Abstract
Although mitochondrial disease research in general is robust, adequate treatment of these life-threatening conditions has lagged, partly because of a persistence of clinical anecdotes as substitutes for scientifically and ethically rigorous clinical trials. Here I summarize the key lessons learned from some of the “first generation” of randomized controlled trials for genetic mitochondrial diseases and suggest how future trials may benefit from both past experience and exciting new resources available for patient-oriented research and training in this field.
Keywords: clinical trials, coenzyme Q10, dichloroacetate, mitochondria, mitochondrial diseases
1. What is the problem?
The ubiquitous mitochondrion is one of the most exhaustively studied components of animal cells. These pleotropic organelles are unquestionably vital to the welfare of the host; indeed, research over the last 30 years has linked mitochondrial dysfunction to a continuously growing spectrum of acquired and congenital disorders (Chinnery et al. 2002; DiMauro S et al. 2006; Gvozdjakova 2008) and to the inexorable decline of various functions associated with aging (Balaban et al. 2005; Finkel et al. 2009; Lenaz et al. 2006). The elucidation of the mitochondrial DNA (mtDNA) genome (Anderson et al. 1981) and the subsequent discovery of disease-causing mutations in mtDNA (Holt et al. 1988; Wallace et al. 1988) heralded an explosion of mitochondrially-oriented research, including rapid advances in the diagnosis, pathogenesis and epidemiology of genetic mitochondrial diseases.
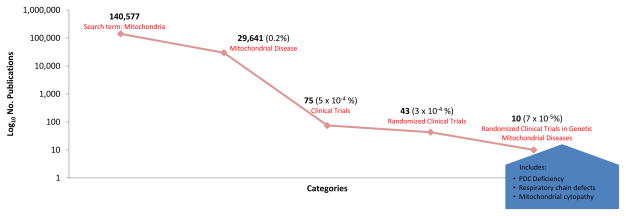
However, distillation of mitochondria-related research is telling. A review conducted on February 11, 2011 of PubMed citations listed 140,577 under the category “mitochondria” (Fig. 1); of those, 29,641 (0.2%) were classified as pertaining to “mitochondrial disease” and 75 reported results of clinical trials (5×10−6%). Forty-three (57%) of these published trials were randomized controlled trials (RCTs) that were double-blinded and most compared active treatment to placebo. However, only 10 of the RCTs (13%), representing 7×10−5% of all citations, were conducted in patients with congenital causes of mitochondrial disease due to proven enzymatic and/or molecular genetic defects in the pyruvate dehydrogenase complex (PDC) or in one or more respiratory chain complexes or in subjects with histological or immunochemical criteria consistent with “mitochondrial cytopathy.” All studies were single-center trials that evaluated dichloroacetate (DCA; 3 studies) (Duncan et al. 2004; Kaufmann et al. 2006; Stacpoole et al. 2006), a naturally occurring molecule (vitamin, cofactor or food; 5 studies) (Glover et al. 2010; Klopstock et al. 2000; Kornblum et al. 2005; Liet et al. 2003; Tarnopolsky et al. 1997) or a mixture of nutraceuticals (1 study) (Rodriguez et al. 2007). Five trials reported a positive effect of treatment on one or more clinical or biochemical primary endpoints (Duncan et al. 2004; Glover et al. 2010; Rodriguez et al. 2007; Stacpoole et al. 2006; Tarnopolsky et al. 1997). When specified, funding for these trials came from peer-reviewed, extramural grants and/or from private foundations. No publication listed support for the trial from pharmaceutical or biotechnology industry sources, except for one trial in which a nutritional supplement was provided by the manufacturer. None of the trials involving DCA was preceded by meetings with the Food and Drug Administration (FDA) and none resulted in the filing of a New Drug Application. Not included among these studies is an ongoing multicenter, randomized, placebo-controlled trial of coenzyme Q10 that is supported by the Orphan Products Division of the FDA and by the product manufacturer (Tishcon Corp.) (Kurtz 2008). Thus, an infinitesimal proportion of peer-reviewed publications on mitochondria relates to randomized controlled trials (RCTs) and the diversity of therapeutic interventions employed in these trials is alarmingly small. Why is this so?
Fig. 1.
PubMed citations as of February 11, 2011.
2. Analysis of the problem
It could reasonably be argued that the relative paucity of RCTs involving genetic mitochondrial diseases reflects the fact that late-phase interventional trials for any disease rests on a deep foundation of laboratory- and clinically-oriented investigation of the disease and putative therapies. This process may span decades, often terminating prematurely because of unanticipated toxicity, inefficacy, or both. However, it is instructive to consider lessons learned from the genesis, implementation and outcome of some of the few RCTs that have focused on genetic mitochondrial diseases, lest ignorance beget repetition.
