Abstract
Trauma-hemorrhage (T-H) causes hypoxia and organ dysfunction. Mitochondrial dysfunction is a major factor for cellular injury due to T-H. Aging also has been known to cause progressive mitochondrial dysfunction. In order to study the effect of aging on T-H-induced mitochondrial dysfunction, we recently developed a rodent mitochondrial genechip with probesets representing mitochondrial and nuclear genes contributing to mitochondrial structure and function. Using this chip we recently identified signature mitochondrial genes altered following T-H in 6 and 22 month old rats; augmented expression of the transcription factor c-myc was the most pronounced. Based on reports of c-myc-IL6 collaboration and c-myc-Sirt1 negative regulation, we further investigated the expression of these regulatory factors with respect to aging and injury. Rats of ages 6 and 22 months were subjected to T-H or sham operation and left ventricular tissues were tested for cytosolic cytochrome c, mtDNA content, Sirt1 and mitochondrial biogenesis factors Foxo1, Ppara and Nrf-1. We observed increased cardiac cytosolic cytochrome c (sham vs T-H, p<0.03), decreased mitochondrial DNA content (sham vs T-H, p<0.05), and decreased Sirt1 expression (sham vs TH, p<0.05) following T-H and with progressing age. Additionally, expression of mitochondrial biogenesis regulating transcription factors Foxo1 and Nrf-1 were also decreased with T-H and aging. Based upon these observations we conclude that Sirt1 expression is negatively modulated by T-H causing downregulation of mitochondrial biogenesis. Thus, induction of Sirt1 is likely to produce salutary effects following T-H induced injury and hence, Sirt1 may be a potential molecular target for translational research in injury resolution.
Keywords: hemorrhage, trauma, aging, blood loss, trauma-hemorrhage, cardiac function, senescence
1. INTRODUCTION
Aging is associated with increased cellular senescence and decreased mitochondrial function [1,2]. Methods to improve mitochondrial function have demonstrated to prolong longevity and cell viability [3,4]. Aging has also been demonstrated to worsen outcome following injury [5–7]. However the mechanisms by which aging adversely affect injury outcome are not fully elucidated. In the injury model, trauma-hemorrhage (T-H), we have recently demonstrated increased endoplasmic reticulum stress, decreased left ventricular performance and decreased mitochondrial function [8–10]. Hemorrhagic shock causes a whole body hypoxia/reoxygenation injury, leading to dysregulation of biochemical pathways and multiple organ dysfunction syndrome [11–17]. A significant decrease in cardiac output, cardiac contractility and stroke volume is observed following T-H [18–22]. According to published reports, though onset of several age-associated diseases increases with aging, injury remains one of the top ten leading causes of death in all age groups [23]. After acute life-threatening injury, such as severe hemorrhage, patients are at risk for systemic inflammatory response syndrome [24] and also, age has been found to be an independent risk factor for mortality following sepsis [25]. Additionally, in spite of the advancements in therapy and care there is approximately 50% mortality following septic shock [26,27]. The molecular mechanisms underlying organ dysfunction and death are still unclear and studies towards identifying alterations in molecular pathways and regulation of energetics following injury are expected to address this problem.
Mitochondria are the main source of ATP in the cell and mitochondrial function is known to decline with aging and injury [28–32]. Among the molecular consequences of mitochondrial dysfunction is increased production of reactive oxygen species (ROS) and ROS in turn cause more mitochondrial damage triggering a vicious cycle. It is suggested that age-associated decline in mitochondrial function might play an important role in cell aging, particularly in mitochondria-rich cells such as heart muscle, and cumulative damage to mtDNA due to ROS might be involved in cellular aging process [6,7,31–34]. Therefore determining the mechanism of mitochondrial damage following injury and development of methods to improve mitochondrial function are of translational significance.
