Abstract
This study explored the involvement of N-methyl-D-aspartate (NMDA) in the effects of mu opioid agonists. A hot plate procedure was used to assess antinociception and tolerance in mice in which the NR1 subunit of the NMDA receptor was reduced (knocked down, KD) to approximately 10% and in mice treated with the NMDA antagonist, LY235959 [(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboylic acid]. The mu opioid agonists, morphine, l-methadone and fentanyl, were approximately 3-fold less potent in the NR1 KD mice than in wild type (WT) controls; however, the development of morphine tolerance and dependence did not differ markedly in the NR1 KD and WT mice. Acute administration of the NMDA antagonist, LY235959 produced dose-dependent, leftward shifts in the morphine dose-effect curve in WT mice, but not in the NR1 KD mice. Chronic administration of LY235959 during the morphine tolerance regimen did not attenuate the development of tolerance in the NR1 KD nor the WT mice. These results indicate that the NR1 subunit of the NMDA receptor does not play a prominent role in mu opioid tolerance.
Keywords: antinociception, tolerance, withdrawal, NMDA antagonist, mu opioids, hot plate procedure, NR1 knock down mouse
INTRODUCTION
In the central nervous system, glutamate is the most prominent excitatory neurotransmitter and is known to work through several different receptor types, including three different classes of ionotropic receptors: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate as well as metabotropic glutamate (mGlu) receptors. Among these receptor types, there is substantial evidence to support a modulatory role for the NMDA receptor in the effects of opioids.
Several laboratories have shown that NMDA receptor antagonists can potentiate the acute antinociceptive effects of opioid agonists, both in squirrel monkeys (Allen and Dykstra, 2001; Allen et al., 2003) and rodents (Kozela and Popik, 2002; Nemmani et al., 2004; Craft and Lee, 2005; Fischer et al., 2005; Grisel et al., 2005). Moreover, administration of NMDA receptor antagonists in combination with morphine reduces the development of tolerance to its antinociceptive effects (Allen and Dykstra, 1999; 2000; Trujillo, 2000; Bryant et al., 2006; Mendez and Trujillo, 2008).
Despite a wealth of evidence implicating the involvement of NMDA receptors in morphine antinociception and tolerance, the specific role of the NMDA receptor in mediating these effects remains unclear. Studies with antagonists specific to the NMDA receptor represent one way to investigate the role of NMDA receptors in morphine’s effects. Another way to examine these interactions is to take advantage of genetically-modified mice. Therefore, the current study examined the effects of mu-opioid agonists in mice with a hypomorphic allele for the gene encoding the NR1 subunit of the NMDA receptor (Mohn et al., 1999). Whereas mice with a full knockout of NR1 protein are not viable, the mice used here express approximately 5–10% of the normal level of NR1, which is sufficient for survival into adulthood. Consistent with the reduced NR1 gene expression, these mutants display less than 10% of functional NMDA receptors in the brain, compared to WT controls. Therefore, these NR1 knockdown (NR1 KD) mice provide a possible model for assessment of the role of the NR1 subunit of the NMDA receptor in the neurobiology of the antinociceptive effects of morphine.
Specifically, the present study used a hotplate nociceptive assay to measure the acute antinociceptive effects of morphine and two other mu opioid agonists, l-methadone and fentanyl. Effects of morphine were examined in NR1 KD mice and compared to WT controls, as well as in mice that were treated both acutely and chronically with the NMDA antagonist, LY235959 [(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboylic acid]. The hotplate procedure was selected here in order to make direct comparisons with data collected previously in mice that had been treated with NMDA antagonists. In addition, the development of tolerance to and dependence upon morphine was examined in NR1 KD and WT mice. Based on the results of several studies reporting that NMDA antagonists can increase the antinociceptive effects of morphine under certain conditions, as well as attenuate the development of opioid tolerance, we hypothesized that similar effects would be observed in mice in which the NR1 subunit was knocked down.
METHODS
Subjects
Adult male homozygous NR1 receptor deficient mice and wildtype controls were either provided by Dr. Beverly Koller (UNC, Department of Genetics) or bred and genotyped in the animal facilities of the Psychology Department at the University of North Carolina at Chapel Hill. All mice were generated as described previously (Mohn et al., 1999) and weighed between 23 and 34 g when experiments were conducted. Previous investigations have characterized these mice as NR1 hypomorphs, given that the NR1 gene is not eliminated in these mice, but rather under-expressed. Previous investigations indicate that the NR1 subunit is greatly reduced in all brain regions of these mutant mice (Duncan et al., 2002). The behavioral phenotype of these mice includes reduced habituation in activity chambers (Mohn et al., 1999) as well as increased acoustic startle responses and deficits in prepulse inhibition (Duncan et al., 2004).
The mice were group housed 4–5/cage in standard Plexiglas cages in a colony room maintained on a 12-hr reversed light/dark cycle (lights off at 07:00h). All mice had continuous access to food and water throughout the study. Mice were also handled and adapted to the testing environment for two days prior to initiation of an experiment. All testing procedures were conducted between 09:00 and 14:00h. Animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill and the 2010 Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH).
Antinociceptive procedure
Antinociception was assessed using a hot plate analgesia meter (25.3 × 25.3 cm) (Columbus Instruments, Columbus, OH) maintained at 56±0.1°C. The antinociceptive response was evaluated by recording the latency to lick or flutter the hind paw(s), or to jump from the hotplate surface. Responses were measured using a stopwatch to the nearest 0.1 sec. To prevent tissue damage, a cutoff time of 20 s was defined as the maximal response. Immediately following the termination of a trial, mice were removed from the hotplate surface.