2. 1. Dichloroacetate
DCA is the only small molecule xenobiotic that has entered into phase 3 clinical trials for genetic mitochondrial diseases. It faced several significant obstacles at the beginning of its clinical development (Stacpoole 1989). First, DCA holds an almost unique position at the interface of environmental health and allopathic medicine that has raised serious questions about its safety to humans. It has long been considered a potential health hazard by environmental toxicologists, owing to its ubiquity in our biosphere as a byproduct of water chlorination and as a metabolite of certain industrial solvents, such as the Superfund chemical trichloroethylene (Stacpoole 2011). Moreover, doses 10 thousand-fold higher than those to which humans receive from environmental exposure have been administered when DCA has been used for therapeutic purposes. Yet, doses similar to those employed clinically have been associated with serious end-organ toxicity, including liver cancer and peripheral neuropathy, in various animal species (Stacpoole et al. 1998). Second, although DCA is a synthetic chemical, its structural simplicity and long history of laboratory-based pharmacodynamic research made discovery patents based on its synthesis impossible, thereby severely limiting interest by industry. Consequently, almost all its pharmaceutical development has occurred in university settings and RCTs involving DCA have been supported exclusively by federal or foundation grants and by investigator INDs.
DCA was not initially “targeted” for use in mitochondrial diseases, although the mitochondrion is the cellular target of its dynamic action. The drug is a prototypic inhibitor of pyruvate dehydrogenase kinase, thereby activating the pyruvate dehydrogenase complex (PDC) and stimulating oxidative phosphorylation in mammalian cells (Stacpoole 1989). Following the first description of its use in a child with congenital lactic acidosis (Coude et al. 1978), DCA became the subject of many case reports describing beneficial effects from its acute or chronic administration to patients with genetic mitochondrial diseases (Stacpoole et al. 1997).
2. 2. Lesson 1: The DCA/CLA Clinical Trial
Based on these encouraging anecdotes, a multicenter, 5-year, double-blind, placebo-controlled, crossover trial was submitted to the National Institutes of Health in the early 1990s, in which quality of life (QOL), various functional neurological and neurobehavioral tests and blood and cerebral spinal fluid lactate comprised the major outcome measures of efficacy and safety. The study population included children with congenital lactic acidosis (CLA) due to biochemically or genetically proven defects in the PDC or in one or more complexes of the respiratory chain, or a pathological mutation in nDNA or mtDNA. This was to be the first controlled trial of any therapy for congenital lactic acidosis and received strong encouragement from the Director of the Institute from which funding was sought. A Special Emphasis Panel of peer reviewers conducted an all-day site visit at the principal investigator’s institution. The proposal received a score of 230, which at the time, placed it in the “Very Good” (aka, unfundable) range. Specific criticisms included the unnecessary application of formal nerve conduction testing, in lieu of clinical neurological examination, to detect DCA neurotoxicity (at that time, the high prevalence of peripheral neuropathy in genetic mitochondrial diseases was underappreciated); the questionable applicability of neurological outcome measures to the population in question (there were no previously validated QOL or neurobehavioral tools for congenital lactic acidosis); and the absence of survival as a primary outcome measure.
The proposal was revised to include a parallel design, thereby allowing survival to be included as an endpoint. However, this design change significantly expanded the requisite number of randomized subjects and the number of study sites and increased the cost of the trial three-fold (from about $5 million to over $15 million). Other modifications in the application attempted to address the reviewers’ concerns regarding the outcome measures, while defending the retention of nerve conduction testing. A repeat site visit was held that included virtually all the original reviewers. The score improved less than 10 points, with complaints about the size of the budget and insufficient movement on improving the clinical neurological outcome measures. The second revision generated a review by teleconference between reviewers and a few key investigators, without significant changes in the outcome.
At this point, almost three years had elapsed since the first application was submitted, and the multicenter collaborative group disbanded. However, investigators at two centers persisted, secured a small grant from the Muscular Dystrophy Association, incorporated the toxicological assessments (including nerve conduction testing) for the trial into a successful Superfund Program Project grant at the principal investigator’s institution and, subsequently, secured a 3-year grant from the FDA’s Orphan Products Division to undertake a single-center, phase 3 parallel trial, with the collaborating center functioning as a key diagnostic resource.
By far the most ironical consequence of this protracted exercise was that the principal investigator was contacted on five separate occasions between site visits by some of the NIH reviewers to request “compassionate use” DCA for their own patients. In retrospect, one may interpret such actions charitably as being consistent with the state-of-the-art at that time, namely, an over-reliance on anecdote and a naiveté, laced with suspicion, of the strategic importance of controlled clinical trials in advancing the treatment of pediatric mitochondrial diseases. However, the impact of this unfortunate behavior was further magnified by the coincident implementation of an open label trial of DCA in a similar heterogeneous group of mitochondrial disease patients at another institution (Barshop et al. 2004).
