Abstract
Distorted interval timing is a common feature of the cognitive impairment observed in patients with schizophrenia. The neural circuits which are required for interval timing and those thought to be compromised in schizophrenia overlap and include the cortico-striatal pathways. Here, we suggest that a focus on temporal information processing offers a window into understanding the cognitive deficits of schizophrenia and how deficits might contribute to a variety of symptoms. A disruption in the functioning of the cortico-striatal pathways may lead to cognitive deficits which in turn lead to impaired processing of temporal information. Disrupted temporal processing may also contribute to a variety of other symptoms associated with the disorder. Because interval timing is a cognitive/behavioral phenotype that can easily be assessed in animals it can be used as a sensitive screen for deficits in animal models. Using a recently developed transgenic mouse that models increased D2 receptor upregulation in the striatum similar to that observed in patients with schizophrenia we illustrate the utility of an interval timing approach in assessing cognitive impairment. We further discuss how variants of timing procedures can be used to assess attention and working memory performance as well as other necessary components of adaptive cognitive function.
Distortions in timing are well documented in patients with schizophrenia. In addition to historical anecdotal reports of temporal distortions (Freedman, 1974; Lewis, 1932), a growing literature has demonstrated empirically the existence of distorted temporal information processing in a variety of experimental paradigms in individuals diagnosed with schizophrenia (e.g., Clausen, 1950; Densen, 1977; Johnson & Petzel, 1971; Lhamon & Goldstone, 1956; Tysk, 1983, 1984, 1990; Davalos et al., 2003; Elvevag et al., 2004; Elvevag et al., 2003; Penney et al., 2005; Todd, 2006; Yang et al., 2004). In the present review, we document that altered temporal processing is a general cognitive phenotype in schizophrenia and discuss how studying temporal information processing can facilitate identification of cognitive deficits in animal models. We first briefly review data on timing deficits in patients diagnosed with schizophrenia. We then discuss why a focus on temporal information processing is likely to lead to advances in understanding the nature of the cognitive deficits in animal models of schizophrenia. We conclude with an example that describes the recent analysis of the potential mechanisms underlying an interval timing deficit in a transgenic mouse model of schizophrenia.
Interval timing
Timing and time perception are critical for the survival of humans and other animals. Temporal information processing takes place on many timescales, from milliseconds to hours to days (see Balsam et al., 2009; Buhusi & Meck, 2005). For the most part, timing that occurs on these different timescales appears to be governed by separable neural mechanisms. Millisecond timing, as is required in the execution of fine motor movements, appears to arise in some cases directly from the intrinsic properties of motor control circuits (Ivry & Schlerf, 2008). On the opposite extreme is circadian timing, which among other things regulates daily sleep and wake patterns. Circadian activity is coordinated in large part by the suprachiasmatic nucleus (see Antle & Silver, 2009, for review). The focus of this paper is on a third type of timing that differs from these other two, and that also appears to be governed by distinct neural mechanisms. This type of timing is referred to as interval timing, and occurs on the order of seconds to minutes (Cordes & Gallistel, 2008). A large literature indicates that the fundamental aspects of interval timing are conserved across a broad range of species, including bees, fishes, turtles, birds, rodents, non human primates, and children and adult humans, and considerable research effort has been directed towards understanding the neural bases of interval timing (see Buhusi & Meck, 2005, for review).
Distorted interval timing in schizophrenia
Patients diagnosed with schizophrenia display timing deficits. The exact nature of the deficits, however, varies across studies. For example, patients have been reported to overestimate interval duration when they are asked to verbally report the duration of a presented stimulus (e.g., Clausen, 1950; Densen, 1977; Johnson & Petzel, 1971; Lhamon & Goldstone, 1956; Tysk, 1983, 1984, 1990) and in repetitive tapping tasks (Carroll et al., 2009a). Patients have also been reported to underestimate interval duration during production tasks, which require the participant to respond when a target stimulus has been present for the appropriate amount of time (Clausen, 1950; Johnson & Petzel, 1971; Tysk, 1983, 1990; Wahl & Sieg, 1980). Greater differences in time estimation of visual vs. auditory signals has also been reported in patients when compared to controls (Penney et al., 2005). When all data are considered together, however, the most reliable finding across paradigms and studies is that patients are more variable in their timing of temporal intervals than controls (Carroll et al., 2008; 2009b; Davalos et al., 2003; Elvevag et al., 2004; Elvevag et al., 2003; Todd, 2006; Yang et al., 2004). In addition to these deficits in timing in individuals diagnosed with schizophrenia, those at risk for developing the disease have also been reported to show interval timing deficits. Penney et al. (2005) compared time perception in the offspring of parents diagnosed with schizophrenia to the offspring of parents diagnosed with major affective disorder and to non-patient controls and found greater variability in timing only in offspring of parents who had been diagnosed with schizophrenia. Thus, distorted time perception may share genetic risk factors with schizophrenia and may be a useful marker in identifying individuals at risk for schizophrenia but not at risk for all psychiatric diseases.
