Abstract
Infectious diseases are often initiated by microbial adherence that is mediated by the binding of attachment molecules, termed adhesins, to cell surface receptors on host cells. We present an experimental system, oblique-incidence reflectivity difference (OI-RD) microscopy, which allows the detection of novel, low-affinity microbial attachment mechanisms that may be essential for infectious processes. OI-RD microscopy was used to analyze direct binding of the onco-pathogen, Helicobacter pylori (H. pylori) to immobilized glycoconjugates in real time with no need for labeling tags. The results suggest the presence of additional Lewis b blood group antigen (Leb) binding adhesins that have not been detected previously. OI-RD microscopy also confirmed the high-affinity binding of H. pylori outer-membrane protein BabA to Leb. The OI-RD microscopy method is broadly applicable to real-time characterization of intact microbial binding to host receptors and offers new strategies to elucidate the molecular interactions of infectious agents with human host cells.
1. Introduction
The first step in the pathogenesis of a mucosal infectious agent is typically adherence mediated by the binding of microbial attachment proteins to specific host cell surface carbohydrates. Examples include binding of adhesins on the influenza virus to sialylated carbohydrates on a host cell surface and binding by the G-adhesin of P-fimbriated Escherichia coli to the P blood group antigens in urinary tract epithelium. Methods that detect the specific binding of microbial adhesins to host glycans have the potential to enhance our understanding of microbial pathogenesis and may lead to translational applications to prevent or treat infectious diseases.
Carbohydrate microarrays are used to probe putative glycan binding proteins, yet few have been used to examine the binding properties of intact microbes. Moreover, standard microarray methods utilize affinity tags and fluorescent labels for detection; these alter the microbial interaction with glycans [1,2], often in unknown ways. Oblique-incidence reflectivity difference (OI-RD) scanning microscopy (Fig. 1) is a recently developed method for the analysis of label-free biomolecular binding to immobilized targets [3–9]. OI-RD microscopy measures small changes in the phase and amplitude of a reflected optical wave from a solid surface due to the reaction of a solution-phase probe (in this study, microbial cells and adhesins) with target molecules (here, immobilized glycans). OI-RD microscopy has been utilized successfully in several biomolecular binding assays, including DNA hybridization [3], antigen-antibody interactions [5–8], and screens of small molecule libraries for protein ligands [9].
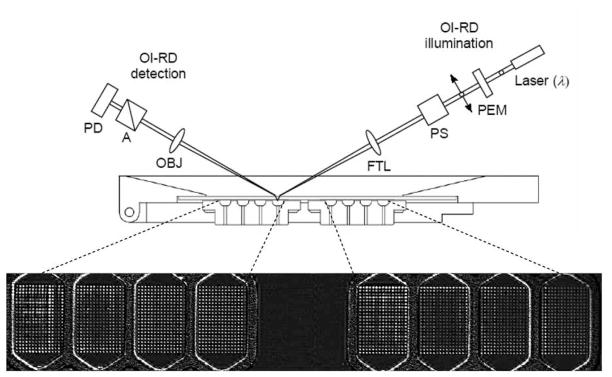
Figure 1. Oblique-incidence reflectivity difference (OI-RD) scanning microscope demonstrates that H. pylori binds to the Leb antigen also in a BabA-independent manner.
OI-RD scanning microscope equipped with a combination of a y-scan mirror and an x-scan linear stage. A 1 in × 3 in glass slide printed with 8 microarrays, each over an area of 3 mm × 4.5 mm, was assembled with a fluidic system with 8 chambers, each of which contained a microarray (A). The fluidic system was mounted on the x-scan linear stage. PEM: photo-elastic modulator. PS: phase shifter. FTL: encoded scan mirror for y-scan. OBJ: objective lens. A: polarization analyzer. PD: photodiode detector. The OI-RD image displays eight protein microarrays, each comprised of 315 bovine serum albumin (BSA) spots (see Supplementary Information, Text S1).