An additional experiment was run in order to determine whether the NR1 receptor knockdown mice and their wild type littermates differed in their baseline responses on the hot plate. In this experiment, responses to a full range of temperatures were examined in NR1 KD and WT mice, from 44 ±0.1°C to 56±0.1°C.
Baseline response latencies on the hot plate were determined twice prior to the beginning of drug administration and spaced 30 min apart. Data from these determinations were averaged to yield one baseline value. Following baseline latency measurements, responding on the hot plate was examined over multiple cycles, spaced either 15 min (fentanyl) or 30 min (morphine, l-methadone) apart. Drugs were administered at the start of each cycle and latency on the hot place was determined during the last min of the cycle. Drug doses were increased cumulatively, with the dose administered increasing in one-quarter or one-half log unit increments prior to each cycle.
Tolerance Regimen
Data collected during the acute phase of the study (described above) indicated that the ED50s for morphine in the NR1 KD mice were larger than those in the WT mice. Therefore, the tolerance regimen was adjusted based on individual sensitivities of the WT and NR1 KD mice, rather than the administration of the same dose of morphine to all mice. The appropriateness of employing a tolerance regimen which is adjusted to each animal’s individual sensitivity is described by Barrett et al. (2001).
Specifically, morphine dose-effect curves were first obtained on the morning of day 1 (prechronic). On the basis of these prechronic morphine dose-effect curves, an individual ED50 value was determined for each mouse. The tolerance regimen was then initiated, with morphine doses adjusted on the basis of each mouse’s prechronic ED50 value. According to this regimen, each mouse received 5× its individual ED50 on the morning and evening of day 2; 10× its individual ED50 on the morning and evening of day 3; 15× its individual ED50 on the morning and evening of day 4 and 20× its individual ED50 on the morning and evening of days 5–13. On the next day (day 14), the postchronic morphine dose-effect curve was determined, coinciding with the time of the regularly scheduled morning injection. Given this regimen, it should be noted that the NR1 KD mice actually received larger absolute doses than the WT mice; however, the functional dose was the same for each mouse.
In an additional set of experiments, mice received a dose of 1.0 mg/kg of LY235959 throughout the tolerance regimen. These experiments paralleled the tolerance regimen described above. That is, on day one, a morphine dose-effect curve was determined for each mouse and individual ED50 values were calculated. The tolerance regimen was then initiated, based on each mouse’s ED50 value; however, mice received 1.0 mg/kg of the NMDA antagonist, LY235959, in combination with increasing doses of morphine throughout the 12 day morphine tolerance regimen as well as on test day.
Withdrawal Jumping
Immediately after determining the postchronic morphine dose-effect curve, mice were given an additional morphine injection to raise their cumulative morphine levels to 20 × their original ED50. Naltrexone (1.0 mg/kg) was administered 30 min later, and mice were placed in 4 L glass beakers (Pyrex, Lowell MA, USA) for a 30 min period. The number of jumps, paw flutters and “wet-dog shakes” were recorded for each 5-min interval.
Drugs
Morphine sulfate was provided by the National Institute on Drug Abuse (Bethesda, MD, USA); l-methadone and LY235959 by Lilly Research Laboratories (Indianapolis, IN, USA) or purchased from Tocris Bioscience (Ellisville, MO, USA). Fentanyl and naltrexone were purchased from Sigma Chemical Co (St. Louis, MO, USA). Drugs were dissolved in 0.9% saline or distilled water to yield all concentrations. All doses were injected subcutaneously at a volume of 0.1 ml/10 g.
Data analysis
Drug effects were expressed as a percentage of the maximal possible effect (%MPE) using the following formula:
Data are expressed as the mean %MPE (± S.E.M.). ED50 values and 95% confidence limits were calculated using PHARM/PCS version 4 (Tallarida and Murray, 1987). When appropriate, statistical analyses were conducted using a t-test or repeated-measures analysis of variance (ANOVA). Post-hoc tests were then conducted to assess the specific group differences. Fisher's protected least significant difference (PLSD) analysis was conducted to determine points that were significantly different from baseline. All analyses were conducted with an alpha level of significance set at p≤ 0.05.
