generalization did not occur in all mice. Potencies of each of these two drugs for generalization, however, exhibited up to 2-fold differences across training drug, with THC and CP55,940 being more potent at producing THC-like (vs. methanandamide-like) discriminative stimulus effects. In addition to supporting previous research in rats, these results are consistent with our recent report that THC generalizes to anandamide in FAAH−/− mice trained to discriminate anandamide from vehicle (Walentiny et al., 2011). Notably, anandamide also generalized to methanandamide even in the absence of metabolism inhibition, albeit it was slightly less efficacious than the training drug. Anandamide generalization to (R)-(+)-methanandamide has also been previously reported in rats (Järbe et al., 2001). Together, the results here demonstrate an asymmetrical pattern of cross-generalization for traditional and anandamide-like cannabinoids in mice, in which traditional cannabinoids generalized to some extent to methanandamide in methanandamide-trained mice, but anandamide-like cannabinoids failed to generalize to THC. In addition, the demonstration that these techniques produce similar results across species (when training dose is set to allow for maximum specificity) suggests that cannabinoid discrimination will serve as a useful tool in work with mutant mice to uncover CB1 and non-CB1, non-CB2 receptor mechanisms that may underlie endocannabinoid action.
While the asymmetrical pattern of cross-generalization is undoubtedly related, in part, to the high training doses used in this study, failure of rimonabant challenge to reverse the discriminative stimulus effects of methanandamide in the methanandamide-trained mice also suggests a possible contribution of a non-CB1 receptor mechanism. Indeed, rimonabant alone (but not PCP, a negative control) increased responding on the methanandamide-associated lever at doses that did not affect response rates. Responding on the methanandamide-associated lever was enhanced by co-administration of methanandamide, albeit that the combination also substantially reduced response rates at higher (10 and 30 mg/kg) doses of rimonabant. These results are in contrast with rimonabant reversal of the discriminative stimulus effects of anandamide analogs reported in previous studies in rats (Järbe et al., 2001. 2006a, 2009; Wiley et al., 2004). Interestingly, Järbe et al. (2001) demonstrated that reversal of the discriminative stimulus effects of R-(+)-methanandamide by 1 mg/kg rimonabant was insurmountable by administration of higher doses of agonist, a result that was attributed to lack of antagonism of the agonist-induced response rate suppression and consequent elimination of responding as R-(+)-methanandamide dose was increased substantially beyond the training dose. Further, rimonabant accentuation of R-(+)-methanandamide-induced operant response rate suppression and open-field effects have been noted previously (Järbe et al., 2003a,b), suggesting the possibility that different mechanisms may be responsible for mediation of discriminative stimulus and response rate effects of anandamide analogs. In the present study, methanandamide-induced suppression of response rates occurred at doses that were only slightly higher than the training dose, suggesting that the training dose was within the dose range with more prominent non-CB1 receptor-mediated activity. Previous work has shown that TRPV1 receptor activation is involved in anandamide-induced reduction in locomotion (de Lago et al., 2004) and offers one possible non-CB1 receptor mechanism that may contribute to decreases in response rates that are not fully rimonabant reversible.
Explanation of the partial substitution of rimonabant for methanandamide is less straightforward; however, it is of note that THC, but not R-(+)-methanandamide, attenuated the discriminative stimulus effects of rimonabant in a discriminated taste avoidance procedure (Järbe et al., 2004, 2008), suggesting potential differences in interactions with the sites of action that these two cannabinoid agonists share with rimonabant (e.g., CB1 receptor). Rimonabant has also been shown to have CB1 receptor-independent actions that may have contributed to its partial substitution for methanandamide in the present study. For example, Bass et al (2002) reported that stimulation of locomotor activity by rimonabant and some of its analogs was not mediated by CB1 receptor antagonism or inverse agonism.
In conclusion, considerable research has been directed towards determination of the nature of pharmacological differences between structural classes of cannabinoids, particularly anandamide-like cannabinoids and traditional plant-derived cannabinoids such as THC. While cannabinoids in these classes share many pharmacological effects, distinct differences also are apparent. Notably, exogenously administered anandamide-like cannabinoids are less THC-like than synthetic cannabinoids such as CP55,940 and WIN55,212-2 in a variety of pharmacological assays, including drug discrimination as shown here in mice. Further, the lack of rimonabant reversal of the discriminative stimulus effects of methanandamide suggests that non-CB1 receptor mechanisms may mediate some of the variant effects of endocannabinoid analogs. These results suggest that some of the neurochemical systems affected by endogenous cannabinoids may differ from those involved in marijuana intoxication and emphasize the utility of using cannabinoid discrimination in mice to distinguish between traditional and anandamide-like cannabinoids.
