Abstract
The adaptor protein Dab1 interacts with amyloid precursor protein (APP) and decreases its pathological processing, an effect mediated by Fyn tyrosine kinase. Fyn is highly enriched in lipid rafts, a major site of pathological APP processing. To investigate the role of Fyn in the localization and phosphorylation of APP and Dab1 in lipid rafts, we isolated detergent-resistant membrane (DRM) fractions from wild-type and Fyn knock-out mice. In wild-type mice, all of the Fyn kinase, 17% of total APP, and 33% of total Dab1 were found in DRMs. Nearly all of the tyrosine phosphorylated forms of APP and Dab1 were in DRMs. APP and Dab1 co-precipitated both in and out of DRM fractions, indicating an association that is independent of subcellular localization. Fyn knock-out mice had decreased APP, Dab1, and tyrosine-phosphorylated Dab1 in DRMs but increased co-immunoprecipitation of DRM APP and Dab1. Expression of phosphorylation deficient APP or Dab1 constructs revealed that phosphorylation of APP increases, while phosphorylation of Dab1 decreases, the interaction between APP and Dab1. Consistent with these observations, Reelin treatment led to increased Dab1 phosphorylation and decreased association between APP and Dab1. Reelin also caused increased localization of APP and Dab1 to DRMs, an effect that was not seen in Fyn knock-out neurons. These findings suggest that Reelin treatment promotes the localization of APP and Dab1 to DRMs, and affects their phosphorylation by Fyn, thus regulating their interaction.
Keywords: amyloid, tyrosine kinase, adaptor protein, reelin, lipid raft
INTRODUCTION
Alzheimer’s disease (AD), an age-related neurodegenerative disease, is characterized by accumulation of the amyloid beta (Aβ) peptide, which is derived from the pathogenic cleavage of the amyloid precursor protein (APP). APP is sequentially processed by either α- or β-secretase, generating secreted APPα and the α-C-terminal fragment (α-CTF) or secreted APPβ and β-CTF, followed by γ-secretase. This second cleavage event results in the release of either a non-toxic p3 fragment or the amyloidogenic Aβ peptide. APP can be preferentially cleaved by either α- or β-secretase depending on its localization within the cell. Evidence suggests that the majority of α-secretase cleavage occurs at the cell surface (Parvathy et al. 1999), while β- and γ-secretases are thought to act predominantly intracellularly within the endosomal pathway (Huse et al. 2000, Vetrivel et al. 2004).
Epidemiological studies have demonstrated a strong association between high cholesterol levels and Aβ deposition or risk for AD (Kivipelto et al. 2001, Pappolla et al. 2003, Wellington 2004). These studies have been supported by both in vitro and in vivo studies involving cholesterol treatment of cultured cells (Frears et al. 1999) or high-cholesterol diets in transgenic mice (Refolo et al. 2000), implicating cholesterol as an important mediator of APP processing. Recent studies suggest that APP and its secretases are at least partially localized in cholesterol-rich membrane microdomains, termed lipid rafts (Vetrivel et al. 2005, Vetrivel et al. 2004, Parkin et al. 1999). Lipid rafts can be biochemically isolated via several methods; here, we isolated detergent-resistant membrane (DRM) microdomains using buffers with 1% Triton-X. Specific signaling properties of some proteins, especially those with glycosylphosphatidylinositol (GPI) anchors, are regulated through differential localization to DRMs (Brown & Rose 1992, Simons & Toomre 2000). It has been hypothesized that, due to the localization of β- and γ-secretases to DRMs, these membrane microdomains are a site for the pathological processing of APP (Riddell et al. 2001, Parkin et al. 1999). These data highlight the importance of understanding APP trafficking, as both the trafficking of APP to the cell surface, as well as the lateral movement of APP in and out of membrane microdomains, may alter the amyloidogenic processing of APP.
Our lab has previously found that APP trafficking and processing are altered by Dab1, an effect mediated by Fyn tyrosine kinase (Hoe et al. 2008, Hoe et al. 2006b). Dab1 increased cell surface APP, increased α-cleavage of APP, and decreased Aβ production in vitro, and Fyn potentiated these effects. Interestingly, Fyn localizes primarily to DRMs where it associates with GPI-anchored proteins (Kramer et al. 1999, Filipp et al. 2003). Therefore, we hypothesized that Fyn-mediated Dab1 effects on APP trafficking and processing could be regulated through localization of these proteins to DRMs.
In this study, we investigated the distribution of total and tyrosine-phosphorylated APP and Dab1 in DRMs from wild-type and Fyn knock-out mice. We report that nearly all of the tyrosine-phosphorylated APP and Dab1 are localized to DRMs, and this localization is decreased in Fyn knock-out mice. Furthermore, we utilized phosphorylation-deficient constructs of APP and Dab1 to show that Fyn phosphorylation of APP increased, while Fyn phosphorylation of Dab1 decreased, the association between APP and Dab1 in cultured cells. Finally, we show that Reelin treatment, which activates Fyn, increased localization of APP and Dab1 to DRMs in primary neurons. These data demonstrate that Fyn is an important regulator of APP trafficking in vivo by promoting its presence in DRMs and altering its association with Dab1.
EXPERIMENTAL PROCEDURES
Constructs
The constitutively active Fyn construct containing a Y531F substitution, which cannot be inactivated through phosphorylation of the C-terminal negative regulation site, was a generous gift from Dr. Katsuya Nagai (Osaka University, Osaka, Japan). Dab1 wild-type and mutant (2F) constructs were a kind gift from Dr. Brian Howell (SUNY Upstate Medical University, Syracuse, NY). APP wild-type and Y757A mutant constructs were kindly provided by Dr. Guojun Bu (Washington University, St. Louis, MO).
Reagents and Antibodies
We used antibodies anti-Fyn (Upstate, Lake Placid, NY), anti-Dab1 (from Dr. Andre Goffinet), C1/6.1 recognizing the C-terminal of APP (provided by Dr. Paul M. Mathews), antibody 369 recognizing the C-terminal of APP (provided by Dr. Sam Gandy), anti-flotillin (BD Biosciences, San Jose, CA), anti-phosphotyrosine (Fitzgerald Industries, Concord, MA), anti-FE65 (from Dr. Suzanne Guenette), anti-X11 (BD Biosciences), anti-Src (Upstate), anti-Yes (Cell Signaling, Danvers, MA), anti-BACE1 (Millipore, Billerica, MA), and anti-presenilin-1 (Chemicon, Temecula, CA). Reelin-conditioned medium or control medium was prepared from either a stable cell line (HEK293) expressing Reelin or normal HEK293 cells. Medium was collected and concentrated by centrifugation at 4000 × g for 20 min through an Amicon Ultra 50K Nominal Molecular Weight Limit (NMWL) filter device (Millipore).
Fyn knock-out mice
Fyn knock-out mice were obtained from Jackson Laboratories, as well as wild-type B6129S controls. For isolation of total protein, mouse brains were homogenized in RIPA buffer containing 50mM Tris-HCl, pH 7.4, 1% NP-40, 150mM NaCl, 1mM EDTA, with protease (Roche) and phosphatase (Sigma) inhibitors.
