Abstract
In light of the adverse side effects of opioids, cannabinoid (CB) receptor agonists may provide an effective alternative for the treatment of cancer pain. The present study examined the potency and efficacy of synthetic CB1 and CB2 receptor agonists in a murine model of tumor pain. Intraplantar (i.pl) injection of the CB1 receptor agonist ACPA (ED50 of 18.4 µg) reduced tumor-related mechanical hyperalgesia by activation of peripheral CB1 but not CB2 receptors. Similar injection of the CB2 receptor agonist AM1241 (ED50 of 19.5) reduced mechanical hyperalgesia by activation of peripheral CB2 but not CB1 receptors. Both agonists had an efficacy comparable to that of morphine (i.pl), but their analgesic effects were independent of opioid receptors. Isobolographic analysis of the co-injection of ACPA and AM1241 determined that the CB1 and CB2 receptor agonists interacted synergistically to reduce mechanical hyperalgesia in the tumor-bearing paw. These data extend our previous findings that the peripheral cannabinoid receptors are a promising target for the management of cancer pain and mixed cannabinoid receptor agonists may have a therapeutic advantage over selective agonists.
Keywords: cannabinoid, hyperalgesia, cancer, mouse
INTRODUCTION
Pain associated with cancer and tumor growth is often difficult to manage. It has been estimated that over half of cancer patients experience tumor-related pain, and approximately two-thirds of patients experience pain with advanced disease (van den Beuken-van Everdingen et al., 2007), particularly with metastases to bone (Coleman, 2006). Although tumor-associated pain may incorporate components of inflammation and nerve injury that are consistent with tumor biology and growth that may impinge upon peripheral nerves, several lines of evidence indicate that tumor pain is distinct from pain resulting from inflammation or nerve injury. In primary somatosensory neurons that innervate a tumor-bearing limb, tumor growth produces phenotypic changes that differ from those associated with a similarly localized site of peripheral inflammation or nerve injury (Honore et al, 2000). Further, chemical mediators released from fibrosarcoma cells in vitro increase the expression of proteins in primary somatosensory neurons that contribute to increased neuronal excitability (Khasabova et al., 2007; Schweizerhof et al., 2009). These data provide evidence that chemicals released from tumor cells are sufficient to promote hyperalgesia that may differ from that experienced in other pain syndromes.
Cannabis sativa has a long history of use for management of pain. Although Cannabis contains many bioactive compounds, the component Δ9-tetrahydrocannabinol is most noted for its activation of cannabinoid (CB) receptors that were identified almost 2 decades ago (Matsuda et al., 1990; Munro et al., 1993). Endogenous and synthetic agonists of CB1 and CB2 receptors were subsequently reported to be effective in alleviating hyperalgesia in models of pain associated with nerve injury and inflammation (see Fox and Bevan, 2005 and Guindon and Hohmann, 2008 for reviews), and more recently tumor growth (Curto-Reyes et al., 2010; Guerrero et al, 2008; Hamamoto et al., 2007). Previously, we found that local injection of AEA reduced mechanical hyperalgesia by a CB1 receptor-dependent mechanism in a murine model of tumor pain (Khasabova et al., 2008). Increased expression of CB1 receptors in the somatosensory neurons innervating the tumor-bearing limb contributed to the effect. We also found that local injection of a non-selective cannabinoid receptor agonist decreased tumor-related hyperalgesia through peripheral CB2, as well as CB1, receptor-dependent mechanisms (Potenzieri et al., 2008).
Investigations of the efficacy of CB2 receptor agonists in models of hyperalgesia have escalated in the last several years with the development of several CB2 receptor selective agonists (A-796260: Yao et al., 2008; A-8336339: Yao et al, 2009; AM1421: Ibrahim et al., 2005, Lozano-Ondoua et al., 2010, Curto-Reyes et al., 2010, Hsieh et al., 2010, Quartilho et al, 2003; GW405833: Leichsenring et al., 2009, Whiteside et al., 2005; HU308: Hanus et al., 1999; JWH133: Yamamoto et al., 2008b). An advantage of CB2 receptor-agonists is that they are effective analgesics that lack the remaining components of the tetrad of cannabinoid pharmacology mediated by CB1 receptors: sedation, motor impairment and hypothermia (Malan et al, 2001). To date, no study has addressed whether co-activation of CB1 and CB2 receptors by synthetic agonists has a synergistic effect in an assay of hyperalgesia.
In light of evidence that CB2 receptor ligands reduce mechanical hyperalgesia in models of inflammation and nerve injury, and evidence that tumors have interactions with sensory neurons that are different from those of other forms of peripheral injury, we determined whether a CB2 receptor agonist reduced tumor-evoked mechanical hyperalgesia through a peripheral mechanism and whether a beneficial effect may occur by the co-administration of a CB1 and a CB2 receptor agonist. Since there is evidence that the release of endogenous opioid peptides from skin mediates the effect of CB2 agonists in the periphery (Ibrahim et al., 2005), we also determined whether naloxone blocked the effect of the CB2 agonist. All cannabinoid agonists had efficacy comparable to that of morphine. Moreover, a synergistic interaction of the agonists in reducing mechanical hyperalgesia provides a rationale for development of peripherally restricted dually active CB1–CB2 receptor agonists for the management of cancer pain.
METHODS
Subjects
Adult male C3H/HeNCr MTV− mice (National Cancer Institute; 25–30 g) were used throughout this study. Mice were housed 4 per cage, allowed free access to food and water, and maintained on a 12-hour light/dark schedule. All behavioral testing was performed during the light cycle. Experiments adhered to the guidelines set forth by the Committee for Research and Ethical Issues of the IASP, and procedures were approved by the Animal Care Committee at the University of Minnesota.
Maintenance and implantation of fibrosarcoma cells
NCTC clone 2472 fibrosarcoma cells (American Type Culture Collection, Manassas, VA, USA) were maintained as described previously (Khasabova et al., 2008). This clone was derived from a connective tissue tumor in a C3H mouse, thus the fibrosarcoma cells are syngeneic with C3H/He mice (Wacnik et al., 2001). Under isoflurane (2%) anesthesia, fibrosarcoma cells (2×105 cells in 10 µl of phosphate buffered saline, pH 7.3) were injected into and around the calcaneus bone of the animal’s left hind paw. Histological studies documented that this approach produces a tumor with bone osteolysis (Wacnik et al., 2001).
