Abstract
Drug discrimination has been an important technique in behavioural pharmacology for at least 40 years. The characteristics of drug-produced discriminative stimuli are influenced by behavioural and pharmacological variables, including the doses used to establish discriminations. This review covers studies on the effects of varying the training dose of a drug in a search for general principles that are applicable across different drug classes and methodological approaches. With respect to quantitative changes, relationships between training dose and rate of acquisition or magnitude of stimulus control were found for most drug classes. Acquisition accelerated with dose up to a point beyond which drug-induced impairments of performance had a deleterious impact. Sensitivity to the training drug as measured by ED50 values typically increased when the training dose was reduced. Qualitative changes were more complex and appeared to fall into three categories: (i) changes in profiles of generalisation between partial and full agonists; (ii) reduced specificity of some discriminations at small training doses and (iii) changes in the relative salience of actions mediated through different neurotransmitter systems or from central and peripheral sites. Three-lever discrimination procedures incorporating ‘drug versus drug’ or “dose versus dose” contingencies enabled detection of more subtle differences than the simple ‘drug versus no drug’ approach when applied to the opioid, hallucinogen and barbiturate classes of drugs. These conclusions have implications for the interpretation of data from studies that utilise either within- or between-subject designs for studying the discriminative stimulus effects of drugs.
Keywords: Drug discrimination, review, psychomotor stimulants, ethanol, opioids, nicotine, GABA-mimetics, hallucinogens, cannabinoids
Introduction
Drug discrimination has served as an important technique in behavioural pharmacology for at least 40 years, with applications in drug discovery, behavioural pharmacology and testing for abuse liability. The characteristics of drug-produced discriminative stimuli are not fixed and invariant, but are influenced by behavioural and pharmacological variables. The dose of drug used to establish a discrimination is thought to influence both its qualitative and its quantitative characteristics. Dose selection is therefore critically important in study design; different studies of the same drug may not use the same training dose and may therefore yield varying results. When using drug discrimination procedures in, for example, drug discovery or drug abuse research, incomplete knowledge of training dose effects can lead to incorrect assumptions about receptor mechanisms and abuse liability.
For many classes of discriminable drug systematic, within-experiment, studies of the role of training dose have been published and these can be an invaluable guide for planning research. This article reviews such studies in order to identify any general principles that may emerge. Experiments of this type were published from the early 1970s onwards, the first apparently being Cameron and Appel (1973), followed by Hirschhorn and Rosecrans (1974) and Overton (1975). These studies covered lysergic acid diethylamide (LSD), nicotine, dissociative anaesthetics and pentobarbital and they were soon followed by investigations with amphetamines, benzodiazepines, opioids and alcohol.
This review covers the majority of relevant studies deemed to be of adequate quality; systematic variation of training dose within a study is the key criterion for inclusion alongside other standard indicators such as peer-review publication, adequate numbers of subjects, descriptions of methodology, etc. The drug discrimination database, searched with the keyword “training dose varied”, was a major bibliographic source (Meisch et al. 2011). Several previous articles contain useful but less comprehensive reviews on this subject (e.g. Järbe, 1989). The quantitative characteristics of drug discriminations include numbers of training sessions to establish a discrimination, maximum attainable discrimination accuracy, and ED50 values when dose-response curves are determined. The ED50 value is commonly used as a measure of subjects’ sensitivity to drugs and it is widely believed that lower training doses are associated with lower ED50 values; the evidence for this assumption will be assessed. Additionally, training dose may impact upon qualitative aspects of a discrimination, as defined by changes in the drugs to which generalisation occurs, and sensitivity to antagonists. Evidence from studies directly comparing different training doses will be used to assess support for the view that lower training doses lead to reduced pharmacological specificity of the resulting cue. A different perspective is that as training dose is reduced, the relative salience of the different stimulus elements in a complex, drug-produced stimulus changes, leading to increases in generalisation to some drugs and reduced generalisation to others. The concept of drug-induced discriminative stimulus complexes was discussed explicitly in seminal papers by Francis Colpaert (Colpaert et al., 1976a,b) and it underlies much later thinking in the area.
This review aims to consider whether any general principles relating to the impact of training dose can be identified, in terms of similarities of findings across different drug classes, methodological approaches and other variables. It is subdivided into sections covering different drug classes, with the classes studied most intensively with respect to training dose generally appearing before the classes subjected to fewer investigations. The final section aims to draw together the findings from the different drug classes.
Psychomotor stimulants
Cocaine
Rodent and monkey studies utilising within-subjects designs revealed quantitative differences in the cocaine stimulus when fading procedures were used to establish low dose discriminations (Colpaert and Jansen, 1982; Terry et al., 1994; Spealman and Bergman, 1994) but not when training doses were increased (Craft and Stratmann, 1996). ED50 valuesdecreased at low doses, often accompanied by decreased specificity. Colpaert and Janssen (1982) reported that as training dose decreased, so too did the slope of the generalisation curve. Between-subjects studies also revealed quantitative differences: typically, sensitivity of discrimination was increased at lower doses, with lower ED50 values (Colpaert and Jansen, 1982; Callahan et al., 1992; Kantak et al., 1995, 1999; Schechter, 1997; Costanza et al., 2001; Wilcox et al., 2001; Quinton et al., 2006). Colpaert and Janssen (1982) reported steeper generalisation gradients at lower doses which mirrored within-subjects comparisons in the same study. However, others have not reported variations in slope with training dose. The study of Colpaert and Janssen (1982) is also noteworthy because it presented direct comparisons of error rates in drug and non-drug training sessions; at certain training doses, the response after cocaine was acquired more rapidly and thus a relatively large proportion of errors occurred in non-drug sessions.
Most studies of qualitative differences between high and low dose cocaine cues have utilised between-subjects designs in rodents. Dopamine-releasing agents generalised fully at all training doses (Schechter, 1997; Cunningham et al., 2006; Quinton et al., 2006). The selective dopamine reuptake inhibitor GBR12909 and dopamine D1 receptor agonists fully generalised at all training doses (Kantak et al., 1995). However, competitive NMDA antagonists substituted for low but not high doses of cocaine (Kantak et al., 1995). Apomorphine, a mixed D1/D2 agonist, substituted more fully at low cocaine doses (Colpaert and Jansen, 1982; Kantak et al. 1995). Conversely, D2 agonists substituted to a greater extent at high doses whereas D2 antagonists and inverse agonists shift the generalisation curve rightwards at both low and high doses (Costanza et al., 2001). Together these studies suggest a divergence of the dopaminergic substrates mediating the stimulus properties of low and high cocaine doses.
In squirrel monkeys, Spealman (1995) reported GBR 12909 substituted for cocaine at low and high doses, selective noradrenaline reuptake inhibitors (SNRI) substituted for the low dose only, while a selective serotonin (5-HT) reuptake inhibitor (SSRI) and noradrenaline α1, α2 and β2 agonists did not substitute at any dose. Pretreatment with an α1 antagonist attenuated cocaine generalisation at both doses and the low-dose cocaine-like effects of the noradrenaline but not the dopamine reuptake inhibitor. Pretreatment with a non-selective α2 and β antagonist did not influence cocaine generalisation at any dose. Pretreatment with a low dose of the SNRI talsupram potentiated high and low dose cocaine-like responding after GBR 12909. Together these findings suggest that noradrenaline reuptake and α1 mechanisms play a role in the stimulus effects of cocaine. In line with this hypothesis, agents with noradrenergic activity, including cocaine analogues and modafinil, fully substitute for low but not high cocaine doses in rodents (Wilcox et al., 2001; Cunningham et al., 2006; Dopheide et al., 2007).
Cocaine-trained subjects often generalise partially to opiate agonists: morphine produced additive effects upon drug-lever responding at a low dose of cocaine, an effect thought to be mediated by morphine-induced release of dopamine (Kantak et al. 1999). Kappa selective agonists shifted the low-dose cocaine generalisation curve to the right but did not fully generalise with the cocaine cue, and delta-selective agonists did not generalise with or alter the cocaine cue at any of the training doses used in monkeys (Spealman and Bergman, 1994). Finally, nicotinic agonists fully substitute for both low and high cocaine doses (Cunningham et al., 2006).
Kleven and Koek (1997, 1998) trained rats to discriminate high doses of cocaine from low doses and found that high cocaine doses elicited more accurate responding, i.e. more correct lever-selections. Administration of non-selective β-adrenoceptor antagonists, but not β1 or 5-HT1A antagonists, together with the low dose of cocaine, switched responding from the low to the high dose lever, suggesting a role of a β2 mechanism in the high dose cue. SSRIs and SNRIs also increased high dose responding in combination with the low cocaine dose, indicating a role of 5-HT and noradrenaline in the stimulus effects of high cocaine doses.
In summary, the discriminative effects of cocaine are influenced both quantitatively and qualitatively by training dose. At low doses, discriminative effects are probably mediated by dopaminergic and noradrenergic systems involving D1, D2 and α2 receptors respectively. At high doses, effects at D2, β2, and 5-HT (but not 5-HT1A) receptors may be more important.
Amphetamines
In humans, amphetamine discrimination was readily acquired (Kollins and Rush, 1999). Stimulus control was significantly better at higher training doses in the sense that vehicle produced more drug lever responding in the low dose group than the high dose group. However, generalisation curves were shifted leftwards at lower doses. Interestingly, there were similar leftward shifts in the dose-response functions for some self-reported subjective effects including drug liking and sociability, but not for others such as euphoria or anxiety, suggesting some dissociation between the discriminative stimulus and subjective effects of amphetamine in humans.
Most studies have utilised between-subjects designs with rodents to study quantitative differences in the stimulus properties of amphetamine as a function of training dose. Some have shown that generalisation curves are steeper at low doses (Stadler et al., 2001) while others have reported steeper curves at high doses (Stolerman and D’Mello, 1981). Overall, the accuracy of discrimination was enhanced at higher doses, yet at lower doses ED50 values were often lower, albeit non-significantly, indicating enhanced sensitivity i.e., an ability to discriminate smaller doses of drug from vehicle (Stolerman and D’Mello, 1981; Barrett and Steranka, 1983; Snoddy and Tessel, 1983; Sanger, 1988; Stadler et al., 2001). For example, Stolerman and D’Mello (1981) reported increases in drug-lever responding after 0.06 mg/kg in rats trained with 0.4 mg/kg, while those trained at 1 mg/kg did not show increased drug-lever responding at doses of amphetamine below 0.5 mg/kg.
Cocaine fully substituted for amphetamine and its ED50 was lower at low training doses (Stadler et al., 2001; Stolerman and D’Mello, 1981). Para-hydroxyamphetamine, which is highly lipophilic and thus penetrates poorly to the central nervous system, showed greater generalisation at low training doses but conversely, apomorphine substituted more at higher doses (Colpaert et al., 1976c; Stolerman and D’Mello, 1981). Gaiardi et al. (2001) found that dizocilpine, a non-competitive NMDA antagonist, generalised more with a low dose while CGP 43487, a competitive antagonist, did not.