The demise of the multicenter collaboration and the head-to-head competition with an open-label study made patient recruitment and retention to the so-called DCA/CLA Clinical Trial challenging. Maintaining the trial’s visibility to both the lay and professional communities required the continuous engagement of advocacy groups, such as the United Mitochondrial Disease Foundation (UMDF), the National Organization for Rare Diseases (NORD) and Exceptional Parent (EP). However, a major potential bottleneck was in transporting patients and accompanying family members across hundreds or thousands of miles for quarterly visits to the General Clinical Research Center at the University of Florida in Gainesville, which is serviced by a small, regional airport. Thus, an alliance between the investigative team and Mercy Medical Airlift (MMA) became an essential component of the study’s success. Founded in 1972 (Rhodes 2008), MMA began as a nationwide charitable private pilots’ association to provide free transportation of patients to specialized treatment centers; however, it had not previously been engaged in facilitating travel for clinical trials. Through its subsidiary, Angel Flight of Florida, MMA provided several hundred thousand dollars in in-kind support to enable free round-trip transportation of patients throughout the U.S. for the duration of the trial.
Many lessons were learned from conducting the first phase 3 pediatric drug trial for genetic mitochondrial diseases (Stacpoole et al. 2006) (Table 1). Investigators failed to anticipate the response of their peers to the potential evolution in the standard of care of mitochondrial disease patients, from reliance on personal, but untested, treatment preference, to a double-blind, placebo-controlled trial. Restricting the trial to a single center was a major limitation for at least three reasons: 1) despite MMA’s efforts, it created logistical hardships for families of participants; 2) it diffused the nascent – and at that time unprecedented – network of mitochondrial disease specialists that had contributed to the initial stimulus for the trial; and 3) it would have decreased enthusiasm by the FDA, had a New Drug Application been submitted with results from only one study site. Nevertheless, the experience provided investigators with new knowledge about how to develop critical infrastructure to organize and sustain an RCT, including strategies for enabling patient transport, developing study venues and maintaining ongoing communication with such oversight groups as Institutional Review Boards, Data Safety and Monitoring Boards and the FDA. Moreover, it encouraged collaborators to reach consensus about the potentially contentious issues of clinical and biochemical diagnostic and inclusion criteria. Furthermore, it brought to the forefront certain major obstacles that future trials would face in choosing validated primary endpoints to determine clinical efficacy and safety. Such assessment tools had to be sufficiently straightforward and robust to be relevant to a patient population known for its clinical heterogeneity and to be applicable at multiple centers. Finally, the trial taught the importance of entrepreneurism in seeking assistance with funding, publicity and patient transportation.
Table 1.
Lessons Learned from the DCA/CLA Clinical Trial
|
2. 3. Lesson 2: The DCA/MELAS Clinical Trial
Shortly after the DCA/CLA Clinical Trial commenced, investigators at Columbia University, in collaboration with those at the University of Florida, secured funding through a Program Project grant from the National Institute of Child Health and Human Development (NICHD) to initiate a phase 3 clinical trial of DCA in patients with the syndrome of mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS), due to the common A3243G point mutation in mtDNA (Kaufmann et al. 2006). This was also a single-center trial in which patients received the same oral dose of DCA and dosing interval as did patients in the Florida trial. However, despite certain similarities, there were important differences between these studies both in design and, particularly, in outcome (Table 2).
Table 2.
A Tale of Two Trials: Congenital Lactic Acidosis vs. MELAS
| Characteristic | DCA/CLA Trial | DCA/MELAS Trial |
|---|---|---|
| Clinical site | U. Florida | Columbia U. |
| Funding | Multiple sources | NIH Program Project |
| Pre-trial FDA meeting | No | No |
| Study design | Parallel | Crossover |
| DCA dose | ||
| mg/kg | 12.5 mg/kg/12 hr | 12.5 mg/kg/12 hr |
| mg/m2 | 320 mg/m2/12h | 500 mg/m2/12h |
| Patient diagnosis | Varied | A3243G mutation |
| Number of subjects | 43 | 30 |
| Mean age at entry | 5.6 yrs | 30 yrs |
| Outcome | Biochemical, but not clinical, benefit; treatment well-tolerated | Terminated prematurely because of (reversible) peripheral neuropathy |
| Reference | Stacpoole et al. 2006 | Kaufmann et al. 2006 |
In both trials, dosing was based on body mass (kg). To account for allometric scaling, consider that a child at the 50th percentile who is 5.6 years old (the mean age of entry in the DCA/CLA trial) would weigh about 20 kg, be about 112 cm in height and have a body surface area of approximately 0.8 m2 (http://www.globalrph.com/bsa2.cgi). A 30-year-old adult at the 50th percentile is about 80 kg and 178 cm, yielding a body surface area of approximately 2.0 m2. Thus, accounting for allometric scaling indicates a discrepancy in dosing between the two trials, which might, in part, account for the greater toxicity of DCA observed in adults.