Measurement of mechanical hyperalgesia
Mechanical hyperalgesia was selected as the dependent measure in the study because of its high reliability and resolution in our hands in measuring evoked responses in mice. Moreover touch-evoked pain is more prominent than heat-evoked pain in neuropathic pain syndromes in humans (Backonja and Stacey, 2004) and our model of tumor pain produces a peripheral neuropathy (Cain et al., 2001). Mechanical hyperalgesia in the tumor-bearing paw was defined as an increase in withdrawal frequency in response to a standard mechanical stimulus: a von Frey monofilament (0.4 g) that delivers a force of 3.9 mN. Animals were placed on an elevated wire mesh platform, covered individually with glass containers, and allowed to acclimate for 30 minutes prior to testing. The monofilament was applied to the plantar surface of each hind paw ten times, and the withdrawal frequency was calculated as the (number of withdrawal responses/total stimuli) X 100% for each paw.
The baseline (pre-tumor) withdrawal frequency for each hind paw was measured on 3 consecutive days preceding implantation of fibrosarcoma cells. The mean baseline withdrawal frequency evoked by the 3.9 mN monofilament was 13%. Following implantation of fibrosarcoma cells, the development of mechanical hyperalgesia was monitored daily. Consistent with previous studies (Wacnik et al., 2001) an increase in the withdrawal frequency of the tumor-bearing paw occurred in response to the test stimulus. By 10 days after implantation, the mean paw withdrawal frequency increased to 78% in the tumor-bearing paw. Approximately 15% of mice did not display mechanical hyperalgesia after implantation of fibrosarcoma cells. On the day of drug injections (day 10 or 11 after implantation), only mice that exhibited a withdrawal frequency ≥70% were used in the experiments. Following intraplantar drug injections, the withdrawal frequency of each hind paw was measured every 30 minutes for 3.5 h. The individual scoring behavioral responses following injection of drugs was blinded to the treatment of the animal in all experiments, and at least 2 drug groups were tested in each session.
Drug solutions and administration
The CB2 receptor agonist AM1241 [(2-iodo-5-nitrophenyl)-(1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl)methanone; Cayman Chemical, Ann Arbor, MI, USA] was dissolved in Tocrisolve:dimethylsulfoxide (Tocris, Ellisville, MO, USA, DMSO; 2:1; 10 µg/µl). The CB1 receptor agonist ACPA (arachidonylcyclopropylamide; Tocris) was prepared in Tocrisolve™ 100 (10 µg/µl). The CB1 receptor antagonist AM281 [1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-mo rpholinyl-1H-pyrazole-3-carboxamide; Tocris] and the CB2 receptor antagonist AM630 [6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl)methanone; Tocris] were each prepared in DMSO (10 µg/µl). Each drug exhibits more than a 100-fold difference in affinity for CB1 and CB2 receptors (Lan et al., 1999; Ross et al., 1999). Morphine sulfate (Sigma-Aldrich, St.Louis, MO, USA; 22 µg/µl) and naloxone hydrochloride (Sigma-Aldrich; 5 µg/µl) were prepared in water.
All drugs were diluted to the appropriate dose in saline for injection in a volume of 10 µl. The highest concentration of DMSO contained in a dose was used as the vehicle control. Drugs or vehicle were injected subcutaneously into the plantar surface (intraplantar, i.pl.) of the hind paw.
Analysis of dose-response effects
The percent maximum drug effect was calculated for each dose of a drug in order to determine the dose-response relationship for the drug.
Calculating maximum drug effect produced a limited number of values greater than 100 or less than 0, which occurred at high and low doses, respectively. These values were adjusted to 100 and 0, respectively, to address only the anti-hyperalgesic effect of each drug. The software FlashCalc (developed by M. Ossipov; www.u.arizona.edu/~michaelo/jflashcalc.html) was used to compute the ED50.
Statistical Analyses
All data are presented as the group mean ± S.E.M. Results were compared between groups and across time using one-way analyses of variance followed by Bonferroni's multiple comparisons test. The isobolographic analysis of data for AM1241 and ACPA were conducted with FlashCalc and graphed as described by Fairbanks and coworkers (2000). For all statistical analyses, a probability value <0.05 was considered significant.
RESULTS
The CB2 receptor agonist AM1241 reduced mechanical hyperalgesia in tumor-bearing mice
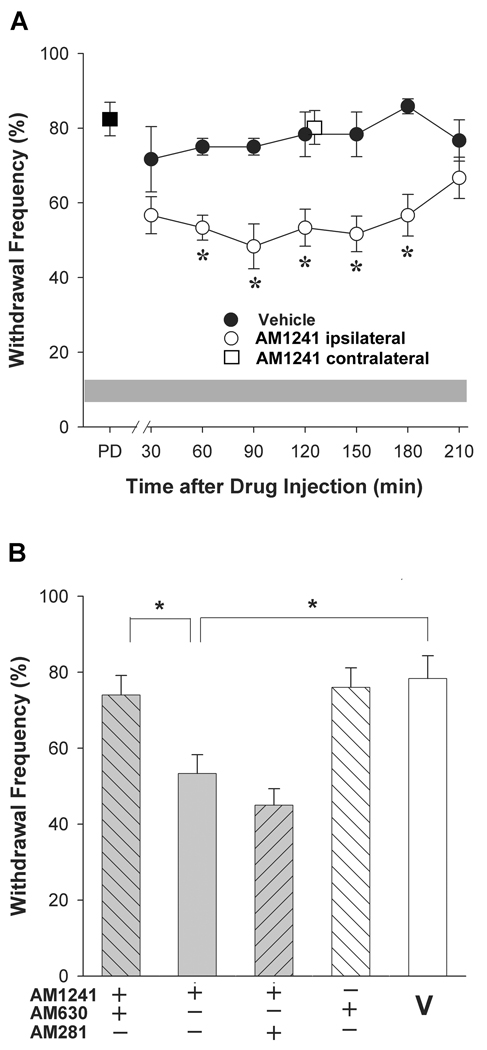
The CB2 receptor agonist AM1241 was chosen for study because of its high selectivity for CB2 over CB1 receptors in rodent tissues compared to other agonists (>80-fold; Ibrahim et al., 2003; Marriott and Huffman, 2008). Intraplantar injection of the CB2 receptor agonist AM1241 ipsilateral to the tumor-bearing paw decreased mechanical hyperalgesia in a time-dependent manner. The reduction in mechanical hyperalgesia persisted from 60 to 180 min after agonist injection when compared to vehicle (Tocrisolve:DMSO 2:1, i.pl., Fig. 1A) and dissipated after 3 h. Intraplantar injection of vehicle alone did not affect mechanical hyperalgesia at any time point assayed. Injection of AM1241 contralateral to the tumor did not alter hyperalgesia in the tumor-bearing paw at the representative time point of 120 min post injection (p=0.90 compared to vehicle, Student’s t-test, n=4–6 mice/group). These data indicate that the anti-hyperalgesic effect of AM1241 injected ipsilateral to the tumor was mediated by an action at a local site and not a site within the central nervous system.