Neurobiology of Schizophrenia and interval timing
We further suggest that focusing on an interval timing phenotype in schizophrenia is likely to be useful because of the underlying neural circuitry. Recent reviews of the literature on the neural bases of timing (Buhusi and Meck, 2005; Ivry and Schlerf, 2008; Ivry and Spencer, 2004) reveal a remarkable overlap in the circuits and brain structures that are affected by schizophrenia (Csernansky and Bardgett, 1998; Harrison, 2005; Kerns et al., 2008; Laviolette, 2007; Stahl, 2004; Wright et al., 2005). Although a number of brain regions have been implicated in both interval timing and schizophrenia, as we describe below, the strongest link between the hypothesized pathophysiology of schizophrenia and the neural basis of interval timing is the role of the striatal dopaminergic system and the interconnectivity between the striatum and the prefrontal cortex (PFC).
Although the specific neural underpinnings of schizophrenia are not precisely known, one of the most general and replicable findings is hyperactivity of dopamine (DA) D2 receptors in the striatum of patients. All effective antipsychotic drugs antagonize D2 receptors (e.g., Seeman et al., 1976), critically implicating them in the disease. Post mortem studies have reported an upregulation in striatal D2 receptors in drug-free patients (Davis et al., 1991). These data were confirmed later in PET studies, which demonstrate about a 12% increase in DA D2 receptor density in the striatum of drug free and drug naïve patients, though not all patients show this increased density of D2 receptors (see Laruelle, 1998, for meta-analysis and review). Patients also display increased occupancy of striatal D2 receptors by DA (e.g., Abi-Dargham et al., 2000). Thus, the preponderance of evidence confirms that there is a hyperfunction of the striatal DA D2 system in schizophrenia, suggesting it is an important part of the pathophysiology of the disease. Furthermore, PFC hypoactivity has also been reported (Barch, 2005; Glahn et al., 2005), and the PFC has long been a target of schizophrenia research, given the well documented distortions in working memory, which requires a functional PFC (e.g., Goldman-Rakic & Selemon, 1997; Lewis et al., 1999; Goldman-Rakic et al., 2004). Abnormal PFC and striatal functioning is thought to compromise the integrity of cortico-striatal circuits, leading to functional impairments (see Simpson, Kellendonk, & Kandel, 2010, for review and discussion on how excessive striatal D2 activity could lead to PFC dysfunction).
In terms of the neural basis of interval timing, there is a large literature from behavioral, pharmacological, genetic, and imaging studies that indicates a critical role for the basal ganglia in temporal information processing, particularly the striatum and its’ connections with the PFC (see Buhusi & Meck, 2005, for review). Many studies indicate that temporal information processing can be distorted by manipulations that target the DA system, specifically through D2 receptor activity (Buhusi & Meck, 2005; Maricq & Church, 1983). For example Meck (1986) demonstrated that the dose of a neuroleptic that was required to distort a rats’ perception of a time interval by 10-15% was negatively correlated with the drugs’ affinity for DA D2 receptors. More recently, it has been suggested that striatal medium spiny neurons may serve as a monitor of neural activity in cortico-striatal circuits, and that coincident activation of these neurons by inputs from the PFC communicates the pattern of activity in working memory during interval timing tasks (Mattel & Meck, 2004). According to this view, a pulse of DA at the beginning of the to-be-timed interval signals the period during which medium spiny neurons should monitor oscillations in the firing of PFC neurons to detect patterned activity. When a second pulse of DA signals the end of the to-be-timed interval (usually via delivery of a reward), the spatial pattern of firing in cortical neurons is stored via Hebbian strengthening of the currently active synapses, thus providing a memory of the temporal interval against which subsequent intervals can be compared (see Meck, Penney, & Pouthas, 2008, for discussion). Although substantiation of this theory awaits further empirical work, there is evidence that accurate interval timing requires an intact PFC, as PFC lesions produce distortions in timing (Meck et al., 1987; Olton, 1989; Olton et al., 1988; Picton et al., 2006) and modulate the disruptive effects of drugs that target striatal D2 receptors (Meck, 2006). Together, these results indicate that interval timing requires the integrity of cortico-striatal circuits. Thus, the research to date on the pathophysiology of schizophrenia and on the neural basis of interval timing indicates that the brain circuits implicated in schizophrenia and those necessary for accurate temporal information processing are strikingly similar.