Other findings suggest a role for noradrenergic mechanisms in the stimulus effects of low amphetamine doses. Nisoxetine, a noradrenaline reuptake inhibitor, generalised with the interoceptive effects of a low but not a high training dose of amphetamine in mice, and pretreatment with nisoxetine shifted the low dose generalisation gradient for amphetamine leftwards (Snoddy and Tessel, 1983). In contrast, a study in rats did not report any influence of α2 antagonists upon the discriminative effects of high or low amphetamine doses (Sanger, 1988). Thus, the differential involvement of α1 and β adrenoceptors as a function of training dose remains to be clarified in rodents.
In summary, both human and animal studies illustrate quantitative differences in the stimulus effects of high and low training doses of amphetamine. At low doses stimulus control was weaker but subjects exhibited greater sensitivity to small doses of amphetamine. There is limited evidence for qualitative variation in the discriminative stimulus properties of low and high amphetamine doses, although suggestions that peripheral stimulation may be more important at low doses warrant further investigation.
Caffeine
Human studies demonstrate that caffeine is readily discriminated from placebo at doses as low as 10 mg and even at 1.8 mg in some individuals (Griffiths et al., 1990; Mumford et al., 1994). These studies show that sensitivity to the stimulus effects of caffeine is positively correlated with the number and magnitude of self-reported subjective effects, indicating a functional relationship between these behavioural properties of caffeine. Findings also suggest that low caffeine doses are discriminated based upon the alerting and positive mood effects.
Studies with rodents demonstrate that the interoceptive effectsof caffeine vary quantitatively with training dose (Holtzman, 1986; Mumford and Holtzman, 1991; Powell et al., 1999). At high training doses, discrimination was acquired faster and the generalisation gradient shift rightward. The stimulus properties of caffeine vary qualitatively with dose. Other stimulant drugs, including cocaine, amphetamine, methylphenidate and ephedrine substituted more fully at lower doses, as did other methylxanthines (Holtzman, 1986; Mumford and Holtzman, 1991). The low-dose cue partially generalised to dopamine agonists and may be attenuated by α-noradrenaline or dopamine antagonists, by adenosine analogues, and diverse behavioural depressants. In contrast, the high-dose caffeine cue was harder to characterise; the only agent that fully generalised was theophylline (Mumford and Holtzman, 1991) and dopamine antagonists did not block caffeine (Powell et al., 1999). Agents which produce anxiety-like behaviours in animals, such as benzodiazepine inverse agonists, and the adrenoceptor antagonist yohimbine did not generalise at high training doses. The NMDA antagonist phencyclidine (PCP) also did not generalise.
In summary, there is close agreement between the results of human and animal studies which demonstrate that the caffeine cue varies quantitatively and qualitatively with training dose. At low doses, discrimination appears to be based upon states of alertness or arousal mediated by adrenergic and dopaminergic systems. However, the substrates mediating discrimination at high doses remain unclear.
Opioids
A study in human subjects trained to discriminate progressively lower doses of the μ-opioid agonist hydromorphone, considered together with data from animal studies on buprenorphine, indicated that the progressive reduction of the training dose most likely enhanced discrimination sensitivity (Preston and Bigelow, 1998). A firmer conclusion was not possible, in part because dose-response curves for hydromorphone at the different training doses were not determined. Variation in the training dose of opioids has been examined more extensively in rats and pigeons.
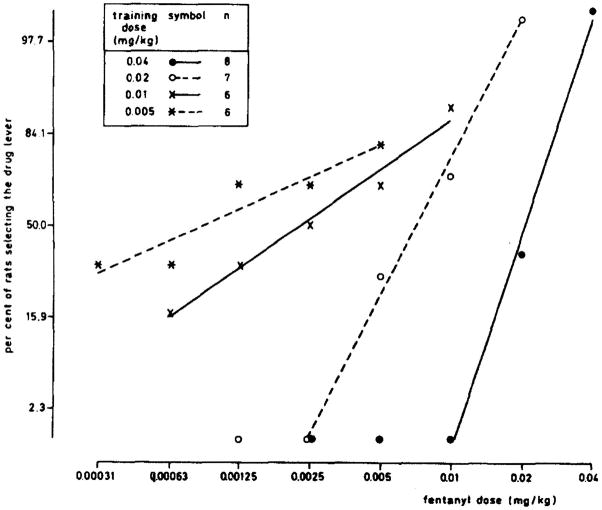
Overton and Batta (1979) used a T-maze procedure to train rats at several doses of various opioids; the numbers of sessions to criterion was inversely related to training dose. Investigations using two-lever procedures showed that ED50 values for full opioid agonists decreased at lower training doses, both in between-group studies (Shannon and Holtzman, 1979; Colpaert et al., 1980a, 1980b; Koek and Slangen, 1982; Krimmer et al., 1984; Picker et al., 1990; Young et al., 1992; Picker et al., 1992; Zhang et al., 2000) and in the same rats after retraining at a lower dose (Craft et al., 1996). Figure 1 shows results from a classic study on fentanyl. In contrast, changes in ED50 values were not seen after training with the κ-opioid U50,488 (Picker et al., 1990), or the partial (mixed) agonists buprenorphine (Holtzman, 1997) and nalbuphine (Walker and Young, 1993). However, full and partial agonists do not seem to have been compared directly within a study.
Fig 1.
Log-probit plot of dose-response curves in different groups of rats trained to discriminate fentanyl from saline at doses of 0.005, 0.01, 0.02 or 0.04 mg/kg in a two-lever, operant conditioning procedure with an FR 10 schedule of food reinforcement. The typical leftward shifts of the dose-response curve at lower training doses can be seen, as well as flattening of dose-response curves as the training dose approached levels that were too low to be discriminable (reproduced from Colpaert et al. 1980b, with permission from Elsevier).
Studies in rodents have also yielded evidence for qualitative changes in patterns of generalisation at different training doses. Full generalisation to partial agonists was apparent in rats at low training doses of the full μ-agonists morphine and fentanyl, whereas at higher training doses there was often partial or no generalisation to partial opioid agonists (Shannon and Holtzman, 1979; Picker et al., 1990; Young et al., 1992; Picker et al., 1992; Zhang et al., 2000). Findings were similar with morphine discrimination established by taste aversion conditioning (Grabus et al., 1999). Comparisons of generalisation profiles to non-opioid compounds produced varying results, with some studies suggesting retention of specificity at low training doses (Koek and Slangen, 1982) whereas others indicated reduced specificity (Shannon and Holtzman, 1979; Colpaert et al., 1980b; Picker et al., 1990). Holtzman (1997) showed increased generalisation to the partial agonists pentazocine and nalbuphine in rats trained with a low dose of buprenorphine. At a high training dose of the κ-agonist U50,488, rats generalised to other κ-agonists but not to μ-agonists, whereas at a lower training dose both μ- and κ-agonists generalised, together with increased responding to non-opioid agents (Picker et al. 1990). In contrast, Broqua et al. (1998) did not find generalisation to morphine from a low training dose of U50,488. In pigeons, increased sensitivity at low training doses was seen consistently with the full and partial agonists (Koek and Woods, 1989; Gauvin and Young, 1989; Picker et al., 1993; Cook and Picker, 1998); in these studies, specificity was sometimes, but not always, retained.
There is also evidence for qualitative changes in opioid discrimination in pigeons as a function of training dose. Picker (1991) identified a naloxone-sensitive opioid component in discrimination of low doses of (−)-NANM (N-allylnormetazocine, SKF-10,047) whereas a large dose was predominantly PCP-like. Interestingly, by comparing data for different training doses of fentanyl, Picker et al. (1993) were able to identify three groups of opioids, i.e. (i) those substances that generalised fully at both doses, with steep dose-response curves and few inter-animal differences; (ii) those generalising fully with low training doses but to a slightly lesser extent with a large dose, with shallower slopes and marked inter-animal differences and (iii) those generalising with the low dose only, with shallow dose-response curves, large inter-animal differences and able to antagonise a high training dose of fentanyl. These different response patterns correlated with differences in intrinsic efficacy at μ-opioid receptors (Picker et al., 1993). Similarly, when subjects were trained on a low dose of morphine, increased generalisation to cyclazocine and (−)-NANM emerged (Cook and Picker, 1998). An absence of full generalisation from a high training dose of fentanyl to meperidine and profadol may have been a consequence of rate-decreasing effects of these substances mediated through non-opioid mechanisms (Picker et al., 1994). Quantification of antagonism has also been valuable in this area; for example, naltrexone antagonised both low and high doses of fentanyl with pA2 values of 7.6 and 7.5 respectively, suggesting that similar receptors were involved (Zhang et al., 2000).
Studies of dose versus dose discrimination also shed light on the role of training dose. Partial μ- and κ-agonists typically generalised with a low training dose in rats trained with fentanyl or bremazocine but antagonised a higher training dose (Colpaert and Janssen, 1986; Smith and Picker, 1995). Results compatible with those of the preceding studies were obtained in elegant three-response procedures in pigeons and humans trained to discriminate between saline and each of two doses of a μ-agonist (Vanecek and Young, 1995; Jones et al., 1999).
To summarise, quantitative changes associated with training dose were apparent, with leftward shifts in dose-response curves seen in almost all studies of training with full μ-opioid agonists in rats and pigeons and with a partial agonist in pigeons. Results were inconsistent for partial agonist training in rats, and a shift was not seen in one study involving training with a κ-opioid in pigeons. Qualitative changes were also seen in both species, consisting primarily of increased generalisation to partial agonists when the training dose of a full agonist was low. However, specificity of stimulus control was not always preserved at low intensities of opioid receptor stimulation.
Alcohol
Ethanol is the most studied form of alcohol in drug discrimination procedures. The effect of ethanol training dose on quantitative and qualitative aspects of ethanol discriminative stimulus effects have been well documented both within and between subjects. Between subjects, the acquisition of ethanol discrimination consistently required fewer training session at higher doses in both rats (Overton, 1974; Grant and Colombo, 1993a; Colombo et al., 1995; Green et al., 1997; Green and Grant, 1998, 1999; Bowen et al., 1999a; Quertemont and Grant, 2002; Quertemont et al., 2003; Szeliga and Grant, 1998; York, 1978) and monkeys (Grant et al., 2000). The widest range of training doses studied under identical conditions was with a T-maze by Overton (1974) and encompassed 0.06–2.5 g/kg, requiring 500 and 20 trials, respectively (fig. 2). A very similar effect of dose range (0.33–0.99) on trials (100–20) was found with a two-lever operant procedure (York, 1978). Typically, there was an inverse relationship between ethanol training dose (range 0.8–2.0 g/kg) and the ED50 of ethanol substitution, although the differences were generally not statistically significant (Colombo et al., 1995; Grant et al., 1997; Green and Grant, 1998, 1999; Quertemont and Grant 2002; Quertemont et al., 2003; Ginsberg and Lamb, 2005). Further, in the studies cited above, the ethanol training dose generally did not have a significant effect on rates of responding, except with training doses over 2.25 g/kg (Overton, 1974).
