Abstract
Since the discovery of the ABCA4 gene as the cause of autosomal recessive Stargardt disease/fundus flavimaculatus much has been written of the phenotypic variability in ABCA4 retinopathy. In this review the authors discuss the findings seen on examination and the disease features detected using various clinical tests. Important differential diagnoses are presented and unusual presentations of ABCA4 disease highlighted.
Introduction
Since the discovery of the ABCA4 gene as the cause of autosomal recessive (ar) Stargardt disease/fundus flavimaculatus (STGD/FF)1 much has been written of the phenotypic variability in ABCA4 retinopathy, from age-related macular degeneration2 in heterozygous carriers to bull's eye maculopathy (BEM),3,4 ar-cone-rod dystrophy (CRD) and ar-retinitis pigmentosa (RP).5-9 In this review the authors discuss the clinical manifestations of ABCA4 retinopathy and highlight unusual presentations of disease as well as important differential diagnoses.
The prevalence of STGD has been estimated between 1 in 8,000 and 1 in 10,000,10 although given that the carrier frequency for a mutation in the ABCA4 gene may be as high as 1:20, the true prevalence of ABCA4 retinopathy is likely much higher.11,12 The process of deriving comprehensive genotype/phenotype correlations has long troubled ophthalmologists and geneticists, not only due to the marked phenotypic variability, the autosomal recessive nature of the disease or the description of over 600 disease causing mutations in the ABCA4 gene, but also because the 3 most common mutations account for less than 10% of the disease phenotypes.13 A common method for genotyping involves use of the ABCA4 microarray. This method detects all currently known disease-associated genetic variants (>600) in the ABCA4 gene. Current detection rates for the array in patients with STGD/FF run between ~ 65% and 75% of all disease–associated alleles. This compares to a detection rate of ~80% with direct sequencing.11 The detection rate is lower in patients with ar CRD at between 30% and 65%,7,14,15 and 16% in patient with arRP.15 A detection rate of 35% has been reported in patients with BEM.3 Beyond their marked heterogeneity, mutations in the ABCA4 gene demonstrate a number of interesting features. Complex alleles are prevalent. The most common, L541P/A1038V, has been reported as a founder mutation in Hungaro-German populations.14,16,17 Furthermore “ethnic group-specific” ABCA4 alleles have been described in other populations also, C1490Y and R602W in South African patients,18 and N965S in a Danish population19 among others.20
In an attempt to explain the variability seen in ABCA4 retinal phenotypes and to correlate this with individual mutation effect, a model was proposed which correlated disease severity with residual ABCA4 function.14, 21 Maugerie et al. classified ABCA4 mutant alleles as “mild”, “moderate”, and “severe” based on the predicted effect of the mutation on the transport function of the protein i.e. the more severe the effect of the mutation on ABCA4 function, the more aggressive the disease phenotype. The various biochemical defects underlying certain mutations have been assessed in vitro using recombinant proteins,22 while the pathogenic effect of mislocalization of the ABCA4 protein was demonstrated in transgenic tadpoles.23 N retinylidene-phosphatidylethanoliamne (NRPE), formed by the binding of all-trans retinal to the phospholipid phosphatidylethanoliamne (PE), is removed from the disc space to the cytoplasm of the rods and cone photoreceptors by normally functioning ABCA4 protein. Failure of this process leads to a build-up of NRPE in the disc spaces and thus facilitates the binding of a second molecule of alltrans retinal to NRPE leading to the formation of A2E24 which is a component of lipofuscin. Lipofuscin accumulates in the retinal pigment epithelium (RPE) through a process of disc shedding and phagocytosis and is toxic to the RPE through a number of mechanisms, including effects on membrane permeability, lysosomal dysfunction, and the detachment of pro-apoptotic proteins leading to a cell-death pathway. A2E also inhibits 11-cis retinal regeneration by binding to retinoid isomerase RPE65.25 Despite improvements in our understanding of pathogenesis in ABCA4 disease, it remains to be determined which occurs first: photoreceptor degeneration or RPE atrophy?26,27
Natural History
Patients with ABCA4 disease commonly deny family history and the earliest symptoms are consistent with slowly progressive central vision loss. Age of onset can be indicative of the disease severity i.e. the earlier the onset the more aggressive the disease course, however caution must be exercised when interpreting this parameter as it will be heavily dependent on the presence or absence of foveal sparing (Figure 1). Also, an asymptomatic patient can be diagnosed before the onset of symptoms due to the diagnosis of a symptomatic relative. Patients with STGD/FF have an age of onset typically between ages 10-20 years.28 Later ages of onset and have been associated with a more favorable visual prognosis,29-31 while earlier ages of onset have been reported in patients with electrophysiological features of cone or cone-rod dysfunction compared to those patients who have a normal full-field electroretinogram (FF ERG).29,32 Because age of onset is a subjective marker it is recommend that age at examination is documented in all patients at each visit as a surrogate marker for disease duration. Patients often report varying patterns of dyschromatopsia.29,33,34 Photoaversion may be reported with isolated cone dysfunction, while nyctalopia develops more commonly in CRD.