Interval timing as a window on cognitive impairments
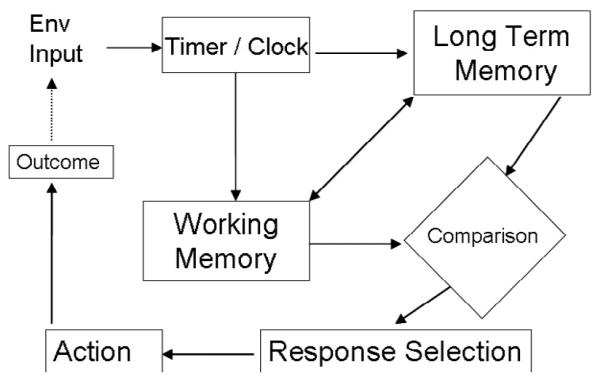
Cognitive impairments are a hallmark of schizophrenia and include disturbances in working memory, attention, and executive function (Kerns et al., 2008), all of which are essential for accurate and precise timing. Figure 1 shows the cognitive processes that underlie performance on interval timing tasks. To perform well the subject needs to perceive, attend to, and remember time, decide whether a given interval has elapsed by comparing the elapsed time during a trial with the memory for previously experienced durations and act on this comparison by executing or inhibiting responses. Specifically, an environmental event must first trigger a timing mechanism. Information about elapsed time is stored in short term or working memory and when the event ends or another event happens the value of that duration is stored in long-term memory. On subsequent occasions when this event is encountered the elapsing time can be compared to the remembered time and when the comparison crosses response thresholds selection of appropriate behavior occurs. Consequently, accurate temporal performance is affected by perception, learning, short-term memory, long-term reference memory and decision processes. Moreover, this process is further influenced by attention and motivation. Attention is thought to impact the latency to trigger the timing mechanism and the consistency with which time accumulates over the course of an interval (Buhusi & Meck, 2006; Lejuene et al., 1999), while motivation is thought to affect the speed with which the timing mechanism accumulates information about duration (Bizo & White, 1994).
Figure 1.
It should be clear, then, that the behavioral manifestation of accurate interval timing requires proper functioning in a great many cognitive processes that are also affected in patients. Dysfunction in any of these areas will be manifest as an interval timing deficit. We therefore suggest that assessing performance on interval timing tasks might serve as a sensitive screen for deficits in cognitive function in animal models. In the next section we illustrate how this strategy is a productive one for understanding cognitive and behavioral deficits in animal models.
Analyzing cognitive/behavioral deficits in animal models
A major drawback to commonly used test batteries of cognitive/behavioral function in animal models is that the assays of specific functions typically differ along many dimensions. Under the general category of tasks which assess learning and memory, one might assay working memory in a radial arm maze with food reward, spatial cognition in a Morris water maze, and long term memory in fear conditioning with shock, and sustained attention in a vigilance task in an operant chamber. If one finds that a manipulation affects one type of task but not others it is typically interpreted as indicating a difference in specific cognitive function. However, the tasks also differ in the perceptual demands of contextual and punctuate stimuli; the nature of the response that is measured, the motivational system activated by the task, and the history of exposure and training required to perform each task. Thus, accurate characterization of deficits in cognitive function is complicated by this kind of heterogeneous testing strategy. By focusing on variants of basic interval timing tasks, it is possible to hold motivational and task requirements constant while assaying specific cognitive processes using the same basic methodology in which mice press a lever to earn a reward. In addition, there is a rich empirical and theoretical literature from which to draw for the purposes of designing experiments and analyzing data that can be used to study the roles of specific cognitive processes in interval timing. This approach has great potential as a tool for isolating the effects of genetic or other manipulations on specific cognitive processes in animal models of schizophrenia.
We have recently used this approach to characterize the cognitive/behavioral deficits in a transgenic mouse model of the negative and cognitive symptoms of schizophrenia. In an effort to model the increased occupancy and density of striatal D2 receptors in patients discussed above, Kellendonk et al. (2006) generated transgenic mice which selectively and reversibly overexpress DA D2 receptors in the striatum congenitally, modeling developmental overexpression or hyperactivity of D2 receptors in patients. The mice display a 15% increase in binding capacity compared to their control littermates, similar to the increase in D2 activity observed in patients with schizophrenia (Laruelle, 1998). In addition, Kellendonk et al. demonstrated that developmental overexpression of striatal D2 receptors is sufficient to produce persistent deficits in PFC function, as D2OE mice have increased DA levels, decreased DA turnover, and greater D1 receptor activation in the PFC. The increased striatal D2 activity, coupled with the PFC dysfunction in this model, make the D2OE mouse an excellent tool for the study of the cognitive deficits in schizophrenia, including their molecular underpinnings.