The DCA/CLA trial showed no differences in adverse effects between treatment groups (Stacpoole et al. 2006), a finding that permitted continuation of open label DCA in a majority of participants for several years beyond the placebo-controlled period (Stacpoole et al. 2008). In striking contrast, the MELAS trial was stopped prematurely because of new onset or exacerbation of pre-existing peripheral neuropathy in patients receiving DCA (Kaufmann et al. 2006). This cast a pall over the drug’s future as a therapeutic agent for chronic administration, but begged questions regarding the marked difference in susceptibility to DCA toxicity between the two patient populations. Six of the 43 participants in the Florida trial had MELAS, implying the underlying disease was not accountable for the variance in response to treatment. However, upon further examination of the kinetics of DCA in each population, a significant inverse relationship was found between subject age and rate of plasma clearance of the drug, a finding that was replicated in rats (Shroads et al. 2008). Furthermore, employing a rat model of DCA peripheral neuropathy, animal age was found to correlate inversely with onset of toxicity (Calcutt et al. 2009).
The DCA/MELAS trial was instructive because it served to highlight two well-recorded but frequently ignored tenets of clinical pharmacology: 1) the importance of patient demographics in predicting response to a therapeutic intervention and 2) the non-linear relationship between body weight and body surface area with development (Table 2). Had the relationship between age and toxicity not been elucidated, the therapeutic potential of DCA for mitochondrial and other chronic diseases (c.f. Stacpoole 2011) might have suffered a premature obituary. Application of the principal of allometric dose scaling should also be considered in future trials, particularly involving adults, to mitigate over-dosing and toxicity.
2. 4. Coenzyme Q10
It is axiomatic that patients with suspected or proven genetic mitochondrial disease will self-administer and/or have recommended to them by their physician some mixture of over-the-counter commercial preparations of vitamins, cofactors or other nutritional supplements. The administration of these compounds has been rationalized by the unproven hope that they might stimulate residual enzyme activity or circumvent the enzyme defect. Carnitine, creatinine, thiamine, biotin, lipoate, riboflavin, coenzyme Q10 (CoQ10), tocophenol and vitamin K plus ascorbate have been the most commonly used agents (Kerr 1995). However, clear benefit has been reported in only a very few individuals.
CoQ10 may be expected to benefit children with disorders of the mitochondrial respiratory chain by several mechanisms that are not mutually exclusive. The first would be CoQ10 (ubiquinone) deficiency, which is a clinically heterogeneous disorder detected by low muscle CoQ10, concentrations (Lamperti et al. 2003; Musumeci et al. 2001; Ogasahara et al. 1989). The second mechanism could be facilitating electron transport by circumventing a block in the electron transport chain. For example, if the subject had a block in complex III, supplemental CoQ10 could accept electrons from either the normal ubiquinol binding site or the FeS site of complex I (or both), and donate them to complex IV via ascorbate, which is the rationale for adding ascorbate to the standard supplement. This mechanism was originally demonstrated in an individual with complex III deficiency treated with vitamin K3 (menadione) and ascorbate (Arogov et al. 1986). The third mechanism is that CoQ10 can serve as an antioxidant, accepting electrons from disrupted electron transport and reducing the risk of formation of reactive oxygen species that might cause damage to mitochondrial membranes, proteins, lipids and DNA (Beal 2003; Geromel et al. 2002; Turunen et al. 2004). This is the most general mechanism, potentially applicable to any defect of electron transport and presumably the implicit rationale for widespread current use of CoQ10 in the management of patients with mitochondrial disorders.
Most of the clinical trials of CoQ10 in mitochondrial diseases were reviewed recently (Haas 2007) and only a few were found to qualify as an RCT. Last year, Glover et al. (Glover et al. 2010) reported the first Level I RCT (c.f. Sackett 1993) of CoQ10 in 30 adults (15 with MELAS, mean age 48 years; 15 with other mitochondrial diseases, mean age 56 years) with genetic mitochondrial diseases. Patients received 1,200 mg/d (averaging 19.5 mg/kg/d) CoQ10 as ubiquinol (Qgel; Tishcon) or placebo in a crossover design trial in which each treatment was administered for 60 days. Mitochondrial disease-specific activities of daily living and QOL questionnaires, validated for adult patients, were administered and tests of motor strength and endurance, NMR spectroscopy of the brain and various biochemical blood analyses were performed. Glover, et al. did not report the occurrence of adverse effects from CoQ10 administration, which achieved plasma levels ~5.5-fold above those measured during the placebo phase (5.5 μg/ml vs. 1.0 μg/ml). CoQ10 treatment was associated with an attenuated rise in blood lactate after bicycle ergonometry and transiently increased V02/kg lean mass after 5 minutes of cycling, but did not affect other clinically or biochemically meaningful variables, such as muscle strength, activities of daily living, QOL or brain lactate.
