Abstract
Activation of the dopaminergic system underlies the behavioral effects of (+)-amphetamine, and plays the major role in its discriminative stimulus properties. Although serotonin receptors modulate dopamine levels in the brain, and 5-HT1A and 5-HT2 receptor agonists do not mimic (+)-amphetamine, pretreatment with 5-HT2A/2C agonists significantly potentiates the (+)-amphetamine cue. Further, 5-HT2 antagonists do not modify the discriminative stimulus effect of (+)-amphetamine, but 5-HT1A antagonists have never been tested in (+)-amphetamine-trained rats. The present study sought to characterize the effects of the 5-HT1A antagonist WAY 100635 on (+)-amphetamine-induced discriminative stimulus effects. Male Sprague-Dawley rats were trained in a two-lever, fixed ratio (FR) 50, food-reinforced task with (+)-amphetamine sulfate (1.0 mg/kg, i.p., 30 min pretreatment time) as the discriminative stimulus. Substitution and combination tests with WAY 100635 were then performed. WAY 100635 did not produce substitution in amphetamine-trained rats, but significantly increased behavioral disruption. In combination tests 0.4 and 5.4 mg/kg doses of WAY 100635 potentiated the amphetamine cue. We suggest that low doses of WAY 100635 potentiated the (+)-amphetamine cue by blockade of 5-HT1A receptors, but stimulation of the dopamine D4 receptor by higher doses of WAY 100635 may be responsible for potentiation of amphetamine-induced behavioral sensitization. The high percentage of behavioral disruption in substitution tests might suggest that rats trained to discriminate (+)-amphetamine from saline show behavioral sensitization that is not detectable by the drug discrimination assay but may be expressed as hyperactivity and stereotypic behavior that disrupts operant behavior.
Keywords: WAY 100635, (+)-amphetamine, behavioral sensitization, drug discrimination, rat, locomotor activity, flex-field, dopamine D4 receptor, 5-HT2A
INTRODUCTION
The monoaminergic psychomotor stimulant drug, amphetamine, increases extracellular levels of synaptic dopamine (DA) in the mesocorticolimbic dopaminergic system and thus indirectly serves as a dopaminergic agonist. It is a useful medication in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, excessive daytime sleepiness, and obesity (Howell and Kimmel 2008). The dopamine transporter plays a primary role in the reinforcing and behavioral-stimulant effects of amphetamine in both laboratory animals and humans (Howell and Kimmel 2008), although amphetamine also is a substrate for the other monoamine (SERT, NET) and vesicular monoamine (VMAT) transporters.
The most prominent acute behavioral effects of amphetamine are an increase in locomotor activity, development of behavioral stereotypy, anorexia, and hyperthermia. Amphetamine-induced hyperlocomotion and stereotypy are progressively augmented by repeated administration of amphetamine (Kuczenski et al. 1982, Post et al. 1980, Robinson and Becker 1986, Paulson and Robinson 1991, Robinson and Berridge 2000), an effect known as behavioral sensitization, which, once established, is persistent and is thought to represent a permanent change in the neurobiology of the organism (Kalivas and Stewart 1991). Repeated administration of amphetamine, however, also results in the development of tolerance to some of the amphetamine-induced effects, including anorexia and stereotypy (Demellweek and Goudie 1983, Lewander 1971, Steigerwald et al. 1994), as well as to its discriminative stimulus properties (Barrett et al. 1981).
Although the reinforcing and behavioral stimulant effects of amphetamine do not appear to depend directly on serotonergic systems, the results of drug interaction studies clearly demonstrate that pharmacological modulation of serotonergic systems can alter the behavioral and neurochemical effects of amphetamine (West et al. 1995). In general, the blockade of central 5-HT transmission potentiates the stereotypic behavior induced by amphetamine (Dickson and Curzon 1983). Recently, several studies have been focused on the modulatory role of 5-HT2 receptors in controlling the dopaminergic effects of psychostimulants. For example, data have been reported showing that 5-HT2A receptor activation increases the release of dopamine into the synaptic cleft, whereas 5-HT2C receptor activation inhibits it (Alex and Pehek 2007, Popova et al. 2010). We previously have shown that pre-administration, but not coadministration, of 5-HT2A/2C agonists to rats potentiates the discriminative cue of amphetamine (Marona-Lewicka and Nichols 1997).
Activation of 5-HT1A somatodendritic autoreceptors located on serotonergic cells in the raphe nuclei acutely decreases forebrain 5-HT, blocks amphetamine-induced hyperactivity (Przegalinski et al. 1997), and also attenuates the increase in limbic forebrain dopamine levels produced by amphetamine administration (Ichikawa et al. 1995, Kuroki et al. 1996). Similarly, coadministration of a 5-HT1A agonist with psychostimulants (amphetamine, methamphetamine, or methylphenidate) produces an antihyperkinetic and/or calming effect in mice (Tsuchida et al. 2009), consistent with a decreased ability of amphetamine to increase dopamine levels.
Although (+)-amphetamine is widely used in drug discrimination studies, sensitization to the discriminative stimulus effect of (+)-amphetamine has never been reported with the standard dosing regimen (two to three times per week). Nonetheless, discrimination training with (+)amphetamine in pigeons, followed by suspension of training while administering repeated high doses of (+)-amphetamine, resulted in an approximately 7-fold increase in the dose of (+)amphetamine required for amphetamine-associated key responding, demonstrating development of tolerance to the discriminative stimulus effects of (+)-amphetamine (Young et al. 1992). In rats, tolerance to the discriminative stimulus effect of (+)-amphetamine has also been observed, but only after training was suspended while amphetamine treatment was administered, followed again by discrimination training (Caul et al. 1989). We hypothesized that during drug discrimination training with amphetamine some plasticity should occur (i.e. sensitization) that might modify behavioral responses, but that such adaptation would be hard to detect using the drug discrimination assay.