Fig 2.
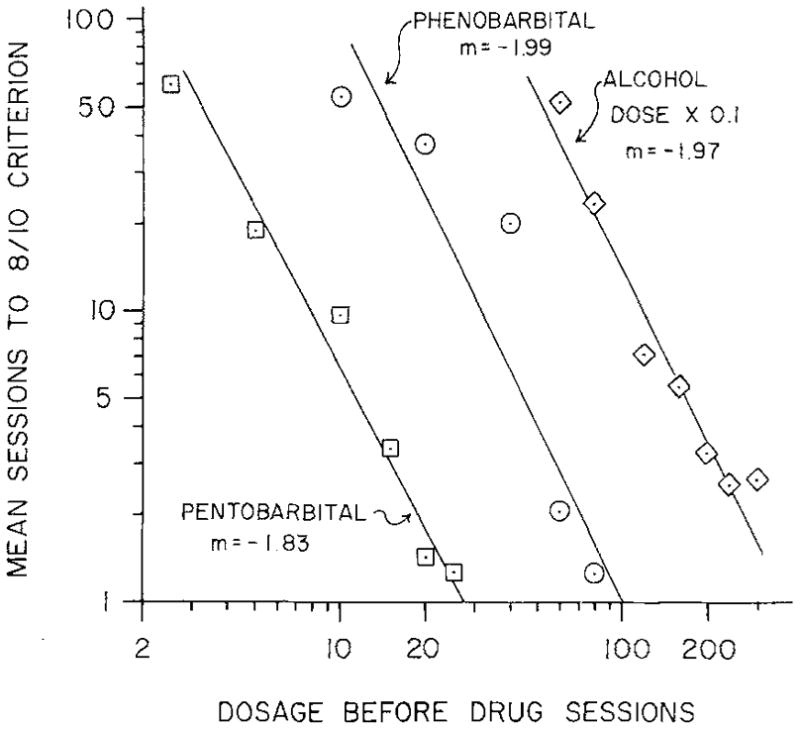
Relationship between training doses and sessions to a criterion of discrimination performance for pentobarbital, phenobarbital and ethanol administered i.p. at the doses shown for rats in a T-maze shock-escape task. Criterion performance was eight correct first-trial choices during ten consecutive training sessions (fig. 1 from Overton, 1977, reproduced with kind permission of Springer Science+Business Media).
Ethanol is a unique drug for examining the qualitative effects of training dose because the range of compounds that generalise to alcohol encompasses many pharmacological classes, and this lower specificity is not necessarily restricted to a lower training dose. A composite of most discrimination outcomes shows that GABAA positive modulators, NMDA antagonists and 5-HT agonists all substitute for ethanol (reviewed in Grant, 1999). However, direct GABAA agonists (Grant and Colombo, 1993a), neuroactive steroids with activity at NMDA receptors (Bowen et al., 1999a, 1999b), taurine (Quertemont et al., 2003), acetaldehyde (Quertemont et al., 2002), morphine (Grant and Colombo, 1993c, Szeliga and Grant, 1998), voltage-sensitive calcium antagonists (Green and Grant, 1999; Green-Jordan and Grant, 2000) and γ-hydroxybutyric acid (Helms et al., 2008b) fail to generalise across a range of training doses of ethanol. Thus, although ethanol is a mixed discriminative stimulus, it still retains pharmacological specificity, and this specificity is not linear with training dose.
The drugs that substitute for lower but not higher ethanol training doses are barbiturates (Grant and Colombo, 1993a; Grant et al., 1993; York, 1978), neuroactive steroids (Bowen et al., 1999), γ-hydroxybutyrate (Colombo et al., 1995) and 5-HT agonists (Grant and Colombo, 1993b; Grant et al., 1997; Green and Grant, 1998) in rats, and benzodiazepines (Grant et al., 2000; Helms et al., 2008b) in monkeys. However, this was not the case for the GABAA neuroactive steroid anaesthetic alphaxalone, which showed substitution at both lower and higher training doses in rats (Ginsberg and Lamb, 2005). In contrast to this substitution pattern, NMDA antagonists appeared to generalise more at higher than lower training doses in rats (Grant and Colombo, 1993, Grant et al., 1993) and monkeys (Vivian et al., 2002) but again, with some exceptions (Green and Grant, 1998).
Ethanol training dose also affects the ED50 of substitution differentially, with a lower ethanol training dose resulting in lower ED50 for pentobarbital (Grant and Colombo, 1993c; Green and Grant, 1998), the 5-HT agonists mCPP, TFMPP and Ru24969 (Grant and Colombo, 1993b; Grant et al., 1997) and the neuroactive steroids alphaxalone (Ginsberg and Lamb, 2005) and allopregnanolone (Bowen et al., 1999a). Similar to these findings in rats, monkeys also exhibited lower ED50 values for pentobarbital and allopregnanolone, using 1.0 g/kg as compared with 2.0 g/kg ethanol as a training dose (Grant et al., 2000, Helms et al., 2008a). In contrast to the GABAA positive modulators, higher ED50 doses were associated with 1.0 g/kg ethanol training as compared with 2.0 g/kg ethanol training in the substitution of the NMDA antagonists (+)3-(2-carboxy-piperazin-4-propyl)-1-propenyl-1-phosphonic acid (CPP) and dizocilpine; this was also the case for the dissociative anaesthetics ketamine and PCP in rats (Grant and Colombo, 1993a; Green et al., 1997), but not in monkeys (Vivian et al., 2002). In general, both qualitative and quantitative aspects of stimulus generalisation suggested a differential effect of ethanol training dose, with lower training dose cues mediated by positive GABAA and 5-HT1B/2C modulation and higher training dose cues more sensitive to the effects of dissociative anaesthetics.
Within-subject differences in the training dose of ethanol have been primarily assessed in rats trained to discriminate ethanol in a 3-lever ‘dose versus dose versus no drug’ procedure, where neuroactive steroids that are GABAA positive modulators show greater qualitative and quantitative substitution for 1.0 g/kg ethanol compared to 2.0 g/kg ethanol (Bowen et al., 1999b). Another approach to within-subject effect of training dose was to train rats initially to a low dose of ethanol versus water and than retrain to a higher dose, or vice-versa (Green and Grant, 1998). Raising the training dose increased the ED50 of pentobarbital and dizocilpine, while eliminating the substitution of TFMPP, whereas lowering the training dose lowered the ED50 of pentobarbital and dizocilpine and revealed substitution of TFMPP. This was taken as evidence for an effect of training dose on overshadowing among the component elements of the ethanol cue (Green and Grant, 1998). Overshadowing is said to occur if conditioning to a relatively weak stimulus is attenuated when it is always presented in combination with a more intense stimulus (Mackintosh, 1974); application of the concept in this area is based upon evidence for overshadowing of one drug by another in experiments where mixtures of dissimilar substances served as training stimuli (Mariathasan and Stolerman 1993; White and Stolerman 1996). An interesting twist on the within-subject approach was to test the substitution of ethanol during different phases of the menstrual cycle in monkeys, in order to address the potential contribution of circulating progesterone on sensitivity to ethanol (Grant et al., 1997). Specifically, in the luteal phases where progesterone is elevated the ED50 of ethanol substitution in lower compared to the follicular phase, where progesterone is very low (Grant et al., 1997), but this effect held only for 1.0 g/kg training dose of ethanol and not for a higher ethanol training dose or for the substitution pattern of midazolam (Green et al., 1997). In summary, within-subject studies of ethanol training dose reflect the general findings of drug versus vehicle discriminations and further allow the investigation of learning or additive physiological factors in both qualitative and quantitative discriminative stimulus effects.
GABA-mimetics
Barbiturates
The most widely studied barbiturate across training doses was pentobarbital, but limited evidence also exists for phenobarbital. Both rats (Overton, 1975, 1977) and gerbils (Järbe and Swedberg, 1998) consistently reached discrimination criteria in fewer sessions when trained on relatively high doses of pentobarbital. An exception was noted with the highest pentobarbital training dose in rats, a discrepancy probably attributable to the marked suppression of response rates (Ator and Griffiths, 1989). An assessment across phenobarbital training doses in rats revealed a similar inverse relationship between training dose and sessions to criteria (fig. 2). Greater discrimination accuracy was obtained with higher pentobarbital training doses, regardless of whether a ‘drug versus no drug’ procedure in parallel groups (De Vry and Slangen, 1986b; Järbe and Johansson, 1984) or a three-choice ‘dose versus dose versus no drug’ approach (McMillan et al., 2001) was utilised. Low pentobarbital training doses frequently engendered leftward shifts in dose-response curves (or lower ED50 values) when compared to higher ones (Ator and Griffiths, 1989; McMillan et al., 2001; De Vry and Slangen, 1986b).
McMillan et al. (2001) trained pigeons to discriminate pentobarbital in a three-lever ‘dose versus dose versus no drug’ procedure. Partial to full substitution by the benzodiazepine chlordiazepoxide was observed, but only for responding on the low dose-appropriate lever. In contrast, two-lever ‘drug versus no drug’ discrimination procedures in rats demonstrated that chlordiazepoxide and lorazepam fully substituted for both low and high pentobarbital training doses, with the generalisation curve for both benzodiazepines shifted to the left as the magnitude of the training dose decreased (Ator and Griffiths, 1989; De Vry and Slangen, 1986b). An additional ‘drug versus drug’ T-maze procedure in gerbils featured three training groups with low, medium or high pentobarbital doses discriminated from a fixed chlordiazepoxide dose (Järbe and Swedberg, 1998). Notably, the 5 and 15 mg/kg training doses of pentobarbital occasioned a high percentage of correct first-trial choices whereas the medium dose (10 mg/kg) was indistinguishable from the chlordiazepoxide training dose. The three-lever procedures suggest that the qualitative characteristics of pentobarbital change as a function of training dose.
