Abstract
The nuclear receptor related 1 (Nurr1) transcription factor contributes to the development and maintenance of dopamine (DA) neurons in the brain. We found that heterozygous Nurr1 knock-out (Nurr1 +/−) influenced the age-dependent decline in the number of DA neurons and influenced DA signaling. We examined the DA marker, tyrosine hydroxylase, using immunohistochemistry, and we measured DA signaling using fast-scan cyclic voltammetry in 3 age groups of wild-type (Nurr1 +/+) and mutant (Nurr1 +/−) mice: 3–6, 9–12, and 15–23 months old. Prior to significant loss of DA neurons and to the onset of parkinsonian symptoms, young Nurr1 +/− mice (3–6 months) exhibited a decrease in peak evoked DA release that was partially countered by a decrease in the rate of DA reuptake. As peak evoked DA release declined with age for both the wild-type and Nurr1 +/− mice, both genotypes manifested decreased DA reuptake. As the DA release fell further with age, decreased DA reuptake eventually could not adequately compensate the Nurr1 +/− mice. The results indicated that Nurr1 deficiency led to impaired DA release even before significant DA neuron loss.
1. Introduction
The major motor and cognitive impairments associated with Parkinson’s disease (PD) usually arise from the degenerative loss of dopamine (DA) inputs to the striatum. PD is characterized by a severe depletion of striatal DA release and a decline in DA neuron biomarkers, such as the DA transporter (DAT) (Bannon and Whitty, 1997; Cruz-Muros, et al., 2009; Hebert and Gerhardt, 1999; Uhl, et al., 1994) and the DA D1 and D2 type receptors (Ichise, et al., 1998; Rinne, et al., 1990; von Euler and Hedlund, 1995; Wang, et al., 1998). Interestingly, the clinical symptoms of PD (e.g., resting tremors, bradykinesia, rigidity, and postural instability) are not present until the majority of DA neurons are lost (Morrish, et al., 1998; Scherman, et al., 1989). Changes in DA denervation and DA content, for example, can occur as early as 20–30 years prior to the onset of motor impairments (Riederer and Wuketich, 1976; Scherman, et al., 1989). In animal models, significant alterations in DA signaling may precede any gross pathological abnormalities (Abeliovich, et al., 2000). These results indicate there is a relatively long preceding period during which disease-related alterations in DA function accumulate until dysfunction reaches a critical threshold where parkinsonian symptoms are expressed (Gaig and Tolosa, 2009; Sossi, et al., 2006).
Recently, Nurr1 mutant mice became a potential model for PD research (Jiang, et al., 2005; Jiang, et al., 2004). Nurr1 is an orphan nuclear receptor expressed predominantly in the central nervous system in limbic areas and the ventral midbrain, including DA neurons (Backman, et al., 1999; Sacchetti, et al., 2001; Zetterstrom, et al., 1996). Mutant mice lacking Nurr1 (−/−) have disrupted development of DA neurons and die (Castillo, et al., 1998; Saucedo-Cardenas, et al., 1998; Zetterstrom, et al., 1997). Nurr1 also plays a protective role in DA neuron maintenance through its prevention of microglia and astrocyte activation (Kadkhodaei, et al., 2009; Saijo, et al., 2009). Heterozygous Nurr1 knock-out (Nurr1 +/−) mice display age-dependent morphological, biochemical, and behavioral phenotypes that resemble the progressive degeneration observed in PD (Imam, et al., 2005; Jiang, et al., 2005; Le, et al., 1999a; Le, et al., 1999b). Young Nurr1 +/− mice do not display overt movement disorders, but after 15 months of age, Nurr1 +/− mice develop locomotor and balance deficits indicative of DA dysfunction (Jiang, et al., 2005; Le, et al., 1999a). Human deficiency of the Nurr1 gene also has been found to be associated with PD (Chu, et al., 2006; Grimes, et al., 2006; Le, et al., 2003), indicating that Nurr1 may be a risk factor underlying the generation and development of PD. However, the physiological mechanism by which Nurr1 influences DA signaling in the dopaminergic system is not known. Furthermore, the changes in DA signaling associated with the age-dependent onset of PD symptoms in the Nurr1 +/− mice (Jiang, et al., 2005) have not been evaluated.
In Nurr1 +/− mice, we quantified the age-dependent decline in DA neuron number in the substantia nigra compacta and characterized electrically evoked DA signals within the striatum over the same age range using fast-scan cyclic voltammetry. The Nurr1 +/− mice showed a decline in peak evoked DA release in the dorsal striatum prior to the onset of significant DA neuron loss. The decrease in DA release was offset by a compensatory decline in DA reuptake. Eventually, however, the age-dependent DA decline crossed a threshold that could not be compensated and parkinsonian symptoms emerged (Jiang, et al., 2005).
2. Methods
2.1. Genotyping Nurr1 mutant mice
Nurr1 deficient mice were generated by Dr. O.M. Conneely’s laboratory as previously described (Saucedo-Cardenas, et al., 1998). Homozygous Nurr1 −/− deletion mice die after birth. This study examined Nurr1 +/− mice and their wild-type littermates, Nurr1 +/+, as controls for the same genetic background. The genotype of the Nurr1 +/− or Nurr1 +/+ mice were analyzed with PCR by using mouse tail DNA (Saucedo-Cardenas, et al., 1998). For all experiments, the Nurr1 mice were separated into 3 age groups: 3–6, 9–12, and 15–23 months. Mice older than 24 months were not used because other age-related diseases are common after this point (Jonec and Finch, 1975). Mice were housed and handled in accordance with the guidelines set forth by the animal care committee at Baylor College of Medicine.
2.2. Tyrosine hydroxylase (TH) immunohistochemistry
Control and Nurr1 +/− mice were transcardially perfused with ice-cold phosphate-buffered saline (pH 7.4), and the striatum and midbrain were dissected for DA biochemistry and TH immunostaining. The midbrains were fixed, rapidly frozen, and sectioned into 30 μm slices. The slices were systematically chosen at 150 μm intervals. Free-floating sections were incubated successively for 15 min with 0.05% H2O2 in 0.1 M PBS to remove endogenous peroxidase activity, for 1 h with 2% goat serum/0.1% Triton X-100 in 0.1 M PBS to block non-specific binding sites, and for 24 hours at 4°C with the primary antibodies, rabbit anti-tyrosine hydroxylase (TH, 1:4000; Protos Biotech, New York, NY, USA) to detect DA neurons. After washing, sections were then incubated for 2 h at a routine time with the appropriate biotinylated secondary antibody (anti-rabbit, 1:200; Vector Laboratories Inc., Burlingame, CA, USA). The avidin–biotin method was used to amplify the signal (ABC Kit; Vector Laboratories Inc.) and 3,3’-diaminobenzidine tetrachloride (DAB) was used to visualize bound antibodies.