In all drug discrimination studies, qualitative and quantitative aspects of the training drug cue are a function of a number of variables, including the specific drug, the specific dose, and the route and timing of administration. Using the drug discrimination paradigm, one can study the degree to which other drugs produce cue states similar to the training drug cue, as well as the degree to which other drugs might block or potentiate the training drug-induced cue in combination tests. Thus, 5-HT2A/2C agonists do not substitute for amphetamine, nor do 5-HT2 antagonists block the amphetamine cue (West et al. 1995), but pretreatment with 5-HT2A/2C agonists produces a leftward shift in the amphetamine dose-response cue (Marona-Lewicka and Nichols 1997). Pre-administration or coadministration of 5-HT1A agonists had no effect on the amphetamine cue, nor did 5-HT1A agonists substitute for amphetamine (Przegalinski et al. 1997), but there are no data reported regarding any influence of 5-HT1A antagonists on the amphetamine cue in the drug discrimination assay.
WAY-100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl-N-(2-pyridinyl)cyclo-hexanecarboxamide trihydrochloride) is a 5-HT1A antagonist ligand that has been widely used over the past decade (Fletcher et al. 1996) and its effect in behavioral studies is well documented. For example, in drug discrimination experiments, WAY-100635 potently blocked the cue produced by full or partial 5-HT1A agonists (Gommans et al. 2000, De Vry et al. 1998, Kleven and Koek 1998, Marona-Lewicka and Nichols 2004, Sanchez et al. 1996). Nevertheless, we recently demonstrated that at higher doses WAY 100635 produces a robust discriminative stimulus that is insensitive to 5-HT1A agonist or antagonist treatment, but which can be blocked by dopamine D4 antagonists (Marona-Lewicka and Nichols, 2009). Moreover, WAY100635 potentiates the temporally-delayed interoceptive cue produced by LSD that is mediated by activation of dopamine D4 receptors (Marona-Lewicka et al. 2009). Further, WAY 100635 is a potent and efficacious dopamine D4 agonist in heterologous cell expression systems (Chemel et al. 2006), although Martel et al. (2007) have emphasized that WAY-100635 has much higher selectivity for the h5-HT1A versus the dopamine D4 receptor.
Thus, we decided to test WAY 100635 in substitution and combination tests in rats trained to discriminate (+)-amphetamine from saline. For combination tests we chose doses of WAY 100635 previously employed in the drug discrimination assay in our laboratory (Marona-Lewicka and Nichols 2004, 2009): 0.4 mg/kg as the dose that completely blocks a 5-HT1A-mediated discriminative cue, and 5.4 mg/kg as the dose that generated a robust discriminative stimulus effects in rats that is mediated by activation of dopamine D4 receptors (Marona-Lewicka and Nichols 2009). We decided also to test several dopamine D4 receptor selective compounds in substitution and combination tests in (+)-amphetamine-trained rats.
METHODS
Subjects
Male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 180–200 g at the beginning of the study were used as subjects. Rats were trained to discriminate saline from S-(+)-amphetamine sulfate (SAMP; 5.4 μmol/kg, 1.0 mg/kg, i.p.) with a 30 min pretreatment time, using a two-lever, food-reinforced operant conditioning task. All experimental conditions and the feeding procedure were described in detail in our previous paper (Marona-Lewicka and Nichols 2007). None of the rats had previously received drugs or behavioral training. Water was freely available in the individual home cages and a rationed amount of supplemental feed (LabDiet-5001, PMI, Nutrition International, LLC, Brentwood, MO) was made available after experimental sessions so as to maintain rats at approximately 80% of their free-feeding weight. Lights were on from 07:00 to 19:00h. The laboratory and animal facility temperature was 22–24 °C and the relative humidity was 40–50%. Experiments were performed between 09:00 and 17:00 each day, Monday–Friday
Apparatus
Six standard operant conditioning chambers (model E10-10RF, Coulbourn Instruments, Lehigh Valley, PA) consisted of modular test cages enclosed within sound-attenuated cubicles with fans for ventilation, and background white noise. A white house light was centered near the top of the front panel of the cage, which also was equipped with two response levers, separated by a food hopper (combination dipper pellet trough, model E14-06, module size 1/2), all positioned 2.5 cm above the floor. Solid state logic in an adjacent room, interfaced through a Med Associates (Lafayette, IN) interface to a personal computer, controlled reinforcement and data acquisition with locally written software.
Procedure
A fixed ratio (FR) 50 schedule of food reinforcement (45 mg dustless pellets, Research Diets, Inc., NJ) in a two-lever paradigm was used. The details of the drug discrimination procedure have been described elsewhere. (Marona-Lewicka and Nichols 1994). At least one drug and one saline session separated each test session. Rats were required to maintain the 85% correct responding criterion on training days in order to be tested. In addition, test data were discarded when the accuracy criterion of 85% was not achieved on either of the two training sessions following a test session. Training sessions lasted 15 min, and test sessions lasted 5 min and were run under conditions of extinction, with rats removed from the operant chamber when 50 presses were emitted on either lever. In a test session, if 50 presses on one lever were not completed within 5 min the session was ended and scored as a disruption. For substitution tests, drugs were administered i.p. 30 min prior to test sessions. Combination tests were carried out by administering two different doses of WAY 100635 (0.4 or 5.4 mg/kg) 30 min before different doses of the training drug, that is, 60 min before tests.
Drugs
(+)-Amphetamine sulfate used as the training drug (1 mg/kg; 5.4 μmol/kg) was purchased from Sigma (St. Louis, MO). WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide) (Forster et al. 1995), A-36958 ([2-[4-(2-cyanophenyl)-1-piperazinyl]-N-(3-methylphenyl) acetamide), ABT-724 (2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole), and A-381393 (2-[4-(3,4-dimethylphenlyl)piperazin-1-ylmethyl]-1H benzoimidazole) were synthesized in our laboratory, and L-745870 (3-([4-(4-chlorophenyl)piperazin-1-yl]methyl)-1H-pyrrolo[2,3-b]pyridine) was purchased from TOCRIS (Ellisville, MO). All drug solutions were prepared by dissolving the compounds in sterile saline (0.9% NaCl) at a concentration that allowed the appropriate dose to be given in a volume of 1 ml/kg, identical to the volume of the saline injection.