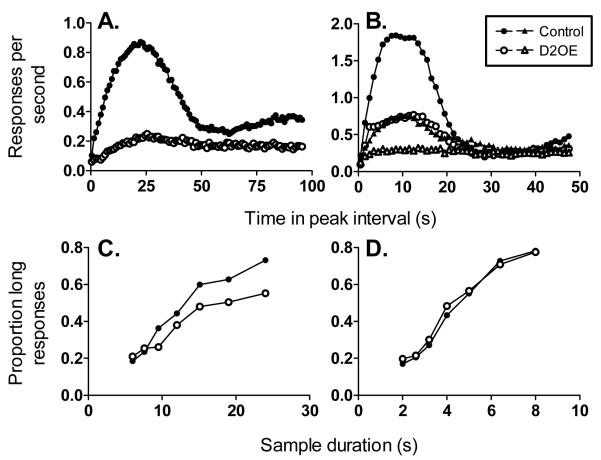
We first tested the D2OE mice on an interval timing task (Drew et al., 2007) known as the peak interval procedure (Roberts, 1981). This procedure assesses how well the mice can time the duration of an ongoing interval. On some trials, called fixed interval (FI) trials, the trial begins with insertion of a response lever into the conditioning chamber and mice are rewarded for the first response that occurs after a fixed interval (e.g., 24 s) has elapsed. This procedure produces a low response rate at the beginning of the trial, followed by an increasing likelihood of responding as the target time is approached. Once the mice are well trained on FI trials, peak trials are introduced. On these trials, the trial begins with insertion of the response lever as in FI trials, but the reward at the usual time is omitted and the trial continues for much longer than usual. On these peak interval trials, the rate of responding across the trial gives an indication of how accurately the mouse is able to time the temporal interval on FI trials. Generally, the average rate of responding increases up to a peak at or around the usual time of reinforcement, and then decreases (e.g., Roberts, 1981). The resulting response distributions can be subjected to quantitative analysis to extract measures of timing performance. The height of the response rate distribution indexes motivation, the location of the maximal rate of responding gives an index of how accurately the mice were able to time the temporal interval, and the spread of the response rate distribution is a measure of the precision (variability) of interval timing. As shown in Figure 2A, D2OE mice performed worse on this task than control mice. Specifically, the performance of D2OE mice indicated decreased motivation (lower overall response rates), less accurate (peak shifted to the right of controls) and more variable (broader response rate distribution) interval timing (see Drew et al., 2007, for statistical analyses). The source of the increased timing variability in D2OE mice is uncertain. According to Scalar Expectancy Theory, the most prominent theoretical account of interval timing, there are several sources of possible variability, including variability in the generation of the temporal signal, transfer of information about interval duration from working to reference memory, and in response thresholds (Gibbon, 1977; Gibbon & Church, 1984; Gibbon & Church, 1992).
Figure 2.
The interval timing deficit in D2OE mice could arise from a number of cognitive impairments. The evidence of decreased motivation, however, poses problems for interpretation of the results from the peak interval procedure because the index of timing is response rate, and response rate is greatly influenced by motivational factors (e.g., Roberts, 1981), making it difficult to separate motivational from cognitive deficits. Furthermore, in a subsequent experiment (Ward et al., 2009), we explicitly manipulated motivation in the peak interval procedure and found that accuracy and precision of interval timing was modulated by motivation in both control and D2OE mice. Figure 2B shows that decreasing the percentage of rewarded FI trials decreased motivation to respond (peak height) and precision of timing (peak spread) for both control and D2OE mice. Note that controls rewarded on 10% of the FI trials time as poorly as D2OE mice rewarded on 100% of FI trials (see Ward et al., 2009 for details). These data strongly suggest a motivational contribution to the timing deficits observed in the peak procedure in D2OE mice.