We used stereological methods to evaluate the number of TH-positive DA neurons in the substantia nigra (SN) using a stereological system (Axioskop 2; Carl Zeiss Inc., Thornwood, NY, USA). Counting was performed with the computer-assisted stereological toolbox software program, Stereo Investigator 7.0 (MicroBrightField, Inc., Willston, VT, USA). The SN was outlined under 2.5 X magnification, and the TH-positive cells in the outlined region were counted with a 40 X lens and analyzed using a sampling design generated with the following stereologic parameters: grid size, 200 X 200 μm; counting frame size, 150 X 150 μm; and dissector height, 14 μm.
2.3. Determination of striatal DA content
The concentration of DA was quantified in striatal tissue by HPLC. Striatal tissue was homogenized (10% wt/vol) by sonication in ice-cold 0.1 M perchloric acid. Homogenates were centrifuged at 10,000 g for 10 min at 4°C, and the supernatant was collected and filtered through acro-disc filters (0.25 μm; Fisher Scientific, Pittsburgh, PA, USA), subjected to HPLC (HTEC-500; Eicom, Kyoto, Japan) with the SC-3ODS column (EICOMPAK; Eicom), and detected by an electrochemical detector (AD Instruments Pty Ltd., Castle Hill, NSW, Australia). The mobile phase consisted of 0.1 mM citric acid, 0.1 M sodium acetate, 220 mg/L octane sulfate sodium, 5 mg/L EDTA, and 20% methanol, and then the pH was about 3.5.
2.4. Fast-scan Cyclic Voltammetric measurement of DA signals from striatal brain slices
Under deep anesthesia (a combination of ketamine, xylazine, and acepromazine), mice were decapitated and the brains were rapidly dissected out. Horizontal slices (350 μm) were cut on a vibratome, and they were incubated at 32 ± 0.5 °C for 30 min (Zhou, et al., 2001). Then they were held at room temperature for >30 min, and studied at 34 ± 1 °C in 125 mM NaCl, 2.5 mM KCl, 1.3 mM MgCl2, 2.5 mM CaCl2, 26 mM Na2HPO3, 1.25 mM NaHCO3 and 10 mM glucose saturated with 95% O2 and 5% CO2. The bath (1 ml) was continually perfused at a rate of 1.5 ml/min. That perfusion was used to administer drugs onto the slice, such as GBR-12909 (GBR), which was used to inhibit the DA transporter (DAT). The GBR was washed into the slice for > 20 min to achieve equilibration before testing for any GBR-induced changes in DA release.
Fast-scan cyclic voltammetry was performed using homemade carbon-fiber microelectrodes (10 μm diameter and approximately 100 μm exposed length; P55s, Amoco Polymers, Greenville, SC) that were placed in the dorsolateral striatum (L. Zhang, et al., 2009; Zhou, et al., 2001). The potential applied to the carbon-fiber microelectrode was linearly scanned (12 ms duration, 10 Hz) from 0 to -400 to 1000 to -400 to 0 mV against a silver/silver chloride reference electrode at a rate of 300 mV/ms. An Axopatch 200B amplifier, a Digidata 1320 interface, and a pClamp 8 system (Axon Instruments Inc., Union City, CA) were used to acquire and analyze the data. The voltammograms were sampled at 50 kHz, and the background current was subtracted digitally. The peak amplitude oxidation currents for DA in each voltammogram (at approximately 600 mV) were converted into DA concentration from a post-experimental calibration of the carbon-fiber electrode against fresh solutions of 0.5 to 10 μM DA.
Intrastriatal stimuli were delivered using a bipolar tungsten electrode. The two poles of the stimulating electrode were about 150 μm away from each other. The tip of the carbon-fiber recording electrode was about 200 μm away from each of the two poles of the stimulating electrode. Burst stimulations were applied at intraburst frequencies ranging from 5 to 80 Hz with 5 pulses per burst train. Each pulse was 1 ms in duration and about 0.6 mA of constant current.
The electrically evoked DA response was measured as the peak amplitude (μM) or was measured as the area under the curve of DA concentration (μM-s). The relative DA signal was calculated by comparing the burst evoked DA signal to the single-pulse evoked DA signal, and these data are presented as the means ± SEM. Two-way ANOVA was used to judge the changes in frequency-dependent DA release between genotypes.
3. Results
3.1. Midbrain TH-positive neurons and striatal DA levels in Nurr1 +/− mice
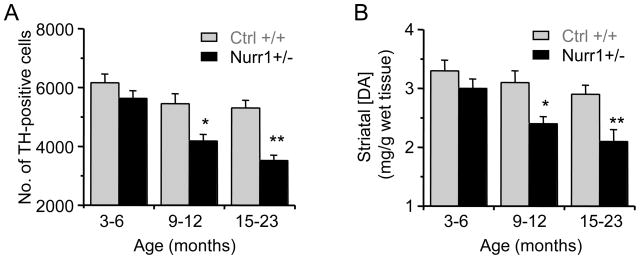
The number of TH-positive DA neurons in the SN of Nurr1 +/− and Nurr1 +/+ (control) mice at different ages was determined with an unbiased stereological optical dissector using the Cavalieri principle (Pakkenberg, et al., 1991). In mice aged 3–6 months, there was no significant difference in the number of TH-positive neurons between the Nurr1 +/− mice and their littermate controls (Fig. 1A). In the 9–12 month age group, the number of TH-positive cells in the midbrain of Nurr1 +/− mice (4190 ± 223, n = 48 slices from 6 mice) was less than in the control mice (5455 ± 340, n = 48 slices from 6 mice; p < 0.05). In the 15–23 month age group, the number of TH-positive cells in the midbrain of Nurr1 +/− mice (3520 ± 182, n = 45 slices from 5 mice) was less than in the control mice (5312 ± 256, n = 45 slices from 5 mice; p < 0.01).
Fig. 1.