Data Analysis
Data from the drug discrimination study were scored in a quantal fashion, with the lever on which the rat first emitted 50 presses in a test session scored as the “selected” lever. The percentage of rats selecting the drug lever (%SDL) for each dose of test compound was determined. The method of Litchfield and Wilcoxon (1949) was used to determine the ED50 and 95% confidence interval (95% C.I.). All dose-dependent curves of (+)-amphetamine used for determination of ED50s were later tested for parallelism using a locally-written computer program. For a comparison of response rates, the mean and SEM were calculated for all (+)amphetamine-trained rats from 22 consecutive drug or saline training sessions (data published in Marona-Lewicka et al. 2005). Student’s t-test was employed for statistical comparison of response-rates between saline and (+)-amphetamine training sessions.
RESULTS
Our colonies of rats trained to discriminate (+)-amphetamine from saline generated a dose-dependent discriminative stimulus effect that was stable over time. Table 1 presents examples of ED50 values calculated twice per year for the last five years for (+)-amphetamine-trained rats. All dose-dependent curves from which the ED50 was calculated were parallel to each other (lack of significant difference between slopes) and none of the ED50 values are significantly different.
Table 1.
The ED50 values and 95% Confidence Limits (95% C.L.) for the past five years, calculated twice per year for the same group of rats, trained to discriminate (+)-amphetamine (1 mg/kg; 5.4 μmol/kg, i.p.) from saline. N = 8–16 rats per group. “a” – the ED50 calculated from tests immediately after rats passed the criterion for accuracy (see details in Methods section), and “b” –Calculated based on results from tests run 6–8 months later for the same group of rats. The ED50 was calculated according to the method of Litchfield and Wilcoxon (1949).
| Year | ED50 | 95% C.L. | ED50 | 95% C.L. |
|---|---|---|---|---|
| μmol/kg | mg/kg | |||
| 2004a | 1.31 | 0.88–1.97 | 0.24 | 0.16–0.37 |
| 2004b | 1.34 | 0.93–1.95 | 0.24 | 0.17–0.36 |
| 2005a | 1.55 | 1.04–2.31 | 0.29 | 0.19–0.46 |
| 2005b | 1.60 | 1.03–2.20 | 0.30 | 0.19–0.46 |
| 2006a | 1.43 | 0.93–2.21 | 0.26 | 0.17–0.41 |
| 2006b | 1.77 | 1.42–2.22 | 0.33 | 0.26–0.41 |
| 2007a | 1.23 | 0.81–1.85 | 0.23 | 0.15–0.34 |
| 2007b | 1.25 | 0.84–1.87 | 0.23 | 0.16–0.35 |
| 2008a | 1.31 | 0.89–1.22 | 0.24 | 0.17–0.34 |
| 2008b | 1.45 | 0.89–2.38 | 0.27 | 0.16–0.44 |
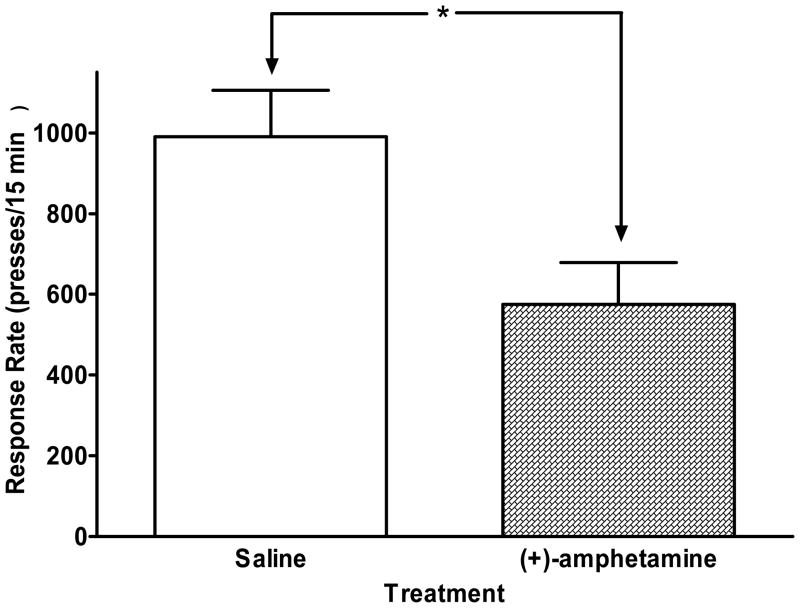
Rats trained to discriminate (+)-amphetamine administered 30 min before training, however, emitted fewer presses per training session after drug administration than after saline treatments (Fig 1., p < 0.01, Student’s t test, (+)-amphetamine-training vs. saline-training comparison). Similar response rate data in amphetamine-trained rats as of 2004 were presented as part of Fig 1 in Marona-Lewicka et al. (2005), where we showed a comparison of response rates for several training drugs used in our laboratory.
Figure 1.
Comparison of the mean response rate for rats trained to discriminate (+)-amphetamine (5.4 μmol/kg; 1 mg/kg) from saline, calculated from 22 consecutive sessions for each treatment. The open bar represents the number of presses after saline pretreatment during 15 min training sessions; the filled bar shows the number of presses after treatment with (+)-amphetamine, taken from 15 min training sessions. Similar response rate data as of 2004 were presented in Marona-Lewicka et al (2005). n = 12. *p < 0.01 (Student’s t-test).