Images A and B show wide-field FAF views of a patient with widespread involvement of the retina in STGD. White arrows point to the foveal regions which demonstrate uniform fluorescence bilaterally i.e. relative foveal sparing. SD-OCT image C corresponds with the white dashed line seen in image B through the central macula. The black doubled-headed arrow indicates relative ISOS sparing in the fovea and parafoveal macula. The white double-headed arrows overlie regions of loss of ISOS. Images D and E are the fundus photos correlating with images A and B respectively. The extent of disease and sparing is underestimated by the fundus photos. 165×173mm (72 × 72 DPI)
Best Corrected Snellen Visual Acuity (BCVA)
BCVA continues to be a useful marker of disease progression and even prognosis, depending on the age at presentation. Presenting visual acuities of 20/40 or better were associated with foveal sparing, and those patients who presented later than 20 years of age were more likely to maintain visual acuities of better than 20/200 over the course of their lifetime compared to patients presenting before 20 years of age. Only 4% of patients from a cohort of 361 with STGD had visual acuity of 20/400 recorded.30 Poorer visual acuities have been reported in a greater proportions of patients with widespread disease (as measured by retinal structural changes or effects on the FF ERG) compared to those with central macular disease only.29,32
Clinical Examination and Fundus Photography
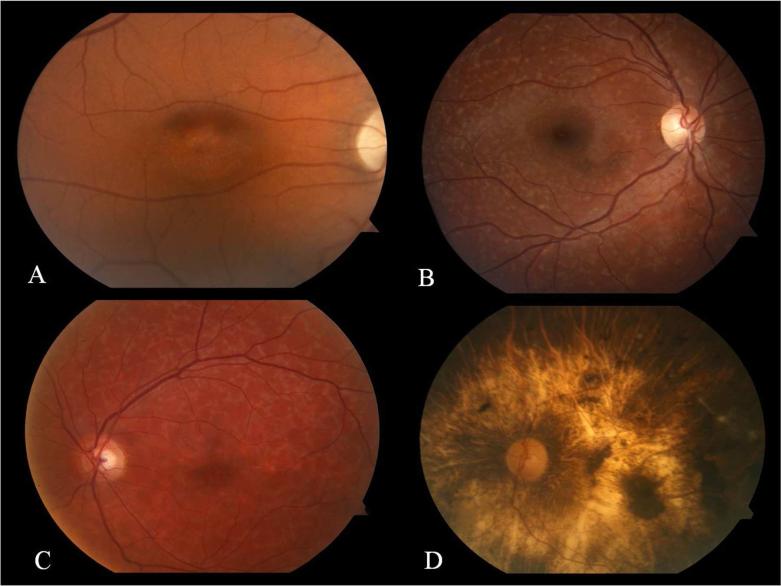
Fishman's seminal description of STGD in 4 stages was based on clinical findings, and electrophysiological and psychophysical testing (Figure 2).34 In Stage I the disease is confined to the fovea or parafoveal macula with pigmentary changes giving a “beaten-metal” or “snail-slime” appearance. The RPE in this region eventually becomes atrophic. A discontinuous ring of flecks approximately 1 disc diameter in size often encircles the fovea. The electro-oculogram (EOG) and dark adaptation as measured with the ERG are normal in Stage I disease. With Stage II disease the flecks were more widespread extending anterior to the vascular arcades and/or nasal to the optic disc. These may be partially or totally resorbed. Subnormal cone and rod responses may be observed in this group with delayed dark adaptation. A relative central scotoma may be present concurrent with macular involvement. Resorbtion of the flecks is seen in Stage III, with widespread atrophy of the choriocapillaris. EOG testing demonstrates subnormal ratios for light peak to dark trough and dysfunction of the cone or cone and rod photoreceptors may be evident. Visual field testing will demonstrate central visual field defects as well as increasing involvement of the peripheral field as the disease moves centrifugally. In stage IV disease there is further resorbtion of flecks with extensive choriocapillaris and RPE atrophy. Progression of visual field changes can be expected and there is marked abnormality of both cone and rod systems detected with the ERG.33 Importantly, these phenotypes do not imply a rigid phenotypic pathway of disease progression. Patients with an early onset (within the first decade of life) will be expected to demonstrate more widespread disease with time, especially in terms of fundus autofluorescence changes that may not be visible on fundus photography or bio-microscopy. Aaberg described a method for classifying STGD and FF to demonstrate the common characteristics these phenotypes shared, to evaluate progression and to highlight interfamilial co-existence in an attempt to unify them as a single disease entity.35 BEM is considered distinct from the maculopathy seen in STGD, although the former may also be caused by mutations in the ABCA4 gene. The central macular atrophy seen in STGD is discontinuous, as opposed to the continuous atrophy seen in classic BEM.36 While BEM may be present as an isolated presentation of localized ABCA4 disease, it may also be an early manifestation of a progressive retinopathy with cone or cone-rod dysfucntion.32 Two phenotypes have been described in CRD based on fundus appearance. The most common exhibited extensive pigment clumping while the other demonstrated little or no clumping. These changes have been associated with specific mutations in the ABCA4 gene.7 Fundus photography allows accurate and convenient documentation of the appearance of the macula and extramacular retina. It is also useful for assessing and documenting the preferred retinal location (PRL) in patients where unstable fixation precludes accurate perimetry by asking the patient to focus on the tip of the target of the fundus camera and acquiring 3 photographs of the PRL in each eye.
FIG. 2.
Images A-D demonstrate the fundus appearance of patients with Fishman stage I-IV STGD respectively. Subtle foveal atrophy is seen in image A. There are no flecks seen in this case of Stage I disease. Image B demonstrates widespread flecks extending outside the vascular arcades in a patient with Stage II disease. Resorbed flecks and developing chorioretinal atrophy are seen in image C of a patient with Stage III disease. Widespread chorioretinal and RPE atrophy is seen in a patient with Stage IV disease. 152×114mm (300 × 300 DPI)
The Electroretinogram
Given the progressive nature of the fundus changes over time and in an effort to determine concordance between family members with different ages of onset and retinal appearances, a classification based solely on the focal (F), pattern (P) and FF ERG has been described (Figure 3).29 Isolated abnormalities in the FERG and PERG were documented in patients with group I disease with normal photopic and scotopic FF ERG. With group II disease the FERG, PERG and photopic FF ERG were abnormal with a normal scotopic FF ERG. With group III disease abnormalities were demonstrated with FERG, PERG, and scotopic and photopic FF ERG.29 This classification system is of prognostic importance for the patient. In a follow-up study of patient with STGD/FF at a mean interval of 9.3 years, three of 13 patients with group I disease progressed to group II, and 1 to group III. Five of the 8 patients with group II disease progressed to group III. All patients in group III demonstrated progression with electrophysiological testing at follow-up.37 Functional studies correlating ERG progression with peripheral visual field changes are not available and would be challenging to perform, especially in those patients with group III disease where fixation is markedly unstable. It is encouraging that the majority of patients with ABCA4 disease tend to have a normal FF ERG.29,32 Concordance, in terms of ERG changes, has been demonstrated between siblings irrespective of discordance in fundoscopic appearance of the retina, age of disease onset, or visual acuity.38 This intrafamilial phenotype variation in the context of genetic homogeneity may be due to the modifying effects of environmental or non-ABCA4 genetic factors on ABCA4 protein expression and function.39
FIG. 3.