Despite the limitations of these studies of CoQ10 as monotherapy in mitochondrial disease patients, certain noteworthy findings emerge. First, very few pediatric subjects were enrolled in these studies; thus, the chronic safety and efficacy of CoQ10 has never been examined prospectively in children. Second, the doses of CoQ10 employed in the earlier trials were markedly lower (~2 mg/kg/d for a 70 kg adult) than those used in more recent studies, including those involving neurodegenerative disorders other than genetic mitochondrial diseases (Glover et al. 2010; Kaufmann et al. 2009; Mancuso et al. 2010; Stamelou et al. 2008; The Huntington Study Group 2001; The Huntington Study Group 2010). Third, the earlier trials of CoQ10 provided evidence of its safety, at least in adults. Finally, even the low doses used in the earlier trials were associated with at least trends in improvement in biochemical and/or clinical motor function among the treated subjects (Haas 2007).
There have been 3 published trials of CoQ10 administered as part of a “cocktail” of vitamins, cofactors and/or creatine or methylpredinsolone to patients with various types of suspected or proven genetic mitochondrial disease (Matthews et al. 1993; Peterson 1995; Rodriguez et al. 2007). In the only one of these trials to achieve Level II status, according to standard criteria (Sackett 1993), 16 patients who completed a RCT (crossover design) of daily 120 mg CoQ10, 300 mg lipoic acid and 3 g creatine monohydrate for two months achieved lower levels of resting blood lactate and urinary 8-isoprostane and, in some patients, evidence of modest neuromuscular improvement.
2. 5. Lesson 3: The CoQ10 Clinical Trial
Based on the above biochemical rationale and prior clinical experience, an application for conducting a three-year phase 3 trial of CoQ10 in children with genetic mitochondrial diseases was funded in late 2007 by the Orphan Products Division (OPD) of the FDA. The trial has involved three centers in the U.S. and one in Canada and tests the primary hypothesis that oral CoQ10 administration is safe and effective in improving motor function and quality of life. The trial incorporates a crossover design in which CoQ10 (10 mg/kg/d to a maximum of 400 mg/d as ubiquinol) and placebo are each administered for 6 months. Both formulations are provided gratis to patients by Tishcon Corp., which holds an IND and Orphan Product designation for CoQ10 for mitochondrial diseases. The trial is powered such that 40 patients must complete the 12-month treatment period. Eligible patients are between 6 months and 18 years at the time of randomization and must have a biochemically-proven deficiency of complex I–IV of the respiratory chain or have a known pathological mutation in a gene coding for a respiratory chain component (nDNA or mtDNA). Because of the prevalence of nutritional cocktails in the patient population, the investigators attempted to reduce this as a variable by providing each participant with a fixed-dose liquid preparation of vitamin C, riboflavin, thiamine and carnitine to be administered daily.
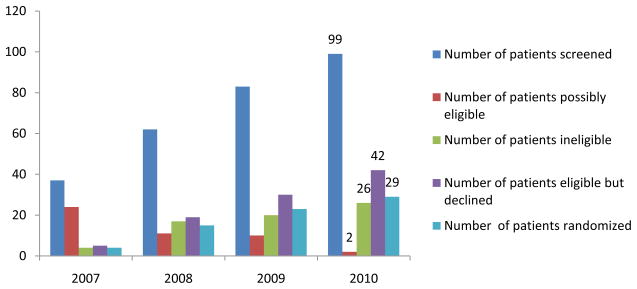
The trial has screened 99 potential subjects and has randomized 29 patients through December, 2010 (Fig. 2). A few individuals are continuing to undergo additional testing to determine eligibility. However, of 71 subjects deemed eligible for inclusion in the trial, only 29 (41%) have been randomized. In contrast, the families of 42 patients (59%) declined further testing. A few eligible families declined to participate in this trial in part or solely because of the logistical challenges associated with travel to a treatment site. However, the principal reason why recruitment to the trial has lagged behind expectations relates to the proportion of patients who were already taking some dose and formulation of CoQ10 as part of their individual nutritional supplementation. In fact, over 90% of potentially eligible subjects were receiving a “cocktail” containing CoQ10. Many families were convinced that CoQ10 was already benefiting their child and refused to participate in a placebo-controlled study.
Fig. 2.
Subject screening and enrollment in the CoQ10 trial, 2007–2010.
New recruitment strategies that include increased publicity of the trial via the UMDF and EP are underway to increase recruitment and retention to the CoQ10 trial. However, the challenges in meeting enrollment reflect at least three fundamental beliefs of affected families and caregivers that can be broadly implicated as major barriers to the advancement of promising treatments for mitochondrial diseases:
“It won’t hurt and it might help”
“I don’t want (my child) to be a guinea pig”
“I want (my child) to get the real treatment, not the placebo”
As with any prejudice, these attitudes reflect learned behavior and originate partly from the professional mitochondrial disease community in general. Its historical passivity in fostering rigorously controlled trials has perpetuated the type of irrational and, ultimately, self-defeating, behavior of a lay community desperate for help.