It is possible to separate deficits in interval timing that result from motivational impairments from those that result from cognitive impairments by using an alternate method for studying timing called the temporal bisection procedure (Church & Deluty, 1977). In the temporal bisection procedure, at trial onset a sample (e.g., tone) is presented for either a short (e.g., 2 s) or long (e.g., 8 s) duration. Following sample presentation, response levers are presented and responses on one lever are only rewarded following presentation of short samples while responses on the other lever are only rewarded following presentation of long samples. Once performance with these anchor durations is learned, mice can be tested on trials in which intermediate duration samples are presented. Accuracy and precision of interval timing performance can be assessed by examining the proportion of choices to the lever corresponding to a “long” sample duration as a function of sample duration. Once performance is stable, the proportion of responses to the long choice option is generally an increasing sigmoidal function of sample duration (Church & Deluty, 1977). The slope of this function indicates precision of interval timing, while the point on the function that corresponds to 50% long choices, or the point of subjective equality, gives an estimate of the accuracy of interval timing. Because only a single response is required each trial, performance in the bisection task is not as affected by motivation to respond as the peak interval procedure.
We tested D2OE and control mice on the bisection task with anchor durations of 6 and 24 s (Ward et al., 2009). As shown in Figure 2C, the proportion of responses to the “long” lever is low for both genotypes at the shortest sample duration, and increases with increasing sample duration, indicating that choice responding was under control of the temporal durations. The flatter function for D2OE mice indicates that they were less accurate at categorizing the long sample durations than controls. In fact, they do no better than chance at classifying the longer interval. To verify this selective impairment on longer duration samples, we again assessed interval timing performance in the bisection task, but changed the anchor durations to 2 and 8 s. With this arrangement, there was no difference between performance of D2OE mice and controls (Figure 2D). This profile of results suggests that the interval timing deficit in D2OE mice is in accurately processing temporal information over longer intervals, a difficulty which could arise from impaired working memory or sustained attention. The interpretation of these results as evidencing a deficit in working memory may be supported by results showing that D2OE mice are slower to acquire a working memory task in a t-maze and radial arm maze paradigm (Kellendonk et al., 2006), although it is not possible to rule out a more general learning impairment as the source of this acquisition deficit.
Interval timing procedures can be readily modified to isolate sustained attention and working memory function. Sustained attention can be studied using variants of either the peak or the bisection procedures. For example, Buhusi and Meck (2006) assessed sustained attention in the peak procedure in rats by presenting distractors, which consisted of presentation of several seconds of white noise during regular peak interval trials. On these trials, the peak of the response rate function was shifted to the right of the function from peak trials without distractors, indicating that the distractors diverted attention away from the to-be-timed interval. Ward and Odum (2007) employed a similar manipulation in the bisection procedure with pigeons and found that distraction (houselight flashing on and off during sample presentation) disrupted accuracy selectively on long sample trials, indicating that attention was diverted from the temporal sample stimuli during distraction, so that long samples were incorrectly perceived as short. Using these types of manipulations, differences in sustained attention can be ascertained.
Working memory can be assessed in a variant of the peak interval procedure known as the gap procedure. The gap procedure is a typical peak procedure with FI and peak trials, except that on some peak trials, the to-be-timed interval is interrupted for some amount of time (the gap) after which it continues (Church, 1984). Typically, on gap trials the response rate increases before the gap, decreases during the gap, and then increases again and peaks at a time that is displaced relative to the peak time on peak interval trials without gaps (Buhusi & Meck, 2006). The magnitude of this displacement has been taken as a reflection of the rate of decay of temporal information in working memory and has been shown to be sensitive to manipulation of gap duration (e.g., Cabeza de Vaca et al., 1994).
Further support for the involvement of working memory in the gap procedure comes from a study by Meck, Church, and Olton (1984) in which they assessed the effects of lesions of the fimbria fornix on interval timing in the gap procedure in rats. This manipulation disrupts normal hippocampal function, and while it has been reported to produce a number of behavioral effects, the most commonly reported are memory impairments, including impairments in spatial working memory (e.g., Galani et al., 2002; Meck et al., 1984). On peak trials, rats showed a peak time at about 20 s, the time at which reward was available on FI trials. During gap trials, 10 s after it began, the to-be-timed signal was interrupted for 5 s. On these trials, the peak time of control rats was shifted about 5 s later than in trials without a gap. By contrast, the peak time of rats with fimbria fornix lesions was shifted about 15 s later than the usual peak time (the duration of the pre-gap interval + the gap), indicating degraded memory for the 10 s pre gap interval. Thus, the gap procedure appears sensitive to manipulations that affect maintenance of working memory, and is likely to be a useful method for assaying working memory function in animal models of schizophrenia and for screening potential pharmacotherapies. For example, Buhusi and Meck (2007) reported that acute administration of the atypical antipsychotic clozapine (2 mg/kg) improved the ability of rats to maintain the duration of the pre gap interval in working memory, evidenced by a decreased magnitude of peak displacement compared to saline treated rats. Although the data on cognitive enhancement by clozapine and other atypical antipsychotics in schizophrenia are mixed (e.g., Keefe et al., 2005; McGurk et al., 2005; Meltzer & McGurk, 1999), thereby making the applicability of these results to schizophrenia unclear, these results nevertheless illustrate that cognitive processes of interest in the study of schizophrenia are amenable to elucidation and pharmacological manipulation in interval timing procedures.