Benzodiazepines
The prototypical benzodiazepines used in studies examining training dose as an independent variable are chlordiazepoxide, diazepam, midazolam, and lorazepam. Two stand-alone investigations provide preliminary insights into the role of training dose for the α1-subunit selective agonist alpidem (Sanger and Zivkovic, 1994) and the partial inverse agonist β-CCE (Rowlett et al., 1999). Numerous studies demonstrated that benzodiazepine training dose magnitude was inversely proportional to the number of sessions to discrimination criteria in rats (Bruner and Anderson, 2009; De Vry and Slangen, 1986b; Shannon and Herling, 1983; Tang and Franklin, 1991; Sanger and Zivkovic, 1994; Rowlett et al., 1999) and gerbils (Järbe and Swedberg, 1998). It is similarly well documented that lower benzodiazepine training doses are associated with a leftward shift in the dose-response curve (i.e., lower ED50) when compared to higher doses of the same training drug in rats (Ator and Griffiths, 1989; De Vry and Slangen, 1986b, 1986b; Bruner and Anderson, 2009; Sannerud and Ator, 1995a, 1995b; Shannon and Herling, 1983; Tang and Franklin, 1991; Sanger and Zivkovic, 1994; Rowlett et al., 1999) and humans (Rush et al., 1995). When reported, maximal discrimination accuracy was found to be greatest for the higher benzodiazepine training doses (Bruner and Anderson, 2009; De Vry and Slangen, 1986b; Shannon and Herling, 1983).
Generalisation of the barbiturate pentobarbital for chlordiazepoxide (De Vry and Slangen, 1986b) and diazepam (Shannon and Herling, 1983; Tang and Franklin, 1991) was found at both low and high training doses, whereas pentobarbital only partially substituted for a low training dose of lorazepam while producing no high dose-appropriate responding (Ator and Griffiths, 1989). This discrepancy may be explained by the relatively higher potency of lorazepam for the benzodiazepine binding site. A few studies suggested that low training doses of chlordiazepoxide (Bruner and Anderson, 2009), midazolam (Sannerud and Ator, 1995a), and the partial inverse agonist β-CCE (Rowlett et al., 1999) are less selective because negative pharmacological controls (i.e., psychostimulants) were found to partially substitute for the low but not the high training doses. Additional evidence for qualitative differences across training dose magnitude stems from antagonism tests. The antagonist flumazenil (Ro-1788) partially substituted for a low chlordiazepoxide training dose, but was also demonstrated to block a high chlordiazepoxide training dose to a greater extent than a low one (De Vry and Slangen, 1986a). A similar scenario was reported for the antagonist-like compound U-78875 and training doses of diazepam (Tang and Franklin, 1991). In summary, the cue produced by high training doses of agonists and partial inverse agonists exhibits greater specificity for benzodiazepine receptors.
γ-Hydroxybutyric acid
Gamma-hydroxybutyric acid is an endogenous ligand that acts through specific GHB receptors as well as GABAA and GABAB receptors following its metabolic conversion to GABA. Three studies established GHB discriminations in both rats and pigeons using food-reinforced T-maze and 2-lever discrimination procedures, respectively. Higher training doses of GHB were associated with more rapid acquisition of discrimination criteria and rightward shifts in dose-response curves (Koek et al., 2006; Colombo et al., 1995, 1998). It was also reported that the highest GHB training dose in pigeons achieved the greatest maximal discrimination accuracy (Koek et al., 2006). While GABAB receptor involvement persisted across training doses (as evidenced by both substitution with an agonist and blockade with an antagonist), partial substitution with diazepam-sensitive and -insensitive GABAA receptors agonists waned as the training dose increased (Colombo et al., 1998; Koek et al., 2006). These findings suggest that the pharmacological specificity of GHB discrimination narrows with higher training doses. The antagonist NCS-382 completely blocked GHB-appropriate responding at two training doses in rats (Colombo et al., 1995). while in pigeons NCS-382 failed to block either training dose and even partially substituted at low training doses (Koek et al., 2006).
Nicotine
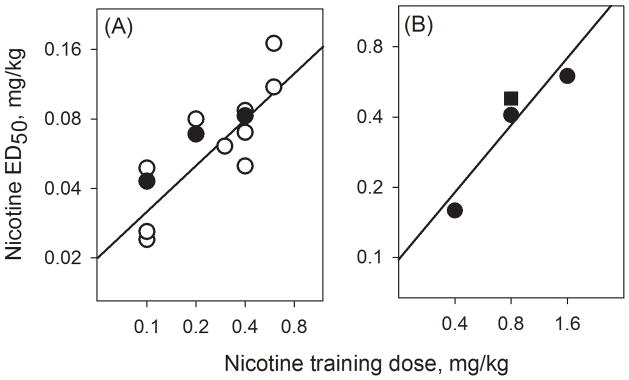
Studies in rats, mice and humans have examined the impact of varying the training dose of nicotine. Discrimination of higher doses of nicotine in rats was acquired more rapidly and remained more accurate than discrimination of low doses, indicating a quantitative effect related to training dose (Hirschhorn and Rosecrans, 1974; Chance et al., 1977; Stolerman et al., 1984; Gasior et al., 1999). In most studies ED50 values also increased with training dose (Chance et al., 1977; Meltzer et al., 1980; Stolerman et al., 1984). Figure 3 illustrates this relationship using ED50 data for rats and mice from multiple publications. One study did not at first detect any differences between ED50 values, but a difference was found when the dose-response curves were re-determined in the same animals (Gasior et al., 1999). Possible qualitative differences in nicotine discrimination as a function of training dose have been explored to a limited extent. The nicotine antagonist mecamylamine was approximately equipotent in blocking discriminations maintained at two dose levels of nicotine in rats (Hirschhorn and Rosecrans, 1974). Chance et al. (1997) found partial generalisation to amphetamine at each of three nicotine training doses. Stolerman et al. (1984) observed more generalisation to the nicotinic agonists cytisine and anabasine at a low training dose of nicotine, an observation that was attributed to actions of the low dose on high-affinity nicotinic receptor subtypes; there was no generalisation at the low training dose to several non-nicotinic drugs, suggesting that specificity of the discrimination may not have been lost. Gasior et al. (1999) carried out tests with selective dopamine agonists and uptake inhibitors but did not find evidence for a qualitative change in nicotine discrimination.
Fig 3.
Rat and mouse ED50 values from diverse studies showing the relationship with the training dose of nicotine. (A) results from ten studies in Sprague-Dawley rats showing that training with lower doses of nicotine was associated with lower ED50 values (○, data from Batman et al. 2005; Bondarev et al. 2003; Desai et al. 2003; Gasior et al. 1999; Le Foll & Goldberg 2005; Mansbach et al. 2000; Young & Glennon 2002 ; Young et al. 2006) and a within-study comparison of training doses in Lister hooded rats (●, Stolerman et al. (1984). (B) results from Stolerman et al. (1999) showing relationship of ED50 values with training dose in C57BL/6 mice (●), and results at a single training dose for DBA/2 mice (■). This figure has been modified from a similar one in Smith and Stolerman (2009) with kind permission of Springer Science+Business Media.
The experiments discussed above used conventional, two-lever drug discrimination procedures. Murray and Bevins (2007) trained rats with different doses of nicotine as conditioned stimuli in a task requiring entries into a receptacle where sucrose solution was presented. The procedure appears functionally similar to one-lever operant conditioning techniques although it was interpreted in Pavlovian terms as a goal-tracking experiment. Rates of acquisition and ED50 values for nicotine were similar at all training doses, but potential differences may have been obscured by unconditioned drug-induced changes in responding; one-lever operant discrimination procedures were abandoned long ago due to the confounding of discriminative and response rate effects.
Mice of the C57BL/6 strain acquired nicotine discrimination more rapidly at higher training doses (Stolerman et al., 1999, 2004; Shoaib et al. 2002). Mecamylamine blocked nicotine discrimination at all three training doses (Stolerman et al. 1999).
Human subjects are able to discriminate nicotine delivered by means of a nasal spray. Perkins et al. (1996) compared two training doses of nicotine and obtained results that were strikingly similar to the findings discussed above for animals.
Glutamate Receptor Ligands
The dissociative anaesthetics PCP and ketamine are thought to act primary through antagonism of NMDA glutamate receptors. PCP is the most widely studied drug of this class across training doses. The relevant studies all employed a two-lever operant conditioning procedure in rats with training of ‘drug versus no drug’ discriminations unless otherwise noted. PCP discriminations met acquisition criteria more rapidly as a function of increasing training dose (Beardsley et al., 1987; Beardsley and Balster, 1988; Jackson and Sanger, 1988). A similar relationship was observed for both PCP and ketamine training doses within a T-maze shock-escape procedure (Overton, 1975). Leftward shifts in dose-response curves were demonstrated for low PCP training doses compared to high ones (Beardsley et al., 1987; Beardsley and Balster, 1988; Jackson and Sanger, 1988), and this shift in potency held regardless of whether comparisons were made between training dose groups or within the same training group of pigeons that were serially re-trained with progressively lower PCP training doses (fig. 4). A heightened discriminative accuracy (or decline in percentage response error) for PCP at high training doses versus lower ones was reported in two studies (Beardsley et al., 1987; Mansbach and Balster, 1991), while no difference was demonstrated in a third study with pigeons (Koek et al., 1987).
Fig 4.
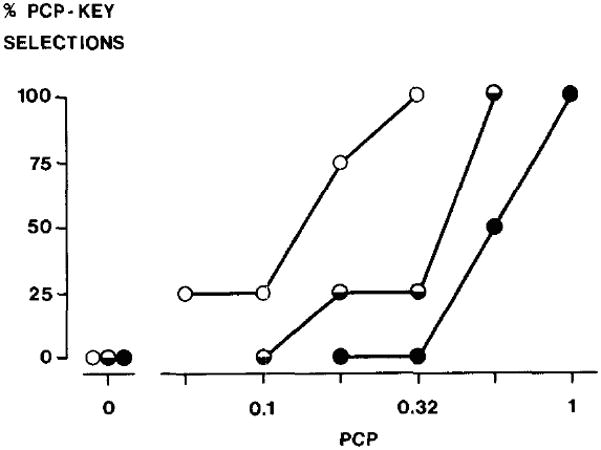
Leftward shifts of dose--response curves as a function of training dose in pigeons trained to discriminate between phencyclidine and saline. The training dose was lowered progressively and a dose-effect curve was established at each training dose level in pigeons that met discrimination criteria at all training doses. Vertical axis: percentage of drug-key selections; horizontal axis: dose (mg/kg, i.m.). Training dose: ● 1.0 mg/kg;
 0.56 mg/kg; ○ 0.32 mg/kg (reproduction of fig. 1 from Koek et al., 1987, with kind permission of Springer Science+Business Media).
0.56 mg/kg; ○ 0.32 mg/kg (reproduction of fig. 1 from Koek et al., 1987, with kind permission of Springer Science+Business Media).
The NMDA receptor antagonists dizocilpine and AP-5, the sigma agonist NANM, and the PCP derivative metaphit all generalised regardless of PCP training dose magnitude, and leftward shifts in the generalisation curves were observed at lower training doses in rats (Jackson and Sanger, 1988) and pigeons (Koek et al., 1987). In contrast, the competitive NMDA receptor antagonist CPP partially generalised to low and medium PCP training doses (0.56 and 1.25 mg/kg), but not to the highest training dose studied (3.0 mg/kg; Mansbach and Balster, 1991). While no generalisation to pentobarbital was observed for any training dose of PCP in pigeons (Koek et al., 1987), this barbiturate did fully substitute for the lowest, but no other, training dose of PCP in rats (Mansbach and Balster, 1991). Collectively, these findings suggest that high training doses of PCP impart the greatest pharmacological specificity.