Unilateral electroretinogram tracings of patients with groups I - III Stargardt Disease and a normal control. Normal amplitudes and implicit times are seen in both rod and cone photoreceptor systems in ERG Group I. Patients with group II disease have a reduction in the photopic waveforms and a significant implicit time delay but normal scotopic responses. Subnormal amplitudes in both photopic and scotopic responses and delay in implicit times are seen in group III disease. Note the difference in scaling required for visualization of the waveforms for Group III vs. I or II. While these waveforms were not “extinguished” they were markedly reduced. (A similar figure has been previously published in Experimental Eye Research, 2010;91(5): p.592-600. Burke T. et al., Loss of peripapillary sparing in non-Group I Stargardt Disease, Copyright Elsevier). 152×173mm (300 × 300 DPI)
Fluorescein Angiography (FA)
The presence of a “dark” or “silent” choroid on FA has long assisted ophthalmologists in making the clinical diagnosis of STGD. The most commonly quoted frequency for this sign is 85.9%.40 Importantly, its absence does not rule out a diagnosis of ABCA4 disease. The masking of background choroidal fluorescence occurs due to a build-up of lipofuscin in the RPE causing absorption of short wave length light. The G1961E missense mutation, the most commonly detected mutation in the ABCA4 gene,31,33,41 may not be associated with the presence of a dark choroid, as well as a having more localized and less severe disease phenotype.42-44 Where extensive disease is present there can be difficulty identifying the presence or absence of a silent choroid on FA in the presence of numerous window defects and staining of the flecks during the course of angiography.45 The presence of a ring of hypofluorescence in the peripapillary region on FA has been reported in 37% of patients in a cohort of patients with STGD.45 It was detected at a higher frequency in patients with more severe disease, and was associated with poorer visual acuity and greater visual field defects.
Fundus Autofluorescence (FAF)
FAF (Figure 4) allows a qualitative assessment of the buildup and distribution of lipofuscin in ABCA4 disease and also allows detection of changes in the function of the RPE before these can be appreciated on fundus biomicropscopy.46,47 BEM has been divided into 3 phenotypic subtypes based on the appearance on FAF. With Type A there is central hypofluorescence surrounded by a hyperfluorescent ring, while type B demonstrates only central hypofluorescence without surrounding hyperfluorescence. Type C lacks central hypofluorescence and instead has a speckled pattern of alternating hypo- and hyperfluorescence.36 Those patients with type C BEM, where the genetic cause was unknown, were reported to have the latest age of onset and best visual acuity of the three BEM groups and a greater proportion of these patients had photoreceptor dysfunction confined to the macula.36 While the frequency of type C BEM is the lowest of the 3 subtypes reported in the literature, visual acuity less than 20/200 is also less frequently reported in this group.3,36,42
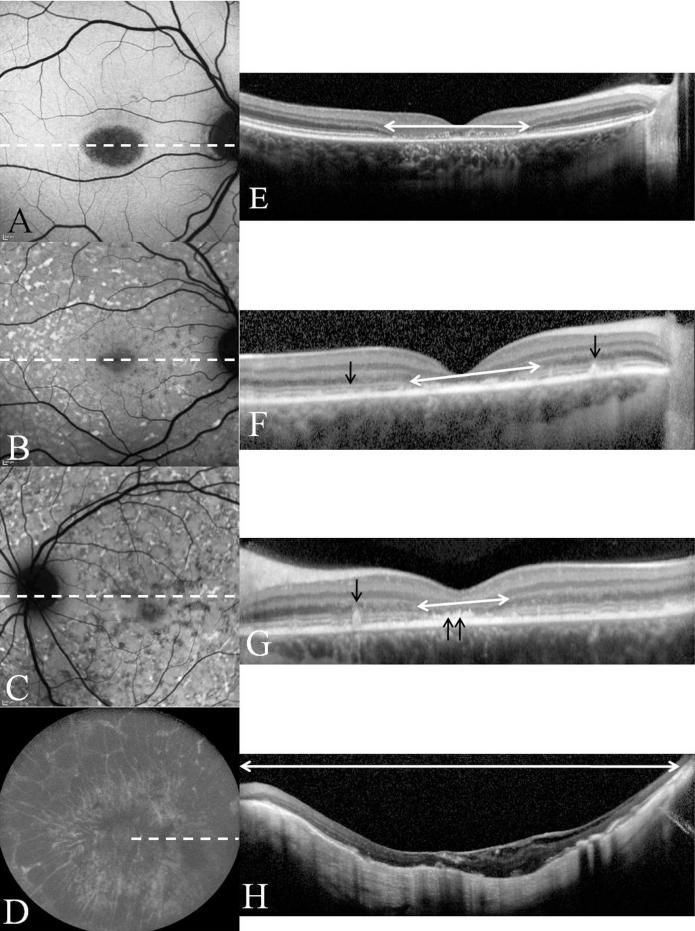
FIG. 4.