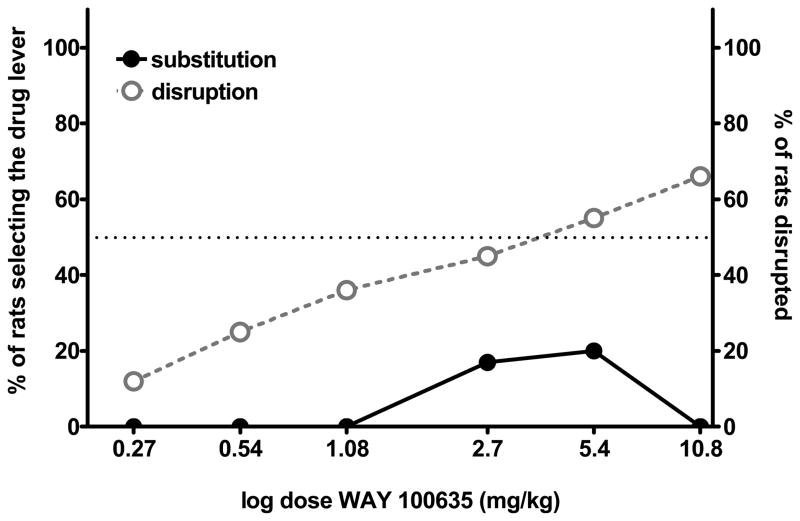
Figure 2 shows the results of substitution tests for WAY 100635 in (+)-amphetamine-trained rats. WAY 100635 did not mimic (+)-amphetamine at any of the doses tested (20% of maximum %SDL at 5.4 mg/kg), although WAY 100635 produced a dose-dependent increase in behavioral disruption. At WAY 100635 doses of 5.4 mg/kg and higher, more than half of the (+)-amphetamine-trained rats were unable to emit 50 presses during the 5-min test session. All rats treated with WAY 100635 were hyperactive and hyper-reflexive, with intense stereotypic sniffing; this hyperactivity is the basis for the inability of the rats to respond in the operant task. Importantly, in amphetamine-naïve rats, WAY 100635 has no such locomotor stimulant effects (Marona-Lewicka and Nichols, 2009). Table 2 shows results from substitution tests for two selective dopamine D4 receptor agonists, A-36958 and ABT-724. Neither D4 agonist mimicked (+)-amphetamine at any of the doses tested, nor did they induce significant disruption of behavior (Table 2).
Figure 2.
Results from substitution tests with WAY 100635 performed in rats trained to discriminate (+)-amphetamine (1 mg/kg; 5.4 μmol/kg, i.p.) from saline. WAY 100635 was administered 30 min before tests. n = 8–12 rats per data point. The right vertical axis and gray-open symbols show the percentage of rats disrupted.
Table 2.
Results from substitution tests of selective dopamine D4 receptor agonists A-36958 and ABT-724 in rats trained to discriminate (+)-amphetamine (1 mg/kg; 5.45 μmol/kg, i.p.) from saline. N – Number of rats tested; %D – percentage of rats disrupted; %SDL – percentage of rats selecting the drug appropriate level.
| TEST DRUG | DOSE
|
N | % D | % SDL | |
|---|---|---|---|---|---|
| mg/kg | μmole/kg | ||||
| A-36958 | 0.11 | 0.3 | 9 | 0 | 0 |
| 0.19 | 0.5 | 11 | 9 | 0 | |
| 0.37 | 1.0 | 9 | 22 | 0 | |
|
| |||||
| ABT-724 | 0.040 | 0.1 | 9 | 33 | 0 |
| 0.080 | 0.2 | 9 | 22 | 0 | |
| 0.205 | 0.5 | 11 | 27 | 0 | |
| 0.410 | 1.0 | 10 | 30 | 14 | |
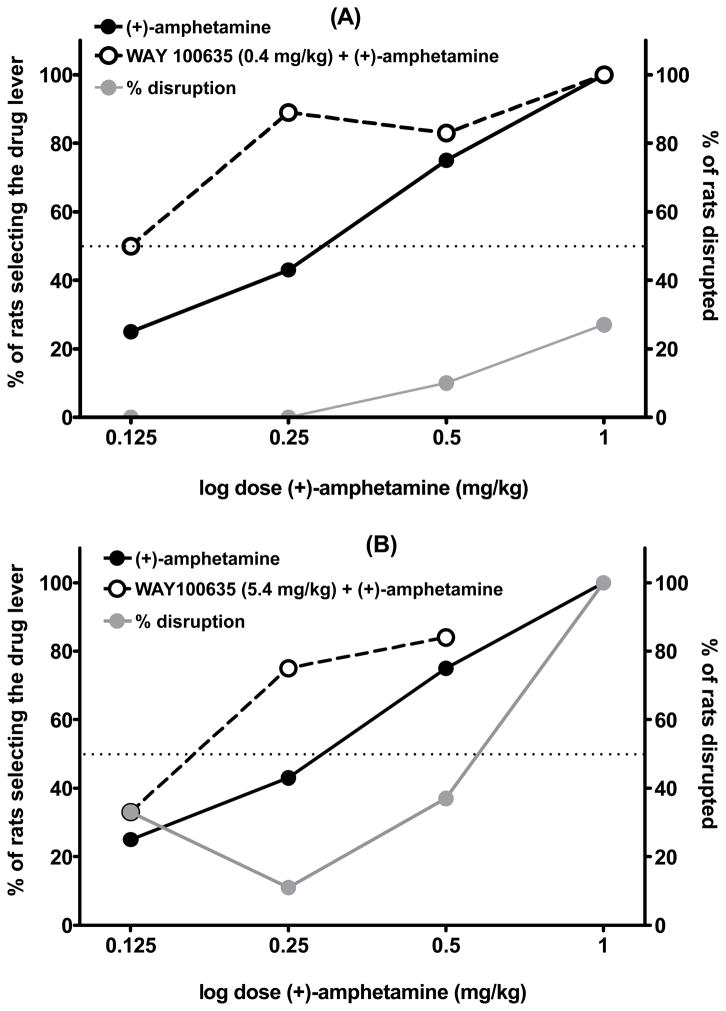
Results from combination tests with two different doses of WAY 100635 are presented in Figure 3. Fig 3A shows a dose-response curve for 0.4 mg/kg of WAY 100635 injected 30 min before different doses of (+)-amphetamine, and Fig 3B represents the combination of 5.4 mg/kg of WAY 100635 with different doses of (+)-amphetamine. Both doses of WAY 100635 increased selection of the drug appropriate lever compared to (+)-amphetamine alone. Combination of the low dose of WAY 100635 with lower doses of amphetamine (0.125 and 0.25 mg/kg) potentiated the discriminative stimulus effect without significantly affecting response rate. The training dose of amphetamine was not affected by pretreatment with 0.4 mg/kg of WAY 100635 because amphetamine produced a ceiling response at this dose: 100% selection of the drug appropriate lever. Interestingly, however, response rates were elevated up to 26% by this combination. The ED50 for the combination of 0.4 mg/kg of WAY 100635 with (+)amphetamine is 0.11 (95% C.L., 0.06–0.22) mg/kg, and for the combination of 5.4 mg/kg of WAY 100635 with (+)-amphetamine is 0.14 (95% C.L., 0.08–0.25). In both cases the ED50 values are lower than for (+)-amphetamine alone (see table 1; ED50 = 0.24, 95% C.L. 0.17–0.34 mg/kg), although neither result is significantly different compared to (+)-amphetamine alone. Pretreatment with 5.4 mg/kg of WAY 100635, followed by the training dose of amphetamine, produced 100% disruption; that is, none of the animals tested were able to emit the required 50 presses during the 5 min test period. All rats receiving this combination showed visible hyperactivity and stereotypic behavior that lasted for at least one hour after the test period (data not shown).