Primary cortical neuronal culture
Primary cortical neurons from P0 wild-type or Fyn knockout mice were cultured in poly-D-lysine coated 10cm dishes at 8 million cells per dish. Cells were maintained in Neurobasal medium containing B27 serum supplement (Invitrogen), 1mM glutamine, gentamycin, and Ara-C (5μg/mL, Sigma) as previously described (Qiu et al. 2004).
Cell lines and culture conditions
COS7 cells were maintained in Opti-MEM (Invitrogen) with 10% fetal bovine serum (FBS, Life Technologies, Inc.) in a 5% CO2 incubator. COS7 cells were transiently transfected with 0.5–1 μg of plasmid in FuGENE6 (Roche) according to the manufacturer’s protocol and cultured 24 hrs in DMEM containing 10% FBS. For co-transfections, cells were similarly transfected with 0.5–1 μg of each plasmid in FuGENE6 and cultured 24 hrs in DMEM with 10% FBS.
Cells were lysed in buffer containing 50 mM Tris-HCl (pH 8.0), 0.15 M NaCl, 1% Nonidet P-40, and phosphatase and protease inhibitors. Proteins were separated by SDS-PAGE on 10% polyacrylamide gels, transferred electrophoretically to PVDF membranes, and blocked with 5% nonfat dry milk. The blots were incubated with antibodies at room temperature for 1 hour. Horseradish peroxidase-conjugated secondary antibodies were visualized by an ECL detection system and exposed to film. Bands on the film were quantified using BioRad QuantityOne software.
DRM fractionation
DRMs were isolated from 1% Triton-X brain or primary neuronal lysates by sucrose density gradient fractionation. Briefly, brains were homogenized in a 10X volume of cold 1% Triton-X buffer containing 25mM Tris and 140mM NaCl at pH 8.0 with a Dounce homogenizer. Cell lysates were harvested with 1mL Triton-X buffer and then homogenized by passing through a 21-gauge needle. 1mL brain or 0.5mL cell lysates were mixed with an equal volume of 90% sucrose to yield 2mL (for brain) or 1mL (for primary neurons) of 45% sucrose. A discontinuous sucrose gradient was layered above, comprising 4mL of 35% and 5% sucrose for brain, or 2mL of 35% and 5% sucrose for primary neuronal lysates. The samples were centrifuged at 140,000 × g in a Sw-40 (for brain) or Sw-55 (for primary neurons) rotor (Beckman Coulter Inc.) for 12 h at 4°C. The sucrose gradients were harvested in 1mL or 0.5mL fractions for brain or cell lysates, respectively, and the distribution of flotillin, to identify DRM fractions, was detected by Western blot. For quantification, each value of APP, Dab1, or p-Dab1 was normalized to the sum of the total levels from both DRM and non-DRM fractions, allowing comparisons of the fraction of protein in the DRM fractions.
Co-immunoprecipitations
Cell lysates from COS7 cells, primary neurons, or brain were incubated overnight at 4°C with antibody and protein G-Sepharose beads (Amersham Biosciences). The beads were then washedthree times with 4°C lysis buffer and resuspended in SDS sample buffer. The samples were separated by SDS-PAGE as described above.
Aβ ELISA
Levels of endogenous mouse Aβ1–40 from DRM fractions were quantified using sandwich ELISA as previously described (Nishitomi et al. 2006). Briefly, a 96-well plate (Maxisorp) was coated with an anti-Aβ40 antibody, clone 1A10, overnight at 4°C. After blocking for 2 hrs, standards (synthetic mouse Aβ peptide 1–40) and samples were loaded and incubated overnight at 4°C. The plate was incubated with HRP-coupled detection antibody, 14F1, and visualized using a 3,3′,5,5′-tetra methyl benzidine (TMB) substrate.
Statistical analyses
All data were analyzed using ANOVA with Graphpad Prism 4 software, using Tukey’s Multiple Comparison test for post-hoc analyses with significance determined as p<0.05. Descriptive statistics are displayed as an expressed mean ± S.E.M. All experiments were conducted a minimum of three times unless otherwise noted.
RESULTS
Fyn, APP and Dab1 are preferentially localized to DRMs
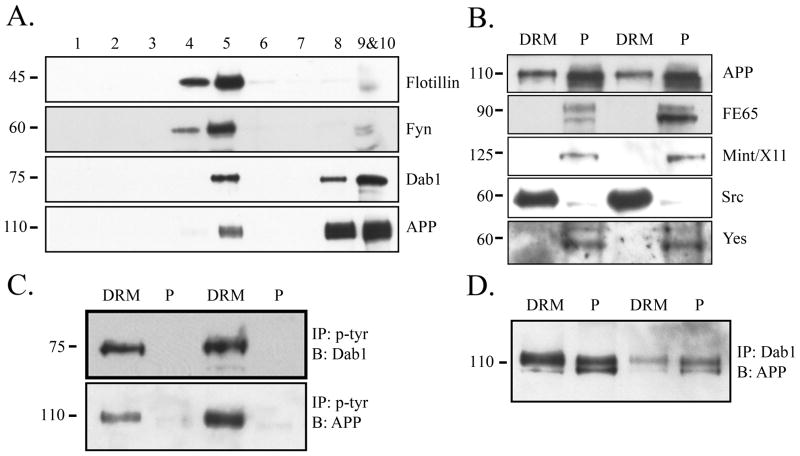
To determine the localization of APP and Dab1 in cholesterol-enriched membrane microdomains, we isolated DRMs from wild-type mouse brains homogenized in 1% Triton-X. We used an antibody against Flotillin, a marker of DRMs, and determined that DRMs were present only in fractions 4 and 5 (Fig. 1A, top panel). As reported (Filipp et al. 2003), we found that the majority of Fyn was localized to DRM fractions (Fig. 1A, second panel). A significant fraction of Dab1 and APP were also present in DRMs (Fig. 1A, bottom panels). We determined whether other APP adaptor proteins or tyrosine kinases were similarly present in DRMs, combining the DRM fractions 4 and 5 (LR) and the protein pellet fractions 9 and 10 (P). The APP adaptor proteins FE65 and Mint/X11 were present only outside DRMs (Fig. 1B). Of the other Src family tyrosine kinases known to be highly expressed in the brain (Inomata et al. 1994, Umemori et al. 1992), Yes was not detected in DRMs, but Src showed a similar localization pattern to Fyn, suggesting that some of the functions regulated by Fyn within DRMs may be shared with Src (Fig. 1B).
Figure 1.