RESULTS
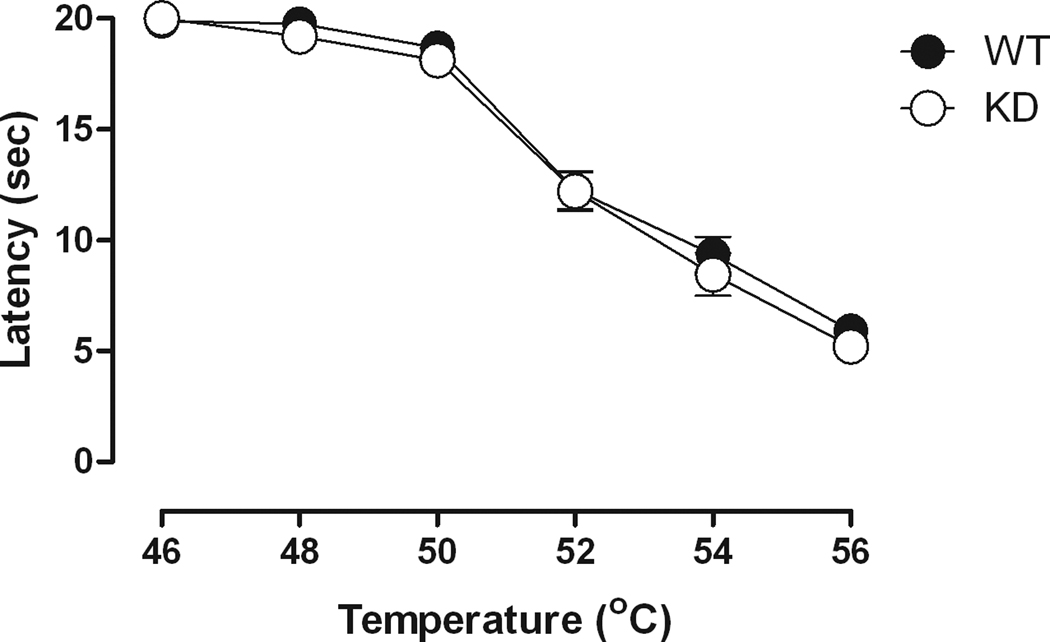
Fig. 1 shows a temperature-effect curve for NR1 KD mice and the WT controls. ANOVA confirmed an effect of temperature, but no effect of genotype. Pairwise comparisons of the temperature effects indicated that response latencies at the lower temperatures (i.e., 46°, 48° and 50°C) were significantly different (p<0.05) from the response latencies at the higher temperatures (i.e., 52°, 53° and 56°C). No differences were observed in baseline response latency between NR1 KD and WT mice across all temperatures [F(1,25)=1.19, NS]. Similarly, a separate examination of baseline response latencies on a 56°C hot plate over a 2-hr test period, revealed no differences between the genotypes following saline administration [F(1,19)=3.03, p=.098] (data not shown). Although response latencies did not differ between the NR1 KD and WT mice when examined on the hot plate, informal observations indicated that the NR1 KD mice did show increased activity levels in their cages and also when they were handled for injections.
Fig. 1.
Temperature-dependent effects on the hot plate in NR1 KD and WT mice. Horizontal axis: hot plate temperature in degrees Celsius. Vertical axis: latency to respond on the hot plate in seconds. Temperature-effect determinations were conducted at 15-min intervals. (n = 7 or 8)
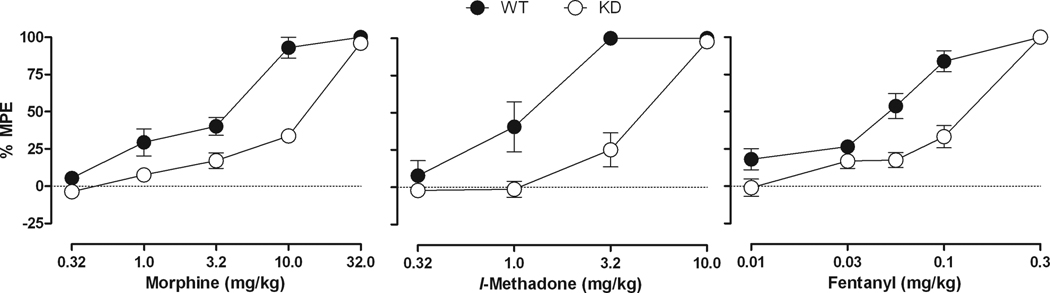
Fig. 2 shows the effects of morphine, l-methadone and fentanyl in the NR1 KD mice and their WT controls. The ED50 value (95% confidence limits) for morphine in WT mice was 2.54 mg/kg (1.76 – 3.67) and 10.39 mg/kg (8.57 – 12.40) in the NR1 KD mice, yielding a potency ratio of 4.09, indicating that morphine was approximately 4 times more potent in WT mice than in the NR1 KD mice. The effects of l-methadone were similar to those observed with morphine, with l-methadone being approximately 4 times more potent in the WT mice than in NR1 KD mice (potency ratio =3.85). Specifically, the l-methadone ED50 value (95% confidence limits) was 1.02 mg/kg (0.72 – 1.44) in the WT mice and 3.93 mg/kg (3.03 – 5.09) in the NR1 KD mice. The profile of effects obtained for fentanyl was similar to those observed with morphine and l-methadone. That is, the ED50 for fentanyl in the WT mice was 0.05 mg/kg (0.04 – 0.06), whereas the ED50 for fentanyl in the NR1 KD mice was 0.12 mg/kg (0.11 – 0.14), yielding a potency ratio of 2.36 (1.93 – 2.89). In both groups of mice, fentanyl was more potent than methadone, which was more potent than morphine.
Fig. 2.
Dose-effect curves for morphine (0.32–32 mg/kg), l-methadone (0.32–10 mg/kg) or fentanyl (0.01–0.3 mg/kg) in NR1 KD and WT mice. Horizontal axis: dose of morphine, l-methadone or fentanyl in milligrams per kilogram. Vertical axis: antinociception quantified as %MPE. Dose-effect determinations were conducted cumulatively at 30-min (morphine and l-methadone) or 15min (fentanyl) intervals. (n = 6 or 7).
Morphine and l-methadone dose-effect curves were subsequently redetermined following pretreatment with the opioid receptor antagonist naltrexone. A dose of 1.0 mg/kg naltrexone attenuated the antinociceptive effects of both morphine and l-methadone in a comparable manner, producing a 3 to 5-fold rightward shift in the morphine and l-methadone dose-effect curves in both NR1 KD and WT mice. The ED50 (95% confidence limits) values for morphine and l-methadone alone and in combination with naltrexone are shown in Table 1.
Table 1.