Figure 1.
The synthetic CB2 receptor agonist AM1241 attenuated tumor-related mechanical hyperalgesia. A. AM1241 (60 µg, i.pl.) reduced mechanical hyperalgesia over 3 h compared to vehicle (Tocrisolve:DMSO, 2:1). Intraplantar injection of AM1241 contralateral to the tumor did not modulate hyperalgesia in the tumor bearing paw (□). The grey band represents the mean±S.E.M. of the withdrawal response of naïve mice. *Different from vehicle at p<0.05 (n=6 mice/group; two-way ANOVA with Bonferroni’s multiple comparisons test;). PD=pre-drug response to the test filament (3.9 mN). B. The CB2 receptor antagonist AM630 (4 µg, i.pl.) blocked the anti-hyperalgesic effect of AM1241. *Different at p<0.05 (n=5–6 mice/group; one-way ANOVA with Bonferroni’s multiple comparisons test;). V=vehicle. Data in B represent effects 120 min post drug injection.
The receptor selectivity of AM1241 was confirmed by co-administration with either AM630 or AM281. Co-administration with the CB2 receptor antagonist AM630 (4 µg, i.pl.) blocked the anti-hyperalgesic effect of AM1241 (p<0.05, one-way ANOVA), but the anti-hyperalgesia following AM1241 was unaffected by co-administration with the CB1 receptor antagonist AM281 (10 µg; p=0.67, one-way ANOVA, Fig. 1B). Importantly, the efficacy of this dose of AM281 was demonstrated in its blockade of the anti-hyperalgesic effect of the CB1 agonist ACPA (see below). Neither AM630 (Fig. 1B) nor AM281 (Fig. 2B) administered alone in tumor-bearing mice reduced mechanical hyperalgesia compared to vehicle (p=0.963, one-way ANOVA). Together, these data indicate that local injection of AM1241 into the hind paw ipsilateral to the tumor in tumor-bearing mice reduced mechanical hyperalgesia through a CB2 receptor-dependent mechanism.
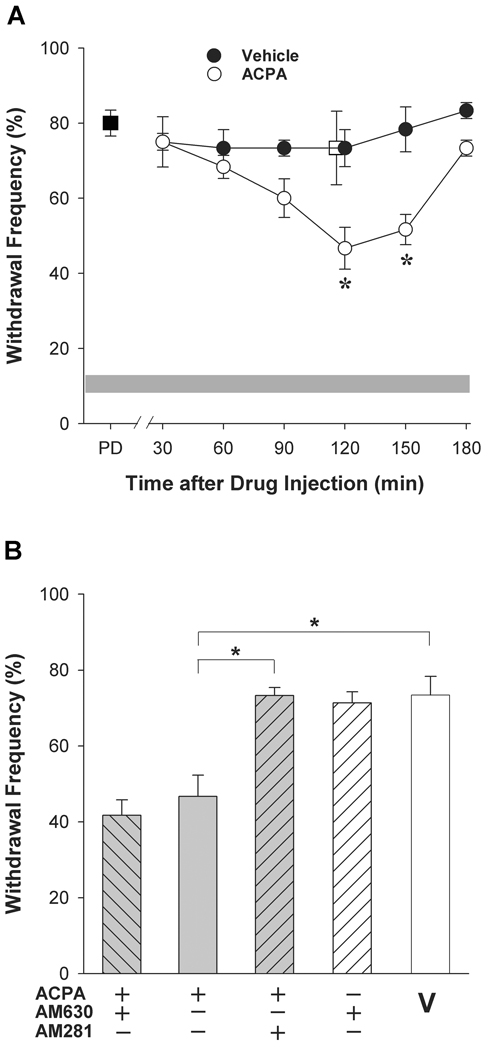
Figure 2.
The synthetic CB1 receptor agonist ACPA attenuated tumor-evoked mechanical hyperalgesia. A. Time course of the anti-hyperalgesic effect of ACPA (60 µg, i.pl.). PD=pre-drug response to a Von Frey filament of 3.9 mN. Intraplantar injection of ACPA contralateral to the tumor did not modulate hyperalgesia in the tumor-bearing paw (□). The grey band represents the mean±S.E.M. of the withdrawal response of naïve mice. *Different from vehicle at respective time point at p<0.05 (n=6 mice/group; two-way ANOVA with Bonferroni’s analysis). B. The anti-hyperalgesic effect of ACPA was blocked by co-administration of the CB1 receptor antagonist AM281 (10 µg) but not the CB2 receptor antagonist AM630 (4 µg). *Different at p<0.001 (n=4–8 mice/group; one-way ANOVA with Bonferroni’s multiple comparisons test). V = vehicle (Tocrisolve:DMSO, 2:1). Data in B represent effects 120 min post drug injection.