Our previous results showed that the left ventricular performance of the aged rats (22 month old) was lower than the younger rats (6 month old) [10]. Interestingly, we observed a decreased propensity for aged animals to alter expression of individual genes and an overall decrease in the total number of genes altered following T-H injury. The comprehensive mitochondrial gene expression profile using our mitochondrial gene chip, RoMitoChip, demonstrated a decreased expression of genes such as Pgc-1α, relating to mitochondrial function following T-H [10]. Others have also demonstrated that specific activation of Ppara, another mitochondrial biogenesis factor, by an agonist can improve liver function following T-H [35]. Interestingly, in both younger (6 month) and the aged (22 month) rats, the most upregulated gene was c-myc, a proto oncogene and pleotropic transcription factor. The enhancement of c-myc following T-H was suggestive of its role in promoting glycolytic process in injury. Furthermore, c-myc activates p53 promoting apoptosis. The increased expression of c-myc is also important in T cell activation and proinflammatory mediators, as it directs a complex inflammatory program [36,37].
Recently it was reported that Sirt1 (silent mating type information regulation 2 homolog 1) may negatively modulate c-Myc [38]. The increased expression of c-myc following T-H irrespective of the age of the animals, and on the established role of Sirt1 in regulating mitochondrial function, prompted us to further investigate the alteration of Sirt1 following T-H injury. In this manuscript, for the first time, we report the relationship between Sirt1 expression and T-H which might possibly help us identify additional molecular targets in developing therapeutic strategies for preventing cardiovascular functional deterioration following T-H.
2. MATERIALS AND METHODS
2.1. Animals
6-month and 22-month old Fisher 344 rats were obtained through the National Institute of Aging, Bethesda, MD. All animal experiments were carried out in accordance with the protocol approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and were consistent with the guidelines of the National Institutes of Health.
2.2. Trauma-Hemorrhage Procedure
This procedure was performed as described earlier [12]. Rats were fasted overnight but allowed water ad libitum. Before surgery the rats were anesthetized with isoflurane (Minrad, Bethlehem, PA) and restrained in a supine position. A 5-cm midline laparotomy was performed and closed asceptically in 2 layers with sutures (Ethilon 6/0, Ethicon, Somerville, NJ). Both femoral arteries and the right femoral vein were asceptically cannulated with polyethylene-10 tubing (Becton Dickinson, Sparks, MD). By attaching one of the catheters to a blood pressure analyzer (Digi-Med BPA-190, Micro-Med Inc., Louisville, KY) the blood pressure was continuously monitored. Upon awakening, animals were bled rapidly through the other arterial catheter to a mean arterial blood pressure of 35 ± 5 mm Hg within 10 minutes. The animals were continued to bleed slowly maintaining the same low arterial pressure until 50% blood volume was removed in approximately 45 minutes. The animals were maintained in shock stage by keeping the blood pressure low for another 45 minutes. During this time 40% of the shed blood volume was given as Ringer’s lactate in small volumes. Following this, the rats were resuscitated with four times the shed blood volume in the form of lactated Ringer's solution over 60 min. The same surgical procedures were conducted on sham-operated animals, but they did not undergo hemorrhage or resuscitation. Two hours following resuscitation, the animals euthanized and left ventricles removed.
2.3. Real-time RT-PCR
Real time RT-PCR was carried out using FAM-labeled Taqmann real time PCR primers for Nrf1, Ppara, FoxO1 and β-actin (ABI, Foster City, CA). The template cDNA was prepared by random priming from RNA isolated from the tissues. The results were expressed in relation to β-actin expression. The PCR reaction was carried out in an ABI 7500 thermal cycler (ABI, Foster City, CA).
2.4 Mitochondrial DNA content assessment
Mitochondrial DNA content was assessed by SYBR green PCR using custom primers. Primers for mtCOI and nuclear β-actin were as described in [39] and were used to amplify mtDNA and nuclear β-actin DNA from total DNA isolated from the ventricular tissue of rat heart.