ED50 values in mg/kg (± 95% confidence limits) and dose ratios for morphine and l-methadone alone and in combination with 1.0 mg/kg naltrexone
| Drug | Genotype | Drug Alone (C.L)1 |
+ NTX (C.L)1 |
Dose Ratio (C.L)1 |
|---|---|---|---|---|
| Morphine | WT | 2.54 (1.76 – 3.67) | 9.67 (8.04 – 11.60) | 3.87 (2.69 – 5.53) |
| NR1 KD | 10.39 (8.47 – 12.73) | 32.54 (26.59 – 39.81) | 3.12 (2.38 – 4.10) | |
| l-Methadone | WT | 1.02 (0.72 – 1.44) | 5.79 (4.29 – 7.83) | 5.81 (3.60 – 8.85) |
| NR1 KD | 3.93 (3.03 – 5.09) | 12.14 (9.09 – 16.20) | 3.10 (2.16 – 4.23) |
C.L.=95% confidence limits
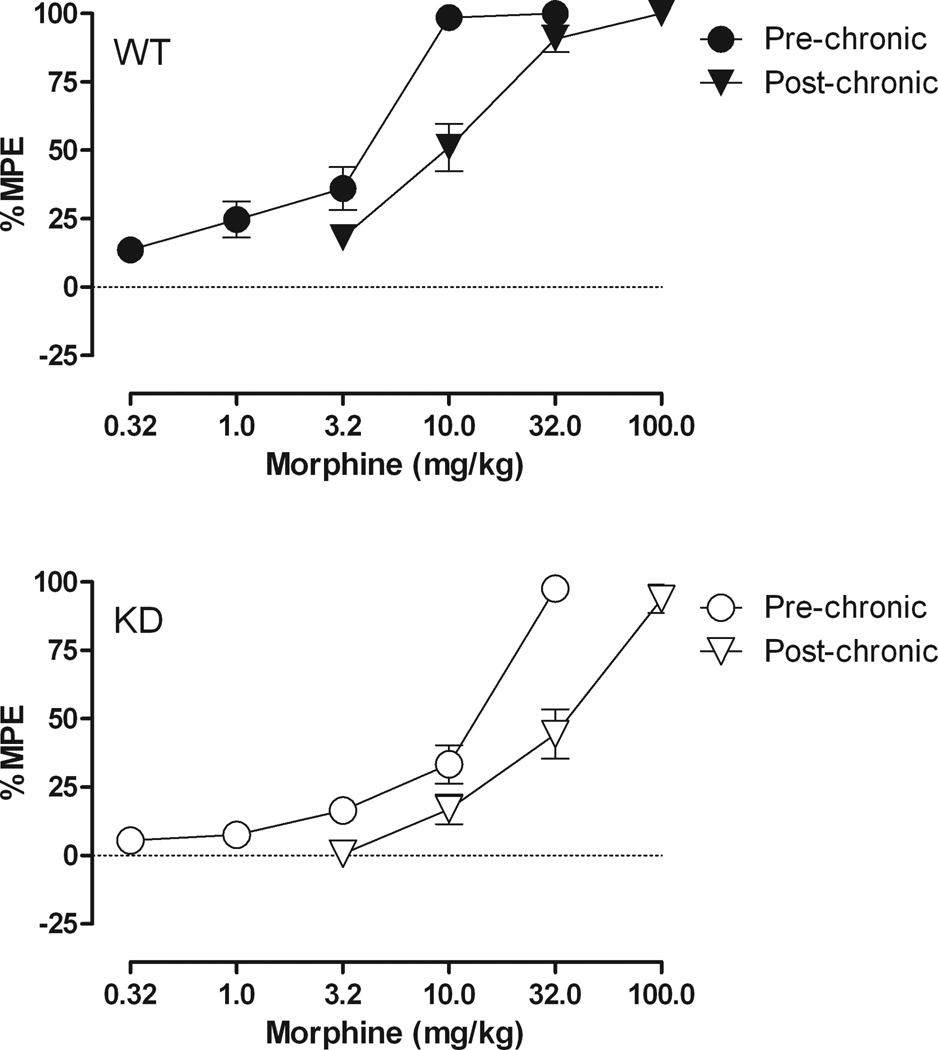
Fig. 3 shows the effects of morphine following a regimen of chronic morphine administration. The effects of morphine prior to tolerance induction were similar to those shown in Fig. 2. That is, morphine produced dose-dependent increases in latency to respond on the hot plate in both NR1 KD and WT mice; however, morphine was less potent in the NR1 KD mice, yielding an ED50 of 7.79 mg/kg (5.86 – 10.36) in the NR1 KD mice and an ED50 of 2.88 mg/kg (2.18 – 3.80) in the WT mice. Following 12 days of chronic morphine administration, with the dose of morphine increased by the same proportion in each individual mouse, the morphine dose-effect curve shifted to the right, yielding an ED50 of 30.15 mg/kg (23.54 – 38.61) in the NR1 KD mice and an ED50 of 9.06 mg/kg (7.16 – 11.47) in the WT mice, with the magnitude of the shift in the morphine dose-effect curve being approximately the same, i.e., 3.1 – 3.9 fold in the WT and NR1 KD mice, respectively.
Fig. 3.
Dose-effect curves for morphine, prior to and following a regimen of chronic administration of morphine for 12 days in WT (top) and NR1 KD (bottom) mice. Horizontal axis: dose of morphine in milligrams per kilogram. Vertical axis: antinociception quantified as %MPE. Dose-effect determinations were conducted cumulatively at 30-min intervals. (n = 8).