Phosphorylated Dab1 and APP are localized to DRMs. A. Sucrose density fractionation was performed on brain homogenates from wild-type mice to isolate DRMs (fractions 4 and 5). Flotillin was used as a marker for DRMs (top). Fyn is localized exclusively to DRM fractions (second panel). Dab1 and APP are localized both in and out of DRMs (bottom two panels). B. The DRM (fractions 4 and 5) or pellet fractions (fractions 9 and 10) from wild-type mouse brains were blotted for receptors, adaptor proteins, or Src family kinases. APP is present both in and out of DRMs (top panel), but adaptor proteins FE65 and Mint/X11 are present only out of DRMs (middle panels). Src is localized primarily to DRMs but Yes is present out of DRMs (bottom panels). Blots show results of two representative experiments. C. DRM or pellet fractions were immunoprecipitated with phosphotyrosine antibody and blotted for Dab1 (top panel) or APP with C1/6.1 (bottom panel). Phosphorylated Dab1 and APP were present predominantly in DRM fractions. D. DRM or pellet fractions were immunoprecipitated with Dab1 antibody and blotted for APP. There was a comparable degree of co-precipitation within the DRM fraction compared to the pellet fraction (comparing lane 1 to 2, or lane 3 to 4).
Dab1 and APP are tyrosine phosphorylated only within DRMs
Because Fyn tyrosine kinase is exclusively localized to DRMs, we investigated the localization of tyrosine-phosphorylated Dab1 and APP. We immunoprecipitated phosphotyrosine proteins from DRM or pellet fractions from wild-type mouse brains and probed for Dab1 or APP. We found that tyrosine-phosphorylated Dab1 and APP were almost exclusively present only within DRMs (Fig. 1C). As a control, lysates were immunoprecipitated with control IgG to ensure antibody-specific pull-down of phosphotyrosine (data not shown). To determine whether tyrosine phosphorylation of Dab1 and APP affected their association, we immunoprecipitated lysates with anti-Dab1 and probed for APP, and found that they co-precipitated to a similar degree in and out of DRMs (Fig. 1D), despite the greater localization of total APP and Dab1 out of DRMs (Fig. 1A). These data suggest that a larger fraction of DRM-associated APP and Dab1 may interact with each other, and that tyrosine phosphorylation is not required for the interaction between Dab1 and APP, as non-DRM APP and Dab1 co-precipitated.
Association between APP and Dab1 is increased in Fyn knock-out mice
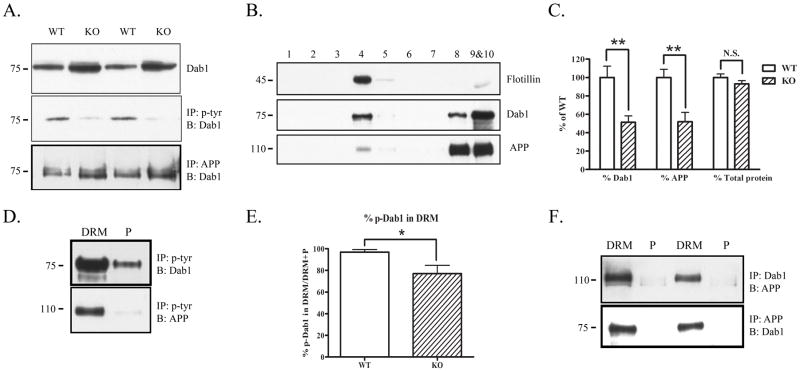
To determine whether the interaction between Dab1 and APP was altered in the absence of Fyn, we homogenized whole brains from wild-type or Fyn knock-out mice. Fyn knock-out mice had increased total Dab1 levels, but decreased tyrosine-phosphorylated Dab1 (Fig. 2A), consistent with a previous study showing that normally Fyn phosphorylation of Dab1 is followed by Dab1’s proteosomal degradation (Bock et al. 2004). To test whether the decreased tyrosine phosphorylation of Dab1 affected its association with APP, we immunoprecipitated whole brain lysates from wild-type and Fyn knock-out mice with anti-APP and probed for Dab1. The association between APP and Dab1 was increased in Fyn knock-out mice (Fig. 2A, bottom panel).
Figure 2.
Fyn knock-out mice have decreased APP and Dab1 in DRMs. A. Brain homogenates from wild-type or Fyn knock-out mice were blotted for Dab1 (top panel), immunoprecipitated with phosphotyrosine and blotted for Dab1 (middle panel), or immunoprecipitated with APP (antibody C1/6.1) and blotted for Dab1 (bottom panel). Fyn knock-out mice had increased total Dab1 (top panel) and decreased phosphorylated Dab1 (middle panel) compared to wild-type mice. Fyn knock-out mice had increased co-precipitation of APP and Dab1 compared to wild-type mice (bottom panel). B. Brain homogenates from Fyn knock-out mice were fractionated and probed for Flotillin to demonstrate presence of DRMs in fraction 4 (top panel). Dab1 (middle panel) and APP (bottom panel) were present both in (fraction 4) and out (fractions 8–10) of DRMs in Fyn knock-out mice. C. The ratio of Dab1 levels in DRM fractions of Fyn knock-out mice (normalized to total Dab1 in all fractions) was decreased by 49% (left, p<0.01), and the ratio of APP levels in DRM fractions (normalized to total APP in all fractions) was decreased by 48% (middle, p<0.01) compared to wild-type mice. There was no significant difference between wild-type and knock-out mice in the ratio of total protein found in the DRM fractions (right). D. DRM or pellet fractions from Fyn knock-out mice were immunoprecipitated with phosphotyrosine and blotted for Dab1 or APP. Phosphorylated Dab1 was present out of the DRM fraction in Fyn knock-out mice (top panel). Phosphorylated APP was present only in the DRM fraction as in wild-type mice (bottom panel). E. The ratio of tyrosine phosphorylated Dab1 in DRMs (normalized to the sum of p-Dab1 from DRM and non-DRM fractions) was significantly decreased in Fyn knock-out mice by 21% (p<0.05). F. DRM or pellet fractions from Fyn knock-out mice were immunoprecipitated with Dab1 and blotted with antibody 369 for APP (top panel), or immunoprecipitated with APP and blotted for Dab1 (bottom panel). Dab1 and APP co-precipitated only in DRM fractions.
APP and Dab1 localize less to DRMs in Fyn knock-out mice
To determine whether Fyn is the major regulator of APP and Dab1 localization to DRMs, we isolated DRMs from Fyn knock-out mouse brains. Some Dab1 and APP was again localized to DRMs (Fig. 2B); however, the fraction of Dab1 or APP in DRMs was significantly lower (by 49% and 48%, respectively) in Fyn knock-out mice compared to wild-type mice (Fig. 2C). The ratio of total protein levels in DRMs was not significantly different (Fig. 2C, right).
Tyrosine-phosphorylated Dab1 is increased out of DRMs in Fyn knock-out mice
To determine whether the localization of tyrosine-phosphorylated Dab1 was altered in Fyn knock-out mice, we immunoprecipitated DRM or pellet fractions from knock-out mice with anti-phosphotyrosine, and probed for Dab1. We found a fraction of tyrosine-phosphorylated Dab1 out of DRMs in Fyn knock-out brains (Fig. 2D, top panel), contrary to wild-type brains where tyrosine-phosphorylated Dab1 (p-Dab1) was exclusively in DRMs (Fig. 1C). The ratio of p-Dab1 in DRMs was quantified and found to be significantly lower (by 21%) in Fyn knock-out mice (Fig. 2E). We did not observe any difference in localization of phosphotyrosine APP in Fyn knock-out mice (Fig. 2D, bottom panel).