Age-dependent changes in DA neurons and DA content changes in Nurr1 +/− and control (Nurr1 +/+) mice. (A) TH-immunostaining of DA neurons in the substantia nigra (SN) in different age groups. The stereological cell counting of TH-positive cells from Nurr1 +/− mice (black bars) showed a significant decrease compared to age matched control mice (gray bars). (C) Striatal DA content decreased in the dorsal striatal in Nurr1 +/− mice. *p < 0.05 and **p < 0.01 vs age-matched Nurr1 +/+ mice with 5–6 mice in each group.
The main target of DA projections from the SNc is the dorsal striatum. When tissue from the dorsal striatum was homogenized and the DA concentration was measured, the results were consistent with the TH cell counts. The Nurr1 +/− mice in the 9–12 month and 15–23 month age groups showed a difference from control mice (n = 5–6 mice, p < 0.05) in the striatal DA concentration (Fig. 1B). In the control mice (Nurr1 +/+), the number of SNc TH-positive neurons and the striatal DA concentration were not statistically different across the three age groups.
3.2. Striatal brain slices from Nurr1 +/− mice have decreased evoked DA release
Nurr1 regulates the expression of TH, aromatic L-amino acid decarboxylase (AADC), DAT, and vesicular monoamine transporter-2 (VMAT-2) (Hermanson, et al., 2003; Jankovic, et al., 2005; Kim, et al., 2002; Sacchetti, et al., 2001; Sakurada, et al., 1999), which are critical for DA synthesis and DA signaling. We examined whether Nurr1 influences electrically evoked DA release in the striatum. In horizontal brain slices, the dorsolateral striatum was stimulated by a single electrical pulse (600 μA, 1 ms), and the real-time extracellular DA concentration was measured using fast-scan cyclic voltammetry (Zhou, et al., 2001). The magnitude of the DA response was quantified by measuring the peak amplitude (μM) and the area under the DA concentration curve (μM x s). The area under the curve subsumes factors arising from the amplitude of the DA release and the rate of DA removal by diffusion and DAT reuptake (Schmitz, et al., 2003; L. Zhang, et al., 2009).
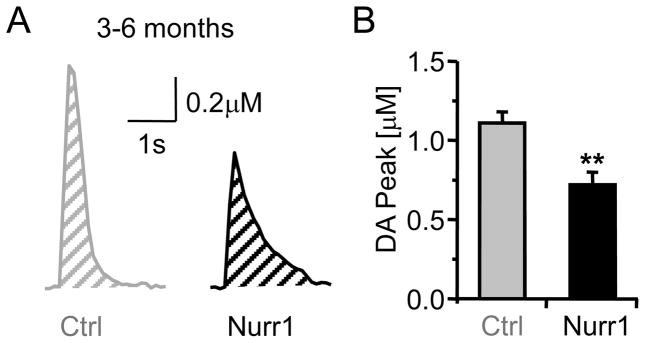
In the youngest age group, the mean amplitude of the single-pulse evoked DA signal was 35% smaller in Nurr1 +/− mice compared to control mice (3–6 months): 0.72 ± 0.08 μM (n = 30) in Nurr1 +/− mice and 1.11 ± 0.07 μM (n = 38, p < 0.01) in age-matched control (+/+) mice (Fig. 2A,B). However, the DA signal, as measured by the area-under-curve was not statistically different between the genotypes (Nurr1 +/− 0.7 ± 0.07, n = 30; control 0.6 ± 0.07, n = 38; p > 0.05), indicating that the DA removal from the extracellular space may have been slowed in the Nurr1 +/− mice. This difference in the removal of DA from the extracellular space can be anecdotally observed in the slower falling phase of the DA signal from the Nurr1 +/− mouse (black trace, Fig. 2A).
Fig. 2.
Single-pulse (lp) evoked DA release in Nurr1 +/+ (Ctrl) and Nurr1 +/− (Nurr1) mice (3–6 months) slices from the dorsal striatum. (A) Representative DA signals from controls (Ctrl, gray) and Nurr1 mice (black). The evoked DA signal from Nurr1 mice was smaller in peak amplitude and showed a prolonged decay phase compared to the control. (B) Mean amplitude of the DA signal in Nurr1 mice and the control (n = 30, 38 respectively; ** p < 0.01).
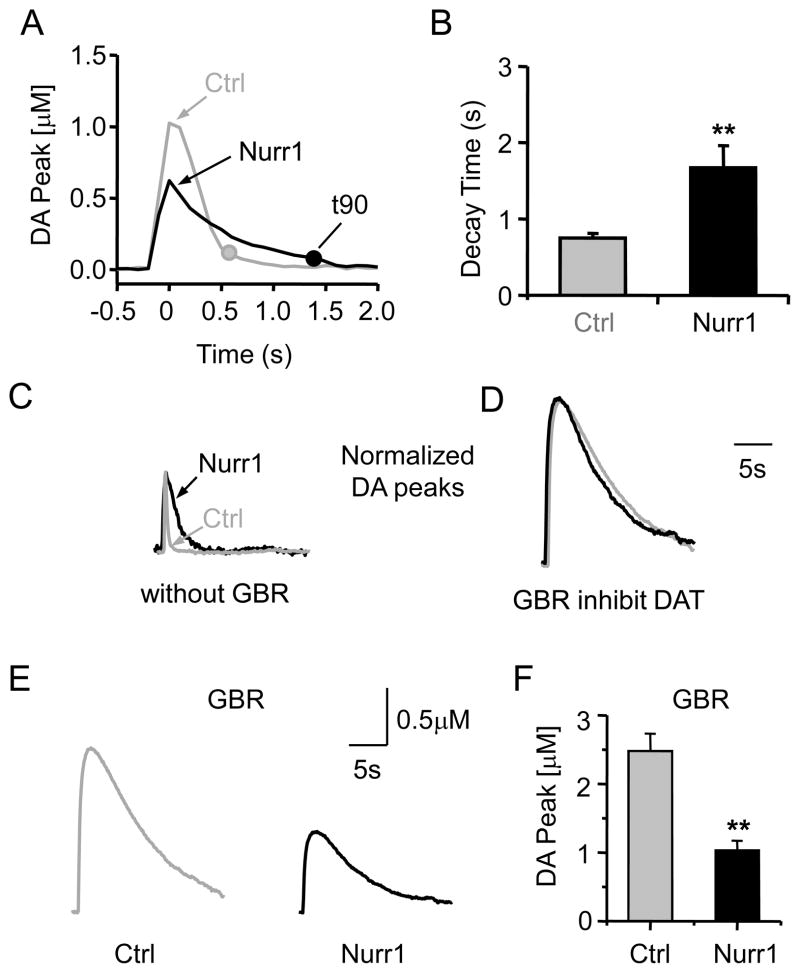
The falling phase was quantified as the time it takes for the DA concentration to fall 90% of the way from the peak to the baseline (t90), as defined by the DA concentration measured before the electrically stimulated release (Fig. 3A). The peak of the evoked DA signal was defined as t = 0, and t90 was 1.66 ± 0.29 s (n = 30) for the Nurr1 +/− mice and was 0.75 ± 0.06 s (n = 38, p < 0.01) for the control mice (Fig. 3A,B). Falling from a smaller peak DA concentration, it takes longer for the DA signal from Nurr1 +/− slices to return to baseline. The slower falling phase suggests that there is a decrease in DAT function in the Nurr1 +/− mice.