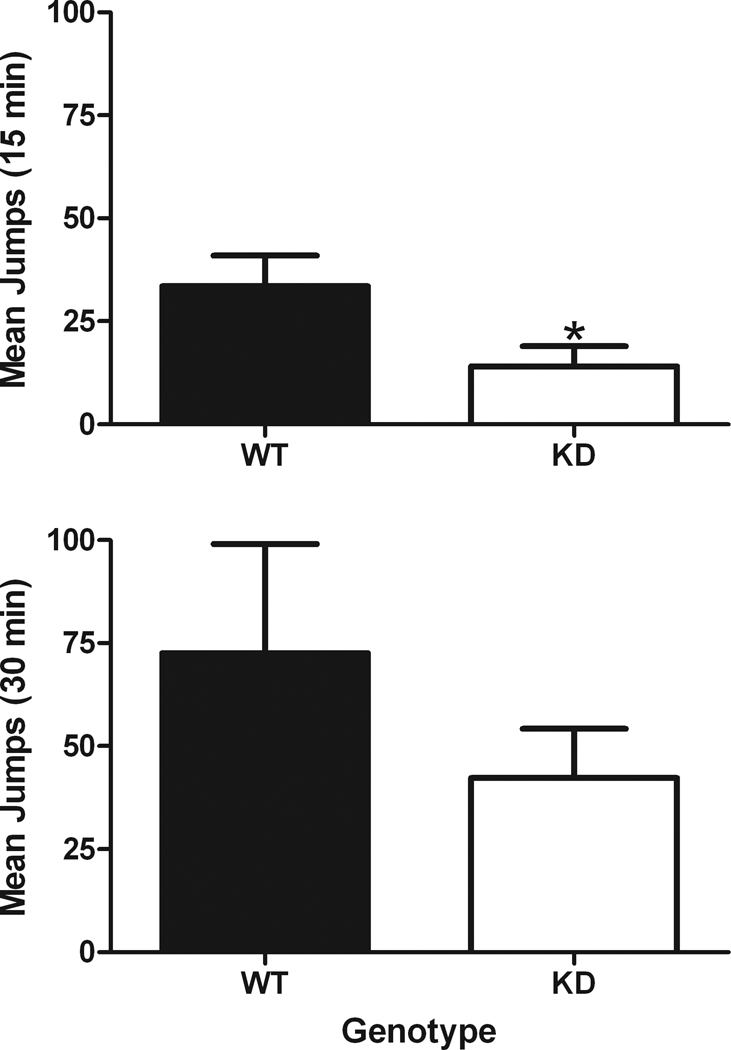
After the post chronic morphine dose-effect curve was determined, mice were administered naltrexone and observed for signs of withdrawal. During the 30-min observation period, typical signs of withdrawal were observed in both WT and NR1 KD mice, including occasional paw tremors and wet dog shakes. The most prominent sign of withdrawal, jumping behavior, is shown in Fig. 4 averaged over a 15-min or a 30-min observation period. The number of jumps that occurred during the first 15-min period of the 30-min observation period were significantly less in the NR1 KD mice as compared to the WT mice (p=.05); however, when the data were averaged across the entire 30-min period, no significant differences were observed between the genotypes (p=.34).
Fig. 4.
Withdrawal jumping in morphine-tolerant mice following administration of 1.0 mg/kg of naltrexone. Data are averaged across two different time periods, either a 15-min or a 30-min observation period. Horizontal axis: data from WT or NR1 KD mice. Vertical axis: total jumps observed during the first 15-min observation period (top) or total jumps observed during the entire 30-min observation period (bottom). Asterisk indicates a significant effect of genotype (p=0.05). (n = 7 or 8)
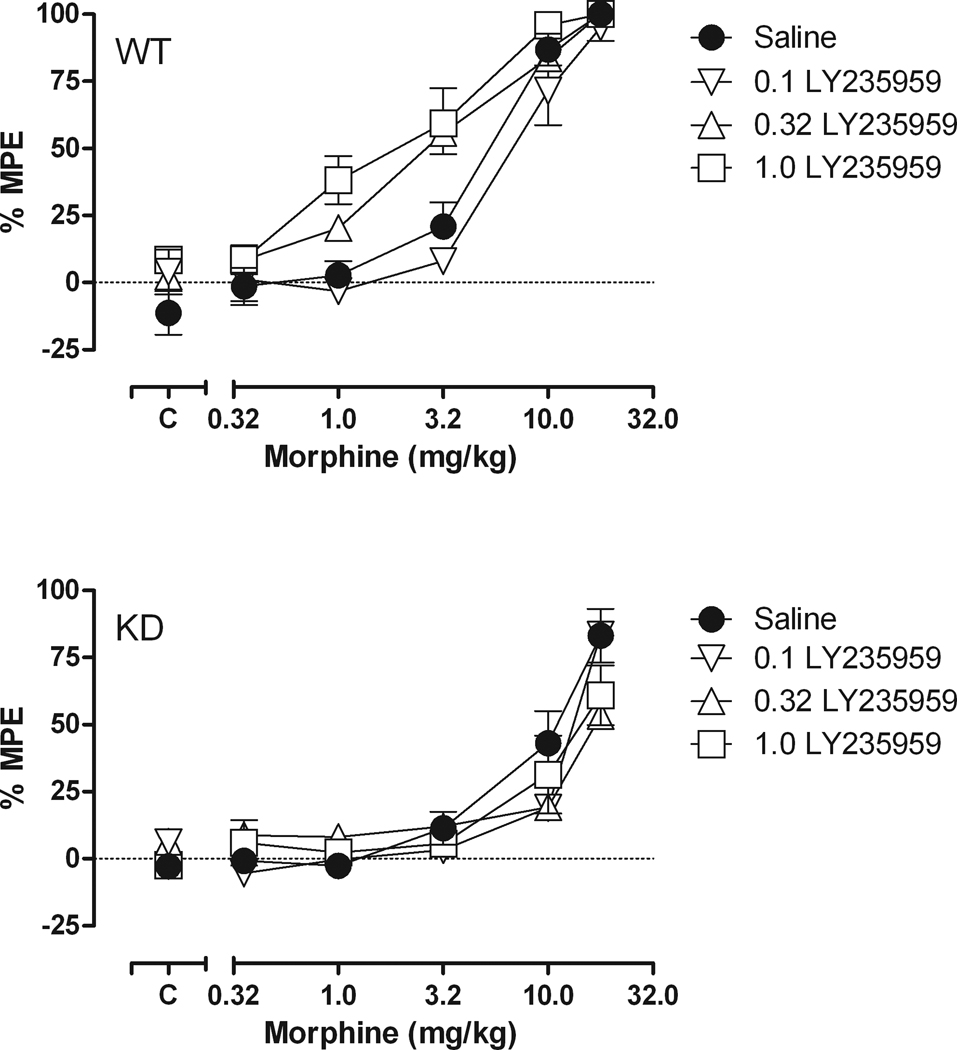
Fig. 5 shows the effects of acute pretreatment with the competitive NMDA receptor antagonist, LY235959 on the morphine dose-effect curve in both WT and NR1 KD mice. The ED50 for morphine alone was 5.54 mg/kg (4.57 – 6.72) in the WT mice (top) and 9.22 mg/kg (6.82 – 12.47) in the NR1 KD mice (bottom). LY235959 did not produce consistent shifts in the morphine dose-effect curve in the NR1 KD mice; however, it did produce leftward shifts in the morphine dose-effect curve in the WT mice. Specifically, the morphine ED50 was 2.82 mg/kg (2.11 – 3.79) following 0.32 mg/kg of LY235959 and 2.02 mg/kg (1.32 – 3.10) following 1.0 mg/kg of LY235959.
Fig. 5.
Dose-effect curves for morphine (0.32–18 mg/kg) in combination with LY235959 (0.1–1.0 mg/kg) in NR1 WT (top) and NR1 KD (bottom) mice. Horizontal axis: dose of morphine in milligrams per kilogram. Vertical axis: antinociception quantified as %MPE. Dose-effect determinations were conducted cumulatively at 30-min intervals. Points above “C” represent tests after saline or LY235959 administration. (n = 8)