2.5. Western Blot
Protein expression of Sirt1 or cytochrome c was analyzed by Western Blot as described [8]. Briefly, total proteins in tissue lysates were resolved using 4–12% Nupage gel (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes. The membranes were saturated with blocking buffer (10 mM Tris, 150 mM NaCl, and 0.05% Tween-20 supplemented with 5% dry milk) for 1 h at room temperature and incubated with the respective primary antibodies: Sirt1 (Santa Cruz Biotechnology, Santa Cruz, CA), cytochrome c (Cell Signaling, Carlsbad, CA) or β-actin (Abcam, Inc, Cambridge, MA). The membranes were then washed five times with TBST (Tris-buffered saline supplemented with 0.05% Tween-20) followed by incubation with appropriate secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) conjugated with horseradish peroxidase for 1 h at room temperature. The membranes were again washed five times with TBST and probed using ECL (Amersham, Piscataway, NJ), and autoradiographed. Cytochrome c was tested using cytosol separated from mitochondria in left ventricular tissues using a tissue mitochondrial isolation kit (Thermo Scientific, Chicago, IL).
2.6. Statistics
Statistical analyses were carried out by non-parametric Mann-Whitney t-test. Graphpad prism software (La Jolla, CA) was used to calculate statistical significance. Observed p values are mentioned in the figure legends with appropriate references on respective figures.
3. RESULTS
3.1. Mitochondrial functional alteration with aging and hemorrhage injury
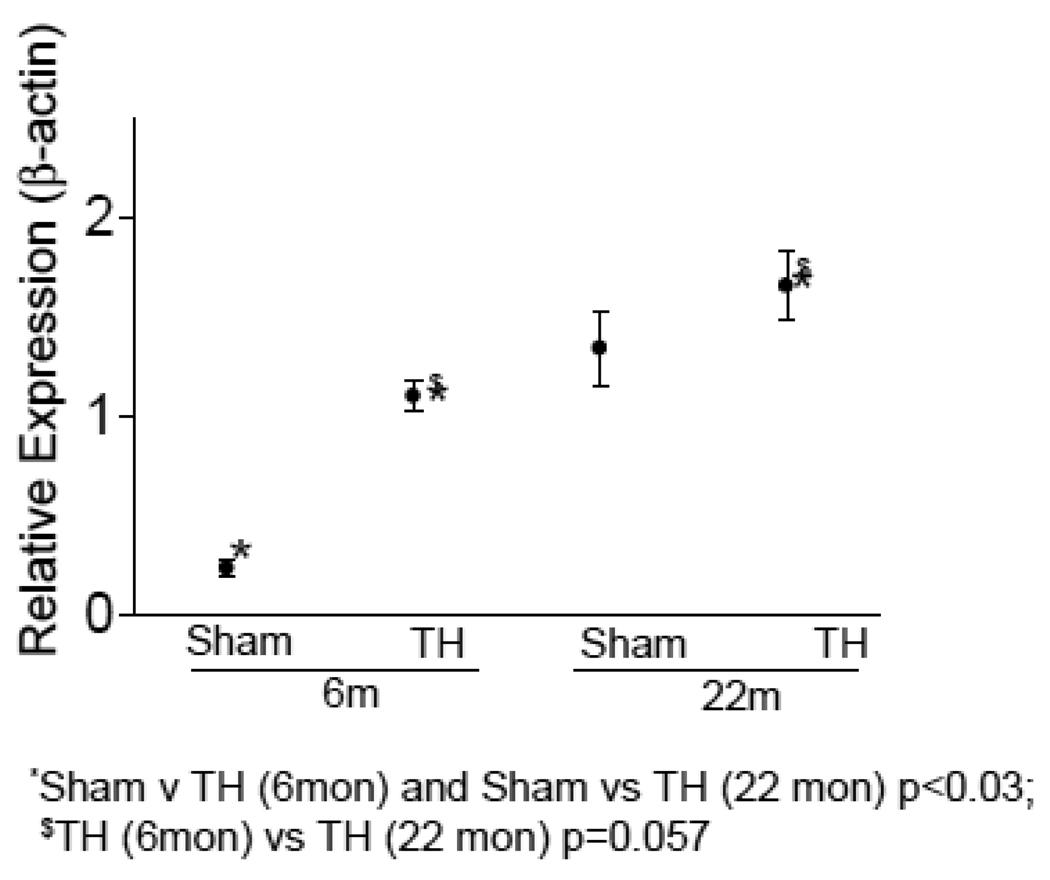
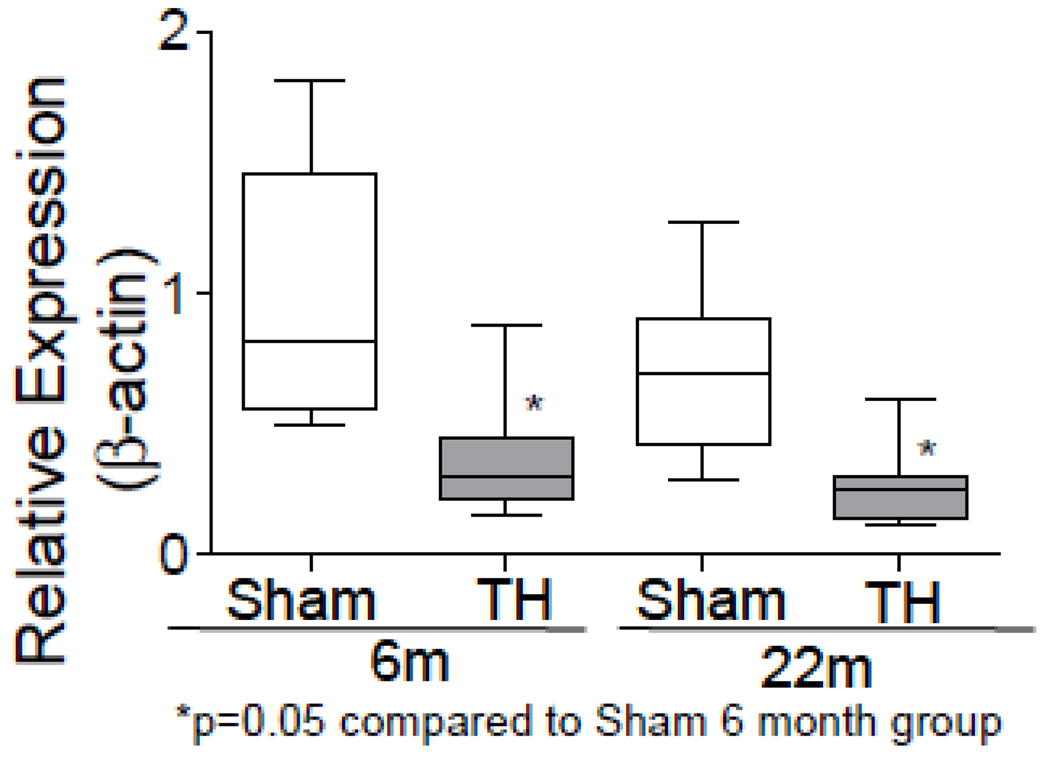
Using our custom mitochondrial gene chip, we studied the gene expression profile of mitochondrial genes in 6 and 22 month old rats subjected to sham or hemorrhage procedure [10]. We observed significant changes in the expression of a total of 142 genes in 6 month old rats and 66 in the 22 month old rats. Among these 36 genes were changed in both age groups. Consistent with the alteration of mitochondrial gene expression, we found a significant increase of cytochrome c protein in the cytosol (Fig. 1) of rats subjected to T-H. As seen in the figure, T-H caused marked increase in cytosolic cytochrome c in both age groups and when the cytochrome c release was compared between 6 month and 22 month-old groups, there was a significantly increased level of cytochrome c in the aged group. Mitochondrial DNA content was assessed by real time PCR using primers specific for mitochondrial COI and nuclear β-actin, and a decline in the mt DNA content was observed with age (Fig. 2).