Images A-D are the FAF images corresponding to the fundus photos seen in figure 2. Corresponding SD-OCT line scans E-H are presented also. Their position on the FAF image is indicated by the white dashed lines. White double headed arrows indicate regions of absence of ISOS. Black arrows point to flecks as seen on SD-OCT as elevations from the RPE. Double arrows in image G point to abnormal thickening of the RPE in the parafoveal region. Image C clearly demonstrates a ring of uniform fluorescence in the peripapillary area consistent with peripapillary sparing. In image D we see marked RPE atrophy in the fundus including the peripapillary area i.e. peripapillary atrophy. 152×199mm (300 × 300 DPI)
The usefulness of FAF in ABCA4 disease has been clearly demonstrated: examination of its pattern and distribution allows determination of the rate of progression of disease. Flecks in STGD are commonly seen as regions of focal hyperfluorescence, while atrophy of the RPE gives hypofluorescence due the absence of fluorophores in this region. Importantly, focal hypofluorescence develops not coincident with, but adjacent to regions of focal hyperfluorescence.48 A mean increase in the area of geographic atrophy (GA) of 30% per year of the 250 micron transition zone has been reported in 7 eyes of 4 patients.48 Another recent series using an automated method for detecting atrophic lesions reported an absolute increase in size of 0.94 (+/-0.87) mm2/year in 30 eyes of 15 patients with STGD.49
Quantifiable parameters of disease progression become vital to identify rapid progressors for inclusion in Phase I and II clinical trials with experimental therapies such as gene therapy, as well as for monitoring treatment effect in patients as well as in the Abca4-/- mouse, the animal model of disease. Correction of the disease phenotype, in terms of a reduction in the RPE A2E levels to those of wild type, has been demonstrated in the Abca4-/- mouse treated with gene therapy delivered via subretinal injection of a lentiviral vector.50 While caution is required when correlating results found in animal models to patients, clearly in vivo measurement of RPE lipofuscin levels is vital as we move to an era of treatment of ABCA4 disease. Using spectrofluorometry, Delori et al. demonstrated the RPE as the origin of FAF and lipofuscin as its major source, thus in vivo quantification of lipofuscin levels over small regions of retina became possible.51 In order to assess the efficacy of a treatment across the entire macula a number of groups have sought to use the scanning laser ophthalmoscope (SLO) to quantify FAF using standardized acquisition protocols.52-54 Significant elevation in levels of FAF were clearly demonstrated and the distribution of fluorescence in the macula reported. Recently our unit described the method of Quantitative autofluorescence (Quant AF) which allows quantification of FAF across the macula and accounts for variations in refractive error, optical media density, laser power and sensitivity at which the image was acquired. Significant elevation (> 400%) in background macular QuantAF levels in patients with STGD relative to age-similar controls were demonstrated.55 Hyperfluorescent flecks gave the highest overall values. Undoubtedly, this method of retinal imaging will allow more accurate monitoring of disease progression, as well as providing a powerful quantitative marker for monitoring treatment effect in the future.
Sparing of the peripapillary retina from the effects of disease in ABCA4 retinopathy has long formed part of the diagnostic criteria for STGD. Furthermore, sparing of peripapillary FAF has been reported in a cohort of patients with ABCA4 retinopathy and this region suggested as an area of interest for monitoring treatment effect in the future.52 Peripapillary atrophy was demonstrated by Hwang et al. in 3 of 150 patients with Stargardt disease based on their FAF findings.56 We recently reported the loss of peripapillary sparing, i.e. presence of hyperfluorescent flecks or various patterns of hypofluorescence, in patients with Group II and III Stargardt disease grouped by ERG. This suggests that loss of sparing correlates with more widespread photoreceptor dysfunction and a poorer visual prognosis.32
Optical Coherence Tomography (OCT)
Time Domain OCT study of the retina demonstrated reduced total macular volume and central foveal thickness in patients with ABCA4 disease compared to controls and a correlation of these measurements with BCVA.17 With the arrival of the Spectral Domain-OCT, improvements in resolution allow visualization of the individual layers of the retina and have allowed more detailed study of the retina in ABCA4 disease. Simultaneous imaging with SLO in infrared or FAF (Spectralis HRA+OCT; Heidelberg Engineering, Dossenheim, Germany) allows point to point correlation of the OCT scan with the en face retinal image, and as such allows for better monitoring of the disease phenotype with perfectly registered images acquired in multiple modalities.
Voigt et al. recently characterized flecks into 5 groups depending on the extent of penetration of hyper reflective material through the various retinal layers. Class A flecks were described as being confined to the outer-segment layer, while class B extended through the inner segment-outer segment (ISOS) junction, with Class C protruded into the outer nuclear layer (ONL). With Class D fleck material was documented only in the ONL, although there is the possibility that the connection of this material to the RPE is not seen due to the spacing between the line scans obtained. Drusen–like pigment epithelial detachments in STGD were described as class E flecks. Although none of the patients had ABCA4 genotyping performed, the presence of a “silent choroid” was an inclusion criterion for this study. Significant correlation between BCVA and complete loss of the inner segment-outer segment junction of the photoreceptors (ISOS) was documented,57,58 but not with the different fleck subtypes.57 Ergun et al. demonstrated a significant correlation between absence of the ISOS and atrophy as seen on FA and FAF also.58 Gomes et al. reported that ISOS loss extended outside areas of GA and into areas of abnormal FAF surrounding the foveal atrophy.26 Furthermore, the ISOS was either fully preserved, or affected to a lesser degree compared to the fovea in the region of the PRL as detected by the Nidek Micoperimeter (MP-1) (see below).26
From a cohort of 11 patients with ar CRD, three of the four patients with mutations detected in the ABCA4 gene demonstrated thinning of the RNFL in at least 1 quadrant of the peripapillary region demonstrating the effect of a predominantly outer retinal disease on the inner retina.59 Examples of SD-OCT correlating with FAF are shown in Figure 4.