2. 6. Applying the lessons: The DCA/PDC Clinical Trial
Both the long-term evaluation (Stacpoole et al. 2008) of the patients originally recruited to the DCA/CLA trial and a review (Berendzen et al. 2006) of the published international experience of DCA in patients with deficiency of a component of the PDC indicated good chronic tolerability to the drug and suggestive evidence of clinical improvement. Consequently, a 1-year planning grant was received from NICHD to organize a pivotal phase 3 trial of DCA in approximately 70 PDC deficient patients (Stacpoole et al. 2011). The planning process is underway and has attempted to incorporate many of the lessons acquired from the preceding trials, namely:
Commitment to a multicenter study, involving 21 potential study sites throughout the U.S. and Canada;
Continuous participation by investigators regarding all aspects of the trial;
Use of a parallel design, incorporating only a few easily applied and validated outcome measures of safety and efficacy;
A focus on early and frequent communication with the potential funding agency and with the FDA and Health Canada, including a pre-trial meeting to address questions about key elements of the study design and its implementation;
Adherence to Good Manufacturing Practices in product formulation, testing and distribution;
Standardization of diagnostic criteria and of the application of assessment tools;
Training of clinical investigators (physicians and non-physicians) in the proper collection and transmittal of data to core facilities;
Establishment of a Data Coordinating Center skilled in electronically-based data acquisition and management and in biostatistical evaluation of outcome data;
Recruitment of patient advocacy and transportation organizations; and
Integration with new infrastructure for mitochondrial disease research and training.
3. Improving Federal Funding Mechanisms
Relatively few disease-specific networks associated with the NIH’s Rare Diseases Clinical Research Network (RDCRN) have conducted phase 3 trials of any drugs or biologics for their constituents. However, NIH grants that establish such consortia are awarded with the tacit assumption that advances will be made toward achieving the goal of conducting such trials. Although RDCRN grants provide basic infrastructure support for clinical trials, additional extramural funds must be sought to implement them. Without significant backing from the pharmaceutical or biotechnology industry, such funding must depend upon federal resources.
Currently, there are three principal mechanisms for funding investigator-initiated clinical trials of rare diseases, in addition to RDCRN grants1. The first is through traditional investigator-initiated grants, such as R01s, or, for some institutes, such as the National Institute of Neurological Diseases and Stroke, through a U-type (Cooperative Agreement) award, in which the institute assumes a more integral role in the study’s infrastructure and operation. The second mechanism for funding clinical trials for rare diseases is through the Orphan Products Division (OPD) of the FDA, the only grant-awarding division of the agency. OPD awards have been instrumental in fostering patient-oriented rare disease research and in garnering FDA approval for new treatments. The third mechanism is the relatively new Rapid Access to Interventional Development (RAID) Program at NIH (NIH-RAID http://commonfund.nih.gov/raid/). RAID accepts applications to request support for producing GMP-quality product (drug, vaccine or biologic), including placebo, for preclinical research and for clinical trials. For common diseases, RAID generally provides product for only early phase trials. However, the limited number of patients recruited to phase 3 trials of rare diseases is such that RAID resources may accommodate such studies. RAID itself is supported by several, but not all, of NIH institutes. Moreover, RAID funding is not provided directly to the successful applicant. Rather, it uses its funds to contract a compounding pharmacy in the private sector to undertake product formulation and testing under GMP conditions and provides the product to the awardee for distribution and use.
However, coordination of effort among NIH, OPD and RAID could be improved to substantially enhance the ability of the academic community to conduct RCTs for rare diseases (Table 3). Standardization of the frequency and timing of grant submissions, protocol formatting and page length and early cross-referencing of joint submissions (for example, cost-sharing drug development with RAID for an NIH or OPD-supported clinical trial) would reduce duplication and achieve better coordination of timelines and milestones associated with a clinical trial.
Table 3.
Federal Funding Mechanisms for Rare Disease Clinical Trials
| Characteristic | NIH | FDA-OPD | RAID |
|---|---|---|---|
| Application receipt datesa | February, June, October | February | January, May, September |
| Proposal page limit | 12b | 25 | 12 |
| Annual budget ceiling | $500,000 direct costsc | $200,000 total costs for phase 1; $400,000 total costs for phases 2 and 3 | None |
| Maximum duration of typical award | 5 years | 3 years for phase 1 4 years for phase 2 or 3 |
Based on time required to fulfill obligations (usually <1 year) |
| Letter of Intent required | Sometimes | No | Yes (must be approved before application can proceed) |
New applications.
Plus Specific Aims page. Certain institutes may allow separate, but linked, 12-page submission for clinical and data coordination and also permit simultaneous submission of a manual of procedures.
May be exceeded with prior permission from institute.
4. The future: The North American Mitochondrial Disease Consortium (NAMDC) and the UMDF
Headquartered at Columbia University, NAMDC is a new member of the NIH’s RDCRN and is also closely affiliated with the UMDF. NAMDC investigators are establishing a web-based patient registry, a data coordinating center, uniform diagnostic criteria for mitochondrial diseases and a biorepository for typing and storage of biological tissues and fluids, including samples from clinical trials. Indeed, the proposed DCA/PDC Clinical Trial aims to be the first NAMDC-affiliated phase 3 trial for mitochondrial diseases and would utilize many of the consortium’s resources. In turn, the trial would provide a mechanism for recruiting new institutions to NAMDC and for beta-testing its emerging resources. As NAMDC’s membership expands, so will the opportunity for clinicians and families affected by mitochondrial diseases to participate as partners in scientifically and ethically rigorous clinical trials and in other longitudinal studies investigating genotype-phenotype relationships, novel disease biomarkers, clinical assessment tools and continuing education programs.