Association between APP and Dab1 is greater in DRMs of Fyn knock-out mice
We next asked whether the change in localization of total Dab1 and APP and tyrosine-phosphorylated Dab1 had an effect on localization of the APP-Dab1 interaction within DRMs. We immunoprecipitated DRM or pellet lysates with an antibody against Dab1 or APP and probed for APP or Dab1, respectively. We found that the association between Dab1 and APP occurred predominantly within DRMs in Fyn knock-out mice (Fig. 2F), contrary to wild-type mice, where there were comparable levels of interaction in and out of DRMs (Fig. 1D).
Tyrosine phosphorylation of Dab1 decreases its interaction with APP
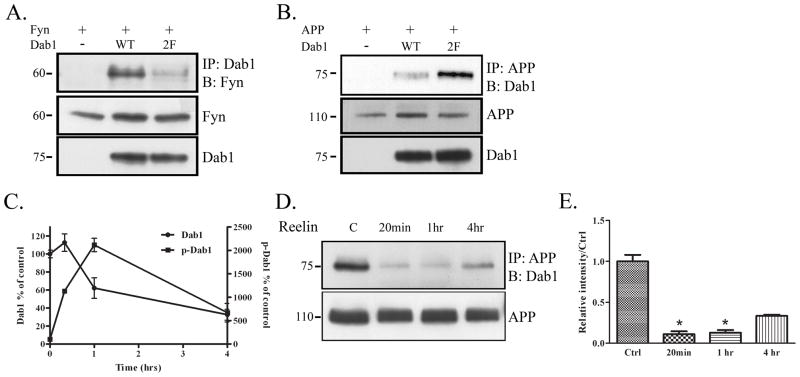
To test whether Fyn phosphorylation of Dab1 altered the association of Fyn with Dab1, we transfected COS7 cells with Fyn and vector, Dab1, or a construct of Dab1 that was mutated at two tyrosine residues known to be phosphorylated by Fyn (Y198,200F, “Dab1(2F)”). In co-immunoprecipitation experiments, we found that Dab1(2F) interacted with Fyn less than wild-type Dab1 did (Fig. 3A), suggesting that the phosphorylation sites of Dab1 are important for its interaction with Fyn.
Figure 3.
Phosphorylation of Dab1 decreases interaction with APP. A. COS7 cells were transfected with Fyn and vector, Dab1 WT, or a Dab1 construct that contained phenylalanine substitutions at two of its tyrosine residues, Dab1(2F). Cell lysates were immunoprecipitated with Dab1 and blotted for Fyn (top panel). Dab1(2F) co-precipitated with Fyn less compared to Dab1 WT. Levels of Fyn and Dab1 remained constant (bottom panels). B. COS7 cells were transfected with APP and empty vector, Dab1 wild-type construct, or Dab1(2F). Cell lysates were immunoprecipitated with APP and blotted for Dab1 (top panel). Dab1(2F) co-precipitated more with APP compared to Dab1 WT. Levels of APP and Dab1 remained constant (bottom panels). C. Primary neurons from wild-type mice (DIV14) were treated with Reelin for 20 min, 1 hr, or 4 hrs. Cell lysates were collected and blotted for Dab1, or immunoprecipitated with phosphotyrosine and blotted for Dab1. Blots of 3 independent experiments were quantified and represented in the graph shown. Total Dab1 decreased over time after 1 hr of Reelin treatment (left axis). Phosphorylated Dab1 increased over time until 1 hr of Reelin treatment (right axis). D. Primary neuronal lysates from the Reelin treatment were immunoprecipitated with APP and blotted for Dab1 (top panel). Co-precipitation between APP and Dab1 decreased with 20 min of Reelin treatment, stayed low at 1 hr, and recovered partially at 4 hrs. Total levels of APP remained constant over time (bottom panel). E. Quantification of data in D (n=2) shows an 89% decrease in APP-Dab1 interaction compared to control at 20 min (p<0.05), an 87% decrease at 1 hr (p<0.05), and a 67% at 4 hrs (p=0.07) of Reelin treatment (by student’s t-test with unequal variance).
Because we had observed that the association between APP and Dab1 was increased in Fyn knock-out mice, we hypothesized that the phosphorylation of Dab1 decreased its interaction with APP (Fig. 2A). We transiently transfected COS7 cells with APP and empty vector, Dab1, or Dab1(2F). The association between APP and the phosphorylation-deficient Dab1(2F) was higher than between APP and wild-type Dab1 (Fig. 3B). Levels of total APP and Dab1 were similar across conditions (Fig. 3B, bottom panels). These results suggest that Fyn phosphorylation of Dab1 specifically increases its interaction with Fyn while decreasing its interaction with APP.
We next undertook a different approach to determine whether phosphorylation of Dab1 decreased its interaction with APP in primary neurons. Reelin, an extracellular matrix protein, can induce Dab1 phosphorylation by binding to the receptors ApoEr2 and VLDLR (Hiesberger et al. 1999), members of the low-density lipoprotein (LDL) receptor family (Herz & Beffert 2000). Reelin-induced multimerization of the receptors results in Fyn activation and Dab1 tyrosine phosphorylation followed by the proteolytic degradation of Dab1 (Arnaud et al. 2003, Bock et al. 2004). We cultured primary neurons from wild-type mice and treated cells at DIV14 with Reelin for varying lengths of time (20 min, 1 hr, 4 hrs). We collected cells at the indicated time points, and measured total Dab1 and p-Dab1 levels. At 20 min of Reelin treatment, there were increased levels of tyrosine phosphorylated Dab1 while levels of total Dab1 remained constant. At 1 hr of Reelin treatment, phosphorylated Dab1 levels were further increased, while total Dab1 levels began to decrease (Fig. 3C). At 4 hrs of Reelin treatment, total levels of Dab1 remained low (about 40% of control levels), and phosphorylated Dab1 began to return to control levels.
This time course allowed us to determine whether the relative amounts of total and phosphorylated Dab1 affected its interaction with APP. We immunoprecipitated cell lysates from Reelin-treated neurons with APP and Western blotted for Dab1 (Fig. 3D). We found that 20 min of Reelin treatment, which increased Dab1 phosphorylation but did not affect Dab1 levels, reduced the interaction between APP and Dab1. This interaction remained low at 1 hr, but by 4 hrs of Reelin treatment, when phosphorylated Dab1 had decreased, there was a slight recovery in the interaction between APP and Dab1, despite continued low levels of total Dab1. Quantification of this data showed an 89% decrease in APP-Dab1 interaction at 20 min (p<0.05), an 87% decrease at 1 hr (p<0.05), and only a 67% decrease by 4 hrs compared to control (p=0.07, not significant) (Fig. 3E). The data support the hypothesis that Fyn phosphorylation of Dab1 decreases its association with APP.