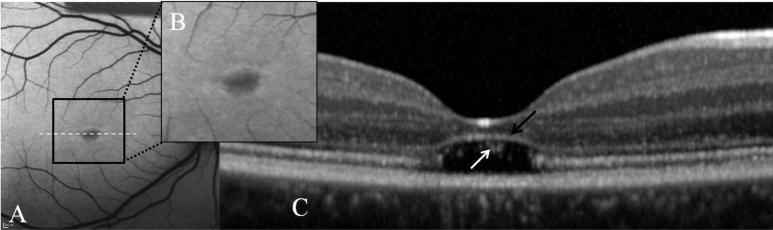
A recently described phenotype of ABCA4 disease, where there is focal absence of the outer segments and ISOS of the photoreceptors at the fovea has been described (Figure 5).26 There was preservation of the external limiting membrane in these cases. Qualitatively, the ONL overlying these regions was thinned.26 There was mild or even no alteration in the pattern of FAF although it had also been reported in association with type A BEM in ABCA4 disease. Patients typically present in their late teens and visual acuity is usually better than 20/150. A similar phenotype, based on SD-OCT finding, has recently been reported in achromatopsia.60
FIG. 5.
Images A and B demonstrate type A BEM i.e. central hypofluorescence surrounded by a ring of hyperfluorescence. Image C is the associated line scan corresponding to the white dashed line. Note the “optical empty space” i.e. absence of photoreceptor outer segments and ISOS. The external limiting membrane appears elevated and encroaches on the thinned ONL in this region. Within the lesion cellular debris/remnants are seen (white arrow). 152×46mm (300 × 300 DPI)
Microperimetry/Visual fields
Monitoring maculopathies which destroy central fixation early in their course, as with ABCA4 disease, makes the use of standard perimetry less reliable as disease progresses. To overcome this problem microperimetry is recommended for use in ABCA4 disease where compensation is made for eye movements as the visual field is assessed. It also allows more accurate structure function correlation for both clinical and research purposes. Careful instruction on continual observation of the target is recommended as patients often attempt to direct their gaze “straight ahead” i.e. so the target falls within their scotoma. Stability of fixation is determined in terms of the percentage of fixation points that fall within a certain circle area i.e. >75% falling within a 2° diameter implies stable fixation, >75% falling within 4° but <75% within 2° diameter implies relatively unstable fixation; and <75% falling within 4° implies unstable fixation.61,62 Superior eccentric fixation is typically seen in ABCA4 disease 61,63 and there is a relationship between increasing eccentricity of the PRL and reduced BCVA. However, a reduction in stability of fixation was not correlated with increased eccentricity of the PRL.61
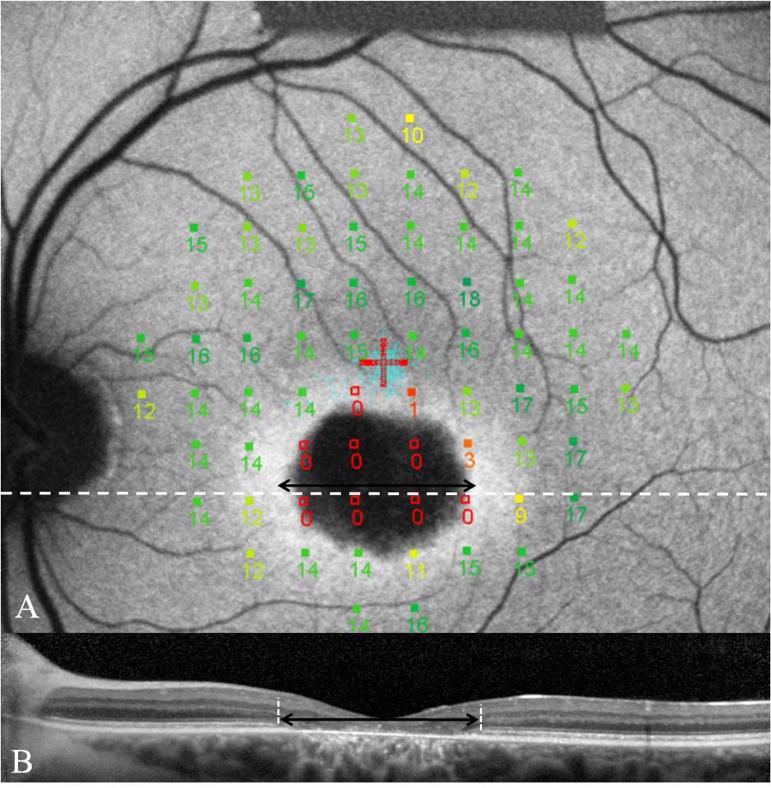
Correlations between FAF images and MP-1 sensitivity results is possible with registration of the imported image using software available on the Nidek MP-1 (Figure 6). The PRL has been reported to fall in retinal areas which have normal FAF, some distance away from the edge of a region of GA,61 with profound reductions in visual sensitivities over regions of hypofluorescence, while regions of hyperfluorescence were not always associated with decreased visual sensitivities. Hyperfluorescence in the transition zone rather than flecks was associated with reduced sensitivities. In patients with advanced disease the peripapillary region or even regions in the nasal retina may be used as the PRL.52 Determination of location of PRL is also important to avoid misinterpretation of defects detected using multi focal ERG and in the visual field.64
FIG. 6.
Image A demonstrates MP-1 results of a 68 point 10-2 field superimposed and registered to an FAF image of a patient with BEM secondary to mutations in ABCA4. This patient has superior eccentric fixation with the PRL located away from the hyperfluorescent transition zone. Note the profound reduction in sensitivities overlying the atrophic area as well as the reduced sensitivity over the transition zone. The SD-OCT image corresponds to the white dashed line. The black double headed arrow indicates the extent of loss of ISOS junction on the SD-OCT image and is duplicated on the AF image. This demonstrates loss of ISOS junction extending into regions overlying structurally preserved RPE. 152×153mm (300 × 300 DPI)
Important Differentials
Drusen
Sporadic or familial drusen may mimic STGD, however, these lesions do not tend to hyperfluorescence to the same level as flecks. Furthermore, visual acuities are invariably normal. On SD-OCT we see that the interior of the lesion, which arise from the RPE, are echolucent compared to typically echodense flecks. While these may be autosomal dominantly inherited, they often occur sporadically and have a benign course (Figure 7).