Fig. 1. Cytochrome c release from mitochondria.
Cytosolic cytochrome c was quantified in the left ventricles of 6 and 22 month old rats subjected to sham or T-H operation. Western blot was carried out using cytosolic fractions of the tissue and quantified in relation to the signal intensity of β-actin. For details see Methods section. Values were normalized to sham levels and represented as mean±SEM.
Fig.2. Mitochondrial DNA following T-H and in relation to age.
Total DNA was isolated from left ventricles of 6 and 22 month old rats. Mitochondrial COI and nuclear β-actin were amplified by real-time PCR using SYBR green. Values expressed in relation to nuclear β-actin DNA were further normalized to sham levels and represented as box and whisker plot.
3.2. Increased cardiac IL-6 with aging and hemorrhage
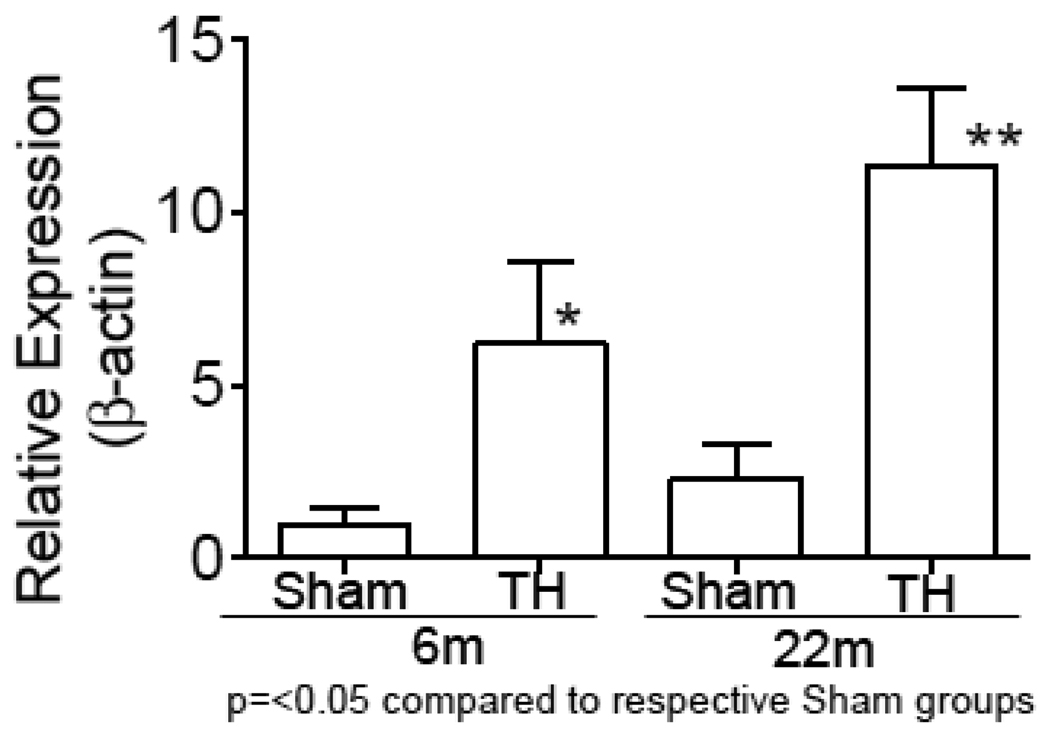
mRNA expression level of IL-6 was tested in the left ventricular tissue of rats at 6 and 22 months of age subjected sham or T-H procedure and as shown in Fig. 3, there was a profound increase in the cardiac IL-6 level after T-H and this was further augmented by the age factor.
Fig.3. Elevated IL-6 following T-H and in relation to age.