A novel application of OI-RD microscopy is real-time analysis of whole bacterial cell binding to surface presented cognate host cell receptors; here we used OI-RD microscopy to analyze the binding of Helicobacter pylori, that is the major cause of peptic ulcer disease and gastric cancer [10] to carbohydrate receptors. H. pylori attachment to the gastric epithelium is mediated in part by the blood group antigen binding adhesin (BabA), which binds with high affinity to the fucosylated ABO blood group antigens and in particular to the Lewis b antigen (Leb) of blood group O. Although the ABO blood group system is based on expression of the ABO antigens on erythrocytes, primary expression of these antigens is on the gastrointestinal epithelium [11,12]. In this study, OI-RD microscopy confirmed that wild-type H. pylori binds specifically to Leb but not to other fucosylated antigens such as Lea, Lex or Ley. Since BabA is a member of a large family of H. pylori outer membrane proteins (OMPs) [13], there are likely additional, unrecognized adhesins with affinity for other glycans expressed on the gastric epithelium. Indeed, OI-RD analysis demonstrated that H. pylori mutants that lack BabA still bind specifically to Leb, albeit with lower binding strength. Hence, OI-RD microscopy not only confirmed the established BabA-mediated binding to Leb, but also revealed the presence of a novel Leb binding mechanism. These results demonstrate that OI-RD microscopy is generally applicable to real-time characterization of both high- and low-affinity microbial binding to host receptors and offers a novel methodology to better investigate and understand microbial cell attachment to human host cells.
2. Materials and Methods
Microarray of Lewis Glycoconjugates
Lewis glycans; Lea-HSA, Leb-HSA, Lex-HSA, and Ley-HSA (Isosep AB; Tullinge, Sweden) were covalently attached to human serum albumin (HSA) at molar ratios of approximately 20 glycans and dispensed in a 384-well plate (Genetix, Charlestown, MA) in concentrations of 2, 4, 8, and 16 μM. The glycoconjugate solutions were spotted into a microarray using an OmniGrid100 Contact-Printing Arrayer (Digilab, Holliston, MA). The microarray consisted of nine replicates of Lewis glycoconjugates at each concentration (in the form of a 3 × 3 lattice), plus two rows of control spots (12 each) printed from 8 μM BSA (Jackson ImmunoResearch Laboratories, PA). A total of 144 target spots and 24 control spots cover a footprint of 3 mm × 4.5 mm. The average diameter of the printed spots is 100 μm, and the center-to-center spacing between the neighboring spots is 300 μm. The printed glycoconjugates were bound covalently to the glass slide by the exothermic reaction of amine residues on HSA and BSA with epoxy groups on the glass surface. Eight glycoconjugate microarrays were printed in separate locations on one 1 in × 3 in glass slide (se Supplementary Information, Fig. S1).
The slide was assembled with a fluidic system with each of the 8 printed microarrays housed in a separate chamber (volume/chamber, 30 μL) as illustrated in Fig. 1. Before reaction, the printed side of the slide was washed with 2 mL of 1×PBS at flow rate of 5 mL/min. The washed surface was then exposed to the BSA solution for 30 minutes and washed again with 2 mL of 1×PBS at 5 mL/min. The blocked microarray surface was imaged again with the OI-RD microscope prior to reaction.
Recombinant BabA
A truncated, soluble BabA derivative lacking a predicted C-terminal beta-barrel structure [13], designated BabA547, was expressed in E. coli with a periplasmatic leader sequence (see Supplementary Information, Text S3). Specific binding of BabA547 to Leb was confirmed by ELISA (see Supplementary Information, Text S4 and Fig. S3).
Binding Reaction of Recombinant BabA (BabA547) with a Lewis Glycoconjugate Microarray
For BabA547 binding assays, the four Lewis glycoconjugates were printed separately at concentrations of 0.64 μM, 3.2 μM, and 16 μM. Each target was printed twice for a total of 24 spots.