The UMDF and NAMDC are also dedicating new resources to support patient-oriented research fellowship training of the next generation of mitochondrial disease investigators and clinical trialists. Consistent with these initiatives, the UMDF has taken the unprecedented step of holding a symposium on clinical trials at its forthcoming annual meeting that will be targeted to an audience of laboratory- and clinically-based researchers and families. The goal is to initiate a process that will forge greater understanding in the professional and lay communities of the essentiality of partnership in advancing translational and clinical research toward the diagnosis, treatment and cure of mitochondrial diseases.
Acknowledgments
This work was supported in part by NIH grant R34 HD065991 and FDA grant R01 FD003032. I thank Kathryn St. Croix for editorial assistance. The author declares that he has no actual or potential competing financial interests.
Abbreviations
- CoQ10
coenzyme Q10
- CLA
congenital Lactic Acidosis
- DCA
dichloroacetate
- EP
Exceptional Parent
- FDA
Food and Drug Administration
- IND
investigational new drug
- MMA
Mercy Medical Airlift
- mtDNA
mitochondrial DNA
- MELAS
mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes
- NICHD
National Institutes of Child Health and Human Development
- NORD
National Organization for Rare Diseases
- NAMD
North American Mitochondrial Disease Consortium
- nDNA
nuclear DNA
- OPD
Orphan Products Division
- PDC
pyruvate dehydrogenase complex
- QOL
quality of life
- RCTs
randomized controlled trials
- RAID
Rapid Access to Interventional Development
- RDCRN
Rare Diseases Clinical Research Network
- TRND
Therapeutics for Rare and Neglected Diseases
- UMDF
United Mitochondrial Disease Foundation
Footnotes
Another NIH resource, the Therapeutics for Rare and Neglected Diseases (TRND) program, was launched in 2009 to provide a means to facilitate preclinical development of new therapeutics, but does not support clinical trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Bankier AT, Barrel BG, De Bruijn MHL, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Arogov Z, Bank WJ, Maris J, et al. Treatment of mitochondrial myopathy due to complex III deficiency with vitamins K3 and C: A 31P-NMR follow-up study. Ann Neurol. 1986;19:598. doi: 10.1002/ana.410190615. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondrial, oxidants and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barshop BA, Naviaux RK, McGowan KA, Levine F, Nyhan WL, Loupis-Geller A, et al. Chronic treatment of mitochondrial disease patients with dichloroacetate. Mol Genet Metab. 2004;83(1–2):138–49. doi: 10.1016/j.ymgme.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann NY Acad Sci. 2003;991:120–31. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Berendzen K, Theriaque D, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Lopez V, Batusta A, Mizisin L, Cunha JM, Shroads AL, et al. Peripheral neuropathy in rats exposed to dichloroacetate. J Neuro Sci Exp Neurol. 2009;68:985–93. doi: 10.1097/NEN.0b013e3181b40217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery PF, Samuels DC, Elson J, Turnbull DM. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet. 2002;360(9342):1323–1325. doi: 10.1016/S0140-6736(02)11310-9. [DOI] [PubMed] [Google Scholar]
- Coude FX, Saudbray JM, DeMaugre F, Marsac C, Leroux JP, Charpentier C. Dichloroacetate as treatment for congenital lactic acidosis. N Engl J Med. 1978;299(24):1365–1366. doi: 10.1056/NEJM197812142992414. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Hirano M, Schon EA. Mitochondrial Medicine. Informa Healthcare; Oxon, United Kingdom: 2006. [Google Scholar]
- Duncan GE, Perkins LA, Theriaque DW, Neiberger RE, Stacpoole PW. Dichloroacetate therapy attenuates the blood lactate response to submaximal exercise in patients with defects in mitochondrial energy metabolism. J Clin Endocrinol Metab. 2004;89(4):1733–1738. doi: 10.1210/jc.2003-031684. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geromel V, Darin N, Chretien D, et al. Coenzyme Q10 and idebenone in the therapy of respiratory chain disorders: rationale and comparative benefits. Molecular Genetics and Metabolism. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- Glover EI, Martin J, Maher A, Thornhill RE, Moran GR, Tarnopolsky MA. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010;42(5):739–748. doi: 10.1002/mus.21758. [DOI] [PubMed] [Google Scholar]
- Gvozdjakova A. Mitochondrial Medicine: Mitochondrial Metabolism, Diseases, Diagnosis and Therapy. Springer Science; 2008. [Google Scholar]
- Haas RH. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion. 2007;7S:S136–S145. doi: 10.1016/j.mito.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Holt IJ, Harding AE, Morgan Huges JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;321:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–30. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Thompson JLP, Levy G, Buchsbaum R, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS. Treatment of lactic acidosis: A review. Int Pediatr. 1995;10:75–81. [Google Scholar]