Tyrosine phosphorylation of APP by Fyn increases its interaction with Dab1
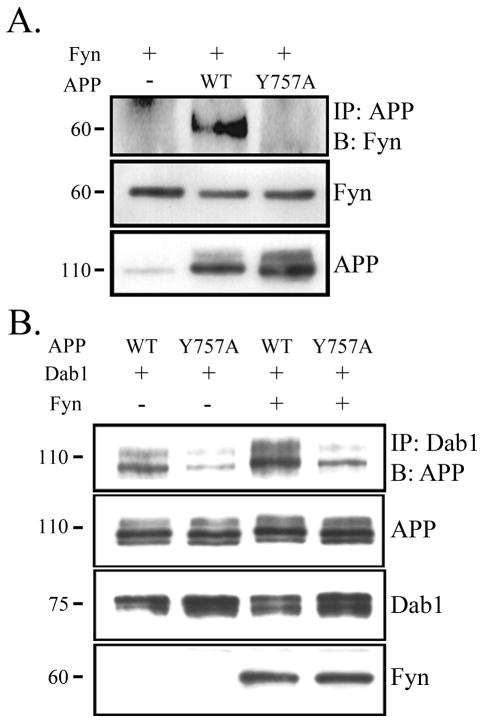
Because Fyn phosphorylates both Dab1 and APP, we next tested whether phosphorylation of APP by Fyn altered its interaction with Fyn or Dab1. First, we co-transfected COS7 cells with Fyn and either vector, wild-type APP, or a construct of APP in which the tyrosine we previously established to be phosphorylated by Fyn was mutated to an alanine (APP Y757A) (Hoe et al. 2008) (Fig. 4A). We co-immunoprecipitated APP and Fyn, and found that wild-type APP did co-IP with Fyn, but APP Y757A did not. These findings suggest that tyrosine 757 of APP is necessary for the interaction of APP with Fyn.
Figure 4.
Phosphorylation of APP increases interaction with Dab1. A. COS7 cells were transfected with Fyn and vector, APP wild-type (WT), or APP mutant construct (Y757A) that contained a substitution mutation at the site of Fyn phosphorylation. Cell lysates were immunoprecipitated with APP and blotted for Fyn (top panel). APP Y757A co-precipitated less with Fyn compared to APP WT. Levels of Fyn and APP remained the same across conditions (bottom panels). B. COS7 cells were transfected with Dab1 and APP WT or APP Y757A in the presence or absence of Fyn. Cell lysates were immunoprecipitated with Dab1 and blotted for APP (top panel). APP Y757A co-precipitated less with Dab1 compared to APP WT both in the presence (right lanes) and absence (left lanes) of Fyn. Levels of APP, Dab1, and Fyn remained similar across conditions (bottom panels).
Next, we included Dab1 in these experiments (Fig. 4B, right lanes). The APP Y757A mutation almost completely abolished the interaction of APP and Dab1 (Fig. 4B, right lanes). Levels of APP, Dab1, and Fyn were constant across conditions (bottom panels). Similar results were seen in the absence of Fyn overexpression (Fig. 4B, left lanes). These data suggest that phosphorylation of APP at tyrosine 757 is important for its interaction with both Dab1 and Fyn.
Reelin effects on APP and Dab1 localization to DRMs depends on Fyn
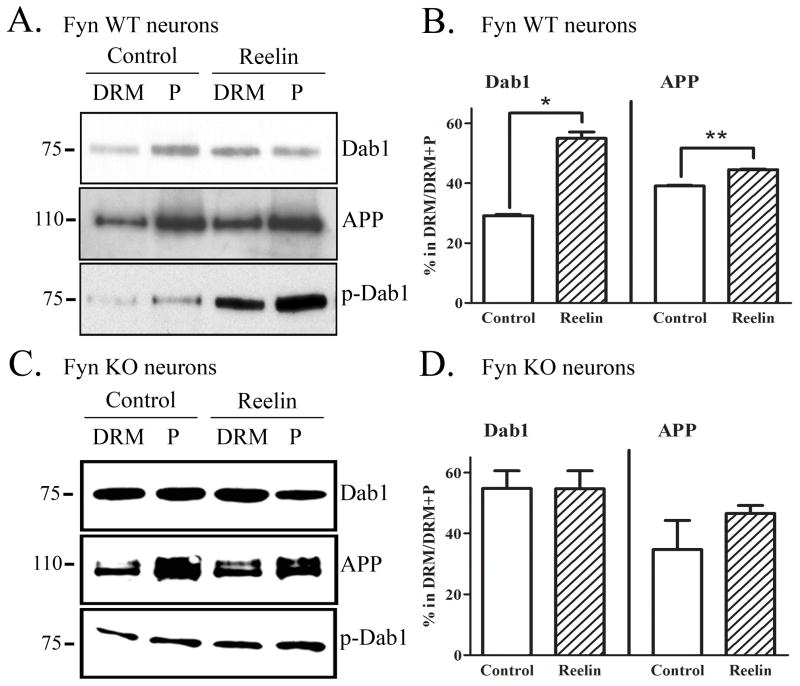
Using Fyn knock-out mice, we demonstrated that reducing Fyn decreases localization of APP, Dab1, and phospho-Dab1 to DRMs (Fig. 2). To test the complementary hypothesis that activating Fyn increases the localization of APP, Dab1, and phospho-Dab1 to DRMs, we treated primary neurons with Reelin and isolated DRMs from the cell lysates. The percentage of DRM Dab1 in control neurons (29%) was similar to that found in wild-type brains (33%), although the percentage of DRM APP was greater in primary neurons (39%) compared to brains (17%). The fractions of Dab1 and APP in DRMs were significantly increased following 20 min of Reelin treatment, by 89% (p<0.05) and 14% (p<0.01), respectively (Fig. 5A & 5B). The ratio of phospho-Dab1 levels in DRMs compared to out was increased following Reelin treatment (Fig. 5A, bottom panel). We observed that although there was almost no phospho-Dab1 outside of DRMs in wild-type brain (Fig. 1C), a phospho-Dab1 band was detected outside of DRMs in primary neurons (Fig. 5A, bottom panel). However, consistent with decreased DRM localization of Dab1 in Fyn knock-out mice (Fig. 2E), we observed that Reelin treatment increased Dab1 localization in DRMs after 20 minutes (Fig. 5B).
Figure 5.
Reelin treatment increases localization of Dab1, APP, and phosphorylated Dab1 to DRMs in primary neurons. A. Primary neurons (DIV14) were treated with control media or Reelin for 20 min. Cell lysates were subjected to a sucrose density gradient and fractionated for DRMs. DRM and pellet fractions were immunoprecipitated for Dab1 and blotted for Dab1 (top panel), immunoprecipitated for APP and blotted for APP (middle panel), or immunoprecipitated for phosphotyrosine and blotted for Dab1 (bottom panel). There was more Dab1, APP, and p-Dab1 present in the DRM fraction following Reelin treatment. B. Quantification of Western blots revealed a significant 89% increase (p<0.05) in the fraction of Dab1 in DRMs following Reelin treatment, and a significant 14% increase (p<0.05) in the fraction of APP in DRM fractions. C. Fyn knock-out primary neurons (DIV14) were treated with control or Reelin for 20 min and similarly probed for Dab1 (top panel), APP (middle panel), and p-Dab1 (bottom panel) in and out of DRMs. D. Quantification of Western blots showed no significant difference in the fractions of Dab1 or APP present in DRMs following Reelin treatment in Fyn knock-out neurons.