Although one study examined the role of training dose in the discrimination of the non-competitive NMDA antagonist dizocilpine, limited testing was conducted and no discernable quantitative or qualitative characteristics were detected between training doses (Smith et al., 1999).
Hallucinogens and other ligands for serotonin receptors
The impact of training dose on the discrimination of hallucinogens such as lysergic acid diethylamide (LSD) was first studied by Cameron and Appel (1973) in rats, and most subsequent studies have also used rats. One group used pigeons to investigate the 5-HT1A agonist 8-hydroxy-(2-di-n-propylamino)-tetralin (8-OH-DPAT) that has been used extensively in studies of hallucinogenic and other drugs (Wolff and Leander, 1997, 1998). Mice were also trained at different doses of LSD (Winter et al., 2005) but there were no systematic comparisons across training doses.
Investigations in rats and humans have found that the dose-response curve for LSD shifts leftward at lower training doses, leading to reduced ED50 values (Greenberg et al., 1975; Nielsen et al., 1982; White and Appel, 1982a, 1982b). In some instances, the slope of dose-response curves was shallower at low training doses (e.g. White and Appel, 1982a; Nielsen et al., 1982). Leftward shifts of the dose-response curve for 8-OH-DPAT have also been found after training with 8-OH-DPAT in pigeons (Wolff and Leander, 1997) and in tests of generalisation from LSD to the 5-HT agonists quipazine, 8-OH-DPAT and 5-OMeDMT in rats (White and Appel, 1982a; Young et al., 1983; Ybema et al., 1993, 1994). After training with low doses of the 5-HT1A partial agonist buspirone, leftward shifts of its dose-response curve were observed in rats (Ator, 1991) and humans (Rush et al., 1995).
Qualitative features of the LSD stimulus also vary with training dose. Notably, the extent of generalisation to quipazine and to 5-HT partial agonists increased at low training doses (White and Appel, 1982a). Nielsen et al. (1982) showed that the full 5-HT2 antagonists pirenperone and ketanserin blocked both high- and low-dose LSD cues, whereas the partial agonist pizotifen completely blocked only the low-dose cue, findings compatible with observations of Colpaert et al. (1982) and Colpaert and Janssen (1983). However, White and Appel (1982a) found that the partial agonists methiothepin and cyproheptadine partially blocked all training doses of LSD. Further tests with 5-HT agonists and antagonists with predominantly peripherally-acting 5-HT ligands suggested that peripheral actions of LSD may have contributed more to the low-dose cue (White and Appel, 1982a). Pizotifen produced full block of 5-OMeDMT when the discrimination was established at a small dose, but even a much larger dose did not block discrimination with a larger training dose; these and other data of Young et al. (1983, 1986) may perhaps be understood by taking account of the partial agonist properties of some of the substances used. The specificity of the LSD cue seems to be retained at low training doses, as determined from generalisation tests with dopamine agonists and antagonists (White and Appel, 1982a).
In an elegant application of drug versus drug discrimination methodology that yielded orderly and readily interpretable data, White and Appel (1982b) trained LSD versus lisuride, a dopamine agonist. Different groups of rats were trained with a fixed dose of LSD against one of three doses of lisuride. Apomorphine generalised fully with lisuride in all groups. Quipazine generalised with LSD except when the training dose of lisuride was so large relative to that of LSD that the latter contributed little to the discrimination.
Cannabinoids
Studies have used between- and within-subjects designs in rodents to examine quantitative differences in Δ9-tetrahydrocannabinol (THC) discrimination with training dose. Some reported smaller ED50 values at lower doses while others did not (Järbe et al., 1998, 2006a, 2006b, 2010). Anandamide does not substitute for THC at any training dose, yet anandamide analogues fully generalise and exhibit smaller ED50 values at lower training doses (Järbe et al., 1998, 2001). CB1 antagonists block THC discrimination and rimonabant may be more potent at lower training doses (Järbe et al., 2006a, 2010). Overall, CP-55940 establishes the most potent CB1 cue, followed by THC, AM-1346 and methanandamide (Järbe et al., 1998, 2006b; De Vry and Jentzsch, 2003). Morphine does not substitute at any training dose yet (+)-amphetamine partially generalises with low doses (Järbe et al., 2006b, 1998, 2009).
Together the findings illustrate equivocal evidence for quantitative and qualitative differences in the stimulus effects of high and low THC training doses yet reveal variation in the interoceptive effects of CB1 agonists.
Antidepressants
Zhang and Barrett (1991) reported no difference in acquisition of imipramine discrimination between groups of pigeons trained at two doses, yet the low dose group exhibited smaller ED50 values. Overall, the high and low training doses did not differ qualitatively. Other tricyclic antidepressants generalised completely in both groups although the low dose group exhibited enhanced sensitivity. Cocaine, amphetamine, nomifensine, tomoxetine and bupropion, but not a selective dopamine reuptake inhibitor, substituted for imipramine, suggesting some importance of both noradrenergic and dopaminergic mechanisms in the imipramine stimulus. Fluoxetine did not generalise, yet 5-HT1A receptor agonists did, indicating that imipramine probably produces some effects similar to these compounds. Finally, scopolamine, a muscarinic antagonist, generalised with the high training dose of imipramine. Jones et al. (1980) reported that acquisition of bupropion discrimination was dose-related; performance at the lower doses was unstable and differences in the bupropion stimulus as a function of training dose were not investigated further.
Antipsychotics
Dose-response curves for discriminative stimulus effects of the second-generation antipsychotic clozapine in rats were shifted leftwards at low training doses (Goudie et al., 2004; Prus et al., 2004). However, studies of the qualitative nature of the clozapine cue have yielded complex results. Low-dose clozapine discriminations generalised fully to the second-generation antipsychotics zotepine and melperone, but not to the first generation antipsychotic haloperidol (Goudie et al., 2004; Porter et al., 1999; Prus et al., 2004). The higher-dose discrimination generalised fully to melperone, but not to zotepine or haloperidol. A low training dose of olanzapine showed no or full generalisation to first generation antipsychotics, whereas a higher dose generalised fully to antipsychotics of both generations (Porter et al., 2000). Studies of three-choice discriminations using two doses of clozapine and vehicle yielded different patterns of results (Prus et al., 2005, 2006). The low training dose generalised fully to quetiapine and sertindole but not to other first and second generation antipsychotics, whereas the higher dose generalised fully to olanzapine but not to any other antipsychotic that was tested. Rather then reflecting antipsychotic actions, the discriminations may have been associated with actions on particular receptors; the anticholinergics scopolamine and trihexyphenidyl and the polyvalent antidepressant mianserin have also been found to generalise (inconsistently) with olanzapine and clozapine (Porter et al., 2000; Prus et al., 2004). Thus, the training dose influences both quantitative and qualitative aspects of cues induced by some antipsychotic drugs, but a general rule relating to the nature of the qualitative changes cannot be discerned.
Conclusions
Quantitative aspects
Relationships between training dose and rate of acquisition or magnitude of stimulus control have been found for diverse drugs. Typically acquisition improves with dose up to a level above which drug-induced impairments of performance may have a deleterious impact. Most often, sensitivity, as measured by ED50 values from dose-response determinations, increases when training dose is reduced. The mechanism of the change in sensitivity is commonly assumed to be a consequence of the training itself rather than of the different overall exposure to drug. Future research could attempt to distinguish such effects from those of training at varying doses, taking into account the larger numbers of training sessions that are sometimes needed with small training doses.
A small number of instances were identified where ED50 values did not decrease with training dose. These include studies with opioid partial agonists, nicotine or cannabinoids. In some cases training dose may not have been varied over a sufficiently wide range. In others, bias may have been introduced; poorly performing subjects are found with increasing frequency as training dose declines and if they are excluded, effects of small training doses on ED50 values and slopes of dose-response curves may be masked. Thus, subject selection may impact on the validity of conclusions about the relationship of sensitivity (ED50) to training dose. It also seems inevitable that slopes will decline as the training dose approaches non-discriminable levels, at which point drug-lever responding will be at chance levels and dose-response curves will be flat. The ratio of the training dose to the ED50 in the same animals is rarely presented but it may be an additional useful metric related to slope. In most investigations subject selection is not an issue because the aim is to obtain subjects that perform well so that reliable data can be obtained during generalisation tests with putative agonists and antagonists.
The reduced ED50 at small training doses has implications for studies where different groups of subjects (e.g. different strains, sexes, genetically modified and wild-type animals) are compared for apparent sensitivity to the training drug. It is conceivable that real group differences may be masked: if one group is less sensitive to the training drug, the expected increase in ED50 might be wiped out by an opposing decrease caused by training with the equivalent of a smaller dose. This is relevant to a study where sophisticated receptor-mechanism explanations were put forward to explain negative results in a comparison of strains of rats that differed in several other procedures (Morgan et al. 1999). In such cases, it seems necessary to use more than one training dose so that the speed of acquisition, which consistently increases with training dose, can be taken as the metric.
Qualitative aspects
Numerous instances of variation in generalisation profiles as a function of training dose have been reported for several species. However, there are many gaps in the evidence; for example, there are studies with rats and pigeons in which the training dose of an opioid is systematically varied but none with monkeys. Nevertheless, several general patterns have emerged from these reports. Firstly, subjects trained on full agonists often show greater generalisation to partial agonists when the training dose is low and these effects are often accompanied by orderly findings for antagonism; drugs that substitute best for low training doses tend to attenuate the stimulus effects of high training doses. This pattern is seen with opioids and benzodiazepines and to some extent with LSD (i.e. in tests with full and partial 5-HT agonists). Secondly, there is a common tendency for specificity to be reduced at low training doses e.g. with opioid partial agonists and PCP, although it is not universal. Subjects maintained on small training doses of PCP generalised to CPP, as expected from their shared NMDA antagonist actions, but there was also generalisation to a barbiturate, suggesting some loss of specificity. Evidence for reduced specificity at low training doses has not yet been obtained for cannabinoids and LSD, whereas the relative dearth of studies with antidepressants and antipsychotics precludes firm conclusions about them.