Fig. 3.
Slower decay time of DA signals in Nurr1 +/− mice slices. (A) Comparison of the decay time of the DA signals obtained from the control (Ctrl, gray) and Nurr1 +/− mice (black). The times when the DA signals decay to 90% of the peak (t90) are shown for each representative trace (filled circles). (B) The t90 was significantly longer in Nurr1 +/− mice (black bar) compared to the control (n = 30, 38; ** p < 0.01). (C) Example of normalized DA signals (100% of control amplitude) showing the longer decay time in Nurr1+/− mice (black trace) compared to the control (gray trace). (D) Blocking the DA transporter with GBR (2 μM) abolished the difference in decay of the control and Nurr1 +/− DA signals (shown in C). The peak amplitudes were again normalized to the control’s peak. (E) When DATs were inhibited by GBR, the difference in peak DA amplitude is accentuated between the control (gray) and the Nurr1+/− (black) mice. (F) The average peak DA amplitude was large and significantly different between the two genotypes (n = 10, 15; ** p < 0.01).
To separate the contribution of diffusion and DAT activity to the falling phase, we inhibited DAT activity selectively with GBR-12909 (2 μM) (T. Zhang, et al., 2009). Without inhibiting the DATs, the DA signal in control mice showed a shorter decay time compared to the Nurr1 +/− mice (Fig. 3C). When the peak of the DA signal was normalized in the Nurr1 +/− (black trace) and control (gray trace) mice, the much slower decay in the Nurr1 deficient mice was easily observed (black trace, Fig. 3C). When the DATs were inhibited, however, the normalized DA signals showed statistically similar falling phases (Fig. 3D): t90 was 20.1 ± 4.6 s (n = 10) in Nurr1 +/− mice and 17.7 ± 3.6 s (n = 15, p > 0.05) in control mice.
The preceding result predicts that DAT activity is higher in the control mice. With DAT activity inhibited (by GBR-12909), the peak single-pulse evoked DA signal was 59% smaller in Nurr1 +/− mice compared to control mice (3–6 months): 1.03 ± 0.14 μM (n = 10) in Nurr1 +/− mice and 2.48 ± 0.25 μM (n = 15, p < 0.01) in control mice (Fig. 3E,F). When DA reuptake is inhibited, there is a larger difference between control and Nurr1 +/− in the peak evoke DA concentration (47%, Fig. 3E,F) than is seen when there is no DAT inhibition (35%, Fig. 2A,B). This result is also consistent with higher DAT activity in control mice.
3.3. Age-dependent differences in DA release in the dorsal striatum
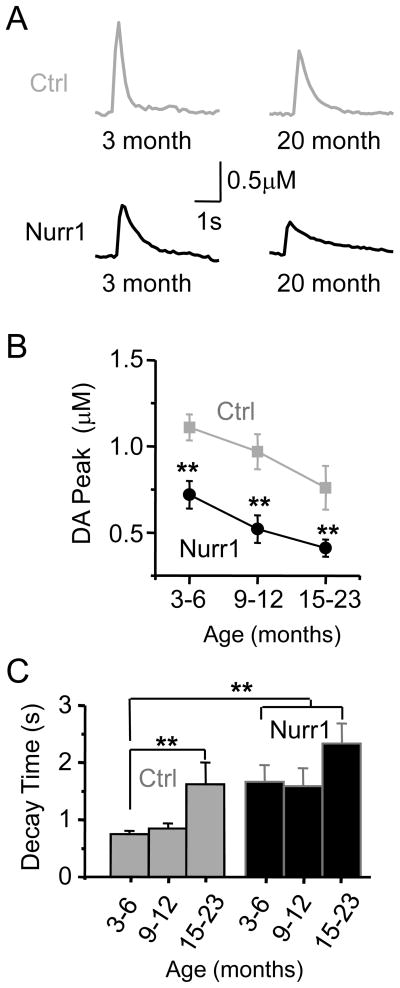
We further examined the relationship between age and DA release in the dorsal striatum using fast-scan cyclic voltammetry. An age-dependent decline in single-pulse evoked DA release was observed in both the Nurr1 +/− mice and the control mice (Fig. 4A). DA peak amplitudes in the dorsal striatum of Nurr1 +/− mice aged 3–6 months, 9–12 months, and 15–23 months were smaller than the age-matched, littermate controls (Fig. 4A, B): Nurr1 +/− mice (0.72 ± 0.08 μM, n = 30; 0.52 ± 0.08 μM, n = 16; and 0.41 ± 0.05 μM, n = 34), control mice (1.11 ± 0.07 μM, n = 38; 0.97 ± 0.08 μM, n = 15; and 0.76 ± 0.12 μM, n = 12). An analysis within each genotype showed that both the Nurr1 +/− mice (F (2,77) = 6.5, p < 0.01) and the control mice (F (2,62) = 3.2, p < 0.05) displayed a significant age-dependent decline in the amplitude of the DA signal. To observe better the changes in DA release amplitude, we normalized the average peak DA amplitude to the value in the 3–6 month control mice. When compared to the control value at 3–6 months, the DA release was reduced to a value of 68 ± 7% in the 15–23 month control mice, and it was reduced to 65 ± 7%, 47 ± 7%, and 37 ± 4% in the three respective age groups of Nurr1 +/− mice.
Fig. 4.
Age-dependent DA release in Nurr1 +/− and control mice slices. (A) Representative DA signals evoked by single pulse stimulation of slices from mice aged 3 months and 20 months. (B) The mean amplitude of the DA signal for each age group was significantly lower in the Nurr1 +/− mice (black data) compared to the control mice (gray data) (n = 12–38, ** p < 0.01). (C) The decay time (t90) was significantly longer across all age groups in the Nurr1 +/− mice when compared to the 3–6 month control. The decay time for the control mice aged 15–23 months was also significantly increased compared to the 3–6 month control.