FIG. 7.
Images A-D are of a 20-year-old patient with drusen. Parental examination revealed no abnormalities. On FAF we see uniform macular fluorescence except for some focal regions of hyperfluorescence extending from the fovea to the temporal macula (images A+B). These discrete yellowish subretinal lesions can be seen on fundus photography also. On SD-OCT (image D), corresponding to the white dashed line in image A, we see these lesions as echolucent elevations of the RPE. The ISOS appears reasonably intact in the regions overlying these lesions. Images E-H are of a patient with pattern dystrophy. On AF we see punctate regions of discrete hyperfluorescence in the central macula (images E+F). These regions are also seen on fundus photography (image G). On SD-OCT (image H), the most striking abnormality is the loss of ISOS junction centrally (white double headed arrow). The RPE demonstrates few focal elevations. 152×106mm (300 × 300 DPI)
Pattern Dystrophy (PD)
Pattern dystrophy is a term used to describe a heterogeneous group. It is important to differentiate these from STGD as the visual prognosis is more favorable and the pattern of inheritance is often autosomal dominant with obvious implications for the offspring of affected individuals.65,66 Mutations in the peripherin/RDS gene have been reported in 12.7% of PD cases.65 The pattern of focal hyperfluorescence tends to be more discrete, punctuate and irregular than that which is observed in the STGD flecks. The “dark” choroid sign is consistently absent in this disorder.66 Furthermore, the ISOS junction disruption is discontinuous with islands of preserved ISOS commonly seen in the central macula. Consequently, visual acuity is often better than in ABCA4 disease.
Conclusion
In summary, the spectrum of disease seen in patients with ABCA4 retinopathy is broad. In order to fully characterize a patient's phenotype, examination using multiple modalities of investigation, as well as careful history and clinical examination is necessary.
Acknowledgements
Supported in part by: NIH/NEI (Bethesda, MD), R01 EY018213 (SHT), Eye Surgery Fund (TB), Foundation Fighting Blindness (Owings Mills, MD), New York Community Trust and an unrestricted grant to the Department of Ophthalmology, Columbia University, from Research to Prevent Blindness Inc. (New York, NY), Foundation Fighting Blindness, Schneeweiss Stargardt Fund, and The Starr Foundation. SHT is a Fellow of the Burroughs-Wellcome Program in Biomedical Sciences, and has been supported by the Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness Award and Foundation Fighting Blindness, Dennis W. Jahnigen Award of the American Geriatrics Society, Crowley Family Fund, Joel Hoffman Fund, Gale and Richard Siegel Stem Cell Fund, Charles Culpeper Scholarship, Schneeweiss Stem Cell Fund, Irma T. Hirschl Charitable Trust, and Bernard and Anne Spitzer Stem Cell Fund, Barbara & Donald Jonas Family Fund, and Professor Gertrude Rothschild Stem Cell Foundation.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt's macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 2.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277(12):1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 3.Michaelidesm M, Chen LL, Brantley MA, Jr., et al. ABCA4 mutations and discordant ABCA4 alleles in patients and siblings with bull's-eye maculopathy. Br J Ophthalmol. 2007;91:1650–1655. doi: 10.1136/bjo.2007.118356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klevering BJ, Deutman AF, Maugeri A, Cremers FP, Koyng CB. The spectrum of retinal phenotypes caused by mutations in the ABCA4 gene. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):90–100. doi: 10.1007/s00417-004-1079-4. [DOI] [PubMed] [Google Scholar]
- 5.Cremers FP, van de Pol DJ, van Driel M, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Gen. 1998;7(3):355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Birch DG, Peters AY, Locke KL, et al. Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4 (ABCR) gene. Exp Eye Research. 2001;73(6):877–886. doi: 10.1006/exer.2001.1093. [DOI] [PubMed] [Google Scholar]
- 7.Fishman GA, Stone EM, Eliason DA, et al. ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol. 2003;121(6):851–855. doi: 10.1001/archopht.121.6.851. [DOI] [PubMed] [Google Scholar]
- 8.Klevering BJ, van Driel M, van de Pol DJ, et al. Phenotypic variations in a family with retinal dystrophy as a result of different mutations in the ABCR gene. Br J Ophthalmol. 1999;83(8):914–918. doi: 10.1136/bjo.83.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Mir A, Paloma E, Allikmets R, et al. Retinitis Pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18(1):11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 10.Blacharski PA. Fundus flavimaculatus. In: Newsome DA, editor. Retinal Dystrophies and Degenerations. Raven Press; New York: 1988. pp. 135–159. [Google Scholar]
- 11.Jaakson K, Zernant J, Külm M, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22(5):395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 12.Yatsenko AN, Shroyer NF, Lewis RA, Lupski JR. Late-onset Stargardt disease is associated with missense mutations that map outside known functional regions of ABCR (ABCA4). Hum Genet. 2001;108:346–355. doi: 10.1007/s004390100493. [DOI] [PubMed] [Google Scholar]
- 13.Allikmets R. Stargardt disease: from gene discovery to therapy. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology. Diagnostics and TherapeuticsHumana Press; 2007. pp. 105–118. [Google Scholar]