Another pattern that has been observed in some studies with low training doses of certain drugs is a decrease in generalisation to agents of one class but an increase to those of another. Such findings imply shifts between the relative salience of stimulus elements originating at different classes of receptor. For example, when the training dose is low, ethanol generalises to agents from several pharmacological classes (barbiturates, benzodiazepines, neuroactive steroids and some 5-HT agonists), but tests with other agents suggest that this is not due to a global loss of specificity. In contrast, large training doses of ethanol show increased generalisation to NMDA antagonists. A rather different pattern emerges with amphetamine: generalisation to apomorphine appears to increase with training dose whereas that to para-hydroxyamphetamine decreases, the latter suggesting a shift from peripheral to central origins for the amphetamine cue, as may also be the case for the LSD cue. Curiously, data for generalisation to para-hydroxyamphetamine at different training doses of cocaine do not seem to be available.
Summary
This review has identified many studies in which the training dose influenced attributes of drug-produced discriminative stimuli. The quantitative changes are easier to explain and comprise increases in the rate of acquisition and robustness of stimulus control at large training doses, accompanied by reduced sensitivity. as shown by larger ED50 values. Further insights may be obtained by more extensive investigations of drug-induced stimuli built around the principles of signal detection theory, as applied to exteroceptive stimuli of different intensities, although altering the dose of a drug may influence not only the intensity of the stimulus that it produces but may also affect its qualitative nature. This review has shown that qualitative changes as a function of training dose are complex and appear to fall into three categories: (i) changes in profiles of generalisation between partial and full agonists; (ii) reduced specificity of some discriminations at small training doses and (iii) changes in the relative salience of actions mediated through different neurotransmitter systems or from central and peripheral sites. It is also notable that three-lever discrimination procedures incorporating ‘drug versus drug’ or ‘dose versus dose’ contingencies enabled detection of more subtle differences than the simple ‘drug versus no drug’ approach when they applied to the opioid, hallucinogen and barbiturate classes of drugs.
Acknowledgments
Preparation of this review was facilitated by financial support from NIH grants AA16849 and DA18165 held by Matthew M Ford, DA04376 held by Richard A Meisch, Ian P Stolerman and Vladimir Tsibulsky and RR000163-51 (Kathleen A Grant).
Financial support was from NIH grants AA16849 and DA 18165 held by Matthew M Ford and from grant DA 04376 held by Richard A Meisch, Ian P Stolerman and Vladimir Tsibulsky.
References
- Ator NA. Discriminative stimulus effects of the novel anxiolytic buspirone. Behav Pharmacol. 1991;2:3–14. [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Differential generalization to pentobarbital in rats trained to discriminate lorazepam, chlordiazepoxide, diazepam, or triazolam. Psychopharmacology (Berl) 1989;98:20–30. doi: 10.1007/BF00442001. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Steranka LR. Drug discrimination in rats: evidence for amphetamine-like cue state following chronic haloperidol. Pharmacol Biochem Behav. 1983;18:611–617. doi: 10.1016/0091-3057(83)90289-7. [DOI] [PubMed] [Google Scholar]
- Batman AM, Munzar P, Beardsley PM. Attenuation of nicotine’s discriminative stimulus effects in rats and its locomotor activity effects in mice by serotonergic 5-HT2A/2C receptor agonists. Psychopharmacology (Berl) 2005;179:393–401. doi: 10.1007/s00213-004-2035-z. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL. Evaluation of antagonists of the discriminative stimulus and response rate effects of phencyclidine. J Pharmacol Exp Ther. 1988;244:34–40. [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Salay JM. Separation of the response rate and discriminative stimulus effects of phencyclidine: training dose as a factor in phencyclidine-saline discrimination. J Pharmacol Exp Ther. 1987;241:159–165. [PubMed] [Google Scholar]
- Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999a;289:405–411. [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. An investigation of endogenous neuroactive steroid-induced modulation of ethanol’s discriminative stimulus effects. Behav Pharmacol. 1999b;10:297–311. doi: 10.1097/00008877-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher M-N, Rivière PJM, Dahl SG. The discriminative stimulus properties of U50,488 and morphine are not shared by fedotozine. Eur Neuropsychopharmacol. 1998;8:261–266. doi: 10.1016/s0924-977x(97)00084-9. [DOI] [PubMed] [Google Scholar]
- Bruner NR, Anderson KG. Discriminative stimulus and time-course effects of kava-kava (Piper methysticum) in rats. Pharmacol Biochem Behav. 2009;92:297–303. doi: 10.1016/j.pbb.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Piercey MF, Cunningham KA. Effects of the putative dopamine autoreceptor antagonists (+)- AJ76 and (+)-UH 232 on the discriminative stimulus properties of cocaine. Psychopharmacology (Berl) 1992;107:73–77. doi: 10.1007/BF02244968. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Appel JB. A behavioral and pharmacological analysis of some discriminable properties of LSD in rats. Psychopharmacologia (Berl) 1973;33:117–134. doi: 10.1007/BF00429082. [DOI] [PubMed] [Google Scholar]
- Chance WT, Murfin D, Krynock GM, Rosecrans JA. A description of the nicotine stimulus and tests of its generalization to amphetamine. Psychopharmacology (Berl) 1977;55:19–26. doi: 10.1007/BF00432812. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Bourguignon J, Fadda F, Lobina C, Maitre M, Reali R, Schmitt M, Gessa GL. Blockade of the discriminative stimulus effects of gamma-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382. Physiol Behav. 1995;58:587–590. doi: 10.1016/0031-9384(95)00086-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Gessa GL. Involvement of GABAA and GABAB receptors in the mediation of discriminative stimulus effects of gamma-hydroxybutyric acid. Physiol Behav. 1998;64:293–302. doi: 10.1016/s0031-9384(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. Factors regulating drug cue sensitivity: limits of discriminability and the role of a progressively decreasing training dose in cocaine-saline discrimination. Neuropharmacology. 1982;21:1187–1194. doi: 10.1016/0028-3908(82)90178-2. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. A characterization of LSD-antagonist effects of pirenperone in the rat. Neuropharmacology. 1983;22:1001–1005. doi: 10.1016/0028-3908(83)90216-2. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. Agonist and antagonist effects of prototype opiate drugs in fentanyl dose-dose discrimination. Psychopharmacology (Berl) 1986;90:222–228. doi: 10.1007/BF00181246. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. The narcotic discriminative stimulus complex: relation to analgesic activity. J Pharm Pharmacol. 1976a;28:183–187. doi: 10.1111/j.2042-7158.1976.tb04127.x. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. Theoretical and methodological considerations on drug discrimination learning. Psychopharmacology (Berl) 1976b;46:169–177. doi: 10.1007/BF00421388. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Kuyps JJMD, Niemegeers CJE, Janssen PAJ. Discriminative stimulus properties of a low dl-amphetamine dose. Arch Int Pharmacodyn Ther. 1976c;223:34–42. [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. Factors regulating drug cue sensitivity: limits of discriminability and the role of a progressively decreasing training dose in fentanyl-saline discrimination. J Pharmacol Exp Ther. 1980a;212:474–480. [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. Factors regulating drug cue sensitivity: the effect of training dose in fentanyl-saline discrimination. Neuropharmacology. 1980b;19:705–713. doi: 10.1016/0028-3908(80)90061-1. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. A drug discrimination analysis of lysergic acid diethylamide (LSD): in vivo agonist and antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, a LSD-antagonist. J Pharmacol Exp Ther. 1982;221:206–214. [PubMed] [Google Scholar]
- Cook CD, Picker MJ. Dopaminergic activity and the discriminative stimulus effects of mu opioids in pigeons: importance of training dose and attenuation by the D3 agonist (±)-7-OH-DPAT. Psychopharmacology (Berl) 1998;136:59–69. doi: 10.1007/s002130050539. [DOI] [PubMed] [Google Scholar]
- Costanza RM, Barber DJ, Terry P. Antagonism of the discriminative stimulus effects of cocaine at two training doses by dopamine D2-like receptor antagonists. Psychopharmacology (Berl) 2001;158:146–153. doi: 10.1007/s002130100872. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 1996;42:27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kalivas PW, Stratmann JA. Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharmacol. 1996;7:764–778. [PubMed] [Google Scholar]
- Cunningham CS, Polston JE, Jany JR, Segert IL, Miller DK. Interaction of lobeline and nicotinic receptor ligands with the discriminative stimulus properties of cocaine and amphetamine. Drug Alcohol Depend. 2006;84:211–222. doi: 10.1016/j.drugalcdep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Intrinsic activity estimation of cannabinoid CB1 receptor ligands in a drug discrimination paradigm. Behav Pharmacol. 2003;14:471–476. doi: 10.1097/01.fbp.0000087739.21047.d8. [DOI] [PubMed] [Google Scholar]
- De Vry J, Slangen JL. Effects of chlordiazepoxide training dose on the mixed agonist-antagonist properties of benzodiazepine receptor antagonist Ro 15- 1788, in a drug discrimination procedure. Psychopharmacology (Berl) 1986a;88:177–183. doi: 10.1007/BF00652236. [DOI] [PubMed] [Google Scholar]
- De Vry J, Slangen JL. Effects of training dose on discrimination and cross- generalization of chlordiazepoxide, pentobarbital and ethanol in the rat. Psychopharmacology (Berl) 1986b;88:341–345. doi: 10.1007/BF00180836. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [3H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Gubellini C, Dall’Olio R, Gandolfi O, Bartoletti M. Effects of N-methyl-D-aspartate agonists and antagonists in rats discriminating amphetamine. Behav Pharmacol. 2001;12:317–324. doi: 10.1097/00008877-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288:1053–1073. [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology (Berl) 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Alphaxalone and epiallopregnanolone in rats trained to discriminate ethanol. Alcohol Clin Exp Res. 2005;29:1621–1629. doi: 10.1097/01.alc.0000179374.39554.04. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Cole JC. Stimulus properties of the “atypical” antipsychotic zotepine in rats: comparison with clozapine and quetiapine. Pharmacol Biochem Behav. 2004;77:163–173. doi: 10.1016/j.pbb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Smurthwaite ST, Riley AL. Nalorphine’s ability to substitute for morphine in a drug discrimination procedure is a function of training dose. Pharmacol Biochem Behav. 1999;63:481–488. doi: 10.1016/s0091-3057(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993a;264:1241–1247. [PubMed] [Google Scholar]