Table 2 shows the effects of chronic administration of 1.0 mg/kg LY235959 throughout the tolerance regimen and on test day. The effects of morphine prior to and subsequent to the development of tolerance were similar to those observed previously. That is, following 12 days of morphine administration in mice that received saline concurrently, the morphine ED50 increased 2.59 and 2.80 fold, in the NR1 KD and WT mice, respectively. In separate groups of NR1 KD and WT mice that received a 1.0 mg/kg dose of LY235959 throughout the tolerance regimen, the morphine ED50 increased 3.24 and 7.03 fold, in the NR1 KD and WT mice, respectively. Therefore, rather than attenuating the development of morphine tolerance, as hypothesized, it appears that chronic administration of 1.0 mg/kg of LY235959 either did not shift the morphine curve or actually shifted the morphine curve further to the right.
Table 2.
ED50 values in mg/kg pre and post chronic morphine in combination with saline or 1.0 mg/kg LY235959
| Genotype | Morphine plus |
Pre Chronic (C.L.)1 |
Post Chronic (C.L.)1 |
Dose Ratio (C.L.)1 |
|---|---|---|---|---|
| WT | saline n=11 | 4.59 (3.79–5.56) | 12.25 (9.20–16.31) | 2.80 (2.02–3.85) |
| LY235959 n=11 | 1.91 (1.48 – 2.48) | 13.14 (10.43–16.55) | 7.03 (5.05–10.28) | |
| NR1 KD | saline n=8 | 10.30 (8.28–12.80) | 24.21 (11.72–50.04) | 2.59 (1.56–4.19) |
| LY235959 n=9 | 9.85 (7.66–12.67) | 31.97 (18.88–54.11) | 3.24 (1.97–5.35) |
C.L.=95% confidence limits
DISCUSSION
The present study employed mice with significantly reduced NR1 subunit expression to examine the role of the NMDA receptor system in mediating the acute antinociceptive effects of a range of opioid agonists as well as the development of tolerance and dependence upon morphine.
Results from the current study regarding the antinociceptive effects of mu opioids in WT mice indicate that fentanyl is more potent than methadone, which is more potent than morphine. This potency relationship is in agreement with well-established findings regarding the efficacy of mu opioids (e.g., Colpaert and Niemegeers, 1975; Colpaert et al., 1976). The same potency relationship was observed in the NR1 KD mice; however, all three mu opioid agonists were approximately 2–4 times less potent in the NR1 KD mice than the WT mice.
The differences in mu opioid potency between the NR1 KD and WT mice cannot be explained by differences in baseline sensitivities since the NR1 KD and WT mice were equally sensitive to a range of temperatures on the hot plate. This observation agrees with another study in which paw withdrawal latencies in response to a noxious thermal stimulus were not different in cortex-specific NR1 knockout mice and C57BL/6 control mice; however, cortex-specific NR1 KD mice did show less nociception than the control mice in a formalin test (Quintero et al., 2007). Moreover, it has been shown that a conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces pain in response to injury (South et al., 2003).