- 14.Maugeri A, Klevering BJ, Rohrschneider, et al. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67(4):960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klevering BJ, Yzer S, Rohrschnedier K, et al. Microarray-based mutation analysis of the ABCA4 (ABCR) gene in autosomal recessive con-rod dystrophy and retinitis Pigmentosa. Eur J Hum Genet. 2004;12(12):1024–1032. doi: 10.1038/sj.ejhg.5201258. [DOI] [PubMed] [Google Scholar]
- 16.Rivera A, White K, Stöhr H, et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67(4):800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargitai J, Zernat J, Somfai GM, et al. Correlation of clinical and genetic findins in Huyngarian patients with Stargardt disease. Invest Ophthal Vis Sci. 2005;46(12):4402–4408. doi: 10.1167/iovs.05-0504. [DOI] [PubMed] [Google Scholar]
- 18.September AV, Vorster AA, Ramesar RS, Greenberg LJ. Mutation Spectrum and Founder Chromosomes for the ABCA4 Gene in South African Patients with Stargardt Disease. Invest Ophthal Vis Sci. 2004;45(6):1705–1711. doi: 10.1167/iovs.03-1167. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg T, Klie F, Garred P, Schwartz M. N965S is a common ABCA4 variant in Stargardt-related retinopathies in the Danish population. Mol Vis. 2007;13:1962–9. [PubMed] [Google Scholar]
- 20.Maugeri A, van Driel MA, van de Pol DJ, et al. The 2588G-->C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999;64(4):1024–1035. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Driel MA, Maugeri A, Klevering BJ, Hoyng CB, Cremers FP. ABCR unites what ophthalmologists divide(s). Ophthal Genet. 1998;19(3):117–122. doi: 10.1076/opge.19.3.117.2187. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26(2):242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- 23.Wiszniewski W, Zaremba CM, Yatsenko AN, et al. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005;14(19):2769–2778. doi: 10.1093/hmg/ddi310. [DOI] [PubMed] [Google Scholar]
- 24.Koenekoop RK. The gene for Stargardt disease, ABCA4, is a major retinal gene: a mini-review. Ophthal Genet. 2003;24(2):75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 25.Moiseyev G, Nikolaeva Chen Y, et al. Inhibition of the visual cycle by A2E though direct interaction with RPE65 and implications in Stargardt disease. Proc Nat Acad Sci. 2010;107(41):17551–17556. doi: 10.1073/pnas.1008769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes N, Greenstein VC, Carlson JN, et al. A comparison of Fundus Autofluorescence and Retinal Structure in Patients with Stargardt Disease. Invest Ophthal Vis Sci. 2009;50(8):3953–3959. doi: 10.1167/iovs.08-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazer LC, Dryja TP. Understanding the etiology of Stargardt's disease. Ophthalmol Clin North Am. 2002;15(1):93–100. doi: 10.1016/s0896-1549(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 28.Fishman GA. Electrophysiologic Testing in Disorders of the Retina, Optic Nerve and Visual Pathway. In: Fishman GA, editor. The Electroretinogram. 2nd ed. The Foundation of the American Academy of Ophthalmology; Singapore: 2001. pp. 54–56. [Google Scholar]
- 29.Lois N, Holder GE, Bunce C, Fizke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119(3):359–369. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 30.Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology. 2003;110(6):1151–1158. doi: 10.1016/S0161-6420(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 31.Simonelli F, Testa F, Zernat J, et al. Genotype-phenotype correlation in Italian families with Stargardt disease. Ophthalmic Res. 2005;37(3):159–167. doi: 10.1159/000086073. [DOI] [PubMed] [Google Scholar]
- 32.Burke T, Allikmets R, Smith RT, et al. Loss of Peripapillary sparing in non-group I Stargardt Disease. Exp Eye Res. 2010;91(5):592–600. doi: 10.1016/j.exer.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerth C, Andrassi-Darida M, Bock M, et al. Phenotypes of 16 Stargardt macular dystrophy/fundus flavimaculatus patients with known ABCA4 mutations and evaluation of genotype-phenotype correlation. Graefes Arch Clin Exp Ophthalmol. 2002;240(8):628638. doi: 10.1007/s00417-002-0502-y. [DOI] [PubMed] [Google Scholar]
- 34.Fishman GA. Fundus Flavimaculatus: A clinical classification. Arch Ophthalmol. 1976;94(12):2061–2067. doi: 10.1001/archopht.1976.03910040721003. [DOI] [PubMed] [Google Scholar]
- 35.Aaberg TM, Han DP. Evaluation of phenotypic similarities between Stargardt flavimaculatus and retinal epithelial disorders. Trans Am Ophthalmol Soc. 1987;85:101–119. [PMC free article] [PubMed] [Google Scholar]
- 36.Kurz-Levin MM, Halfyard AS, Bunce C, Bird AC, Holder GE. Clinical Evaluation in bull's-eye maculopathy. Arch Ophthalmol. 2002;120(5):567–575. doi: 10.1001/archopht.120.5.567. [DOI] [PubMed] [Google Scholar]
- 37.Fujinami K, Michaelides M, Webster AR, et al. A Longitudinal Study of the Electroretinogram Responses in Stargardt-Fundus Flavimaculatus. ARVO. 2010 Poster # A588. [Google Scholar]
- 38.Lois N, Holder GE, Fitzke FW, Plan C, Bird AC. Intrafamilial variation of phenotype in Stargardt macular dystrophy-Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1999;40(11):2668–2675. [PubMed] [Google Scholar]
- 39.Cidecyian AV, Swider M, Aleman TS, et al. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Mutat. 2009;18(5):931–941. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt's macular dystrophy. Ophthalmology. 1987;94(7):809–814. doi: 10.1016/s0161-6420(87)33533-x. [DOI] [PubMed] [Google Scholar]