The CB1 receptor agonist ACPA reduced mechanical hyperalgesia in tumor-bearing mice
Occurrence of CB1 receptors on somatosensory neurons and CB2 receptors on other sites within the region of sensory transduction in the skin raises the possibility of an interaction between CB1 and CB2 receptor agonists in reducing mechanical hyperalgesia. Testing for this interaction required determination of an ED50 for a CB1 receptor agonist. The CB1 receptor agonist ACPA was chosen because it is over 300-fold more selective for CB1 than CB2 receptors (Hillard et al., 1999). Intraplantar injection of ACPA into the tumor-bearing paw attenuated mechanical hyperalgesia in a time-dependent manner (Fig. 2A). When compared to the vehicle control (saline:Tocrisolve 2:1, i.pl.), the reduction in mechanical hyperalgesia persisted for another 30 min, and hyperalgesia returned to the baseline level by 180 min. The latency to the anti-hyperalgesic effect was longer than that observed for AEA (Khasabova et al., 2008). Therefore, we tested another synthetic analog of AEA, methanandamide, in order to determine whether this property was specific to ACPA. Methanandamide (1 µg) exhibited a similar time course of action and did not reduce mechanical hyperalgesia until 120 min after drug injection (p<0.002 compared to pre-drug, one-way ANOVA, Fig. 3). Injection of the vehicle ipsilateral to the tumor did not alter mechanical hyperalgesia at any time point. Injection of ACPA (i.pl) contralateral to the tumor did not affect hyperalgesia in the tumor-bearing paw (p=0.723 at 120 min post drug injection compared to vehicle, Student's t test, n=4–6 mice/group) thereby confirming a local action of ACPA when injected into the tumor-bearing paw.
Figure 3.
Time course of the anti-hyperalgesic effect of MAEA (1 µg, i.pl.). MAEA did not reduce mechanical hyperalgesia until 120 min after drug injection (p<0.005 compared to pre-drug, one-way ANOVA with Bonferroni’s t-test).
The role of CB1 receptors in mediating the effect of ACPA was confirmed by co-administration of the drug with either a CB1 or CB2 receptor antagonist. The CB1 receptor antagonist AM281 (10 µg) abrogated the reduction in mechanical hyperalgesia produced by ACPA (60 µg, i.pl. p<0.001, one-way ANOVA, Fig. 2B). The CB2 receptor antagonist AM630 (4 µg) had no effect. Together these data demonstrate that local injection of ACPA into the tumor-bearing hind paw of mice reduced mechanical hyperalgesia through a CB1 receptor mechanism at 120 min following drug injection.
The anti-hyperalgesic effects of AM1241 and ACPA are dose-dependent
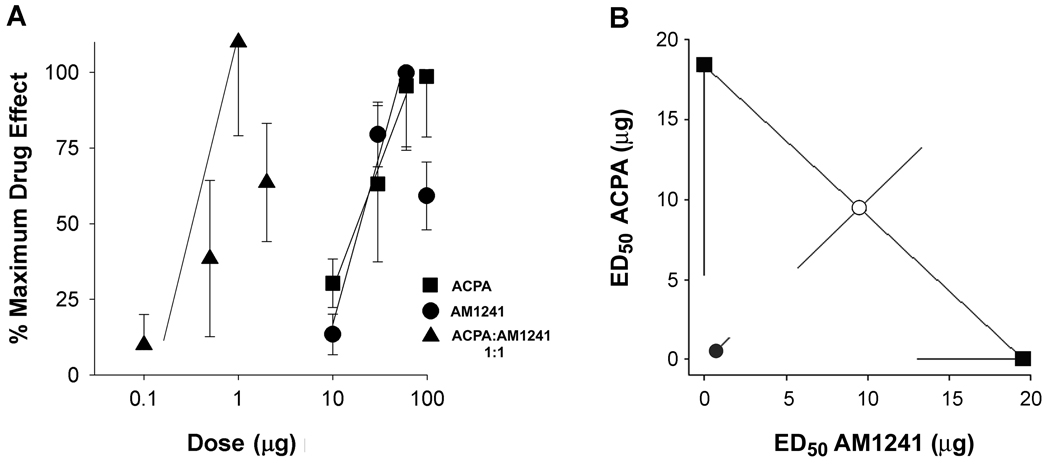
The anti-hyperalgesic effects of the CB2 receptor agonist, AM1241, and the CB1 receptor agonist, ACPA, were dose-dependent (Fig. 4A). Given that the effect of AM1241 was of long duration and that all doses tested were no different than the effect at 120 min post injection, data from the time point of 120 min were used to calculate an ED50 of 19.5 µg (95% CI: 14.0 to 27.1 µg). The dose of 60 µg was most effective, reducing mechanical hyperalgesia by 40% (Table 1). Because the effect of ACPA on mechanical hyperalgesia was maximal at 120 min, the percentage of the maximum effect of ACPA was also determined at this time point across 4 doses of the drug. The maximum effective dose of ACPA (60 µg) reduced mechanical hyperalgesia by 46% (Table 1) at 120 min post drug administration. An ED50 of 18.4 µg (95% CI: 9.0 to 37.7 µg) was calculated from the resulting dose-response relationship
Figure 4.
Co-injection of AM1241 and ACPA reduced mechanical hyperalgesia in a synergistic manner. A. The anti-hyperalgesic effects of AM1241 and ACPA injected individually or co-administered were dose-dependent. AM1241 and ACPA injected individually reduced mechanical hyperalgesia with an ED50 of 19.5 µg (95% CI: 14.0 to 27.1 µg) and 18.4 µg (95% CI: 9.0 to 37.7 µg), respectively. Co-administration of agonists ACPA and AM1241 (1:1 ratio; 1+1 was plotted as 1) reduced mechanical hyperalgesia with an ED50 of 0.69 µg (95% CI: 0.3 to 1.5 µg). Data were acquired from tumor-bearing mice 120 min post drug injection; dose is plotted on a log scale. B. Isobologram demonstrating the synergistic interaction of ACPA and AM1241 in reducing mechanical hyperalgesia (critical t-value=1.9; calculated t-value=5.0). ED50 values and synergy were determined using FlashCalc.
Table 1.
Summary of maximal drug effects.
| Drug | Most Effective Dose | Inhibition of Mechanical Hyperalgesia (%) |
|---|---|---|
| ACPA | 60 µg | 46 ± 12 (6) |
| AM1241 | 60 µg | 40 ± 9 (6) |
| ACPA+AM1241 | 1 ug each | 26 ± 7 (6) |
| Morphine | 7 µg | 53 ± 13 (7) |
Co-administering both CB receptor agonists attenuated mechanical hyperalgesia through a synergistic mechanism
Because intraplantar injection of ACPA and AM1241 individually yielded equivalent ED50 values for the two drugs, they were co-injected at a dose ratio of 1:1 in the protocol to test for an interaction. The time course of reduction in mechanical hyperalgesia following co-administration again demonstrated a maximum effect at 120 min post injection for all effective doses, so this time point was used in subsequent data analyses.