The decay time (t90) for all Nurr1 +/− mice was significantly longer when compared to the 3–6 month control group (Fig. 4C). There was no significant difference in the decay time within the Nurr1 +/− groups. Interestingly, the decay time in the 15–23 month control mice (1.62 ± 0.38 s) increased significantly compared to the 3–6 month control mice (0.75 ± 0.06 s; F (2, 62) = 8.2, p < 0.01) (Ctrl, Fig. 4C). This result suggests that as the DA signaling declines with age in the control mice, then the uptake kinetics slow, as is seen from earlier ages with the Nurr1 +/− mice (as in Fig. 3).
3.4. Tonic and phasic DA signaling in Nurr1 +/− and +/+ mice
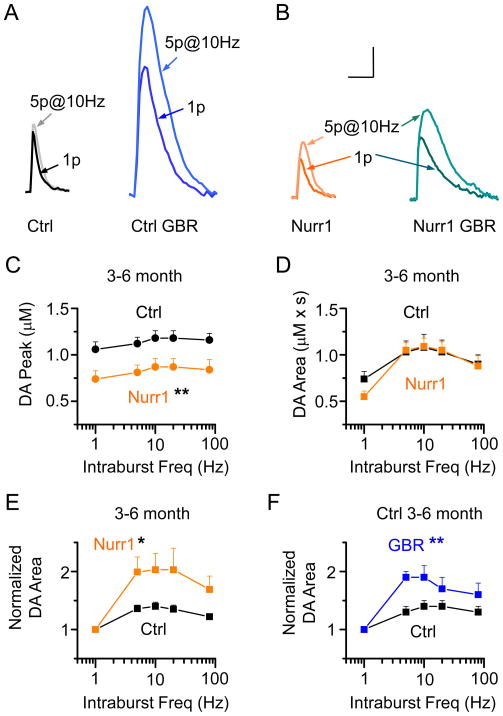
DA neurons fire individual action potentials and phasic bursts of action potentials that can be mimicked by experimentally applied stimulus protocols (Exley, et al., 2008;L. Zhang, et al., 2009). We stimulated the dorsal striatal slices with individual pulses or with trains of 5 pulses (5p) to characterize tonic and phasic patterns of DA release in Nurr1 +/− mice. In the control mice, a train of 5 pulses at 10 Hz did not significantly increase the DA peak amplitude compared to the single pulse evoked signal (118 ± 8 %, p > 0.05, n = 31; Ctrl, Fig. 5A), but modestly increased the area-under-curve to 140 ± 8 % (p < 0.05, Fig. 5A,D). In Nurr1 +/− mice, the DA area-under-curve evoked by the train (5p at 10 Hz) increased 203 ± 28 % (p < 0.01, n = 30; Nurr1, Fig. 5B,D) compared to the single pulse evoked DA area.
Fig. 5.
Frequency dependent DA release in control and Nurr1 +/− mice slices. (A) Representative DA signals evoked by 1-pulse (1p) and 5p trains (at 10 Hz) in the dorsal striatum in the control slices before (Ctrl) and after GBR application (0.2 μM) to control slices (Ctrl GBR, blue traces). (B) Representative DA signals evoked by 1-pulse (1p) and 5p trains (at 10 Hz) in the dorsal striatum in the Nurr1 +/− slices before (Nurr1, orange traces) and after GBR application (0.2 μM) to Nurr1 +/− slices (Nurr1 GBR, teal traces). The Nurr1 +/− and GBR control groups showed greater DA release in response to the 5p stimulation. Scale bars: 3s and 0.5 μM. (C) The DA release evoked by train stimulation (5p at 5–80 Hz) was significantly reduced in the Nurr1 mice compared to the control mice at the age of 3–6 months (n = 30, 31, respectively; ** p < 0.01). In each panel, the left most data points are 1p evoked DA signals, plotted for comparison with the stimulus trains. (D) The area-under-curve for the DA signal evoked by train stimulation was not significantly different between the control and Nurr1 +/− groups. (E) Normalizing the area of the train-evoked DA signal to the 1p evoked DA signal revealed a greater facilitation of DA release in Nurr1 +/− mice (orange data) compared to the control mice (black data). (F) Partial inhibition of the DA transporter using GBR (0.2 μM) on control mice mimicked the release pattern observed in the Nurr1 +/− mice (n = 12, 12; ** p < 0.01).
To examine the frequency range more completely, we applied 5 stimuli ranging in frequency from 5 to 80 Hz. In Nurr1 +/− mice, the peak amplitude of the DA signal was significantly smaller across all frequencies compared to the control (Fig. 5C; two-way ANOVA, p < 0.01). However, the area under the DA concentration curve, which better approximates the overall DA signal, was very similar in the Nurr1 +/− and control mice (Fig. 5D, p > 0.05). When the DA signal was normalized to the DA area evoked by a signal stimulus pulse (1p), then a stimulus train could be seen to cause greater facilitation by the Nurr1 +/− mice (orange data, Fig. 5E) as compared to control mice (black data, Fig. 5E).
Because the DA signal measured by the area under the concentration curve showed more stimulus-train facilitation for Nurr1 +/− mice, we reasoned that the effect could arise from decreased DAT activity causing a more prolonged DA signal. To mimic this possibility, we inhibited the DATs partially using a low concentration of GBR-12909 (0.2 μM) applied to slices from control mice (blue traces, Fig. 5A) and Nurr1 +/− mice (teal traces, Fig. 5B). Under those circumstances, the normalized DA area from the control mice showed similarly sized stimulus-train facilitation (blue data, Fig. 5A,F) to that seen in Nurr1 +/− mice. These findings suggest that decreased DAT activity could produce the altered stimulus-train facilitation of the DA signaling observed in the Nurr1 +/− mice.