The fact that no differences in baseline nociception were observed in the present study, in which the hot plate procedure was used to assess antinociception, may be due to the type of procedure used to examine antinociception or to differences in the specificity of alterations of the NR1 subunit. For example, the studies referred to above (Quintero et al., 2007; South et al., 2003) that have reported decreased nociception in NR1 KD mice in comparison to control mice have examined situations involving tonic nociception (e.g., formalin test; injury induced pain) versus nociception in response to an acute thermal stimulus, as employed in the present study. Moreover, these same studies have examined mice with a conditional and/or cortex-specific deletion of the NR1 subunit rather than a global knockdown of up to 90%, as was the case in the present study.
Although the mu opioids examined here were less potent in the NR1 KD mice than in the WT mice, the mice did not differ when other aspects of opioid pharmacology were examined. The effects of both morphine and l-methadone were antagonized by naltrexone to an equivalent degree in the NR1 KD and WT mice; morphine tolerance developed to the same extent in both NR1 KD and WT mice, and although there was some suggestion that signs of physical withdrawal may have occurred somewhat later in the NR1 KD mice than the WT mice, differences in withdrawal jumping were not significant when withdrawal jumps were observed over a longer time period. Taken together, these data suggest that the mu opioid receptor system is not altered in NR1 KD mice.
The findings regarding the development of morphine tolerance are particularly interesting because previous research has shown that NMDA antagonists can attenuate the development of tolerance. (e.g., Allen and Dykstra, 1999; 2000; Trujillo, 2000; Bryant et al., 2006; Mendez and Trujillo, 2008). Based on these findings, we had predicated that tolerance would be attenuated in the NR1 KD mice. In this context, it is important to emphasize the way in which the tolerance regimen was carried out in the present study.
Typically, when the development of tolerance is examined between different groups of animals, each group receives the same dose and/or administration schedule during a chronic regimen and then post chronic dose effect curves are compared between groups. Since morphine was less potent in the NR1 KD mice, as compared to WT mice, the tolerance regimen in the present study was adjusted, based upon individual morphine ED50s obtained in the WT and NR1 KD mice. As a result, the NR1 KD mice actually received a greater absolute dose of morphine throughout the tolerance regimen, given that their prechronic ED50s were approximately 3 times larger than the ED50s obtained in the WT mice. Since it is well-established that the magnitude of morphine tolerance is greater when larger doses of morphine are administered (Adams and Holtzman, 1990; Hoffman et al., 1998; Colpaert, 1996), one might expect to see a greater degree of tolerance with the NR1 KD mice since they actually received a larger amount of morphine during the tolerance regimen. Nevertheless, tolerance developed to an equivalent degree in both the NR1 KD and WT mice, with the ED50s increasing approximately 3-fold in both the NR1 KD and the WT mice.
At least one other study that examined the development of morphine tolerance in mice lacking the gene encoding the GluRε1 subunit of the NMDA receptor (also referred to as the NR2 subunit) reported that morphine tolerance was attenuated in the genetically-modified mice as compared to C57BL/6J mice (Inoue et al., 2003). Nevertheless, the dose of morphine used in the Inoue et al. study to establish tolerance (10 mg/kg) was the same in both groups, even though the mice lacking the NMDA receptor GluRε1 (or NR2) subunit gene were more sensitive to the antinociceptive effects of morphine than the C57BL/6J controls. Therefore, when comparing the development of tolerance between groups of mice that differ in some way, it is important to accommodate these differences by adjusting the amount of drug administered during the tolerance regimen, based on the acute sensitivity of animals within each group (see Barrett et al., 2001 for a discussion of this issue).
It is also interesting that the differences in potency observed between the NR1 KD mice and their WT littermates were actually in the opposite direction than what was predicted, based on previous studies in our laboratory examining the acute effects of morphine in combination with a range of NMDA antagonists. Indeed, our laboratory has shown that doses of the NMDA antagonist, LY235959 that had no antinociceptive effects when examined alone, produce leftward shifts in the morphine dose effect curve in both squirrel monkeys (Allen and Dykstra, 2001) and mice (Fischer and Dykstra, 2006). This observation was also replicated in the current study in which doses of 0.32 and 1.0 mg/kg of LY235959 shifted the morphine dose-effect curve to the left in wild type mice; however, no shifts were observed in the NR1 KD mice.
There are a number of reasons why differences might be observed between the acute effects of an NMDA antagonist and the prolonged reduction in NMDA receptor function through genetic manipulation. The NR1 KD mice examined in the present study have been described as NR1 hypomorphs given that NR1 binding sites in these mice show both a marked and a global reduction (Mohn et al., 1999; Duncan et al., 2002). Therefore, acute administration of an NMDA antagonist is likely to produce different effects than what might occur when receptor function undergoes long term reduction. In NR1 KD mice, NMDA receptor function is impaired throughout development and remains through adulthood. Clearly, this provides many opportunities for compensatory functions to develop. Indeed, the relatively subtle nature of the metabolic alterations observed in NR1 KD mice, despite a marked reduction in the expression of the NR1 subunit, suggests the presence of compensatory responses (Duncan et al., 2004).
Secondly, although the NR1 subunit is a critical component of the entire NMDA receptor complex, the consequences of a 90% reduction in the NR1 subunit upon other subunits of the NMDA receptor is not well known. For example, significant global reductions in NR2A and NR2B protein levels have been reported in NR1 KD mice (Ramsey et al., 2008). Mice deficient in the NR2A subunit (also called the GluRε1 subunit) of the NMDA receptor did not reveal altered responses to painful stimuli in assays of allodynia or hyperalgesia (Petrenko et al., 2003); however, the antinociceptive effects of morphine in a tail pinch procedure were enhanced in the NR2A (or GluRε1) deficient mice (Inoue et al., 2003). Clearly, additional data are required to determine the contribution of each of these variables (i.e., the specific NR subunit examined, type of analgesic procedure employed, etc.) to interactions between opioid receptor function and the NMDA system.