- 41.Lewis RA, Shroyer NF, Singh N, et al. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64(2):422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella W, Greenstein VC, Zernant-Rajanj J, et al. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull's eye maculopathy. Exp Eye Res. 2009;89(1):16–24. doi: 10.1016/j.exer.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fishman G, Stone EM, Grover S, et al. Variation of Clinical Expression in Patients With Stargardt Dystrophy and Sequence Variations in the ABCR Gene. Arch Ophthalmol. 1999;117(4):504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 44.Genead MA, Fishman GA, Stone EM, Allikmets R. The natural history of Stargardt disease with specific sequence mutation in the ABCA4 gene. Invest Ophthalmol Vis Sci. 2009;50(12):5867–5871. doi: 10.1167/iovs.09-3611. [DOI] [PubMed] [Google Scholar]
- 45.Jayasundera T, Rhoades W, Branham K, et al. Peripapillary Dark Choroid Rinag as a Helpful Diagnostic Sign in Advanced Stargardt Disease. Am J Ophthalmol. 149(4):656–660. doi: 10.1016/j.ajo.2009.11.005. 1010. [DOI] [PubMed] [Google Scholar]
- 46.Boon CJ, Jeroen Klevering B, Keunen JE, et al. Fundus autofluoresecence imaging of retinal dystrophies. Vis Res. 2008;48(26):2569–2577. doi: 10.1016/j.visres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 47.von Rückmann A, Fitzke FW, Bird AC. In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol. 1997;115(5):609–615. doi: 10.1001/archopht.1997.01100150611006. [DOI] [PubMed] [Google Scholar]
- 48.Smith RT, Gomes NL, Barile F, et al. Liposfuscin and autofluorescence metrics in progressive STGD. Invest Ophthalmol Vis Sci. 2009;50(8):3907–3914. doi: 10.1167/iovs.08-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen B, Toscha C, Gorin MC, Nusinowitz S. Analysis of autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp Eye Res. 2010;91(2):143–152. doi: 10.1016/j.exer.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15(19):1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delori FC, Staurenghi G, Arend O, et al. In vivo measurement of lipofuscin in Stargardt's disease--Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36(11):2327–2331. [PubMed] [Google Scholar]
- 52.Cideciyan AV, Swider M, Aleman TS, et al. ABCA4-associated retinal degenerations spare structure and function of the human parapapillary retina. Invest Ophthalmol Vis Sci. 2005;46(12):4739–4746. doi: 10.1167/iovs.05-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lois N, Halfyard AS, Bird AC, Fitzke FW. Quantitative evaluation of fundus autofluorescence imaged “in vivo” in eyes with retinal disease. Br J Ophthalmol. 2000;84(7):741–745. doi: 10.1136/bjo.84.7.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lois N, Halfyard AS, Bird AC, Holder GE, Fitzke FW. Fundus Autofluorescence in Stargardt Macular Dystrophy–Fundus Flavimaculatus. Am J Ophthalmol. 2004;138(1):55–63. doi: 10.1016/j.ajo.2004.02.056. [DOI] [PubMed] [Google Scholar]
- 55.Burke T, Tsang SH, Greenberg J, et al. Absolute Autofluorescence (AbsAF) in Stargardt Disease (STGD). ARVO. 2010 Poster #: A447. [Google Scholar]
- 56.Hwang JC, Zernant J, Allikmets R, et al. Peripapillary atrophy in Stargardt disease. Retina. 2009;29(2):181–186. doi: 10.1097/IAE.0b013e31818a2c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voigt M, et al. Analysis of Retinal Flecks in Fundus Flavimaculatus Using High-Definition Spectral-Domain Optical Coherence Tomography. Am J Ophthalmol. 2010;150(3):330–337. doi: 10.1016/j.ajo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Ergun E, Hermann B, Wirtitsch M, et al. Assessment of central visual function in Stargardt's disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46(1):310–316. doi: 10.1167/iovs.04-0212. [DOI] [PubMed] [Google Scholar]
- 59.Pasadhika S, Fishman GA, Allikmets R, Stone EM. Peripapillary retinal nerve fiber layer thinning in patients with autosomal recessive cone-rod dystrophy. Am J Ophthalmol. 2009;148(2):260–265. doi: 10.1016/j.ajo.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiadens AA, Somervuo V, van den Born LI, et al. Progressive Loss of Cones in Achromatopsia. An Imaging Study using Spectral-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2010;51(11):5952–5957. doi: 10.1167/iovs.10-5680. [DOI] [PubMed] [Google Scholar]
- 61.Greenstein VC, Santos RA, Tsang SH, et al. Preferred retinal locus in macular disease: characteristics and clinical implications. Retina. 2008;28(9):1234–1240. doi: 10.1097/IAE.0b013e31817c1b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujii GY, de Juan E, Jr, Sunness J, et al. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109(9):1737–1744. doi: 10.1016/s0161-6420(02)01120-x. [DOI] [PubMed] [Google Scholar]
- 63.Messias A, Reinhard J, Velasco e Cruz AA, et al. Eccentric fixation in Stargardt's disease assessed by Tübingen perimetry. Invest Ophthalmol Vis Sci. 2007;48(12):5815–5822. doi: 10.1167/iovs.06-0367. [DOI] [PubMed] [Google Scholar]
- 64.Seiple W, Szlyk JP, Paliga J, Rabb MR. Perifoveal function in patients with North Carolina macular dystrophy: the importance of accounting for fixation locus. Invest Ophthalmol Vis Sci. 2006;47(4):1703–1709. doi: 10.1167/iovs.05-0659. [DOI] [PubMed] [Google Scholar]
- 65.Grover S, Fishman GA, Stone EM. Atypical Presentation of Pattern Dystrophy in Two Families with Peripherin/RDS Mutations. Ophthalmology. 2002;109(6):1110–1117. doi: 10.1016/s0161-6420(02)01029-1. [DOI] [PubMed] [Google Scholar]
- 66.Francis PJ, Schultz DW, Gregory AM, et al. Genetic and phenotypic heterogeneity in pattern dystrophy. Br J Ophthalmol. 2005;89(9):1115–1119. doi: 10.1136/bjo.2004.062695. [DOI] [PMC free article] [PubMed] [Google Scholar]