Figure 3.
Results of tests with different doses of (+)-amphetamine combined with 0.4 mg/kg (0.74μmol/kg) WAY 100635 (A, upper panel), or with 5.4 mg/kg (10 μmol/kg) of WAY 100635 (B, lower panel) in rats trained to discriminate (+)-amphetamine (1 mg/kg; 5.4 μmol/kg, i.p.) from saline. WAY 100635 was administered 30 min prior to (+)-amphetamine. Right Y axis and gray symbols show the percentage of rats disrupted. n = 8–12 rats per data point. For the combination of (+)-amphetamine with saline, or (+)-amphetamine alone, the percentage of rats disrupted was always 0% for all doses of (+)-amphetamine.
Results from combination tests with the D4 agonist ABT-724, and the two D4 antagonists A-381393 and L-745870 are presented in Table 3. Administration of ABT-724 prior to amphetamine significantly potentiated drug appropriate responding without increasing the percentage of disruption, and significantly lowered the ED50 compared to the ED50 calculated for amphetamine alone. The D4 antagonists L-745870 and A-381393 partially blocked the discriminative stimulus effects of amphetamine without producing a significant disruptive effect. L-745870 produced a maximum 60% inhibition at 3 mg/kg, whereas A-381393 was able to inhibit the maximum effect of amphetamine response by only 33% (Table 3).
Table 3.
Results from combination tests of the D4 dopamine receptor agonist ABT-724 (0.08 mg/kg; 0.2μmol/kg), and two selective D4 receptor antagonists A-381393 (3.93 mg/kg; 10 μmol/kg) and L-745870 (3 mg/kg; 6.88 μmol/kg), with different doses of (+)-amphetamine, tested in rats trained to discriminate (+)-amphetamine (1 mg/kg; 5.45 μmol/kg, i.p.) from saline. N – number of rats tested; %D – percentage of rats disrupted; %SDL – percentage of rats selecting the drug appropriate level. The ED50 was calculated according to the method of Litchfield and Wilcoxon (1949).
| TEST DRUG | DOSE OF COMBINED DRUG
|
DOSE OF (+)-AMPHETAMINE
|
N | % D | % SDL | ED50 (95% C.I.)
|
|||
|---|---|---|---|---|---|---|---|---|---|
| mg/kg | μmole/kg | mg/kg | μmole/kg | mg/kg | μmole/kg | ||||
| saline | 0 | 0 | 0.13 | 0.68 | 11 | 0 | 18 | 0.24 (0.17–0.36) | 1.34 (0.93–1.95) |
| 0 | 0 | 0.25 | 1.35 | 11 | 0 | 55 | |||
| 0 | 0 | 0.50 | 2.70 | 11 | 0 | 73 | |||
| 0 | 0 | 1.00 | 5.40 | 11 | 0 | 100 | |||
|
| |||||||||
| ABT-724 | 0.08 | 0.2 | 0.13 | 0.68 | 7 | 0 | 71 | 0.09 (0.04–0.22) | 0.49 (0.22–1.19) |
| 0.08 | 0.2 | 0.25 | 1.35 | 9 | 33 | 100 | |||
| 0.08 | 0.2 | 0.50 | 2.70 | 10 | 10 | 100 | |||
| 0.08 | 0.2 | 1.00 | 5.40 | 12 | 17 | 100 | |||
|
| |||||||||
| L-745870 | 3.0 | 6.88 | 0.13 | 0.68 | 11 | 0 | 0 | ||
| 3.0 | 6.88 | 0.25 | 1.35 | 10 | 0 | 20 | |||
| 3.0 | 6.88 | 0.50 | 2.70 | 10 | 0 | 30 | |||
| 3.0 | 6.88 | 1.00 | 5.40 | 11 | 9 | 40 | |||
|
| |||||||||
| A-381393 | 3.93 | 10 | 0.13 | 0.68 | 11 | 0 | 27 | ||
| 3.93 | 10 | 0.25 | 1.35 | 11 | 0 | 36 | |||
| 3.93 | 10 | 0.50 | 2.70 | 11 | 27 | 38 | |||
| 3.93 | 10 | 1.00 | 5.40 | 11 | 18 | 67 | |||
DISCUSSION
Drugs that modify both serotonergic and dopaminergic functions may have profound psychopharmacological properties, and can modulate psychostimulant-mediated effects, even if they themselves do not actually mimic the psychostimulant-mediated cue in drug discrimination experiments (Marona-Lewicka and Nichols 1997, Muller et al. 2007). WAY 100635 possesses very potent 5-HT1A receptor antagonist properties, with about 10-fold weaker agonism at dopamine D4 receptors (Chemel et al. 2006). Although the low-dose 5-HT1A antagonist properties of WAY 100635 in drug discrimination studies are well documented (Gommans et al. 2000, De Vry et al. 1998, Kleven and Koek 1998, Marona-Lewicka and Nichols 2004, Sanchez et al. 1996), doses of WAY 100635 only about ten-fold higher produce a robust discriminative stimulus effect in rats that is mediated by activation of dopamine D4 receptors (Marona-Lewicka and Nichols, 2009).