3.5. Frequency dependent DA release as a function of age
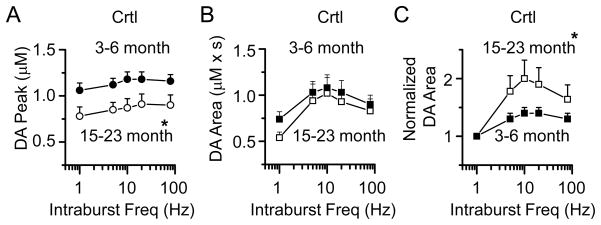
To investigate further the changes in DA signaling with age, we measured the frequency dependence of DA release in the mice across the three age groups. In the control, Nurr1 +/+ mice, the peak amplitude of the DA signal evoked by stimulus trains was significantly lower in the mice aged 15–23 month compared to the 3–6 month group (two-way ANOVA, p < 0.05; Fig. 6A). The DA signal represented by the area, however, was not statistically different between the groups (Fig. 6B). When the area of the DA signal was normalized to 1p, then the older animals did show a significant stimulus-train facilitation of DA release (white squares, Fig. 6C). This result suggests an age-related decline in DAT function to compensate for the age-dependent decrease of DA release with age.
Fig. 6.
Age-dependent DA signals in control (Nurr1 +/+) mice aged 3–6 months and 15–23 months. (A) The peak amplitude of the DA signal evoked by 1p (left most data points in each panel) or train stimulation (5p at 5–80 Hz) was significantly reduced in the 15–23 month old mice compared to the 3–6 month group (n = 19, 31; * p < 0.05). (B) The area-under-curve of the DA signals from control mice aged 15–23 months was not different from the 3–6 month group. (C) The normalized DA area (train evoked DA signal relative to the 1p evoked DA signal) was increased in the 15–23 month old mice compared to the 3–6 month old mice (* p < 0.05).
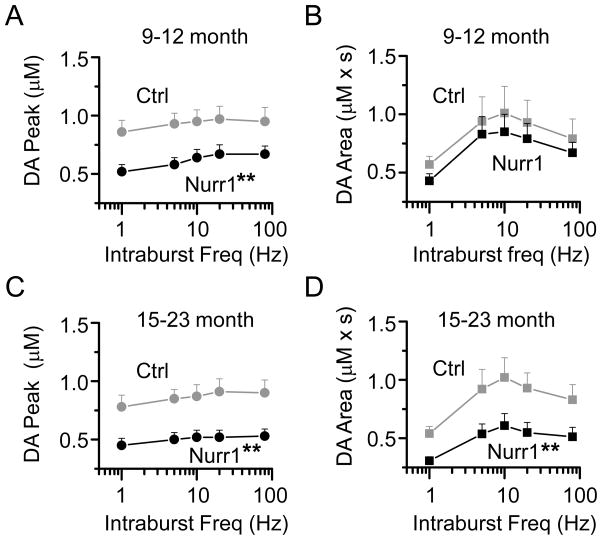
In the Nurr1 +/− mice aged 9–12 months, the peak amplitude of the DA signal was significantly lower than the age-matched littermate control mice (two-way ANOVA, p < 0.01) (Fig. 7A). However, the area of the DA signal was essentially unchanged between the 2 age groups (Fig. 7B), suggesting that the decreased DA release was compensated by decreased reuptake in the 9–12 month age group. The older Nurr1 +/− mice (15–23 months) showed a significantly lower peak amplitude (Fig. 7C) and a decrease in the area of the DA signal compared to the age-matched control mice (p < 0.05) (Fig. 7D). These data suggest that in the older Nurr1 +/− mice (15–23 months), decreased DA reuptake can no longer compensate for decreased DA release, resulting in suboptimal extracellular DA levels.
Fig. 7.
Differences in frequency-dependent DA release in the Nurr1+/− and control mice slices at 9–12 months and 15–23 months. (A) The DA signal evoked by train stimulation (5p at 5–80 Hz) was significantly decreased in the Nurr1 +/− mice aged 9–12 months compared to the control mice (n = 26, 17; ** p < 0.01). In each panel, the left most data points are 1p evoked DA signals, plotted for comparison with the stimulus trains. (B) The area of the DA signal evoked by train stimulation was not significantly different between the two groups. (C) The peak of the DA signal evoked by train stimulation was significantly decreased in the Nurr1 +/− mice aged 15–23 months compared to comparably aged control mice (n = 27, 19; ** p < 0.01). (D) The area of the DA signal was also reduced in the Nurr1 +/− mice aged 15–23 months compared to comparably aged control mice (n = 27, 19; ** p < 0.01).
4. Discussion
4.1. Nurr1 +/− have decreased DA release and DAT activity
Nurr1 is a nuclear receptor expressed in the nigral dopaminergic system and is essential for the induction and the maintenance of DA neuron function in adulthood (Chu, et al., 2006; Kadkhodaei, et al., 2009; Saucedo-Cardenas, et al., 1998). Nurr1 +/− mice display age-dependent morphological, biochemical, and behavioral phenotypes that resemble the progressive dopaminergic degeneration observed in PD (Imam, et al., 2005; Jiang, et al., 2005; Le, et al., 1999a; Le, et al., 1999b). In this study, we examined the changes in DA signaling that arise with age in the Nurr1 +/− mice.
The Nurr1 +/− mice show an accelerated age-dependent decline in DA neuron number and DA content in the striatum by 9–12 months of age, but a decrease in evoked DA release was observed at an earlier age (3–6 months). Interestingly, this significant decrease in the amplitude of the DA signal in Nurr1 mice was offset by slower reuptake kinetics. The DA signal showed a prolonged decay time, and because of that the area under the DA curve was unchanged at a young age. This decrease in reuptake allowed the DA to remain in the extracellular space longer, thereby prolonging the signal in a compensatory manner (Hebert and Gerhardt, 1999). As the DA release declined further in the older Nurr1 +/− mice (15–23 months), the decrease in DA reuptake could not sufficiently compensate for the impairment of DA release. These findings are consistent with the idea that Nurr1 gene is an essential, age-dependent genetic regulator of DA function during the development of PD (Jankovic, et al., 2005).
4.2. The interaction of age and Nurr1 gene on the onset of DA neuron loss
When compared with the wild type littermate controls (Nurr1 +/+), Nurr1+/− mice exhibited an accelerated age-dependent decline in DA neuron number and DA tissue levels in the striatum. Recent evidence has shown that Nurr1 functions to inhibit the expression of pro-inflammatory neurotoxic mediators in both microglia and astrocytes. The reduced expression of the Nurr1 gene may result in exaggerated inflammatory responses (Saijo, et al., 2009) and increased α-synuclein protein expression (Yang and Latchman, 2008), which could lead to DA cell death (Le, et al., 2009).