H. pylori Strains
Wild type (WT) H. pylori strains J166 and J99 were grown for 24 hrs on solid media [25] and harvested into blocking buffer (0.2% BSA, 0.05% Tween 20, and 0.05% NaAzide). The concentration was adjusted to an optical density of 0.10 at 600 nm. Isogenic deletions of babA (ΔbabA) or both babA and sabA (ΔbabAΔsabA) were as described previously [14, 20]. H. pylori CCUG 17875 [20], was used to express recombinant BabA and to detect bacterial cells binding to Leb by fluorescent microscopy.
Whole H. pylori Cell Binding Reaction with Lewis Glycoconjugate Microarrays
For the association phase, we replaced 1×PBS in the fluidic chamber with 0.2 mL of the bacterial solution at 5 mL/min and incubated at room temperature for 66 hrs. For the dissociation phase, we replaced the bacterial solution with 0.3 mL of 1×PBS at 5 mL/min and incubated for 28 hours at room temperature. The long dissociation time is likely due to the presences of multiple adhesin molecules on the bacterial cell surface that bind to multiple receptors. Three bacterial strains were loaded simultaneously into six chambers, each with a glycoconjugate microarray. The microarray in one of the remaining two chambers was exposed only to 1×PBS as a control.
OI-RD Scanning Microscopy for Label-free Detection of H. pylori Binding to Lewis Glycoconjugates
We measured the amount of recombinant BabA proteins and the bacteria captured by the Lewis glycoconjugates (per unit area) with the scanning OI-RD microscope as illustrated in Fig. 1. The working principle of the microscope has been reported previously [7,9], and the key features of the microscope used in the present study are described in Supplementary Information (Text S1.) Briefly, binding of bacterial cells causes changes in the phase and amplitude of an optical beam reflected from the surface of the solid support. These changes arise from differences in the refractive index of the target-probe layer, the solid support, and the aqueous ambient, and depend on whether the optical beam is p-polarized or s-polarized. A scanning OI-RD microscope measures the differential reflection change between two polarizations across a microarray-covered solid surface [4,7,9]. To characterize a glycoconjugate microarray and its subsequent reaction with BabA protein, we measured the differential phase change (see Supplementary Information, Text S1: Eq. (S2) and Eq. (S3)) for contrast. To characterize the H. pylori whole cell binding reaction with a glycoconjugate microarray, we measured differential amplitude change (See Supplementary Information, Text S1: Eq. (S4)) for contrast. The image of the glycoconjugate microarrays was acquired using a step size of 10 μm. By taking a sequence of optical images at two-hour time intervals, we obtained real-time binding curves as well as the end-points of the bacterial reactions.
3. Results
Recombinant BabA Binds Specifically to Surface Presented Leb
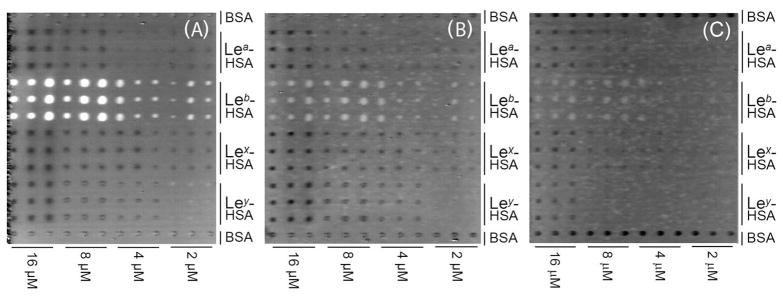
We first used OI-RD to study the binding of recombinant BabA to a Lewis glycoconjugate microarray. Fig. 2A shows the OI-RD image of a Lewis antigen glycoconjugate microarray before the reaction, and Fig. 2B shows the change in the image after the microarray was incubated with 400 μM recombinant BabA547 for 60 min. Recombinant BabA547 binds to Leb but not to the related fucosylated antigens Lea, Lex, and Ley. This observation supports the notion that binding of H. pylori to Leb moieties on a host surface is mediated primarily by the affinity of the BabA adhesin for Leb.