- Grant KA, Colombo G. Substitution of the 5-HT1 agonist trifluoromethylphenylpiperazine (TFMPP) for the discriminative stimulus effects of ethanol: effects of training dose. Psychopharmacology (Berl) 1993b;113:26–30. doi: 10.1007/BF02244329. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Pharmacological analysis of the mixed discriminative stimulus effects of ethanol. Alcohol Alcohol Suppl. 1993c;2:445–449. [PubMed] [Google Scholar]
- Grant KA, Colombo G, Gatto GJ. Characterization of the ethanol-like discriminative stimulus effects of 5-HT receptor agonists as a function of ethanol training dose. Psychopharmacology (Berl) 1997;133:133–141. doi: 10.1007/s002130050383. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Evidence for overshadowing by components of the heterogenous discriminative stimulus effects of ethanol. Drug Alcohol Depend. 1998;52:149–159. doi: 10.1016/s0376-8716(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Effects of L-type voltage-sensitive calcium channel modulators on the discriminative stimulus effects of ethanol in rats. Alcohol Clin Exp Res. 1999;23:806–814. [PubMed] [Google Scholar]
- Green KL, Gatto GJ, Grant KA. The nitric oxide synthase inhibitor L-NAME (N-Nitro-L-arginine methyl ester) does not produce discriminative stimulus effects similar to ethanol. Alcohol Clin Exp Res. 1997;21:483–488. [PubMed] [Google Scholar]
- Green-Jordan KL, Grant KA. Modulation of the ethanol-like discriminative stimulus effects of diazepam and phencyclidine by L-type voltage gated calcium channel ligands in rats. Psychopharmacology. 2000;149:84–92. doi: 10.1007/s002139900344. [DOI] [PubMed] [Google Scholar]
- Greenberg I, Kuhn DM, Appel JB. Behaviorally induced sensitivity to the discriminable properties of LSD. Psychopharmacologia (Berl ) 1975;43:229–232. doi: 10.1007/BF00429255. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, Woodson PT. Low-dose caffeine discrimination in humans. J Pharmacol Exp Ther. 1990;252:970–978. [PubMed] [Google Scholar]
- Helms CM, Rogers LSM, Grant KA. Gamma-hydroxybutyric acid in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Behav Pharmacol. 2008a;19:317–324. doi: 10.1097/FBP.0b013e328308f20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rogers LSM, Waters CA, Grant KA. Zolpidem generalization and antagonism in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Alcohol Clin Exp Res. 2008b;32:1197–1206. doi: 10.1111/j.1530-0277.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn ID, Rosecrans JA. Studies on the time course and the effect of cholinergic and adrenergic receptor blockers on the stimulus effect of nicotine. Psychopharmacologia (Berl) 1974;40:109–120. doi: 10.1007/BF00421360. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus properties of caffeine in the rat: noradrenergic mediation. J Pharmacol Exp Ther. 1986;239:706–714. [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of buprenorphine in the rat. Psychopharmacology (Berl) 1997;130:292–299. doi: 10.1007/s002130050242. [DOI] [PubMed] [Google Scholar]
- Jackson A, Sanger DJ. Is the discriminative stimulus produced by phencyclidine due to an interaction with N-methyl-d-aspartate receptors? Psychopharmacology (Berl) 1988;96:87–92. doi: 10.1007/BF02431538. [DOI] [PubMed] [Google Scholar]
- Järbe TUC. Discrimination learning with drug stimuli. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Neuromethods, Volume 13: Psychopharmacology. Clifton, New Jersey: Humana Press; 1989. pp. 513–563. [Google Scholar]
- Järbe TUC, Johansson B. Interaction between drug discriminative stimuli and exteroceptive, sensory signals. Behav Neurosci. 1984;98:686–694. doi: 10.1037//0735-7044.98.4.686. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Swedberg MDB. Discriminative stimulus functions of CNS sedative drugs assessed by drug versus drug discrimination procedures in gerbils. Psychopharmacology (Berl) 1998;135:201–212. doi: 10.1007/s002130050502. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Makriyannis A, Lin S, Goutopolos A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Lin S, Makriyannis A. (R)-Methanandamide and delta9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta9-THC and (R)-methanandamide in rats. Psychopharmacology (Berl) 2006a;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with delta9-THC or (R)-methanandamide (AM-356) Psychopharmacology (Berl) 2006b;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Li C, Liu Q, Makriyannis A. Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide. Psychopharmacology (Berl) 2009;203:229–239. doi: 10.1007/s00213-008-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Gifford RS, Makriyannis A. Antagonism of the Δ9-THC behavioral effects by rimonabant: time course studies in rats. Eur J Pharmacol. 2010;648:133–138. doi: 10.1016/j.ejphar.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CN, Howard JL, McBennett ST. Stimulus properties of antidepressants in the rat. Psychopharmacology (Berl) 1980;67:111–118. doi: 10.1007/BF00431964. [DOI] [PubMed] [Google Scholar]
- Jones HE, Bigelow GE, Preston KL. Assessment of opioid partial agonist activity with a three-choice hydromorphone dose-discrimination procedure. J Pharmacol Exp Ther. 1999;289:1350–1361. [PubMed] [Google Scholar]
- Kantak KM, Edwards MA, Spealman RD. Effects of n-methyl-d-aspartate antagonists in rats discriminating different doses of cocaine: comparison with direct and indirect dopamine agonists. J Pharmacol Exp Ther. 1995;274:657–665. [PubMed] [Google Scholar]
- Kantak KM, Riberdy A, Spealman RD. Cocaine-opioid interactions in groups of rats trained to discriminate different doses of cocaine. Psychopharmacology (Berl) 1999;147:257–265. doi: 10.1007/s002130051165. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by beta-adrenergic receptor antagonists. Psychopharmacology (Berl) 1997;131:307–312. doi: 10.1007/s002130050297. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther. 1998;284:1015–1025. [PubMed] [Google Scholar]
- Koek W, Slangen JL. The role of fentanyl training dose and of the alternative stimulus condition in drug generalization. Psychopharmacology (Berl) 1982;76:149–156. doi: 10.1007/BF00435269. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH. Partial generalization in pigeons trained to discriminate morphine from saline. Drug Dev Res. 1989;16:169–181. [Google Scholar]
- Koek W, Woods JH, Jacobson AE, Rice KC. Phencyclidine (PCP) like discriminative stimulus effects of metaphit and of 2-amino-5-phosphonovalerate in pigeons: generality across different training doses of PCP. Psychopharmacology (Berl) 1987;93:437–442. doi: 10.1007/BF00207232. [DOI] [PubMed] [Google Scholar]
- Koek W, Chen W, Mercer SL, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate: role of training dose. J Pharmacol Exp Ther. 2006;317:409–417. doi: 10.1124/jpet.105.096909. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR. Effects of training dose on the relationship between discriminative-stimulus and self-reported drug effects of d-amphetamine in humans. Pharmacol Biochem Behav. 1999;64:319–26. doi: 10.1016/s0091-3057(99)00084-2. [DOI] [PubMed] [Google Scholar]
- Krimmer EC, McGuire MS, Barry HI. Effects of the training dose on generalization of morphine stimulus to clonidine. Pharmacol Biochem Behav. 1984;20:669–73. doi: 10.1016/0091-3057(84)90182-5. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Ethanol does not affect discriminative-stimulus effects of nicotine in rats. Eur J Pharmacol. 2005;519:96–102. doi: 10.1016/j.ejphar.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The Psychology of Animal Learning. Academic Press; New York: 1974. [Google Scholar]
- Mansbach RS, Balster RL. Pharmacological specificity of the phencyclidine discriminative stimulus in rats. Pharmacol Biochem Behav. 1991;39:971–975. doi: 10.1016/0091-3057(91)90061-6. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology (Berl) 2000;148:234–242. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, Stolerman IP. Overshadowing of nicotine discrimination in rats: a model for behavioural mechanisms of drug interactions? Behav Pharmacol. 1993;4:209–215. [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M. Discrimination of pentobarbital doses and drug mixtures under fixed-ratio and fixed-interval reinforcement schedules. Behav Pharmacol. 2001;12:195–208. doi: 10.1097/00008877-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Stolerman IP, Tsibulsky V. [accessed March 2011];Comprehensive drug self-administration and discrimination database. 2011 www.drugrefs.org.
- Meltzer LT, Rosecrans JA, Aceto MD, Harris LS. Discriminative stimulus properties of the optical isomers of nicotine. Psychopharmacology (Berl) 1980;68:283–286. doi: 10.1007/BF00428116. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Picker MJ. Sensitivity to the discriminative stimulus and antinociceptive effects of mu opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the mu opioid receptor. J Pharmacol Exp Ther. 1999;289:965–975. [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG. Qualitative differences in the discriminative stimulus effects of low and high doses of caffeine in the rat. J Pharmacol Exp Ther. 1991;258:857–865. [PubMed] [Google Scholar]
- Mumford GK, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, Silverman K, Griffiths RR. Discriminative stimulus and subjective effects of theobromine and caffeine in humans. Psychopharmacology (Berl) 1994;115:1–8. doi: 10.1007/BF02244744. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen EB, White FJ, Holohean AM, Callahan PM, Appel JB. Behavioral and biochemical evidence for serotonergic actions of tetrahydro-beta-carbolines. Life Sci. 1982;31:2433–2439. doi: 10.1016/0024-3205(82)90747-0. [DOI] [PubMed] [Google Scholar]
- Overton DA. Experimental methods for the study of state-dependent learning. Fed Proc. 1974;33:1800–1813. [PubMed] [Google Scholar]
- Overton DA. A comparison of the discriminable CNS effects of ketamine, phencyclidine and pentobarbital. Arch Int Pharmacodyn Ther. 1975;215:180–189. [PubMed] [Google Scholar]
- Overton DA. Comparison of ethanol, pentobarbital and phenobarbital using drug vs. drug discrimination training. Psychopharmacology (Berl) 1977;53:195–199. doi: 10.1007/BF00426492. [DOI] [PubMed] [Google Scholar]
- Overton DA, Batta SK. Investigation of narcotics and antitussives using drug discrimination techniques. J Pharmacol Exp Ther. 1979;211:401–408. [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, D’Amico D, Sanders M, Grobe JE, Wilson A, Stiller RL. Influence of training dose on nicotine discrimination in humans. Psychopharmacology (Berl) 1996;126:132–139. doi: 10.1007/BF02246348. [DOI] [PubMed] [Google Scholar]
- Picker MJ. Discriminative stimulus properties of (+)- and (−)-n-allylnormetazocine: the role of training dose in the stimulus substitution patterns produced by PCP/sigma and mixed action opioid compounds. Behav Pharmacol. 1991;2:497–511. [PubMed] [Google Scholar]