Interestingly, we found that the Nurr1+/− mice did not show a significant reduction in the number of TH neurons in young adult mice (3–6 months old). A significant decline in DA neurons (≈25% decline compared to wild type) occurred only after the Nurr1 +/− mice were 9–12 months old. This result suggests that there is an interaction between Nurr1 deficiency and the natural aging process. Several studies have shown that there is an age-dependent decrease in Nurr1 expression in wild-type animals (Saucedo-Cardenas, et al., 1998; Smits, et al., 2003; Zetterstrom, et al., 1997). Possibly, normal age-related decreases in Nurr1 expression are compounded by the deficiency of the Nurr1 gene in the heterozygous mice we studied.
4.3. Nurr1 gene influences striatal DA release preceding the decline of DA neurons
The estimated premotor period from the onset of neuronal loss in the SNc to the onset of PD motor symptoms is approximately 5 years in humans (Fearnley and Lees, 1991). In Nurr1 +/− mice, there is a meaningful decrease in the number of DA neurons at the age of 9–12 months, which is about 6 months earlier than the onset of motor deficits reported previously in Nurr1 deficient mice (Jiang, et al., 2005). This “premotor” period during which changes in the DA system occurred, could vary according to the species, the methodology employed, and the brain regions. In humans, changes in DA denervation and content in the nucleus caudatus can occur 20–30 years prior to the onset of any motor symptoms (Riederer and Wuketich, 1976; Scherman, et al., 1989). Therefore, it is possible that the period between the onset of pathological changes in PD and the onset of motor symptoms could be longer than previously estimated by classically tracking the loss of DA neuron number in the SNc.
In the Nurr1 +/− mice, decreased DA release was seen at the earliest age tested (3–6 months). At that age, the Nurr1 +/− mice exhibited a significant decrease in the amplitude of the evoked DA signal ( 35%) compared to the control, without showing a change in DA neuron number and striatal DA content. Although the mechanism arising from the Nurr1 deficiency that underlies this change is not known, other studies have shown that the Nurr1 gene may regulate the expression of DAT and TH (Sacchetti, et al., 2001; Sakurada, et al., 1999), which influence DA signaling. Our results indicate that the influence of the Nurr1 gene over striatal DA release is not initially due to a decline in the number of DA neurons in the SN. These findings are consistent with results showing that changes within the DA system can occur earlier than the onset of DA neuron loss (Collier, et al., 2007).
We observed an age-dependent decrease in striatal DA release in older control mice (15–23 months), which is consistent with the normal decline in DA function (Collier, et al., 2007; Hebert and Gerhardt, 1999). This situation was amplified in the Nurr1 deficient mice initially and as they aged (Fig. 4B). A decrease in basal extracellular DA release may serve as an early marker for the onset of DA dysfunction as compared to DA neuron loss (Abeliovich, et al., 2000; Collier, et al., 2007). These results also indicate the utility of voltammetric measurements in detecting the “premotor” symptoms of PD.
4.4. DA depletion induces distinct compensatory regulation of DA uptake
Although older Nurr1 +/− mice (15–23 months) show locomotor abnormalities (Jiang et al., 2005) and a 60% decline in the DA signal, these mice did not generally display any gross physical abnormalities such as tremor or dyskinesia. We hypothesize that the older Nurr1 mice that suffer from deficits in DA neuron number and DA release require a premorbid period that is longer than the life span of the mice. Another possibility is that during this premorbid period several compensatory processes serve to delay the onset of PD symptoms (Cruz-Muros, et al., 2007; Zigmond, 1997). Here, we provide evidence that compensatory alterations in DAT contribute to the delay in PD symptoms.
Two possibilities may account for the change in the DA signal’s decay time: alterations in DAT activity and non-specific diffusion factors. Changes in the diffusion factors seem unlikely because, when we blocked the DATs, the decay phases between the Nurr1+/− mice and the controls were similar. The DATs appear to be down-regulated in response to a decline in the striatal DA levels (Ma, et al., 1999; Salvatore, et al., 2003). We also observed a decline in DA release ( 30%) in the older wild-type mice (15–23 months). Similar to the Nurr1 +/− mice, the decay time was longer in older wild-type mice, which resulted in an area-under-curve for DA that was very similar to the young adults (3–6 months). Thus, the prolonged DA-signal decay time in Nurr1 +/− mice may reflect an adaptive change in DAT activity in response to the genetic manipulation. Consistent with that expectation, it was shown previously that DAT expression is decreased by 25% and 40% in young and old Nurr1+/− mice as compared with age-matched littermate (Nurr1 +/+) mice (Jiang, et al., 2005).
4.5 DAT-induced frequency dependency of DA release
Several studies show that the striatum regulates tonic and phasic DA release via local circuit mechanisms. Many regulatory factors (such as nicotinic acetylcholine receptors (nAChRs), D2 autoreceptors, and DAT activity) are involved in this process (Exley, et al., 2008; Venton, et al., 2003; L. Zhang, et al., 2009). In this study, the Nurr1 deficient mice showed greater facilitation in response to a stimulus train compared to the wild-type, littermate control mice. We hypothesize that this alteration is due, at least in part, to local regulation of DAT activity. Partial blockade of the DATs made the frequency dependence of DA release similar between the Nurr1+/− mice and the control mice, which suggests a prominent role for DAT in the compensatory changes in DA signaling associated with the Nurr1 +/− genotype.
In summary, our findings provide evidence that the Nurr1 gene regulates striatal DA release prior to the onset of classical PD symptoms, such as DA neuron loss. We also demonstrate an adaptation in DA clearance that may compensate for the decline in DA signaling that occurs in the earlier stages of PD. These results help to explain why the classical symptoms of PD do not appear in Nurr1 +/− mice until the decline in the DA system has reached an advanced stage. Because this age-related decline shares similarities to human PD, the Nurr1 +/− mice serve as a valuable animal model to study the age-dependent dopaminergic dysfunction relevant to PD.
Acknowledgments
This work was supported by grants from the National Institutes of Health: NINDS NS21229 and NIDA DA09411. This study also was supported by the Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine.