Acknowledgements
Research supported by NIH/NIDA Grants DA-026449, DA-03672, and DA-09789. The authors thank Anu Mahadevan (Organix, Inc.) for providing methanandamide and anandamide, which were synthesized with support from NIH/NIDA grant DA-09789 (Project 2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Adams IB, Ryan W, Singer M, Razdan RK, Compton DR, Martin BR. Pharmacological and behavioral evaluation of alkylated anandamide analogs. Life Sci. 1995a;56:2041–2048. doi: 10.1016/0024-3205(95)00187-b. [DOI] [PubMed] [Google Scholar]
- Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, Martin BR. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J Pharmacol Exp Ther. 1995b;273:1172–1181. [PubMed] [Google Scholar]
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- Ahn K, Johnson D, Mileni M, Beidler D, Long J, McKinney M, Weerapana E, Sadgopan N, Liimatta M, Smith S, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens R, Cravatt B. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta-9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta-9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Baskfield CY, Martin BR, Wiley JL. Differential effects of delta-9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. J Pharmacol Exp Ther. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- Bass CE, Griffin G, Grier M, Mahadevan A, Razdan RK, Martin BR. SR-141716A-induced stimulation of locomotor activity. A structure-activity relationship study. Pharmacol Biochem Behav. 2002;74:31–40. doi: 10.1016/s0091-3057(02)00945-0. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta-9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta-9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lago E, de Miguel R, Lastres-Becker I, Ramos JA, Fernandez-Ruiz J. Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res. 2004;1007:152–159. doi: 10.1016/j.brainres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Brandi I, Jefferson RG, Winckler RL, Davis JB, Dasse O, Mahadevan A, Razdan RK, Martin BR. Highly selective CB(1) cannabinoid receptor ligands and novel CB(1)/VR(1) vanilloid receptor "hybrid" ligands. Biochem Biophys Res Commun. 2001;281:444–451. doi: 10.1006/bbrc.2001.4354. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essen Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmaco. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of delta-9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992;262:479–486. [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta-9-THC and anandamide. Brain Res. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Mathis DA. Dissociative and discriminative stimulus functions of cannabinoids/cannabimimetics. In: Murphy L, Bartke A, editors. Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, FL: CRC Press; 1992. pp. 425–458. [Google Scholar]
- Järbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Delta-9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe T, Lamb R, Lin S, Makriyannis A. Delta-9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behav Pharmacol. 2000;11:81–86. doi: 10.1097/00008877-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and delta-9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, DiPatrizio NV, Li C, Makriyannis A. The cannabinoid receptor antagonist SR-141716 does not readily antagonize open-field effects induced by the cannabinoid receptor agonist (R)-methanandamide in rats. Pharmacol Biochem Behav. 2003a;75:809–821. doi: 10.1016/s0091-3057(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. (R)-Methanandamide and delta-9-tetrahydrocannabinol-induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol. 2003b;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Harris MY, Li C, Liu Q, Makriyannis A. Discriminative stimulus effects in rats of SR-141716 (rimonabant), a cannabinoid CB1 receptor antagonist. Psychopharmacology (Berl) 2004;177:35–45. doi: 10.1007/s00213-004-1916-5. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with delta-9-THC or (R)-methanandamide (AM-356) Psychopharmacology (Berl) 2006a;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta-9-THC and (R)-methanandamide in rats. Psychopharmacology (Berl) 2006b;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats. Psychopharmacology (Berl) 2008;198:467–478. doi: 10.1007/s00213-008-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe T, Li C, Liu Q, Makriyannis A. Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog. Psychopharmacology. 2009;203:229–239. doi: 10.1007/s00213-008-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner HA, Dingell JV. The metabolism and excretion of delta-9-tetrahydrocannabinol in the rat. Life Sci. 1971;10:49–59. doi: 10.1016/0024-3205(71)90245-1. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Balster RL. Pharmacological specificity of the phencyclidine discriminative stimulus in rats. Pharmacol Biochem Behav. 1991;39:971–975. doi: 10.1016/0091-3057(91)90061-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of delta-9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor M, Rivara S, Lodola A, Plazzi PV, Tarzia G, Duranti A, Tontini A, Piersanti G, Kathuria S, Piomelli D. Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies. J Med Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, D.C: National Academies Press; 2003. [PubMed] [Google Scholar]
- Panlilio LV, Mazzola C, Medalie J, Hahn B, Justinova Z, Drago F, Cadet JL, Yasar S, Goldberg SR. Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats. Psychopharmacology (Berl) 2009;203:529–538. doi: 10.1007/s00213-008-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perio A, Rinaldi-Carmona M, Maruani J, Barth F, Le Fur G, Soubrie P. Central mediation of the cannabinoid cue: Activity of a selective CB1 antagonist, SR 141716A. Behav Pharmacol. 1996;7:65–71. [PubMed] [Google Scholar]
- Ryan JW, Banner WK, Wiley JL, Martin BR, Razdan RK. Potent anandamide analogs: the effect of changing the length and branching of the end pentyl chain. J Med Chem. 1997;40:3617–3625. doi: 10.1021/jm970212f. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of Delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, Vann RE. The endogenous cannabinoid anandamide shares discriminative stimulus effects with delta-9-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–67. doi: 10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Eads M. Synergistic interactions of endogenous opioids and cannabinoid systems. Brain Res. 1999;848:183–190. doi: 10.1016/s0006-8993(99)01908-3. [DOI] [PubMed] [Google Scholar]
- Welch SP, Huffman JW, Lowe J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J Pharmacol Exp Ther. 1998;286:1301–1308. [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of "internal bliss"? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995a;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995b;34:669–676. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995c;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995d;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in Rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of delta-9-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Peterson J, Andersson DA, Chuang HH, Sorgard M, DiMarzo V, Julius D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]