The reduction in mechanical hyperalgesia following co-administration of ACPA:AM1241 (1:1, i.pl.) was dose-dependent across combined drug doses of 0.1, 0.5, 1, and 5 µg of each drug. The dose-response relationship for the combined drugs was shifted nearly 100-fold to the left (Fig. 4A) with an ED50 value of 0.34 µg of each drug. The most effective combined dose was 1 µg of each drug, which reduced mechanical hyperalgesia by 26% when compared to vehicle (saline/Tocrisolve/DMSO). Because the maximum effect of the combined drugs was lower than that of either drug alone, we were concerned that the calculation produced a low estimate of the ED50 for the combined drugs and that this low estimate would bias determination of a synergistic interaction in the isobolographic analysis. Therefore, in a second calculation we adopted a more conservative approach and determined the inhibition of hyperalgesia by the combined drugs as a percent of the maximum effect of ACPA. Using this approach, the ED50 for ACPA: AM1241 (1:1) was 0.69 µg (95% CI: 0.3 to 1.5 µg). The data from the second calculation were used in the isobolographic analysis.
An isobolographic analysis was used to resolve whether the leftward shift of the dose-response curve for the combined drugs was due to an additive or synergistic interaction. The isobologram (Fig. 4B) was constructed from the dose-response data for individual injections of ACPA (ED50 18.4 µg) and AM1241 (ED50 19.5 µg), as well as combined agonists (ED50 0.69 µg), using the method of Tallarida (1992) as executed by FlashCalc. The experimental ED50 of the combined agonists is significantly lower than the theoretical ED50 for an additive effect of the two agonists indicating that the combined CB1 and CB2 receptor agonists (1:1) reduced mechanical hyperalgesia in a synergistic manner (critical t-value = 1.9; calculated t-value = 5.0).
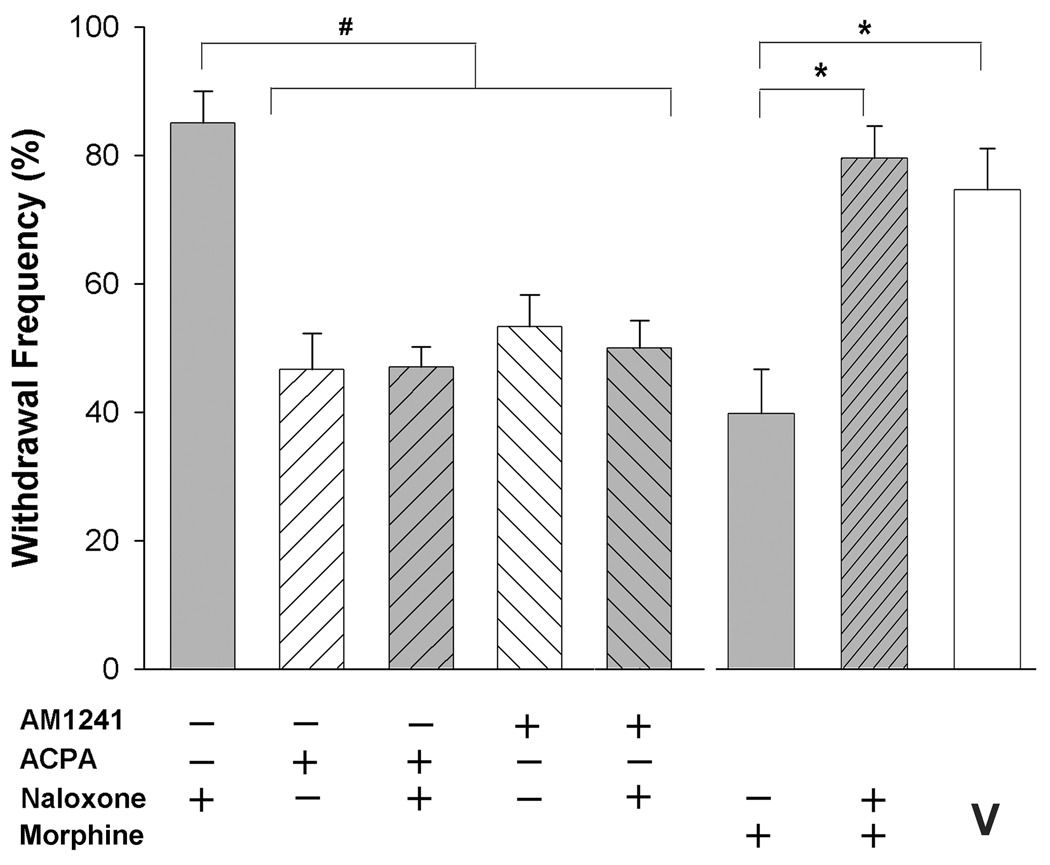
The anti-hyperalgesic effect of cannabinoid agonists was not mediated by opioid receptors
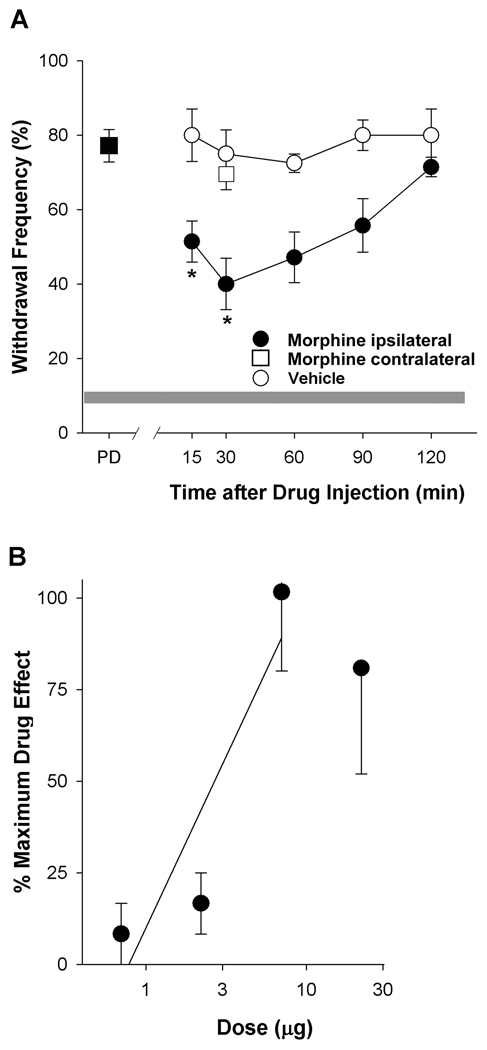
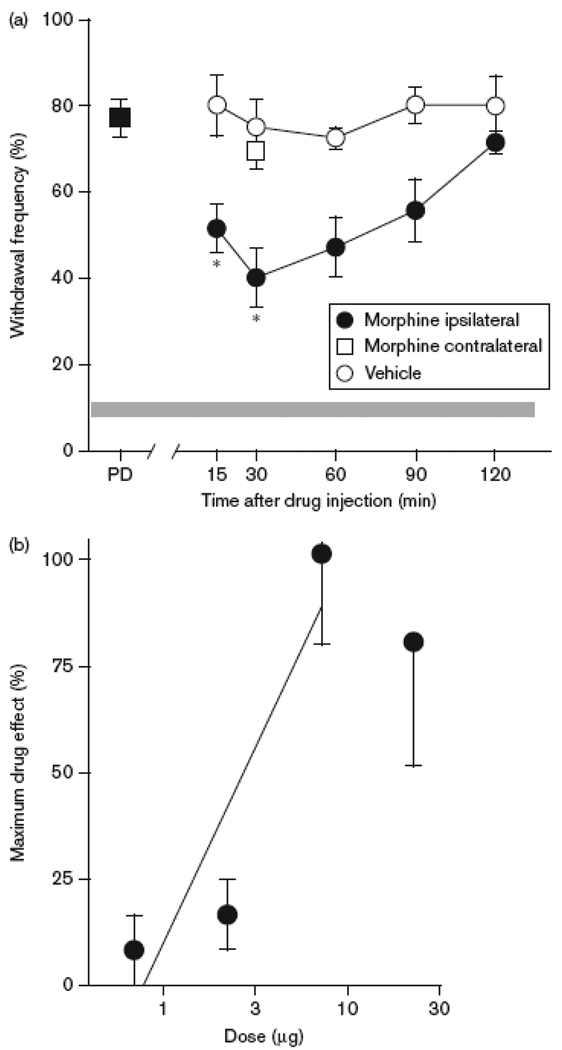
It has been reported that the antinociceptive effect of AM1241 was blocked by the local injection of naloxone in an assay of thermal nociception in rats (Ibrahim et al., 2005). Therefore, we tested whether naloxone altered the effect of AM1241 on mechanical hyperalgesia in tumor-bearing mice. Naloxone (5 µg, i.pl.) did not alter the anti-hyperalgesia produced by either AM1241 (60 µg, i.pl) or ACPA (60 µg, i.pl), nor did it alter mechanical hyperalgesia when injected alone (Fig. 5). Evidence that this dose of naloxone blocked the anti-hyperalgesic effect of morphine (7 µg, i.pl, p<0.001, one-way ANOVA) confirmed its effectiveness in blocking opioid receptors. This dose of morphine had a maximum effect 30 min after drug injection (Fig. 6A) and was the maximally effective intraplantar dose (Fig. 6B), reducing mechanical hyperalgesia by 53% (Table 1).
Figure 5.