Footnotes
Disclosure Statement
There were no conflicts of interest for this study for any of the authors. The animal procedures were appropriate, and the mice were housed and handled in accordance with the guidelines set forth by the animal care committee at Baylor College of Medicine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Backman C, Perlmann T, Wallen A, Hoffer BJ, Morales M. A selective group of dopaminergic neurons express Nurr1 in the adult mouse brain. Brain Res. 1999;851(1–2):125–32. doi: 10.1016/s0006-8993(99)02149-6. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48(4):969–77. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci. 1998;11(1–2):36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- Chu Y, Le W, Kompoliti K, Jankovic J, Mufson EJ, Kordower JH. Nurr1 in Parkinson's disease and related disorders. J Comp Neurol. 2006;494(3):495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26(1):56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Muros I, Afonso-Oramas D, Abreu P, Barroso-Chinea P, Rodriguez M, Gonzalez MC, Hernandez TG. Aging of the rat mesostriatal system: differences between the nigrostriatal and the mesolimbic compartments. Exp Neurol. 2007;204(1):147–61. doi: 10.1016/j.expneurol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Cruz-Muros I, Afonso-Oramas D, Abreu P, Perez-Delgado MM, Rodriguez M, Gonzalez-Hernandez T. Aging effects on the dopamine transporter expression and compensatory mechanisms. Neurobiol Aging. 2009;30(6):973–86. doi: 10.1016/j.neurobiolaging.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33(9):2158–66. doi: 10.1038/sj.npp.1301617. 1301617 [pii] [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114 ( Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gaig C, Tolosa E. When does Parkinson's disease begin? Mov Disord. 2009;24(Suppl 2):S656–64. doi: 10.1002/mds.22672. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Han F, Panisset M, Racacho L, Xiao F, Zou R, Westaff K, Bulman DE. Translated mutation in the Nurr1 gene as a cause for Parkinson's disease. Mov Disord. 2006;21(7):906–9. doi: 10.1002/mds.20820. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Age-related changes in the capacity, rate, and modulation of dopamine uptake within the striatum and nucleus accumbens of Fischer 344 rats: an in vivo electrochemical study. J Pharmacol Exp Ther. 1999;288(2):879–87. [PubMed] [Google Scholar]
- Hermanson E, Joseph B, Castro D, Lindqvist E, Aarnisalo P, Wallen A, Benoit G, Hengerer B, Olson L, Perlmann T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp Cell Res. 2003;288(2):324–34. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Ichise M, Ballinger JR, Tanaka F, Moscovitch M, St George-Hyslop PH, Raphael D, Freedman M. Age-related changes in D2 receptor binding with iodine-123-iodobenzofuran SPECT. J Nucl Med. 1998;39(9):1511–8. [PubMed] [Google Scholar]
- Imam SZ, Jankovic J, Ali SF, Skinner JT, Xie W, Conneely OM, Le WD. Nitric oxide mediates increased susceptibility to dopaminergic damage in Nurr1 heterozygous mice. Faseb J. 2005;19(11):1441–50. doi: 10.1096/fj.04-3362com. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol. 2005;77(1–2):128–38. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wan X, He Y, Pan T, Jankovic J, Le W. Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Exp Neurol. 2005;191(1):154–62. doi: 10.1016/j.expneurol.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wan X, Jankovic J, Christian ST, Pristupa ZB, Niznik HB, Sundsmo JS, Le W. Dopaminergic properties and experimental anti-parkinsonian effects of IPX750 in rodent models of Parkinson disease. Clin Neuropharmacol. 2004;27(2):63–73. doi: 10.1097/00002826-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Jonec V, Finch CE. Ageing and dopamine uptake by subcellular fractions of the C57BL/6J male mouse brain. Brain Res. 1975;91(2):197–215. doi: 10.1016/0006-8993(75)90543-0. [DOI] [PubMed] [Google Scholar]
- Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, Chambon P, Lindqvist E, Larsson NG, Olson L, Bjorklund A, Ichinose H, Perlmann T. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29(50):15923–32. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–6. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Le W, Chen S, Jankovic J. Etiopathogenesis of Parkinson disease: a new beginning? Neuroscientist. 2009;15(1):28–35. doi: 10.1177/1073858408319974. 1073858408319974 [pii] [DOI] [PubMed] [Google Scholar]
- Le W, Conneely OM, He Y, Jankovic J, Appel SH. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. J Neurochem. 1999a;73(5):2218–21. [PubMed] [Google Scholar]
- Le W, Conneely OM, Zou L, He Y, Saucedo-Cardenas O, Jankovic J, Mosier DR, Appel SH. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol. 1999b;159(2):451–8. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33(1):85–9. doi: 10.1038/ng1066. ng1066 [pii] [DOI] [PubMed] [Google Scholar]
- Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999;409(1):25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64(3):314–9. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54(1):30–3. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38(3–4):277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res. 1990;508(2):349–52. doi: 10.1016/0006-8993(90)90423-9. [DOI] [PubMed] [Google Scholar]
- Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76(5):1565–72. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. S0092-8674(09)00086-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126(18):4017–26. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Apparsundaram S, Gerhardt GA. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging. 2003;24(8):1147–54. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A. 1998;95(7):4013–8. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D, Desnos C, Darchen F, Pollak P, Javoy-Agid F, Agid Y. Striatal dopamine deficiency in Parkinson's disease: role of aging. Ann Neurol. 1989;26(4):551–7. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87(2):273–89. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Smits SM, Ponnio T, Conneely OM, Burbach JP, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18(7):1731–8. doi: 10.1046/j.1460-9568.2003.02885.x. 2885 [pii] [DOI] [PubMed] [Google Scholar]
- Sossi V, de la Fuente-Fernandez R, Schulzer M, Adams J, Stoessl J. Age-related differences in levodopa dynamics in Parkinson's: implications for motor complications. Brain. 2006;129(Pt 4):1050–8. doi: 10.1093/brain/awl028. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Mash D, Faucheux B, Javoy-Agid F. Dopamine transporter messenger RNA in Parkinson's disease and control substantia nigra neurons. Ann Neurol. 1994;35(4):494–8. doi: 10.1002/ana.410350421. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87(5):1284–95. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- von Euler G, Hedlund PB. Regional and age-dependent differences in the affinity of dopamine D2 agonist binding in the rat brain. Neurochem Int. 1995;26(4):397–410. doi: 10.1016/0197-0186(94)00136-i. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30(1):56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yang YX, Latchman DS. Nurr1 transcriptionally regulates the expression of alpha-synuclein. Neuroreport. 2008;19(8):867–71. doi: 10.1097/WNR.0b013e3282ffda48. 00001756-200805280-00015 [pii] [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–50. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res. 1996;41(1–2):111–20. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76(2):396–404. doi: 10.1124/mol.109.056317. mol.109.056317 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29(13):4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. 29/13/4035 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4(12):1224–9. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis. 1997;4(3–4):247–53. doi: 10.1006/nbdi.1997.0157. [DOI] [PubMed] [Google Scholar]