Figure 2. Recombinant BabA protein binds specifically to Leb antigen.
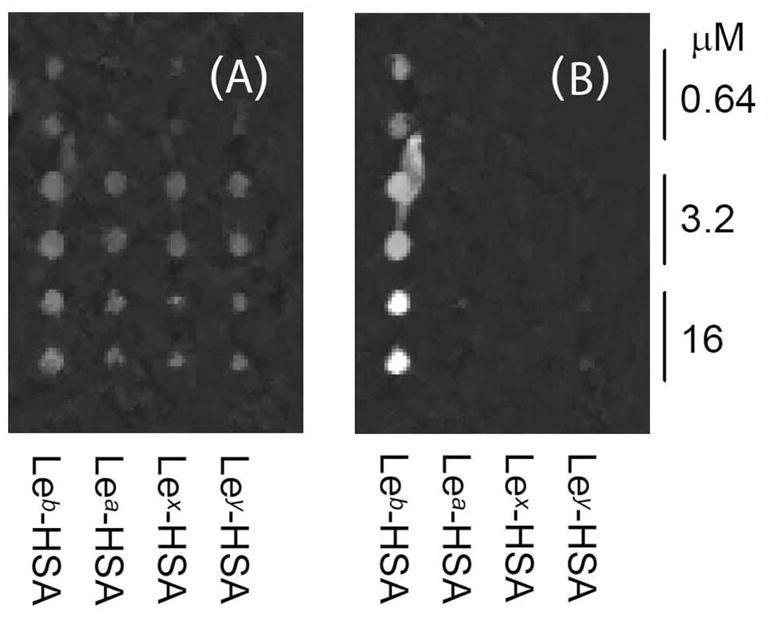
(A) The OI-RD image of a printed Lewis antigen microarray (Leb, Lea, Lex, Ley) with all glycoconjugates visualized before incubation with recombinant BabA547. Each glycoconjugate was printed twice at three target concentrations. (B) Recombinant BabA547 binds to Leb-HSA but does not bind to the closely related Lewis antigens.
H. pylori Binds to Leb in a BabA-independent Manner
We next investigated the binding of H. pylori strain J166 whole cells to the Lewis glycoconjugate microarray. As shown in Fig. 3A, wild-type H. pylori J166 exhibits specific binding to Leb, but not to Lea, Lex, Ley, or to non-glycosylated bovine serum albumin (BSA) controls. Surprisingly, H. pylori J166ΔbabA, which does not express BabA and does not bind to Leb-HSA in solution, also exhibits specific binding to Leb, although binding is reduced compared to binding of the wild-type J166 strain (Fig. 3B). We observed a similar binding pattern for H. pylori strain J99 (Fig. 3C). These observations suggest that the H. pylori strains have previously unrecognized BabA-independent Leb-binding activity.
Figure 3. H. pylori binds to the Leb antigen in a BabA-independent manner.
(A) The J166WT strain, (B) the J166ΔbabA deletion-mutant strain and, (C) the J99ΔbabA deletion-mutant strain (F) were incubated for 66 hours at room temperature on an extended Lewis glycoconjugate microarray (Leb, Lea, Lex, Ley, each at four target concentrations). The oblique-incidence reflectivity difference (OI-RD) images show that both the WT and the babA deletion mutants bind specifically to the Leb antigen, although the WT strain has higher binding strength. The results were obtained by subtracting the images taken before incubation from the images taken after incubation.