The anti-hyperalgesic effects of ACPA and AM1241 were not modulated by opioid receptors. Naloxone (5 µg, i.pl.) did not block the anti-hyperalgesic effects of ACPA or AM1241. #Different at p<0.01 (one-way ANOVA with Bonferroni’s multiple comparisons test; 4–7 mice/group). Data for both cannabinoid agonists represent effects 120 min post drug injection. The same dose of naloxone blocked the effect of morphine (data represent effects 30 min post drug injection) in tumor bearing mice. Different at p<0.005 (one-way ANOVA with Bonferroni’s multiple comparisons test, n=5–7 mice/group). V=vehicle.
Figure 6.
Morphine attenuated tumor-evoked mechanical hyperalgesia. A. Time course of the anti-hyperalgesic effect of morphine (7 µg, i.pl.). A reduction in mechanical hyperalgesia occurred at the earliest time point measured (15 min) and persisted through 1.5 h. *Different from vehicle at respective time point at p<0.01 (n=6–7 mice/group; two-way ANOVA with Bonferroni’s analysis). Intraplantar injection of morphine contralateral to the tumor did not modulate hyperalgesia in the tumor-bearing paw (□). B. The anti-hyperalgesic effect of morphine was dose-dependent with an ED50 of 2.92 µg (95% CI: 1.9 to 5.4 µg). Dose was plotted on a log scale.
Given the clinical use of morphine in the treatment of cancer pain, we compared its maximum efficacy to that of selective cannabinoid receptor agonists administered by the same route in the same model (Table 1). There was no difference in efficacy among these treatments (p=0.49, one-way ANOVA).
DISCUSSION
Approximately half of cancer patients experience pain regardless of the stage of the disease (Portenoy, 1989), and the incidence increases to 70% in patients over 60 years of age (SEER cancer statistics review 1975–2007, National Cancer Institute). Not only is pain prominent in patients with advanced cancer, but over 40% of these individuals experience "breakthrough pain", pain not managed by ongoing palliative treatment (Greco et al., 2010). Clinical evidence of the refractoriness of cancer pain to opiates (Portenoy 1999, Mancini et al., 2004) coupled with preclinical evidence that the expression of mu opioid receptors is lower in somatosensory neurons affected by tumors compared to those of naive animals (Yamamoto et al., 2008a) underscores the need for alternative treatments for cancer pain. Using a murine model of tumor pain, the present study demonstrates that local administration of the synthetic cannabinoid receptor agonist AM1241 reduced mechanical hyperalgesia through a CB2 receptor-dependent mechanism and with an efficacy comparable to that of local injection of morphine. When used in combination with a selective CB1 receptor agonist, ACPA, the agonists exhibited synergy in decreasing the tumor-evoked mechanical hyperalgesia. Together these data support the use of combined CB1 and CB2 receptor agonists in the development of strategies for the treatment of tumor related pain.