There are only a few drug discrimination studies reporting tests of 5HT1A or dopamine D4 agents in amphetamine-trained animals. For example, the discriminative stimulus properties of (+)-amphetamine in rats were not modulated by the 5-HT1A agonist, 8-OH-DPAT (Przegalinski and Filip 1997). This result was later confirmed by Young et al. (2006), where 8-OH-DPAT neither generalized to (+)-amphetamine when given alone, nor antagonized its stimulus effects when given in combination. In a rhesus monkey study, buspirone, gepirone, and 8-OH-DPAT failed to mimic (+)-amphetamine, but all of these 5-HT1A receptor agonists inhibited the discriminative stimulus properties of (+)-amphetamine (Nader and Woolverton 1994). Most studies, however, have reported that 5-HT1A agonists do not substitute for psychostimulant drugs, nor do 5-HT1A antagonists modulate the discriminative stimulus properties of psychostimulants.
We report here that WAY 100635 did not mimic (+)-amphetamine in substitution tests over a range of doses (0.27–10.8 mg/kg); however, in combination tests it potentiated the discriminative stimulus effect produced by (+)-amphetamine. Similar results were obtained for the selective dopamine D4 receptor agonist ABT-724 tested in substitution and combination tests in (+)-amphetamine trained rats, without producing any significant disruption of behavior. The lowest dose of WAY 100635 used in the present experiments was shown previously to be the most effective (100% inhibition) in blocking a 5-HT1A-mediated cue (Marona-Lewicka and Nichols 2004). The highest dose of WAY 100635 used in our combination tests served effectively as a training drug and generated a cue mediated by D4 dopamine receptor activation (Marona-Lewicka and Nichols 2009). Thus, we suggest that the 5-HT1A antagonist effect of low doses of WAY 100635 is responsible for the potentiation of the (+)-amphetamine cue, without affecting response rates, whereas the highest dose of WAY 100635 not only blocked 5-HT1A receptors, but also effectively stimulated dopamine D4 receptors, an effect that became evidentwhen rats showed marked hyperactivity and stereotypic behavior during the drug discrimination tests. We note that these robust changes in behavior significantly interfered with operant behavior; they were visible only in animals exposed for longer times to (+)-amphetamine but not in naïve rats treated acutely with a combination of WAY 100635 and (+)-amphetamine (data not shown).
WAY 100635 is a potent antagonist at pre- and postsynaptic 5-HT1A receptors, and lacks affinity at other 5-HT receptors (Fletcher et al 1996). Although 5-HT1A antagonists induce only mild behavioral activation in rats (Jackson et al. 1998), WAY 100635 significantly increased behaviors resembling those induced by dopamine D1 and D2 agonists (Jackson et al 1994), showing a possible interaction between serotonergic and dopaminergic systems. Indeed, Jackson et al. (1998) later demonstrated that these behaviors could be blocked with the D1 antagonist SCH 23390 or the D2 antagonist raclopride.
In the rat, 5-HT1A receptor agonists elicit a characteristic set of motor behaviors, including the 5-HT syndrome, and under certain conditions can increase locomotor activity (Berendsen et al. 1989, Hillegaart et al. 1989, Evenden and Angeby-Moller 1990, Renyi 1991, Mignon and Wolf 2002). All these behaviors induced by 5-HT1A agonists are reversed by pretreatment with WAY 100635, but not by dopaminergic antagonists (see references above). There are conflicting data, however, about the effects of 5-HT1A agonists on psychostimulant-induced hyperactivity; 8-OH-DPAT inhibits amphetamine-induced hyperactivity (Przegalinski and Filip 1997), whereas it potentiated cocaine-induced hyperlocomotion (Muller et al. 2003). Rats that have been trained to discriminate amphetamine from saline, when treated with WAY 100635 alone or when pretreated with WAY 100635 before amphetamine, show marked, visible changes in behavior, manifested as hyperactivity and elevated stereotypic behavior characteristic of dopaminergic system stimulation, which leads to a high percentage of behavioral disruption (present results). The fact that WAY 100635 does not produce increases in motor behavior in amphetamine-naïve rats (Marona-Lewicka and Nichols, 2009), but only in rats that have been given repeated doses of amphetamine, strongly suggests that the present results may be evidence for amphetamine-induced behavioral sensitization.
WAY 100635 is a potent and efficacious dopamine D4 agonist in heterologous cell expression systems (Chemel et al. 2006), although Martel et al. (2007) have emphasized that WAY-100635 has much higher activity at the h5-HT1A receptor. A low dose (up to 0.4 mg/kg) of WAY 100635 that effectively blocks 5-HT1A-mediated cues did not produce a discriminative stimulus effect by itself, but a ten-fold higher dose of WAY 100635 produces a robust cue that is mediated by stimulation of dopamine D4 receptors (Marona-Lewicka and Nichols 2009). Feldpausch et al. (1998) have demonstrated that the dopamine D4 receptor plays an important role in the induction of behavioral sensitization by amphetamine and accompanying adaptations in pre- and post-synaptic neural systems associated with mesolimbocortical dopamine projections. Moreover, D4 dopamine receptor KO mice show an attenuated response to amphetamine-induced hyperlocomotion compared to WT mice (Thanos et al. 2010). The D4 antagonist A-381393 reversed WAY 100635-induced inhibition of forskolin-stimulated cAMP accumulation (Chemel et al.2006), partially inhibited the discriminative stimulus effects of amphetamine, and right-shifted the amphetamine dose-response cue (Table 3). Although D4 dopamine agonists (A-36958 and ABT-724) do not substitute in amphetamine-trained animals (Table 2), it seems possible that the higher doses of WAY 100635 used in the present experiments potentiate the discriminative stimulus effect of amphetamine through simultaneous inhibition of 5-HT1A receptors and stimulation of dopamine D4 receptors.