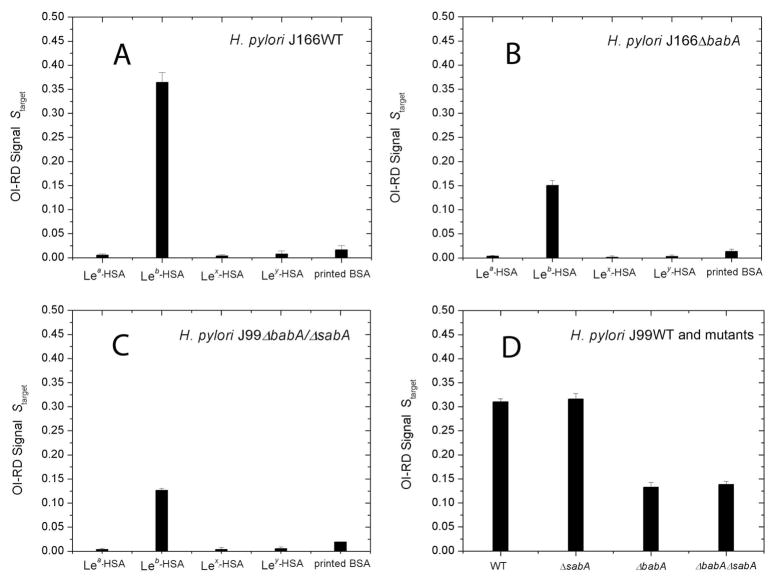
Quantitative analysis confirms BabA-independent binding to Leb
We next quantified the bacterial binding by separating the optical signals due to bacterial binding to glycoconjugates from the signals due to non-specific binding to the blocking agent, BSA. Even at the printing concentration of 16 μM, immobilized glycoconjugates do not fully cover the functionalized solid surface. Specifically, surface coverage of the glycoconjugate targets (Θtarget) is less than unity, and the remaining surface (1 − Θtarget) is covered with the blocking agent, BSA. Thus, both the glycoconjugates and the blocking BSA on the surface contribute to the OI-RD signal shown in Fig. 3. If we designate Sblocking-BSA as the optical signal from the surface fully covered with BSA, and Starget as the signal from the surface fully covered with glycoconjugates (or printed BSA), the total optical signal S in Fig. 3 is expressed as:
| (1) |
We determined Θtarget from the OI-RD microscopy images obtained before and after blocking with BSA (see Supplementary Information, Text S2). With the information on Ttarget and Sblocking-BSA from the unprinted surface region, we calculated Starget using Eq. (1) from the data shown in Fig. 3. Fig. 4A and Fig. 4B show, respectively, the results for H. pylori J166 (WT) and J166ΔbabA binding to the four Lewis glycoconjugates and printed BSA control. Wild-type J166 binds to Leb with high strength and specificity, while the J166ΔbabA mutant shows lower but still specific affinity for Leb. The latter observation supports the notion that H. pylori J166 has both BabA-dependent and BabA-independent Leb-binding adhesin activity. Findings were similar for H. pylori strain J99WT, which differs from J166 in that it in addition to BabA also expresses the sialyl-Lex binding protein, SabA [14]. Both J99WT and the J99ΔsabA mutant bind to Leb with high affinity and specificity, while the J99ΔbabAΔsabA and J99ΔbabA mutants bind to Leb with reduced but still specific affinity (Fig. 4C and 4D). These results suggest that, like J166, the J99 strain also has additional Leb-binding activity due to one or more outer membrane proteins. This complementary adhesin activity is specific for Leb (Fig. 4C) and is not associated with the SabA adhesin, since the ΔsabA mutants exhibit intact Leb binding (Fig. 4C and 4D).
Figure 4. H. pylori binds to the Leb antigen in a BabA- and SabA-independent manner.
Binding of (A) J166WT cells (babA intact), (B) J166ΔbabA cells (babA deleted), and (C) J99ΔbabA/ΔsabA (babA and sabA deleted) cells to Lewis arrays as detected by OI-RD signals Starget. All three strains bind to Leb, but they do not bind Lea, Lex, Ley (albumin glycoconjugates), or to BSA printed on functionalized glass. (D) H. pylori binds more strongly to Leb in the presence of BabA (in the J99WT strain and the J99ΔsabA deletion mutant), whereas the SabA adhesin does not contribute to H. pylori binding to Leb.