- Picker MJ, Doty P, Negus SS, Mattox SR, Dykstra LA. Discriminative stimulus properties of U50, 488 and morphine effects of training dose on stimulus substitution patterns produced by mu and kappa opioid agonists. J Pharmacol Exp Ther. 1990;254:13–22. [PubMed] [Google Scholar]
- Picker MJ, Craft RM, Negus SS, Powell KR, Mattox SR, Jones SR, Hargrove BR, Dykstra LA. Intermediate efficacy Mu opioids: examination of their morphine- like stimulus effects and response rate-decreasing effects in morphine-tolerant rats. J Pharmacol Exp Ther. 1992;263:668–681. [PubMed] [Google Scholar]
- Picker MJ, Yarborough J, Hughes CE, Smith MA, Morgan D, Dykstra LA. Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther. 1993;266:756–767. [PubMed] [Google Scholar]
- Picker MJ, Smith MA, Morgan D. Assessment of the relative intrinsic efficacy of profadol and meperidine in a pigeon drug discrimination procedure: relevance to partial substitution patterns. Behav Pharmacol. 1994;5:61–70. doi: 10.1097/00008877-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Porter JH, Villanueva HF, Rosecrans JA. Role of D1 and D2 dopamine receptors in the discriminative stimulus properties of the atypical antipsychotic clozapine in rats. Drug Dev Res. 1999;46:139–147. [Google Scholar]
- Porter JH, McCallum SE, Varvel SA, Vann RE. The discriminative stimulus properties of the atypical antipsychotic olanzapine in rats. Psychopharmacology (Berl) 2000;148:224–233. doi: 10.1007/s002130050046. [DOI] [PubMed] [Google Scholar]
- Powell KR, Koppelman LF, Holtzman SG. Differential involvement of dopamine in mediating the discriminative stimulus effects of low and high doses of caffeine in rats. Behav Pharmacol. 1999;10:707–716. doi: 10.1097/00008877-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Baker LE, Meltzer HY. Discriminative stimulus properties of 1.25 and 5.0 mg/kg doses of clozapine in rats: examination of the role of dopamine, serotonin, and muscarinic receptor mechanisms. Pharmacol Biochem Behav. 2004;77:199–208. doi: 10.1016/j.pbb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Philibin SD, Pehrson AL, Porter JH. Generalization to atypical antipsychotic drugs depends on training dose in rats trained to discriminate 1.25 mg/kg clozapine versus 5.0 mg/kg clozapine versus vehicle in a three-choice drug discrimination task. Behav Pharmacol. 2005;16:511–520. doi: 10.1097/01.fbp.0000172735.73876.06. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Philibin SD, Pehrson AL, Porter JH. Discriminative stimulus properties of the atypical antipsychotic drug clozapine in rats trained to discriminate 1.25 mg/kg clozapine vs. 5.0 mg/kg clozapine vs. vehicle. Behav Pharmacol. 2006;17:185–194. doi: 10.1097/01.fbp.0000197457.70774.91. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Grant KA. Role of acetaldehyde in the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2002;26:812–817. [PubMed] [Google Scholar]
- Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168:262–270. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Gerak LR, Moerschbaecher JM, Winsauer PJ. Effects of pregnanolone in rats discriminating cocaine. Pharmacol Biochem Behav. 2006;85:385–392. doi: 10.1016/j.pbb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Discriminative stimulus effects of ethyl-beta-carboline-3-carboxylate at two training doses in rats. Psychopharmacology (Berl) 1999;145:324–332. doi: 10.1007/s002130051065. [DOI] [PubMed] [Google Scholar]
- Rush CR, Critchfield TS, Troisi II, JR, Griffiths RR. Discriminative stimulus effects of diazepam and buspirone in normal volunteers. J Exp Anal Behav. 1995;63:277–294. doi: 10.1901/jeab.1995.63-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ. Behavioural effects of the alpha-2 adrenoreceptor antagonists idazoxan and yohimbine in rats: comparisons with amphetamine. Psychopharmacology (Berl) 1988;96:243–249. doi: 10.1007/BF00177568. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Zivkovic B. Discriminative stimulus effects of alpidem, a new imidazopyridine anxiolytic. Psychopharmacology (Berl) 1994;113:395–403. doi: 10.1007/BF02245215. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Ator NA. Drug discrimination analysis of midazolam under a three-lever procedure: dose dependent differences in generalization and antagonism. J Pharmacol Exp Ther. 1995a;272:100–111. [PubMed] [Google Scholar]
- Sannerud CA, Ator NA. Drug discrimination analysis of midazolam under a three-lever procedure II: differential effects of benzodiazepine receptor agonists. J Pharmacol Exp Ther. 1995b;275:183–193. [PubMed] [Google Scholar]
- Schechter MD. Discriminative characteristics of high and low cocaine administration: Effect of other psychostimulants. Pharmacol Biochem Behav. 1997;56:457–463. doi: 10.1016/s0091-3057(96)00301-2. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Herling S. Discriminative stimulus effects of diazepam in rats: evidence for a maximal effect. J Pharmacol Exp Ther. 1983;227:160–166. [PubMed] [Google Scholar]
- Shannon HE, Holtzman SG. Morphine training dose: a determinant of stimulus generalization to narcotic antagonists in the rat. Psychopharmacology (Berl) 1979;61:239–244. doi: 10.1007/BF00432265. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith JA, Boyer-Millar C, Goudie AJ. Does MK-801 discrimination constitute an animal model of schizophrenia useful for detecting atypical antipsychotics? Pharmacol Biochem Behav. 1999;64:429–433. doi: 10.1016/s0091-3057(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychopharmacology. Berlin: Springer-Verlag; 2009. pp. 295–333. [DOI] [PubMed] [Google Scholar]
- Smith MA, Picker MJ. Examination of the kappa agonist and antagonist properties of opioids in the rat drug discrimination procedure: influence of training dose and intrinsic efficacy. Behav Pharmacol. 1995;6:703–717. [PubMed] [Google Scholar]
- Snoddy AM, Tessel RE. Nisoxetine and amphetamine share discriminative stimulus properties in mice. Pharmacol Biochem Behav. 1983;19:205–210. doi: 10.1016/0091-3057(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Spealman RD, Bergman J. Opioid modulation of the discriminative stimulus effects of cocaine: comparison of mu, kappa and sigma agonists in squirrel monkeys discriminating low doses of cocaine. Behav Pharmacol. 1994;5:21–31. doi: 10.1097/00008877-199402000-00003. [DOI] [PubMed] [Google Scholar]
- Stadler JR, Caul WF, Barrett RJ. Effects of training dose on amphetamine drug discrimination: dose-response functions and generalization to cocaine. Pharmacol Biochem Behav. 2001;70:381–386. doi: 10.1016/s0091-3057(01)00616-5. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, D’Mello GD. Role of training conditions in discrimination of central nervous system stimulants by rats. Psychopharmacology (Berl) 1981;73:295–303. doi: 10.1007/BF00422421. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Szeliga KT, Grant KA. Analysis of the 5-HT2 receptor ligands dimethoxy-4-indophenyl-2-aminopropane and ketanserin in ethanol discriminations. Alcohol Clin Exp Res. 1998;22:646–651. doi: 10.1111/j.1530-0277.1998.tb04306.x. [DOI] [PubMed] [Google Scholar]
- Tang AH, Franklin SR. The discriminative stimulus effects of diazepam in rats at two training doses. J Pharmacol Exp Ther. 1991;258:926–931. [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Vanecek SA, Young AM. Pharmacological characterization of an operant discrimination among two doses of morphine and saline in pigeons. Behav Pharmacol. 1995;6:669–681. [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology (Berl) 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Discriminative-stimulus effects of the low efficacy Mu agonist nalbuphine. J Pharmacol Exp Ther. 1993;267:322–330. [PubMed] [Google Scholar]
- White FJ, Appel JB. Training dose as a factor in LSD-saline discrimination. Psychopharmacology (Berl ) 1982a;76:20–25. doi: 10.1007/BF00430748. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB. Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. Science. 1982b;216:535–537. doi: 10.1126/science.7071600. [DOI] [PubMed] [Google Scholar]
- White J-A, Stolerman IP. Reversal of overshadowing in a drug mixture discrimination in rats. Psychopharmacology (Berl) 1996;123:46–54. doi: 10.1007/BF02246280. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Ordway GA, Woolverton WL. Role of the dopamine transporter and the sodium channel in the cocaine-like discriminative stimulus effects of local anesthetics in rats. Psychopharmacology (Berl) 2001;157:260–268. doi: 10.1007/s002130100796. [DOI] [PubMed] [Google Scholar]
- Winter JC, Kieres AK, Zimmerman DM, Reissig CJ, Eckler JR, Ullrich T, Rice KC, Rabin RA, Richards JB. The stimulus properties of LSD in C57BL/6 mice. Pharmacol Biochem Behav. 2005;81:830–837. doi: 10.1016/j.pbb.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Differentiation of 5-HT1A receptor ligands by drug discrimination. Eur J Pharmacol. 1997;333:113–122. doi: 10.1016/s0014-2999(97)01125-4. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Selective serotonin reuptake inhibitors potentiate 8-OH-DPAT-induced stimulus control in the pigeon. Eur J Pharmacol. 1998;345:35–39. doi: 10.1016/s0014-2999(98)00052-1. [DOI] [PubMed] [Google Scholar]
- Ybema CE, Slangen JL, Olivier B, Mos J. Dose-dependent discriminative stimulus properties of 8-OH-DPAT. Behav Pharmacol. 1993;4:610–624. [PubMed] [Google Scholar]
- Ybema CE, Slangen JL, Olivier B. Discriminative stimulus effect of flesinoxan: effects of 5-HT1A antagonists and PCPA. Pharmacol Biochem Behav. 1994;47:957–962. doi: 10.1016/0091-3057(94)90303-4. [DOI] [PubMed] [Google Scholar]
- York JL. A comparison of the discriminative stimulus effects of ethanol, barbital, and phenobarbital in rats. Psychopharmacology (Berl) 1978;60:19–23. doi: 10.1007/BF00429173. [DOI] [PubMed] [Google Scholar]
- Young AM, Masaki MA, Geula C. Discriminative stimulus effects of morphine: effects of training dose on agonist and antagonist effects of mu opioids. J Pharmacol Exp Ther. 1992;261:246–257. [PubMed] [Google Scholar]
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol. 2002;443:113–118. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA. Behavioral effects of 5-methoxy-N,N-dimethyltryptamine and dose- dependent antagonism by BC-105. Psychopharmacology (Berl ) 1983;80:156–160. doi: 10.1007/BF00427960. [DOI] [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA. Further studies on the dose dependent stimulus properties of 5- methoxy-N,N,dimethyltryptamine. Pharmacol Biochem Behav. 1986;25:1207–1210. doi: 10.1016/0091-3057(86)90113-9. [DOI] [PubMed] [Google Scholar]
- Young R, Bondareva T, Wesolowska A, Young S, Glennon RA. Effect of the 5-HT6 serotonin antagonist MS-245 on the actions of (−)nicotine. Pharmacol Biochem Behav. 2006;85:170–177. doi: 10.1016/j.pbb.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barrett JE. Imipramine as discriminative stimulus. J Pharmacol Exp Ther. 1991;259:1088–1093. [PubMed] [Google Scholar]
- Zhang L, Walker EA, Sutherland JII, Young AM. Discriminative stimulus effects of two doses of fentanyl in rats: pharmacological selectivity and effect of training dose on agonist and antagonist effects of mu opioids. Psychopharmacology (Berl) 2000;148:136–145. doi: 10.1007/s002130050035. [DOI] [PubMed] [Google Scholar]