To test whether Fyn was necessary for Reelin effects on Dab1 and APP localization to DRMs, we performed the same experiment in primary neurons cultured from Fyn knock-out mice (Fig. 5C & 5D). We found that Reelin treatment did not alter the localization of Dab1, APP, or phospho-Dab1 in DRMs (Fig. 5C & 5D), suggesting that these effects in wild-type cells require Fyn activation. Thus, Reelin caused a rapid increase in the phosphorylation of Dab1 and its localization in DRMs by a Fyn-dependent mechanism.
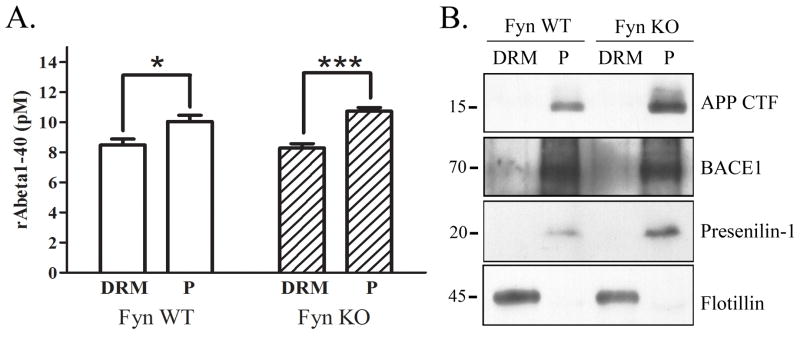
APP processing is not altered in Fyn knock-out mice
We have so far established that Fyn plays a role in the trafficking of Dab1 and APP to DRMs. We took one approach to testing whether Fyn affected APP processing and Aβ production in vivo by examining Fyn knock-out brains. We found no significant difference between wild-type and Fyn knock-out brains in the levels of Aβ in and out of DRMs (Fig. 6A). We observed a significantly greater localization of Aβ out of DRMs compared to within in both types of animals. We also observed the majority of APP CTFs, as well as BACE1 and presenilin-1, localized out of the DRM fractions, suggesting that the majority of APP is processed outside of DRMs in our preparation (Fig. 6B).
Figure 6.
APP processing is unaltered in DRM fractions of Fyn knock-out mice. A. Endogenous mouse Aβ1–40 levels were measured in DRM and pellet fractions from wild-type or Fyn knockout mice. There was no significant difference in Aβ1–40 in either the DRM or pellet fractions in Fyn knock-out mice compared to wild-type mice. There was a significant increase in Aβ1–40 levels in the pellet fraction compared to the DRM fraction of both wild-type (18%, p<0.05, n=5) and Fyn knock-out mice (30%, p<0.01, n=5). B. DRM and pellet fractions from wild-type and Fyn knock-out mice were Western blotted for APP CTF, BACE1, Presenilin-1, and Flotillin. Flotillin localized to the DRM fraction, while APP CTF, BACE1, and Presenilin-1 were almost exclusively localized to the non-DRM fraction.
DISCUSSION
In this study, we combined a number of approaches including knock-out mice, primary cultures, and in vitro transfection experiments to demonstrate that Fyn promotes the localization of APP, Dab1, and phosphorylated Dab1 to DRM fractions. Fyn knock-out mice had decreased localization of APP, Dab1, and phosphorylated Dab1 in DRMs, and increased association between APP and Dab1 within DRMs (Fig. 2). Mutant APP or Dab1 constructs deficient in tyrosine phosphorylation revealed that phosphorylation of APP promotes, while phosphorylation of Dab1 inhibits, their interaction (Fig. 3 & 4). This effect was also observed in primary neurons, where Reelin treatment, which promoted Dab1 phosphorylation, decreased the Dab1 association with APP (Fig. 4). Furthermore, Reelin treatment increased the localization of APP, Dab1, and phosphorylated Dab1 to DRMs, an effect that was not observed in Fyn knock-out neurons (Fig. 5).
Many cytoplasmic proteins, such as FE65, Mint/X11, Dab1, and SNX17, interact with APP and affect its processing based on in vitro overexpression studies (Lau et al. 2000, Borg et al. 1998, Tomita et al. 1999, Sastre et al. 1998, Hoe et al. 2006a, Guenette et al. 1996, Borg et al. 1996, He et al. 2007, Parisiadou & Efthimiopoulos 2007, Lee et al. 2008). Our study reveals that regulation of these interactions occurs at least partially through their compartmentalization in subcellular compartments, an important fact when considering effects on APP processing in an intact cellular system. Adaptor proteins may compete with each other for overlapping binding sites within APP. Adaptor proteins may also compete for binding with proteins other than APP. ApoEr2 and VLDLr share a number of common adaptor proteins with APP that affect their trafficking and processing, including FE65 and Mint/X11 (Hoe et al. 2006a, Gotthardt et al. 2000, Hoe et al. 2008, Hiesberger et al. 1999, Minami et al. 2009). Lipid raft compartmentalization presents one mechanism, in addition to transport among subcellular structures, by which trafficking of proteins may regulate their interactions.
Tyrosine phosphorylation is known to potentiate downstream signal transduction by fostering interactions between proteins containing SH2 and other phosphotyrosine-binding domains (Marengere & Pawson 1994). Here, we demonstrate that tyrosine phosphorylation can both promote and inhibit protein-protein interactions. Tyrosine phosphorylation of Dab1 by Fyn inhibits its interaction with APP, while increasing its interaction with Fyn. Tyrosine phosphorylation of APP enhances its interaction with both Dab1 and Fyn. These findings suggest that it is a balance between phosphorylation of APP and Dab1 that regulates their interaction. Interestingly, DRM localization of phosphorylated Dab1, but not phosphorylated APP, was altered in Fyn knock-out mice, suggesting that Fyn is the major kinase promoting phosphorylated Dab1 in DRMs, but other tyrosine kinases compensate to localize phosphorylated APP to DRMs.
Interaction of extracellular ligands with surface receptors can also regulate interactions with intracellular proteins. Reelin is known to bind its transmembrane receptors ApoEr2 and VLDLr, leading to phosphorylation of Dab1 and Fyn to regulate neuronal migration (Bock et al. 2004). Dysregulation of this pathway results in the inversion of neocortical layers in reeler mutant mice (Goffinet 1979), a phenotype shared by Dab1 mutant mice (Gallagher et al. 1998), and ApoEr2 and VLDLr double knock-out mice (Trommsdorff et al. 1999). These observations support the model that Reelin binds to ApoEr2 and VLDLr extracellularly, promoting signaling through Dab1 intracellularly. Recently, we have shown that Reelin also binds APP and affects its trafficking and processing (Hoe et al. 2009b, Hoe et al. 2006b). Since Reelin can both increase trafficking of APP to the cell surface (Hoe et al. 2009b) and increase localization of APP to DRMs, it may promote both the enhanced α-cleavage at the cell surface and the enhanced β- and γ-secretase cleavage within DRMs (Parkin et al. 1999, Riddell et al. 2001). However, the Reelin-mediated increase in α-processing of APP and decrease in Aβ in vitro suggest that localization of APP to DRMs does not result in pathological processing of APP.