Finally, the current study suggests that the withdrawal signs following chronic administration of morphine were not different between the WT and NR1 KD mice. These findings are similar to those reported by Glass et al., (2008) in which they showed that bilateral local NR1 gene deletion did not alter either the somatic or visceral symptoms of withdrawal precipitated by naloxone in morphine-treated mice.
The results obtained here indicate that alterations in mu opioid antinociception and tolerance in NR1 KD mice do not parallel the effects produced by either the acute or chronic administration of an NMDA antagonist. Taken together these results suggest that mu opioid receptor function is not changed in mice that express low levels of the NR1 subunit of the NMDA receptor.
Acknowledgments
Support was provided by grants from the National Institutes of Health, RO1-DA002749 and T32-DA007244.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Adams JU, Holtzman SG. Tolerance and dependence after continuous morhine infusion from osmotic pumps measured by operant responding in rats. Psychopharmacology. 1990;100:451–458. doi: 10.1007/BF02243995. [DOI] [PubMed] [Google Scholar]
- Allen RM, Dykstra LA. The competitive NMDA receptor antagonist LY235959 modulates the progression of morphine tolerance in rats. Psychopharmacol. 1999;142:209–214. doi: 10.1007/s002130050881. [DOI] [PubMed] [Google Scholar]
- Allen RM, Dykstra LA. Attenuation of mu-opioid tolerance and cross-tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY235959 is related to tolerance and cross-tolerance magnitude. J Pharmacol Exp Ther. 2000;295:1012–1021. [PubMed] [Google Scholar]
- Allen RM, Dykstra LA. N-methyl-D-aspartate receptor antagonists potentiate the antinociceptive effects of morphine in squirrel monkeys. J Pharmacol Exp Ther. 2001;298:288–297. [PubMed] [Google Scholar]
- Allen RM, Granger AL, Dykstra LA. The competitive N-methyl-D-aspartate receptor antagonist (−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboylic acid (LY235959) potentiates the antinociceptive effects of opioids that vary in efficacy at the μ-opioid receptor. J Pharmacol Exp Ther. 2003;307:785–792. doi: 10.1124/jpet.103.055319. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the μ opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R315–R326. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: No tolerance to opiates. Pharmacological Reviews. 1996;48:355–402. [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE. On the narcotic cuing action of fentanyl and other narcotic analgesic drugs. Archives internationales de Pharmacodynamie et de Therapie. 1975;217:170–172. [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJE, Janssen PAJ. The narcotic discriminative stimulus complex: relation to analgesic activity. J. Pharm. Pharmac. 1976;28:183–187. doi: 10.1111/j.2042-7158.1976.tb04127.x. [DOI] [PubMed] [Google Scholar]
- Craft RM, Lee DA. NMDA antagonist modulation of morphine antinociception in female vs male rats. Pharmacol Biochem Behav. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Duncan G, Miyamoto S, Gu H, Lieberman J, Koller B, Snouwaert J. Alterations in regional brain metabolism in genetic and pharmacological models of reduced NMDA receptor function. Brain Res. 2002;951:166–176. doi: 10.1016/s0006-8993(02)03156-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Dykstra LA. Interactions between an N-methyl-D-aspartate antagonist and low-efficacy opioid receptor agonists in assays of schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2006;318:1300–1306. doi: 10.1124/jpet.106.101683. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Carrigan KA, Dykstra LA. Effects of N-methyl-D-aspartate receptor antagonists on acute morphine-induced and l-methadone-induced antinociception in mice. J Pain. 2005;6:425–433. doi: 10.1016/j.jpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Hegarty DM, Oselkin M, Quimson L, South SM, Xu Q, Pickel VM, Inturrisi CE. Experimental Neurology. 2008;213:57–70. doi: 10.1016/j.expneurol.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Allen S, Nemmani KV, Fee JR, Carliss R. The influence of dextromethorphan on morphine analgesia in Swiss Webster mice is sex-specific. 2005 doi: 10.1016/j.pbb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hoffman O, Plesan A, Wiesenfeld-Hallin Z. Genetic differences in morphine sensitivity, tolerance and withdrawal. Brain Res. 1998;806:232–237. doi: 10.1016/s0006-8993(98)00768-9. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRε1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci. 2003;23:6529–6536. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Popik P. The effects of NMDA receptor antagonists on acute morphine antinociception in mice. Amino Acids. 2002;23:163–168. doi: 10.1007/s00726-001-0123-5. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Trujillo KA. NMDA receptor antagonists inhibit opiate antinociceptive tolerance and locomotor sensitization in rats. Psychopharmacology. 2008;196:497–509. doi: 10.1007/s00213-007-0984-8. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Baba H, Sakimura K. Unaltered pain-related behavior in mice lacking NMDA receptor GluRε1 subunit. Neuroscience Research. 2003;46:199–204. doi: 10.1016/s0168-0102(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Quintero GC, Erzurumlu RS, Vaccarino AL. Decreased pain response in mice following cortex-specific knockout of the N-methyl-D-aspartate NR1 subunit. Neurosci Lett. 2007;425:89–93. doi: 10.1016/j.neulet.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey AJ, Laakso A, Cyr M, Sotnikova TD, Salahpour A, Medvedev IO, Dykstra LA, Gainetdinov RR, Caron MG. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacol. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, et al. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. Berlin: Springer; 1987. [Google Scholar]
- Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacol. 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]