Perihperal CB2 receptors reduce mechanical hyperalgesia
The anti-hyperalgesic effect of AM1241 was mediated by CB2 receptors in the present study because the effect was dose-dependent and blocked by a CB2 receptor antagonist that had no effect on the CB1 receptor agonist under the same experimental conditions. Although some components of CB2 receptor-mediated analgesia are mediated through actions on the central nervous system following systemic injection (Curto-Reyes et al., 2010; Hohmann et al., 2005; Hsieh et al, 2010; Yamamoto et al., 2008b), the anti-hyperalgesic effect of AM1241 in the present study was mediated locally. Injection of an effective dose of AM1241 into the paw contralateral to the tumor had no effect on hyperalgesia in the tumor-bearing paw. However, the locus of CB2 receptors that inhibit mechanical hyperalgesia has not been resolved. Progress in this area is hampered by the lack of specific antibodies that can be used in immunohistochemistry and the diversity of cell types surrounding the tumor in situ. In the periphery CB2 receptors are predominately expressed in skin by keratinocytes (Casanova ML et al., 2003; Ibrahim et al., 2005) and Langerhans cells (Oka et al., 2006) as well as by immune cells (Munro et al., 1993). Relevant to the present study, the fibrosarcoma tumors also express CB2 receptors, but to date we have found no evidence that the expression of CB2 receptors is induced in somatosensory neurons that innervate the tumor-bearing limb (unpublished observation). Although our results are consistent with a local, CB2 receptor-mediated effect of AM1241 on mechanical sensitivity in a rat model of inflammation (Gutierrez et al., 2007) and 2AG in a rat model of neuropathic pain (Desroches et al., 2008), a local CB2-receptor mediated effect of AM1241 on mechanical allodynia in a similar model of bone tumor was not confirmed (Curto-Reyes et al., 2010). The difference in results in the two studies of tumor-related pain may reflect differences in the tumor growth at the two sites (calcaneous bone versus tibia), the bioavailability of the two receptor antagonists following local injection, the assays (changes in threshold versus attenuation of a suprathreshold response) or the expression of CB2 receptors at the site of testing for mechanical hyperalgesia in the two models. In the present study, the tumor site was closer to the surface of the hind paw. In light of evidence that keratinocytes are intimately related to the transduction of stimuli in somatosensory neurons (Kozumi et al., 2004) and that factors released from tumors can affect the expression of proteins in neurons (Khasabova et al., 2007; Schweizerhof et al., 2009), we speculate that differential changes in expression of CB2 receptors in skin may contribute to the difference in results of the two models. Alternatively, since immune cells also express CB2 receptors and release pronociceptive chemicals, a difference in the distribution of these cells at the testing site in the two models may also be a factor.
An earlier report provided evidence that AM1241 evoked thermal analgesia in rats and mice by promoting the release of β-endorphin from keratinocytes. Thus, effects of AM1241 were blocked by ablation of the mu opioid receptor either pharmacologically with naloxone or by genetic deletion (Ibrahim et al., 2005). Evidence that systemic injection of naloxone blocks the inhibitory effect of systemic administration of AM1241 on thermal hyperalgesia in a rat model of peripheral inflammation (Yao et al., 2008) but not that of other synthetic CB2 receptor agonists under similar conditions (A-79620, Yao et al., 2008; A-836339, Yao et al., 2009; GW405833, Whiteside et al., 2005) suggests the effect is specific to AM1241. However, given the use of systemic drug administration in these studies, the site of interaction may be within the central nervous system rather than in the periphery. Further, the mu opioid receptor-dependent effect of AM1241 may be limited to attenuation of thermal nociception associated with inflammation. This hypothesis is based on evidence that the anti-allodynic effect of AM1241 was not blocked by a systemic injection of naloxone in a model of neuropathic pain (Hsieh et al., 2010). In the present study, local injection of naloxone did not block the effect of AM1241, thereby excluding the likelihood that its effect was mediated by release of an endogenous opioid receptor ligand. Importantly, the intraplantar dose of naloxone effectively blocked the intraplantar dose of morphine that produced a comparable level of mechanical analgesia, thereby confirming the analgesia was mediated locally.
Synergy of cannabinoid receptor ligands
The present data are the first documentation of a peripherally mediated synergy between a CB1 and CB2 receptor agonist in a nociceptive assay. The results with the synthetic CB1 agonist ACPA are consistent with our earlier report that anandamide decreased mechanical hyperalgesia by approximately 50% in the same model when used in the same experimental protocol (Khasabova et al., 2008). Importantly, control experiments validated that the effect of ACPA was mediated locally through CB1 receptors. Activation of CB1 receptors on sensory neurons innervating the skin decreases the transduction of mechanical stimuli (Argawal et al., 2007; Khasabova et al., 2008; Potenzieri et al., 2008). Our earlier work provided evidence that an increase in the expression of CB1 receptors by a population of somatosensory neurons that is likely to be nociceptive contributes to the anti-hyperalgesic effect of CB1 receptor agonists in this model.
Evidence of synergy in the interaction of CB1 and CB2 receptor agonists supports the therapeutic advantage of peripheral cannabinoid therapy in treating tumor related pain and presents a challenge. AM1241 exhibited decreased efficacy at the highest dose tested alone, and this also occurred at the highest dose tested in combination with ACPA. The decrease in the analgesic effect of the CB2 agonist at the high dose may be mediated by recruitment of eosinophils and exacerbation of release of inflammatory mediators (Oka et al., 2006). It is likely that the effect will decrease by adjusting the dose ratio. This speculation is based on evidence that the non-selective CB receptor agonist WIN 55212-2 reduced mechanical hyperalgesia by 94% in the same model with the same route of administration (Potenzieri et al., 2008).
Conclusion
Currently there is considerable interest in the modulation of pain with CB2 receptor agonists (Anand et al., 2009). We demonstrated that the local administration of a CB2 receptor agonist reduced mechanical hyperalgesia in a murine model of tumor pain. CB2 receptor agonists have several significant pharmacological advantages over CB1 receptor agonists as well as opiates in this model: tolerance does not develop to the antihyperalgesic effect (Hald et al., 2008; Leichsenring et al., 2009; Lozano-Ondoua et al., 2010), the growth of a variety of tumor cell types is impaired (Lozano-Ondoua et al., 2010; Pisanti et al., 2009) and effects on the central nervous system associated with the use of the other drugs are lacking. Moreover, the data address two strategies recently cited for enhancing the therapeutic potential of cannabinoids: targeting multiple cannabinoid receptors and developing peripherally restricted ligands (Pertwee, 2009). Evidence that synergy occurred in the analgesic effects of a CB1 receptor ligand in combination with a CB2 receptor ligand when injected peripherally at the tumor site supports the utility of these strategies in developing novel therapeutics for the management of cancer pain.