We report here for the first time that WAY 100635 did not mimic (+)-amphetamine, but when combined with different doses of (+)-amphetamine was able to potentiate drug-appropriate lever selection, especially with lower doses of (+)-amphetamine. Thus, it seems possible that the dual 5-HT1A antagonist/D4 agonistic properties of WAY 100635 may regulate the extent of dopamine release by (+)-amphetamine, in some way potentiating its behavioral effects. Pretreatment with 10 μmol/kg of WAY 100635 prior to 5.4 μmol/kg of (+)-amphetamine (the training dose) produced complete behavioral disruption (Fig 3B) as a result of a marked increase in locomotor activity. During the combination tests all rats had marked excitation and hyperactivity while being transported, as well as when inside the operant chambers, a behavior that strongly interfered with the learned food-reinforced response. All the animals used in the drug discrimination studies were well adapted to the experimental conditions, all experimental chambers were the identical, and injections were always done by the same person in the same surroundings. Thus, we believe environmental factors can be excluded as an explanation for the observed behavioral excitation. Using the drug discrimination assay, however, it is impossible to test acute effects of compounds because animals are chronically exposed to training drugs for at least several weeks.
Our experience from more than two decades in which we have employed (+)-amphetamine-trained rats indicates that neither tolerance nor sensitization develop during normal, regular training with (+)-amphetamine (Steigewald et al. 1994, and our observations). Our ED50 values determined every six months confirmed the stability of the discriminative stimulus effects of (+)-amphetamine. Even after testing a variety of compounds in amphetamine-trained rats, the ED50 remained unchanged during amphetamine training, suggesting that long-term adaptive changes did not occur, or if they occurred did not affect stimulus control. Further, Steigewald et al. (1994) have shown stabilization of the ED50 for (+)-amphetamine even after testing large doses of this drug.
Although drug discrimination is a very powerful method for investigating mechanisms of action for psychoactive drugs, it is not generally useful for exploration of neurochemical changes occurring over prolonged drug administration. That is, rats exposed to prolonged treatment with (+)-amphetamine may develop adaptive changes that are not detectible by measuring discriminative stimulus effects, whereas another behavioral assay may be necessary to identify abnormalities in their behavior. Further studies are indicated to clarify and explore the effect of 5-HT1A receptor antagonists on amphetamine-induced changes in behavior, as well as the role of the dopamine D4 receptor in behavioral sensitization.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RJ, Leith NJ. Tolerance to discriminative stimulus properties of d-amphetamine. Neuropharmacology. 1981;20:251–255. doi: 10.1016/0028-3908(81)90129-5. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Jenck F, Broekkamp CLE. Selective activation of 5-HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Caul WF, Burgin KL, Barrett RJ. Drug discrimination training during chronic drug treatment affects the development of tolerance. Behavioral Neuroscience. 1989;103:373–377. doi: 10.1037//0735-7044.103.2.373. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- De Vry, Jentzsch KR. Discriminative stimulus properties of the 5-HT1A receptor agonist BAY x 3702 in the rat. Eur J Pharmacol. 1998;357:1–8. doi: 10.1016/s0014-2999(98)00503-2. [DOI] [PubMed] [Google Scholar]
- Demellweek C, Goudie AJ. Behavioural tolerance to amphetamine and other psychostimulants: the case for considering behavioural mechanisms. Psychopharmacology (Berl) 1983;80:287–307. doi: 10.1007/BF00432109. [DOI] [PubMed] [Google Scholar]
- Dickinson SL, Curzon G. Roles of dopamine and 5-hydroxytryptamine in stereotyped and non-stereotyped behaviour. Neuropharmacology. 1983;7:805–812. doi: 10.1016/0028-3908(83)90124-7. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Angeby-Moller K. Effects of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on locomotor activity and rearing of mice and rats. Psychopharmacology (Berl) 1990;102:485–491. doi: 10.1007/BF02247129. [DOI] [PubMed] [Google Scholar]
- Feldpausch DL, Needham LM, Stone MP, Althaus JS, Yamamoto BK, Svensson KA, Merchant KM. The role of dopamine D4 receptor in the induction of behavioral sensitization to amphetamine and accompanying biochemical and molecular adaptations. J Pharmacol Exp Ther. 1998;286:497–508. [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Gommans J, Hijzen TH, Maes RA, Olivier B. Two-lever drug-drug discrimination with the 5-HT1 receptor agonists flesinoxan and eltoprazine. Int J Neuropsychopharmacol. 2000;3:221–228. doi: 10.1017/S1461145700001991. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Wadenberg M-L, Ahlenius S. Effects of 8-OH-DPAT on motor activity in the rat. Pharmacol Biochem Behav. 1989;32:797–800. doi: 10.1016/0091-3057(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Kuroki T, Kitchen MT, Meltzer HY. R(+)-8-OH-DPAT, a 5-HT1A receptor agonist, inhibits amphetamine-induced dopamine release in rat striatum and nucleus accumbens. Eur J Pharmacol. 1995;287:179–184. doi: 10.1016/0014-2999(95)00624-9. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Johansson C, Lindgren LM, Bengtsson A. Dopamine receptor antagonists block amphetamine and phencyclidine-induced motor stimulation in rats. Pharmacol Biochem Behav. 1994;48:465–471. doi: 10.1016/0091-3057(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Wallsten CE, Jerning E, Hu PS, Deveney AM. Two selective 5-HT1A receptor antagonists, WAY-100 635 and NDL-249, stimulate locomotion in rats acclimatised to their environment and alter their behaviour: a behavioural analysis 47. Psychopharmacology (Berl) 1998;139:300–310. doi: 10.1007/s002130050721. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the inhibition and expression of drug-and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus effects of 8-hydroxy-2-(di-n-propylamino)tetralin in pigeons and rats: species similarities and differences. J Pharmacol Exp Ther. 1998;284:238–249. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Weinberger SB, Browne RG. Evidence that a behavioral augmentation following repeated amphetamine administration does not involve peripheral mechanisms. Pharmacol Biochem Behav. 1982;17:547–553. doi: 10.1016/0091-3057(82)90317-3. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Ichikawa J, Dai J, Meltzer HY. R(+)-8-OH-DPAT, a 5-HT1A receptor, agonist, inhibits amphetamine-induced serotonin and dopamine release in rat medial prefrontal cortex. Brain Res. 1996;743:357–361. doi: 10.1016/s0006-8993(96)01111-0. [DOI] [PubMed] [Google Scholar]