The detection sensitivity of the OI-RD microscopy technique was compared to that of conventional microscopy by applying fluorescently labeled H. pylori CCUG 17875 bacterial cells to a fresh Lewis glycoconjugate microarray (see Supplementary Information, Fig. S2). In agreement with the OI-RD results (Fig. 3A), H. pylori demonstrated strong binding affinity to immobilized Leb, in the range of 400 bacterial cells over a single printed spot. Binding to other Lewis glycoconjugates, such as Ley and Lex, was close to background, although Lea and the printed BSA control showed some residual binding. The latter implies a non-specific interaction, which presumably relates to the use of the FITC-tag on the fluorescent labeled bacterial cells.
Real-time Analyses Demonstrate Slow Bacterial Binding Kinetics
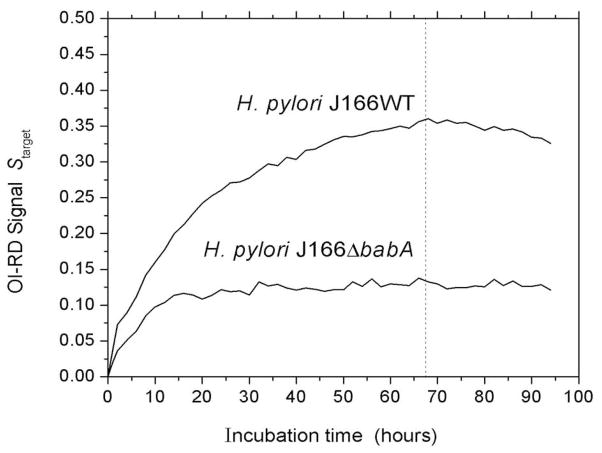
The optical signals shown in Fig. 3 and Fig. 4 were obtained after 66 hours of incubation (before the dissociation phase). Using Eq. (1) and the images taken before and after that time, we obtained Starget as a function of time and constructed the association-dissociation curves of H. pylori-Leb binding (Fig. 5). At a bacterial concentration of 108 cells/mL ([c] = 0.17 pM), the association of H. pylori J166 with immobilized Leb took over 60 hours to level off. The difference in association kinetics for J166 and the J166ΔbabA mutant shows that the association rate is partly limited by mass transport of the bacteria to the microarray-covered solid surface and partly limited by association efficiency with the glycoconjugates. By fitting the association-dissociation curves, we found that Kd for the wild-type J166 to Leb was 0.7 × 10−15 M, while Kd for the J166ΔbabA mutant to Leb was 9.2 × 10−15 M (weaker binder).
Figure 5. Whole bacterial cells exhibit slow binding kinetics.
J166WT binding leveled off after 66 hours, whereas J166ΔbabA mutant binding was faster and leveled off after only 15 hours of incubation. The diagrams depict the association-dissociation curves of J166WT and J166ΔbabA with immobilized Leb-HSA. The dashed line marks the time at which 1×PBS (buffer) replaces the bacterial solution in the fluidic chamber and the time at which the images shown in Fig. 3 were obtained.
4. Discussion
Microbial pathogens that infect mucosal surfaces must negotiate a delicate balance between intimate attachment to host epithelial cells and persistent access to host-derived nutrients vs. the cost of host cell turnover and exposure to the inflammatory mediators of the host immune response. Microbes typically solve this dilemma by expressing adhesins for targeted adherence using a homing mechanism often referred to as tissue tropism. Although the functional interpretation of such glycan-protein interactions has been described for several microbial pathogens, detailed understanding of the underlying biochemistry is difficult. This complexity stems primarily from low-affinity binding, and in particular so because the binding affinity of most adhesins for monosaccharides is exceedingly low, in the mM range, whereas the binding affinity for complex glycans is in the 1 to 10 μM range. Hence, for biological events, multivalent binding often amplifies the relatively low-affinity binding to glycans [15], a phenomenon best illustrated by binding to heavily glycosylated mucin molecules. Similarly, microbes gain binding strength by multivalent presentation of their adhesive subunits, often displaying hundreds of glycan-binding pili or toxins. Examples include the cholera toxin that binds sialylated GM1-glycan in the intestine [16] and, the hemagglutinin molecules on the surface of influenza A virus that bind to sialylated carbohydrates. The importance of low-affinity binding has stimulated strategies for assays based on multivalency in receptor presentation [17–19].