- Klopstock T, Querner V, Schmidt F, Gekeler F, Walter M, Hartard M, et al. A placebo- controlled crossover trial of creatine in mitochondrial diseases. Neurology. 2000;55(11):1748–1751. doi: 10.1212/wnl.55.11.1748. [DOI] [PubMed] [Google Scholar]
- Kornblum C, Schröder R, Müller K, Vorgerd M, Eggers J, Bogdanow M, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo-controlled, double-blind 31P-MRS crossover study. Eur J Neurol. 2005;12(4):300–309. doi: 10.1111/j.1468-1331.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- Kurtz TL for the CoQ10 Trial Investigators. Phase 3 Trial of Coenzyme Q10 in Mitochondrial Diseases. United Mitochondrial Disease Foundation; Indianapolis, IN: 2008. Jun 24–28, [Google Scholar]
- Lamperti C, Naini A, Hirano M, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–08. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Baracca A, Fato R, Genova ML, Solaini G. New insights into structure and function of mitochondria and their role in aging and disease. Antioxid Redox Signal. 2006;8(3–4):417–437. doi: 10.1089/ars.2006.8.417. [DOI] [PubMed] [Google Scholar]
- Liet JM, Pelletier V, Robinson BH, Laryea MD, Wendel U, Morneau S, et al. The effect of short-term dimethylglycine treatment on oxygen consumption in cytochrome oxidase deficiency: a double-blind randomized crossover clinical trial. J Pediatr. 2003;142(1):62–66. doi: 10.1067/mpd.2003.mpd0333. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Orsucci D, Volpi L, Calsolaro V, et al. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr Drug Targets. 2010;11(1):111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- Matthews PM, Ford B, Dandurand RJ, Eidelman DH, et al. Coenzyme Q10 with multiple vitamins is generally ineffective in treatment of mitochondrial disease. Neurology. 1993;43:884–890. doi: 10.1212/wnl.43.5.884. [DOI] [PubMed] [Google Scholar]
- Musumeci O, Naini A, Slonim AE, et al. Familial cerebellar ataxia with muscle Q10 deficiency. Neurology. 2001;56:849–55. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- [Accessed February 14, 2011];NIH Rapid Access to Interventional Development (NIH-RAID) http://commonfund.nih.gov/raid/
- Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalopathy. Proc Natl Acad Sci USA. 1989;86:2379–82. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PL. The treatment of mitochondrial myopathies and encephalomyopathies. Biochim Biophys Acta. 1995;1271:275–280. doi: 10.1016/0925-4439(95)00039-7. [DOI] [PubMed] [Google Scholar]
- Rhodes S. Images of America: Angel Flight Mid-Atlantic. Arcadia Publishing; 2008. [Google Scholar]
- Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Nuscle Nerve. 2007;35(2):235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, et al. Beneficial effects of creatine, CoQ10 and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- Sackett DL. Rules of evidence and clinical recommendations for the management of patients. Can J Cardiol. 1993;9:487–489. [PubMed] [Google Scholar]
- Shroads AL, Guo X, Dixit V, Liu H-P, James MO, Stacpoole PW. Age-dependent metabolism of dichloroacetate in rats: possible relevance to human toxicity. J Pharmacol Exper Ther. 2008;324:1163–1171. doi: 10.1124/jpet.107.134593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW for the DCA/PDC Collaborative Group. Phase 3 Trial of Dichloroacetate for Pyruvate Dehydrogenase Complex Deficiency. Society for Inherited Metabolic Disorders Annual Meeting; Asilomar, CA. February 27-March 2.2011. [Google Scholar]
- Stacpoole PW. The dichloroacetate dilemma: environmental hazard vs. therapeutic goldmine - both or neither? Environ Health Perspect. 2011;119(2):155–158. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, Shuster JJ. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223–e1228. doi: 10.1542/peds.2007-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, et al. A controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. 1998;30:499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Barnes CL, Hurbanis MD, Cannon SL, Kerr DS. Treatment of congenital lactic acidosis with dichloroacetate. Arch Dis Child. 1997;77:535–541. doi: 10.1136/adc.77.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- Stamelou M, Reuss A, Pilatus U, Magerkurth J, et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Movement Disorders. 2008;23(7):942–949. doi: 10.1002/mds.22023. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA, Roy BD, MacDonald JR. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve. 1997;20(12):1502–1509. doi: 10.1002/(sici)1097-4598(199712)20:12<1502::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- The Huntington Study Group. Safety and tolerability of high-dosage coenzyme Q10 in Huntington’s disease and healthy subjects. Mov Disord. 2010;25(12):1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–99. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]