- Lewander T. A mechanism for the development of tolerance to amphetamine in rats. Psychopharmacologia. 1971;21:17–31. doi: 10.1007/BF00403992. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–112. [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan. European Journal of Pharmacology. 1994;258:1–13. doi: 10.1016/0014-2999(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. 5-HT2A/2C receptor agonists potentiate the discriminative cue of (+)-amphetamine in the rat. Neuropharmacology. 1997;36:1471–1475. doi: 10.1016/s0028-3908(97)00106-8. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Aripiprazole (OPC-14597) fully substitutes for the 5-HT1A receptor agonist LY 293284 in the drug discrimination assay in rats. Psychopharmacology. 2004;172:415–421. doi: 10.1007/s00213-003-1677-6. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Further evidence that the delayed temporal dopaminergic effects of LSD are mediated by a mechanism different than the first temporal phase of action. Pharmacol Biochem Behav. 2007;87:453–461. doi: 10.1016/j.pbb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. WAY 100635 produces discriminative stimulus effects in rats mediated by dopamine D(4) receptor activation. Behav Pharmacol. 2009;20:114–118. doi: 10.1097/FBP.0b013e3283242f1a. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 2005;180:427–435. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D(4) receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 2009;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormiere AM, Faucillon V, Danty N, Culie C, Cussac D, Newman-Tancredi A. WAY-100635 has high selectivity for serotonin 5-HT(1A) versus dopamine D(4) receptors. Eur J Pharmacol. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Mignon L, Wolf WA. Postsynaptic 5-HT(1A) receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology (Berl) 2002;163:85–94. doi: 10.1007/s00213-002-1121-3. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Salloum JB, Huston JP. Serotonin1A-receptor agonism attenuates the cocaine-induced increase in serotonin levels in the hippocampus and nucleus accumbens but potentiates hyperlocomotion: an in vivo microdialysis study. Neuropharmacology. 2003;44:592–603. doi: 10.1016/s0028-3908(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Blockade of the discriminative stimulus effects of d-amphetamine in rhesus monkeys with serotonin 5-HT(1A) agonists. BehavPharmacol. 1994;5:591–598. doi: 10.1097/00008877-199410000-00004. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequet intra-accumbens amphetamine challenge in rats. Psychopharmacology. 1991;104:140–141. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renyi L. The involvement of the 5-HT1 and 5-HT2 receptors and of catecholaminergic systems in different components of the 5-HT syndrome in the rat. Pol J Pharmacol Pharm. 1991;43:405–419. [PubMed] [Google Scholar]
- Popova NK, Naumenko VS, Kozhemyakina RV, Plyusnina IZ. Functional characteristics of serotonin 5-HT2A and 5-HT2C receptors in the brain and the expression of the 5-HT2A and 5-HT2C receptor genes in aggressive and non-aggressive rats. Neurosci Behav Physiol. 2010;40:357–361. doi: 10.1007/s11055-010-9264-x. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Filip M. Stimulation of serotonin (5-HT)1A receptors attenuates the locomotor, but not the discriminative, effects of amphetamine and cocaine in rats. Behav Pharmacol. 1997;8:699–706. doi: 10.1097/00008877-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Romero L, Hervas I, Artigas F. The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neuroscience Letters. 1996;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Moltzen EK. Assesment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Signs SA, Boja JW. Stability of the stimulus properties of drugs over time. Pharmacol Biochem Behav. 1989;32:361–364. doi: 10.1016/0091-3057(89)90256-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald ES, Anderson DW, Young AM. Tolerance to discriminative stimulus effects of d-amphetamine. Exp Clin Psychopharmacol. 1994;2:13–24. [Google Scholar]
- Thanos PK, Bermeo C, Rubinstein M, Suchland KL, Wang GJ, Grandy DK, Volkow ND. Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J Psychopharmacol. 2010;24:897–904. doi: 10.1177/0269881109102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida R, Kubo M, Kuroda M, Shibasaki Y, Shintani N, Abe M, Koves K, Hashimoto H, Baba A. An antihyperkinetic action by the serotonin 1A-receptor agonist osemozotan co-administered with psychostimulants or the non-stimulant atomoxetine in mice. J Pharmacol Sci. 2009;109:396–402. doi: 10.1254/jphs.08297fp. [DOI] [PubMed] [Google Scholar]
- West WB, Van Groll BJ, Appel JB. Stimulus effects of d-amphetamine II: DA, NE, and 5-HT mechanisms. Pharmacol Biochem Behav. 1995;51:69–76. doi: 10.1016/0091-3057(94)00361-l. [DOI] [PubMed] [Google Scholar]
- Young AM, Walton MA, Carter TL. Selective tolerance to discriminative stimulus effects of morphine or d-amphetamine. Behav Pharmacol. 1992;3:201–209. [PubMed] [Google Scholar]
- Young R, Bondareva T, Wesolowska A, Glennon RA. Modulation of a (+)amphetamine discriminative stimulus in rats by 8-hydroxy-2-(N, N-di-n-propylamino)tetralin (8-OH DPAT) 64. Pharmacol Biochem Behav. 2006;83:612–617. doi: 10.1016/j.pbb.2006.03.025. [DOI] [PubMed] [Google Scholar]