In the present study, we investigated binding to surface presented blood group antigens by the gastric pathogen, H. pylori. Binding analyses of soluble blood group antigens under conditions of equilibrium have shown that high-affinity binding of H. pylori is mediated by BabA [12, 20]. Further, H. pylori strains that express BabA are isolated more frequently from individuals with peptic ulcers or gastric cancer [21], suggesting that tight mucosal binding is a risk factor for development of overt disease. However, BabA expression is often lost during infection in animal models [22, 23], which implies that H. pylori has complementary adhesins with lower binding affinities that make them difficult to detect.
One such low-affinity adhesin is SabA, which mediates binding to sialylated antigens [14]. During H. pylori infection, the predominantly fucosylated mucosal glycosylation shifts to more sialylated patterns [14, 24]. The SabA adhesin binds to the sialylated mucosal landscape, though with 100-fold lower affinity compared to BabA binding to ABO/Leb. To investigate the presence of a second line of attachment proteins complementary to BabA but with substantially lower binding affinities, we used a solid-phase whole-cell binding assay that takes advantage of the “Velcro effect” of multivalent presentation of receptors. Of particular relevance, ligand presentation is important for interpretation of biological receptor activities. For example, H. pylori binds with similar affinity to a series of free fucosylated oligosaccharides (glycans) such as H-1, H-2, Leb, and Ley (i.e. mono- vs. difucosylated structures) based on either the lacto-series type-1 or type-2 core chains. However, when these glycans are covalently attached to a carrier to make multivalent glycoconjugates, the bacterial affinity for H-1 and Leb increases > 1000-fold, whereas the affinity for H-2, Lea, Lex, and Ley is completely lost [20]. H. pylori babA deletion mutants do not bind soluble Leb conjugates [12,20], which argues that complementary adhesins may bind the ABO/Leb antigens with affinities that are reduced several log-fold. This discrepancy necessitates development of techniques for more sensitive detection and visualization of the binding activity of intact bacterial cells.
In this report we present a novel experimental platform based on a combination of solid-phase immobilized glycoconjugates and OI-RD microscopy for real time detection of H. pylori whole cell binding. On this platform, H. pylori binds with high strength to Leb blood group antigens but not to the closely related Lea, Lex, or Ley antigens (Fig. 3 and Fig. 4). This specificity is in complete agreement with previous studies on H. pylori binding to soluble glycoconjugates. Furthermore, the specificity in binding to the Leb antigen but not to Lea, Lex, or Ley antigens was reproduced using recombinant BabA protein (Fig. 2 and Supplementary Information Fig. S3). Surprisingly OI-RD microscopy revealed that babA-deletion mutants also bind to Leb antigen with high specificity, albeit with lower binding strength (Fig. 3 and Fig. 4). The residual lower-affinity Leb binding properties have escaped previous detection using assays based on soluble adhesin-glycan interactions. This label-free solid-phase binding assay platform is particularly useful for study of these cells, as most H. pylori outer membrane proteins demonstrate high isoelectric points (pI); hence, many labeling reagents react readily with basic charged amino acids that might sterically inhibit binding. Furthermore, OI-RD microscopy can be used in real time to analyze simultaneous binding of intact microorganisms to thousands of receptors. The increased sensitivity, high throughput, and real-time analysis of whole bacterial cell binding to multivalent receptors in solid-supported microarray format may permit identification of discrete attachment mechanisms essential for infectious processes, and perhaps in turn could identify novel targets for drug or vaccine development.