Deficient timing and positive symptoms in schizophrenia
The position taken in the present review is that deficits in cognitive processes, such as working memory and sustained attention, can lead to the behavioral manifestation of impaired interval timing in schizophrenia. We also suggest that, in turn deficient temporal information processing may contribute to a wide range of positive symptoms associated with schizophrenia. Deficient temporal information processing would lead to dysfunctions in the timing or sequencing of mental activity and behavior, producing some classical symptoms of schizophrenia, such as delusions and disorganized behavior (Andreason et al., 1999; Carroll et al., 2008). To illustrate how distorted timing could produce positive symptoms, consider delusional thinking. Distortions in the perception of temporal intervals could lead to failure to correctly perceive the temporal sequence of contiguous events. This in turn could lead to a failure to ascribe volitional control to one’s own actions (lack of agency) or confusion regarding the consequent relation between one’s actions and the outcome of those actions (e.g., flipping a light switch turns on the light), giving the illusion of lack of control by the individual and control by some other entity, a common aspect of paranoid delusional thinking in schizophrenia (Coltheart, Langdon, & McKay, 2010; Hirjak & Fuchs, 2010; Waters & Jablensky, 2009). Given the critical role of dopaminergic signaling in interval timing, together with the documented dopaminergic dysfunction in schizophrenia, this interpretation may be consistent with current conceptualizations of the involvement of dysfunctional dopaminergic signaling in producing positive symptoms via aberrant attribution of incentive salience to otherwise irrelevant stimuli or abnormal reward processing (e.g., Heinz & Schlagenhauf, 2010).
If timing and symptoms are causally related in schizophrenia, one might expect a correlation between severity of positive, negative, and cognitive symptoms and the severity of timing impairment. The few studies we are aware of that have assessed correlations between symptoms and timing performance have found no correlation between symptoms scores on the Positive and Negative Syndrome Scale (PANSS) or general estimates of intellectual functioning and timing performance (Carroll et al., 2008; Carroll et al., 2009a, 2009b). Given that the most robust timing impairment is increased variability of timing, combined with the heterogeneous nature of the presentation of symptoms in schizophrenia across patients, this result is perhaps not surprising. Future studies should correlate indices of timing with individual indices of performance on tasks which measure specific cognitive functions such as working memory and sustained attention. In addition, because interval timing is a sensitive screen for cognitive functioning it may serve as a useful assay for assessing the efficacy of cognitive enhancing treatments in schizophrenia. For example, it would be interesting to assess whether successful treatment of working memory or sustained attention impairments improves performance on interval timing tasks in patients. These types of studies will clarify the nature of the relationship between interval timing and the cognitive symptoms of schizophrenia.
Conclusions
It must be noted that the neuropharmacology of timing is complex (Bizot, 1997; Hampson et al., 2010; Popke et al., 2000; Ward & Odum, 2005; Ward et al., 2009), as is the neuropharmacology of schizophrenia (Allen et al., 2008). We focused on D2 receptors in the present review due to their implication in the pathophysiology of schizophrenia and their prominent empirical role in modulating interval timing behavior as well as in the key role they are thought to play in current neurobiological models of timing. However, a number of other neurotransmitter systems have been implicated in timing behavior, including the serotonergic (see Hampson et al., 2010, for review), nicotinic and muscarinic acetylcholine (Bizot, 1997; Ward et al., 2009), opioid (Popke et al., 2000; Ward & Odum, 2005), as well as the glutamatergic and the gabaergic systems (Popke et al., 2000). Our use of the D2OE model is meant to provide an example of how the methodology used to study interval timing can be applied to characterize the nature of cognitive deficits in animal models of schizophrenia-not to imply that other neurotransmitter systems are uninvolved in timing. Indeed, because interval timing depends on many aspects of corticostriatal functioning, the method proposed is a general framework and is equally applicable to all animal models of schizophrenia as well as other models of psychiatric disease that share a common neurobiological substrate with interval timing, not only those with a strong dopaminergic component.