Co-localization of Dab1 and APP within rafts may also have significant consequences on the functions of APP and Dab1. APP is a synaptic protein that plays a role in synapse formation or stabilization (Hoe et al. 2009a, Wang et al. 2009). Synaptic receptors are present in lipid rafts, altering the local signaling environment (Delint-Ramirez et al. 2010). Localization in and out of rafts may thus regulate APP’s interactions with synaptic proteins. Movement in and out of rafts may also affect Dab1 functions. Dab1 (Stolt & Bock 2006) and APP (Young-Pearse et al. 2007) both affect neuronal migration during development, and APP modulates the effects of Dab1 on neuronal migration (Pramatarova et al. 2008). Here we have found that Fyn regulates the presence of APP and Dab1 in DRMs and alters the subcellular site of their interaction. Fyn is normally found post-synaptically, although its distribution may be altered in disease states (Ittner et al. 2010); we (unpublished data) and others (Shirazi & Wood 1993, Ho et al. 2005) have also observed that it is increased in AD brains. Increased levels and missorting of Fyn may alter the normal functions of APP and Dab1, contributing to the neuronal dysfunction in AD.
We did not observe an effect on Aβ levels in Fyn knock-out mice, although given the dynamic nature of the effects of Fyn on Dab1 phosphorylation and levels after Reelin treatment (Fig. 3), it is difficult to predict how long-term loss of Fyn would alter APP processing. We did observe that the majority of BACE1 and presenilin-1 were localized out of DRMs, contrary to previously published studies (Riddell et al. 2001, Parkin et al. 1999). Interestingly, one study investigated the ability of several detergents to extract different proteins to DRMs, and found that 1% Triton X, which we use here, did not isolate presenilin-1 in the DRM fraction, but 1% CHAPSO did (Vetrivel et al. 2004). Thus, APP secretases may be associated with a specific subset of DRMs that is freed by extraction with specific detergents.
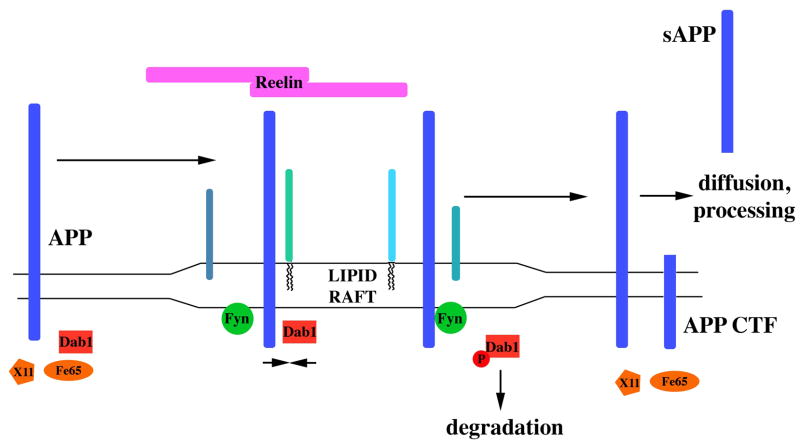
From these data and others concerning APP-Dab1 interaction (Hoe et al. 2006b, Hoe et al. 2008), we have developed a model for Fyn regulating the trafficking of APP (Fig. 7). APP and Dab1 exist both in and out of DRMs, and they interact in both environments (Fig. 1). This interaction occurs when APP is part of a stable complex of proteins, perhaps interacting with other proteins in the extracellular matrix or on the surface of adjoining cells. When neurons are stimulated with Reelin (and other APP/ApoE receptor ligands such as F-spondin (Peterziel et al. 2011, Howell et al. 1999)), the Dab1 in lipid rafts quickly becomes phosphorylated by Fyn. This phosphorylation destabilizes the complexes that contain APP, allowing the Dab1 and the APP to move between lipid raft and non-lipid raft components of the cell. In this state, APP can interact with secretases, primarily α-secretase; we previously found that Fyn increased α-cleaveage of APP only in its active state (Hoe et al. 2008). This destabilization of complexes of APP with other surface proteins may be part of the cellular changes that are necessary for Reelin to promote neurite outgrowth (Hoe et al. 2009b) and long term potentiation (Qiu et al. 2006).
Figure 7.
Model for extracellular and intracellular interactions affecting APP trafficking. APP interacts with Dab1 in and out of lipid rafts, but other APP adaptor proteins (X11, Fe65) are only in non-raft compartments. APP in lipid rafts can interact with other raft components (represented by blue-green shapes). The appearance of extracellular Reelin promotes surface APP localization and Fyn activation. Dab1 is phosphorylated by Fyn, disrupting its interaction with APP. With Dab1 no longer bound, other adaptor proteins can interact with APP and alter its subcellular localization. Dab1 is degraded, but over time returns to normal levels, returning APP to its state before Reelin activation.
In summary, we provide evidence that Fyn promotes the accumulation of phosphorylated APP and phosphorylated Dab1 in DRMs, affecting the distribution of APP and Dab1 in subcellular structures. Furthermore, Fyn regulates the APP-Dab1 association through the reciprocal phosphorylation of both, thus providing a mechanism by which interactions between APP and its adaptor proteins can be regulated. These data support a model where phosphorylation of Dab1 within lipid rafts following Reelin-induced activation of Fyn decreases its association with APP. Phosphorylated Dab1 can then be trafficked out of rafts, where it may interact with any number of proteins before undergoing proteosomal degradation. Fyn may thus regulate the role that APP and Dab1 play in neuronal migration and synaptic signaling.
Acknowledgments
This work was supported by NIH AG14473 (GWR), NIH NS59178 (SSM), and the fund for Alzheimer’s research established in memory of Bill and Marie Drach. We thank Dr. Mark Burns for helpful advice, and Dr. Katsuya Nagai for providing the Fyn plasmid DNA construct, Dr. Brian Howell for Dab1 constructs, and Dr. Guojun Bu for APP constructs. We also thank Dr. Andre Goffinet, Dr. Paul Matthews, Dr. Sam Gandy, Dr. Dudley Strickland, and Dr. Suzanne Guenette for antibodies.