Supplementary Material
Acknowledgments
This study was supported by grants to T.B. from the Swedish Research Council (Grant No. 11218), the Swedish Cancer Foundation, and the J.C. Kempe and Seth M. Kempe Memorial Foundation; to J.V.S. from the National Institutes of Health (R01 AI070803, R01 AI081037); and to X.D.Z from the National Institutes of Health (R01 HG003827-04, R01 GM076360-04S1).
References
- 1.Paulson JC, Blixt O, Collins BE. Nat Chem Biol. 2006;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 2.Liang PH, Wu CY, Greenberg WA, Wong CH. Curr Opin Chem Biol. 2008;12:86–92. doi: 10.1016/j.cbpa.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landry JP, Zhu XD, Gregg J. Opt Lett. 2004;29:581–583. doi: 10.1364/ol.29.000581. [DOI] [PubMed] [Google Scholar]
- 4.Zhu XD, Landry JP, Sun YS, Gregg JP, Lam KS, et al. Appl Opt. 2007;46:1890–1895. doi: 10.1364/ao.46.001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landry JP, Sun YS, Guo XW, Zhu XD. Appl Opt. 2008;47:3275–3288. doi: 10.1364/ao.47.003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fei YY, Landry JP, Sun YS, Zhu XD, Luo JT, et al. Rev Sci Instrum. 2008:79013708. doi: 10.1063/1.2830286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun YS, Landry JP, Fei YY, Zhu XD, Luo JT, et al. Langmuir. 2008;24:13399–13405. doi: 10.1021/la802097z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun YS, Landry JP, Fei YY, Zhu XD, Luo JT, et al. Anal Chem. 2009;81:5373–5380. doi: 10.1021/ac900889p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fei YY, Landry JP, Sun YS, Zhu XD, Wang XB, et al. J Biomed Opt. 2010;15:016018. doi: 10.1117/1.3309743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusters JG, van Vliet AH, Kuipers EJ. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borén T, Falk P, Roth KA, Larson G, Normark S. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 12.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, et al. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 13.Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, et al. Infect Immun. 2000;68:4155–4168. doi: 10.1128/iai.68.7.4155-4168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, et al. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler P, Wood SJ, Lee YC, Lee RT, Petri WA, Jr, et al. J Biol Chem. 1995;270:5164–5171. doi: 10.1074/jbc.270.10.5164. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren J, Lönnroth I, Månsson J, Svennerholm L. Proc Natl Acad Sci USA. 1975;72:2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borén T, Normark S, Falk P. Trends Microbiol. 1994;2:221–228. doi: 10.1016/0966-842x(94)90626-2. [DOI] [PubMed] [Google Scholar]
- 18.Falk P, Borén T, Normark S. Methods Enzymol. 1994;236:353–374. doi: 10.1016/0076-6879(94)36027-8. [DOI] [PubMed] [Google Scholar]
- 19.Aspholm M, Kalia A, Ruhl S, Schedin S, Arnqvist A, et al. Methods Enzymol. 2006;417:293–339. doi: 10.1016/S0076-6879(06)17020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilver D, Arnqvist A, Ögren J, Frick IM, Kersulyte D, et al. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 21.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, et al. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solnick JV, Hansen LM, Salama NR, Boonjakuakul JK, Syvanen M. Proc Natl Acad Sci USA. 2004;101:2106–2111. doi: 10.1073/pnas.0308573100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Styer CM, Hansen LM, Cooke CL, Gundersen AM, Choi SS, et al. Infect Immun. 2010;78:1593–1600. doi: 10.1128/IAI.01297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindén S, Mahdavi J, Semino-Mora C, Olsen C, Carlstedt I, et al. PLoS Pathog. 2008;4:e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solnick JV, Hansen LM, Canfield DR, Parsonnet J. Infect Immun. 2001;69:6887–6892. doi: 10.1128/IAI.69.11.6887-6892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.