Supplementary Material
Acknowledgments
Sources of support: This work was supported by grants from the National Institute for Drug Abuse (DA011471, DAS) and the National Cancer Institute [CA091007 (DAS), CA138684 (VSS)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptor. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60:255–266. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja M-M, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J of Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001;21:9367–9376. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, Jorcano JL, Guzmán M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- Curto-Reyes V, Llames S, Hidalgo A, Menéndez L, Baamonde A. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. Br J Pharmacol. 2010;160:561–573. doi: 10.1111/j.1476-5381.2009.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches J, Guindon J, Lambert C, Beaulieu P. Modulation of the anti-nociceptive effects of 2-arachidonoyl glycerol by peripherally administered FAAH and MGL inhibitors in a neuropathic pain model. Br J Pharmacol. 2008;155:913–924. doi: 10.1038/bjp.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA, Nguyen HO, Grocholski BM, Wilcox GL. Moxonidine, a selective imidazoline-alpha2 -adrenergic receptor agonist, produces spinal synergistic antihyperalgesia with morphine in nerve-injured mice. Anesthesiol. 2000;93:765–773. doi: 10.1097/00000542-200009000-00026. [DOI] [PubMed] [Google Scholar]
- Fox A, Bevan S. Therapeutic potential of cannabinoind receptor agonists an analgesic agesnt. Expert Opin Investig Drugs. 2005;14:695–703. doi: 10.1517/13543784.14.6.695. [DOI] [PubMed] [Google Scholar]
- Greco MT, Corli O, Montanari M, Deandrea S, Zagonel V, Apolone G on behalf of the Writing Protocol Committee and the Cancer Pain Outcome Research Study Group (CPOR SG) Investigators. Epidemiology and Pattern of Care of Breakthrough Cancer Pain in a Longitudinal Sample of Cancer Patients: Results From the Cancer Pain Outcome Research Study Group. Clin J Pain. 2010;2010 doi: 10.1097/AJP.0b013e3181edc250. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Guerrero AV, Quang P, Dekker N, Jordan RCK, Schmidt BL. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett. 2008;422:77–81. doi: 10.1016/j.neulet.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Ding M, Egerod K, Hansen RR, Konradsen D, Jørgensen SG, Atalay B, Nasser A, Bjerrum OJ, Heegaard AM. Differential effects of repeated low dose treatment with the cannabinoid agonist WIN 55,212-2 in experimental models of bone cancer pain and neuropathic pain. Pharmacol Biochem Behav. 2008;91:38–46. doi: 10.1016/j.pbb.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Giridharagopalan S, Simone DA. Acute and chronic administration of the cannabinoid receptor agonist CP 55,940 attenuates tumor-evoked hyperalgesia. Eur J Pharmacol. 2007;558:73–87. doi: 10.1016/j.ejphar.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB(2) receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2010 Sep 28;2010 doi: 10.1111/j.1476-5381.2010.01046.x. (EPub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP., Jr CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, Hanson AE, Simone DA, Seybold VS. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;27:10289–10298. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci. 2008;28:11141–11152. doi: 10.1523/JNEUROSCI.2847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–13565. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Gatley J, Lu Q, Fan P, Fernando SR, Volkow ND, Pertwee R, Makriyannis A. Design and synthesis of the CB1 selective cannabinoid antagonist AM281: a potential human SPECT ligand. AAPS PharmSci. 1999;1:E4. doi: 10.1208/ps010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichsenring A, Andriske M, Bäcker I, Stichel CC, Lübbert H. Analgesic and antiinflammatory effects of cannabinoid receptor agonists in a rat model of neuropathic pain. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:627–636. doi: 10.1007/s00210-008-0386-4. [DOI] [PubMed] [Google Scholar]
- Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, Jimenez-Andrade JM, Mantyh PW, Vanderah TW. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010;86:646–653. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Mancini I, Dumon JC, Body JJ. Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: a pilot study. J Clin Oncol. 2004;22:3587–3592. doi: 10.1200/JCO.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Marriott KS, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor. Curr Top Med Chem. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young CA, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, Nasui M, Sugiura T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–8805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanti S, Malfitano AM, Grimaldi C, Santoro A, Gazzerro P, Laezza C, Bifulco M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract Res Clin Endocrinol Metab. 2009;23:117–131. doi: 10.1016/j.beem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Portenoy RK. Cancer pain. Epidemiology and syndromes. Cancer. 1989;63:2298–2307. doi: 10.1002/1097-0142(19890601)63:11<2298::aid-cncr2820631140>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Portenoy RK. Managing cancer pain poorly responsive to systemic opioid therapy. Oncology (Williston Park) 1999;13:25–29. [PubMed] [Google Scholar]
- Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN 55, 212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, Dickenson A, Simone DA, Kuner R. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Statistical analysis of drug combinations for synergism. Pain. 1992;49:93–97. doi: 10.1016/0304-3959(92)90193-F. [DOI] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:312–320. doi: 10.1016/j.pain.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, Turchin PI, Mark L, Garrison AE, Valenzano KJ. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A. Downregulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience. 2008a;151:843–853. doi: 10.1016/j.neuroscience.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur J Pharmacol. 2008b;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, Salyers AK, Wensink EJ, Honore P, Sullivan JP, Dart MJ, Meyer MD. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Hsieh G, Daza AV, Fan Y, Grayson GK, Garrison TR, El Kouhen O, Hooker BA, Pai M, Wensink EJ, Salyers AK, Chandran P, Zhu CZ, Zhong C, Ryther K, Gallagher ME, Chin CL, Tovcimak AE, Hradil VP, Fox GB, Dart MJ, Honore P, Meyer MD. Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2,2,3,3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)-4,5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2009;328:141–151. doi: 10.1124/jpet.108.145011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.