Abbreviations used
- AD
Alzheimer’s disease
- ApoEr2
apoE receptor 2
- APP
amyloid precursor protein
- CTF
C-terminal fragment
- Dab1
Disabled1
- p-Dab1
tyrosine phosphorylated Dab1
- DRM
detergent-resistant membrane
- VLDLr
very low density lipoprotein receptor
References
- Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–9302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem. 2004;279:33471–33479. doi: 10.1074/jbc.M401770200. [DOI] [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg JP, Straight SW, Kaech SM, de Taddeo-Borg M, Kroon DE, Karnak D, Turner RS, Kim SK, Margolis B. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J Biol Chem. 1998;273:31633–31636. doi: 10.1074/jbc.273.48.31633. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Fernandez E, Bayes A, Kicsi E, Komiyama NH, Grant SG. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci. 2010;30:8162–8170. doi: 10.1523/JNEUROSCI.1792-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipp D, Zhang J, Leung BL, Shaw A, Levin SD, Veillette A, Julius M. Regulation of Fyn through translocation of activated Lck into lipid rafts. J Exp Med. 2003;197:1221–1227. doi: 10.1084/jem.20022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Howell BW, Soriano P, Cooper JA, Hawkes R. Cerebellar abnormalities in the disabled (mdab1-1) mouse. J Comp Neurol. 1998;402:238–251. [PubMed] [Google Scholar]
- Goffinet AM. An early development defect in the cerebral cortex of the reeler mouse. A morphological study leading to a hypothesis concerning the action of the mutant gene. Anat Embryol (Berl) 1979;157:205–216. doi: 10.1007/BF00305160. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Guenette SY, Chen J, Jondro PD, Tanzi RE. Association of a novel human FE65-like protein with the cytoplasmic domain of the beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 1996;93:10832–10837. doi: 10.1073/pnas.93.20.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Cooley K, Chung CH, Dashti N, Tang J. Apolipoprotein receptor 2 and X11 alpha/beta mediate apolipoprotein E-induced endocytosis of amyloid-beta precursor protein and beta-secretase, leading to amyloid-beta production. J Neurosci. 2007;27:4052–4060. doi: 10.1523/JNEUROSCI.3993-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat Rev Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Hashimoto M, Adame A, Izu M, Alford MF, Thal LJ, Hansen LA, Maslish E. Altered p59Fyn kinase expression accompanies disease progression in Alzheimer’s disease: implications for its functional role. Neurobiol Aging. 2005;26:625–635. doi: 10.1016/j.neurobiolaging.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Fu Z, Makarova A, et al. The effects of amyloid precursor protein on postsynaptic composition and activity. J Biol Chem. 2009a;284:8495–8506. doi: 10.1074/jbc.M900141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Lee KJ, Carney RS, et al. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci. 2009b;29:7459–7473. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Magill LA, Fu Z, Vicini S, Rebeck GW. FE65 interaction with the apoE receptor ApoEr2. J Biol Chem. 2006a;281:24521–24530. doi: 10.1074/jbc.M600728200. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Minami SS, Makarova A, Lee J, Hyman BT, Matsuoka Y, Rebeck GW. Fyn modulation of Dab1 effects on amyloid precursor protein and ApoE receptor 2 processing. J Biol Chem. 2008;283:6288–6299. doi: 10.1074/jbc.M704140200. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. Dab1 and Reelin effects on APP and ApoEr2 trafficking and processing. J Biol Chem. 2006b;281:35176–35185. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. J Biol Chem. 2000;275:33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Inomata M, Takayama Y, Kiyama H, Nada S, Okada M, Nakagawa H. Regulation of Src family kinases in the developing rat brain: correlation with their regulator kinase, Csk. J Biochem. 1994;116:386–392. doi: 10.1093/oxfordjournals.jbchem.a124536. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, et al. Dendritic Function of Tau Mediates Amyloid-beta Toxicity in Alzheimer’s Disease Mouse Models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Lau KF, McLoughlin DM, Standen CL, Irving NG, Miller CC. Fe65 and X11beta co-localize with and compete for binding to the amyloid precursor protein. Neuroreport. 2000;11:3607–3610. doi: 10.1097/00001756-200011090-00041. [DOI] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283:11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengere LE, Pawson T. Structure and function of SH2 domains. J Cell Sci Suppl. 1994;18:97–104. doi: 10.1242/jcs.1994.supplement_18.14. [DOI] [PubMed] [Google Scholar]
- Minami SS, Sung YM, Dumanis SB, et al. The cytoplasmic adaptor protein X11{alpha} and extracellular matrix protein Reelin regulate ApoE receptor 2 trafficking and cell movement. FASEB J. 2009 doi: 10.1096/fj.09-138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitomi K, Sakaguchi G, Horikoshi Y, et al. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1553–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Bryant-Thomas TK, Herbert D, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- Parisiadou L, Efthimiopoulos S. Expression of mDab1 promotes the stability and processing of amyloid precursor protein and this effect is counteracted by X11alpha. Neurobiol Aging. 2007;28:377–388. doi: 10.1016/j.neurobiolaging.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Hussain I, Karran EH, Turner AJ, Hooper NM. Characterization of detergent-insoluble complexes containing the familial Alzheimer’s disease-associated presenilins. J Neurochem. 1999;72:1534–1543. doi: 10.1046/j.1471-4159.1999.721534.x. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Cleavage of Alzheimer’s amyloid precursor protein by alpha-secretase occurs at the surface of neuronal cells. Biochemistry. 1999;38:9728–9734. doi: 10.1021/bi9906827. [DOI] [PubMed] [Google Scholar]
- Peterziel H, Sackmann T, Strelau J, Kuhn PH, Lichtenthaler SF, Marom K, Klar A, Unsicker K. F-spondin regulates neuronal survival through activation of disabled-1 in the chicken ciliary ganglion. Mol Cell Neurosci. 2011;46:483–497. doi: 10.1016/j.mcn.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Chen K, Howell BW. A genetic interaction between the APP and Dab1 genes influences brain development. Mol Cell Neurosci. 2008;37:178–186. doi: 10.1016/j.mcn.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Sastre M, Turner RS, Levy E. X11 interaction with beta-amyloid precursor protein modulates its cellular stabilization and reduces amyloid beta-protein secretion. J Biol Chem. 1998;273:22351–22357. doi: 10.1074/jbc.273.35.22351. [DOI] [PubMed] [Google Scholar]
- Shirazi SK, Wood JG. The protein tyrosine kinase, fyn, in Alzheimer’s disease pathology. Neuroreport. 1993;4:435–437. doi: 10.1097/00001756-199304000-00024. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Tomita S, Ozaki T, Taru H, Oguchi S, Takeda S, Yagi Y, Sakiyama S, Kirino Y, Suzuki T. Interaction of a neuron-specific protein containing PDZ domains with Alzheimer’s amyloid precursor protein. J Biol Chem. 1999;274:2243–2254. doi: 10.1074/jbc.274.4.2243. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res Mol Brain Res. 1992;16:303–310. doi: 10.1016/0169-328x(92)90239-8. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang B, Yang L, Guo Q, Aithmitti N, Songyang Z, Zheng H. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington CL. Cholesterol at the crossroads: Alzheimer’s disease and lipid metabolism. Clin Genet. 2004;66:1–16. doi: 10.1111/j.0009-9163.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]