In conclusion, we propose several reasons for why a focus on temporal information processing, with a specific focus on interval timing, will be beneficial in understanding the cognitive deficits associated with schizophrenia: 1) patients with schizophrenia have deficits in interval timing, 2) the neural circuitry implicated in interval timing and schizophrenia has substantial overlap, 3) interval timing depends on cognitive processes that are impaired in schizophrenia, 4) impaired timing can contribute to other positive and cognitive symptoms and 5) the methodological and theoretical approaches used to study interval timing provide a powerful means for studying deficits in specific cognitive processes in animal models of schizophrenia.
Patients with schizophrenia show deficits in interval timing
The neural circuits affected in schizophrenia and mediating interval timing overlap
Studying interval timing is a powerful strategy to understand cognition
Animal models are well suited to do this.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. P. Natl. Acad. Sci. USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat. Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Andreason NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol. Psychiat. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. The neural basis of timing and anticipatory behaviors. Eur. J. Neurosci. 2009;30:1643–1649. doi: 10.1111/j.1460-9568.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam PD, Sanchez-Castillo H, Taylor K, Van Volkinburg H, Ward RD. Timing and anticipation: Conceptual and methodological approaches. Eur. J. Neurosci. 2009;30:1749–1755. doi: 10.1111/j.1460-9568.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Bizo LA, White GW. The behavioral theory of timing: reinforcer rate determines pacemaker rate. J. Exp. Anal. Behav. 1994;61:19–33. doi: 10.1901/jeab.1994.61-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizot JC. Effects of psychoactive drugs on temporal discrimination in rats. Behav Pharmacol. 1997;8:293–308. doi: 10.1097/00008877-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Time sharing in rats: A peak interval procedure with gaps and distractors. Behav. Proc. 2006;71:107–115. doi: 10.1016/j.beproc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Brown BL, Hemmes NS. Interval clock and memory processes in animal timing. J. Exp. Psychol. Anim. B. 1994;20:184–198. doi: 10.1037//0097-7403.20.2.184. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain and Cognition. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain and Cognition. 2009a;70:181–190. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain and Cognition. 2009b;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM. Properties of the internal clock. Ann. NY Acad. Sci. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. J. Exp. Psychol. Anim. B. 1977;3:216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Clausen J. An evaluation of experimental methods of time judgment. J. Exp. Psychol. 1950;40:76–761. doi: 10.1037/h0056354. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Langdon R, McKay R. Delusional belief. Annu. Rev. Psychol. 2010;62:1–28. doi: 10.1146/annurev.psych.121208.131622. [DOI] [PubMed] [Google Scholar]
- Cordes S, Gallistel CR. Intact interval timing in CLOCK mutants. Brain Res. 2008;1227:120–127. doi: 10.1016/j.brainres.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr. Bull. 1998;21:231–248. doi: 10.1093/oxfordjournals.schbul.a033323. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kinsley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiat. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Densen ME. Time perception and schizophrenia. Percept. Motor Skill. 1977;44:436–438. doi: 10.2466/pms.1977.44.2.436. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown GDA, Weinberger DR, Goldberg TE. Duration judgments in patients with schizophrenia. Psychol. Med. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Brown GDA, McCormack T, Vousden JI, Goldberg TE. Identification of tone duration, line length, and letter position: An experimental approach to timing and working memory deficits in schizophrenia. J. Abnorm. Psychol. 2004;113:509–521. doi: 10.1037/0021-843X.113.4.509. [DOI] [PubMed] [Google Scholar]
- Freedman BJ. The subjective experience of perceptual and cognitive disturbances in schizophrenia. Arch. Gen. Psychiat. 1974;30:333–340. doi: 10.1001/archpsyc.1974.01760090047008. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy and Weber’s law in animal timing. Psychol. Rev. 1977;84:279–325. [Google Scholar]
- Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal Cognition. Erlbaum; Hillsdale, NJ: 1984. pp. 465–488. [Google Scholar]
- Gibbon J, Church RM. Comparison of variance and covariance patterns in parallel and serial theories of timing. J. Exp. Anal. Behav. 1992;57:393–406. doi: 10.1901/jeab.1992.57-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum. Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr. Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: Evidence from cognitive dysfunction. Psychopharmocol. 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Hampson CL, Body, Boon FS, Cheung THC, Bezzina G, Langley RW, Fone KCF, Bradshaw CM, Szabadi E. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and D-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behav. Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: Salience attribution revisited. Schizophr. Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjack D, Fuchs T. Delusions of technical alien control: A phenomenological description of three cases. Psychopathology. 2010;43:96–103. doi: 10.1159/000274178. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends Cogn. Sci. 2008;12:273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr. Opin. Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Petzel TP. Temporal orientation and time estimation in chronic schizophrenics. J Clin. Psychol. 1971;27:194–196. doi: 10.1002/1097-4679(197104)27:2<194::aid-jclp2270270210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bull. 1999;205:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore HM, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol. Psychiat. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q. J. Nucl. Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizpr. Bull. 2007;33:971–981. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuene H, Macar F, Zakay D. Attention and timing: Dual-task performance in pigeons. Behav. Processes. 1999;45:141–157. doi: 10.1016/s0376-6357(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Lewis A. The experience of time in mental disorder. P. Roy. Soc. Med. 1932:611–620. doi: 10.1177/003591573202500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TW. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiat. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lhamon LT, Goldstone S. The time sense: Estimation of one second durations by schizophrenic patients. Arch. Neurol. Psychiat. 1956;76:625–629. [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of methamphetamine and haloperidol on time estimation in the rat. Psychopharmacol. 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn. Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch.Gen. Psychiat. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, Schooler NR, Kane JM. The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry. 2005;162:1013–1016. doi: 10.1176/appi.ajp.162.5.1013. [DOI] [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol. Biochem. Behav. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav. Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL, Olton DS. Nucleus basalis magnocellularis and medial septal area lesions differentially impair temporal memory. J. Neurosci. 1987;7:3505–3511. doi: 10.1523/JNEUROSCI.07-11-03505.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr. Opin. Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Olton DS. Frontal cortex, timing, and memory. Neuropsychologia. 1989;27:121–130. doi: 10.1016/0028-3932(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wenk GL, Church RM, Meck WM. Attention and the frontal cortex as examines by simultaneous temporal processing. Neuropsychologia. 1988;26:307–318. doi: 10.1016/0028-3932(88)90083-8. [DOI] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmyer-Kimling L. Interval timing deficits in individuals at high risk for schizophrenia. Brain Cognition. 2005;58:109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: Effects of focal frontal lesions. Neuropsychologia. 44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Comparison of drug effects on the performance of two timing tasks in rats. Pharmacol Biochem Behav. 2000;67:377–85. doi: 10.1016/s0091-3057(00)00380-4. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. J. Exp. Psychol. Anim. B. 1981;7:242–268. [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel ER. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Symptoms and circuits, part 3: Schizophrenia. J. Clin. Psychiat. 2004;65:8–9. doi: 10.4088/jcp.v65n0102. [DOI] [PubMed] [Google Scholar]
- Todd J. Impaired detection of silent interval change in schizophrenia. Neuroreport. 2006;17:785–789. doi: 10.1097/01.wnr.0000220126.93973.31. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time and the subclassification of schizophrenic disorders. Percept. Motor Skill. 1983;57:911–918. doi: 10.2466/pms.1983.57.3.911. [DOI] [PubMed] [Google Scholar]
- Tysk L. A longitudinal study of time estimation in psychotic disorders. Percept. Motor Skill. 1984;5:779–789. doi: 10.2466/pms.1984.59.3.779. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time by patients with positive and negative schizophrenia. Percept. Motor Skill. 1990;71:826. doi: 10.2466/pms.1990.71.3.826. [DOI] [PubMed] [Google Scholar]
- Wahl OF, Sieg D. Time estimation among schizophrenics. Percept. Motor Skill. 1980;50:535–541. doi: 10.1177/003151258005000232. [DOI] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, Kandel ER, Balsam PD. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav. Neurosci. 2009;123:720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Effects of morphine on temporal discrimination and color matching: general disruption of stimulus control or selective effect on timing. J Exp Anal Behav. 2005;84:401–405. doi: 10.1901/jeab.2005.94-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Barrett ST, Johnson RN, Odum AL. Nicotine does not enhance discrimination performance in a temporal bisection procedure. Behav Pharmac. 2009;20:99–108. doi: 10.1097/FBP.0b013e3283242fc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Odum AL. Disruption of temporal discrimination and the choose-short effect. Learn. Behav. 2007;35:60–70. doi: 10.3758/bf03196075. [DOI] [PubMed] [Google Scholar]
- Waters F, Jablensky A. Time discrimination deficits in schizophrenia patients with first rank (passivity) symptoms. Psychiat. Res. 2009;167:12–20. doi: 10.1016/j.psychres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Yang YK, Yeh TL, Chiu NT, Lee IH, Chen PS, Lee LC, Jeffries KJ. Association between cognitive performance and striatal dopamine binding is higher in timing and motor tasks in patients with schizophrenia. Psychiat. Res. 2004;131:209–216. doi: 10.1016/j.pscychresns.2003.07.002. [DOI] [PubMed] [Google Scholar]