IL-6 mRNA was PCR amplified by realtime RT-PCR using Taqman primers for IL-6 and β-actin. Values were normalized to β-actin and represented as Mean±SEM.
3.3. Sirt-1 expression changes with aging and hemorrhage injury
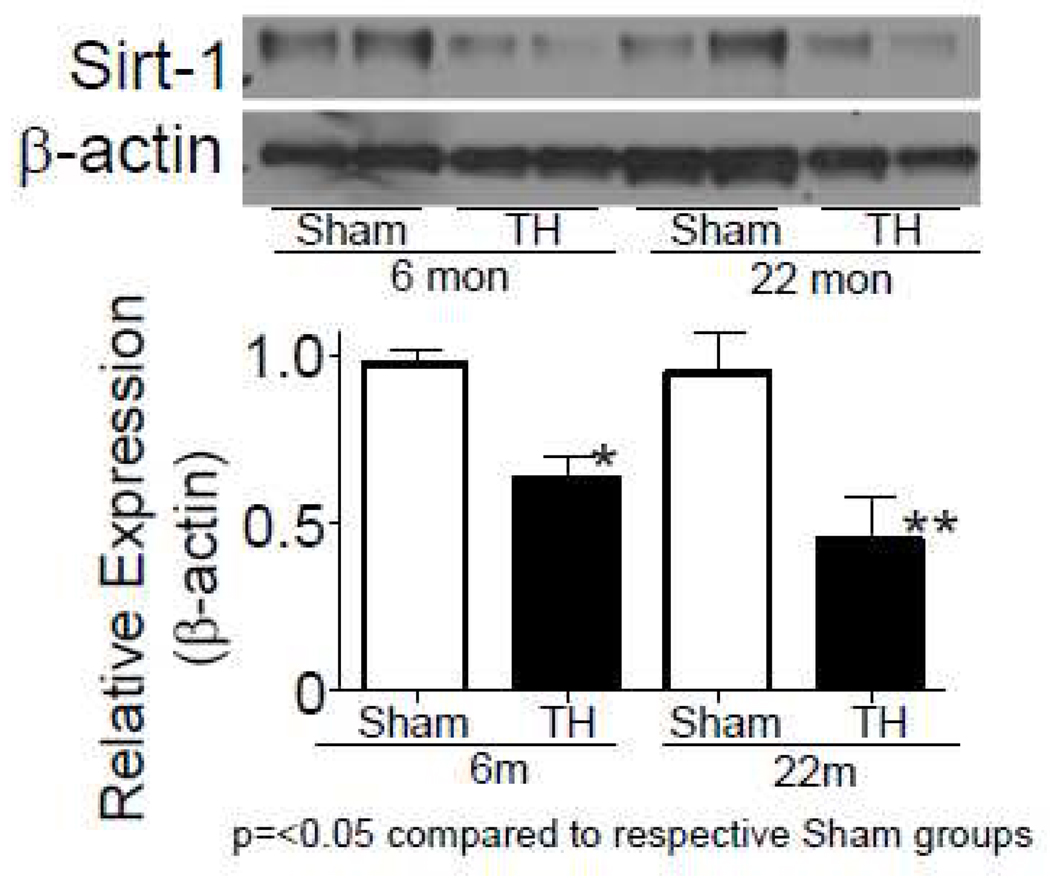
Sirt1 expression with aging and injury was investigated because our previous finding [10] and the data described in this report point towards mitochondrial functional alteration due to aging and injury. This was further prompted by the hypothesis that there could be a myc-Sirt1 negative regulation. Sirt1 protein expression was measured by Western blot and we found a significantly decreased expression following T-H both in 6 and 22 month old rats (Fig. 4).
Fig.4. Sirt1 expression is altered following T-H.
Sirt1 levels were tested by Western blot and the bands were quantified by densitometry. Values expressed in relation to β-actin were further normalized to sham levels and represented as Mean±SEM.
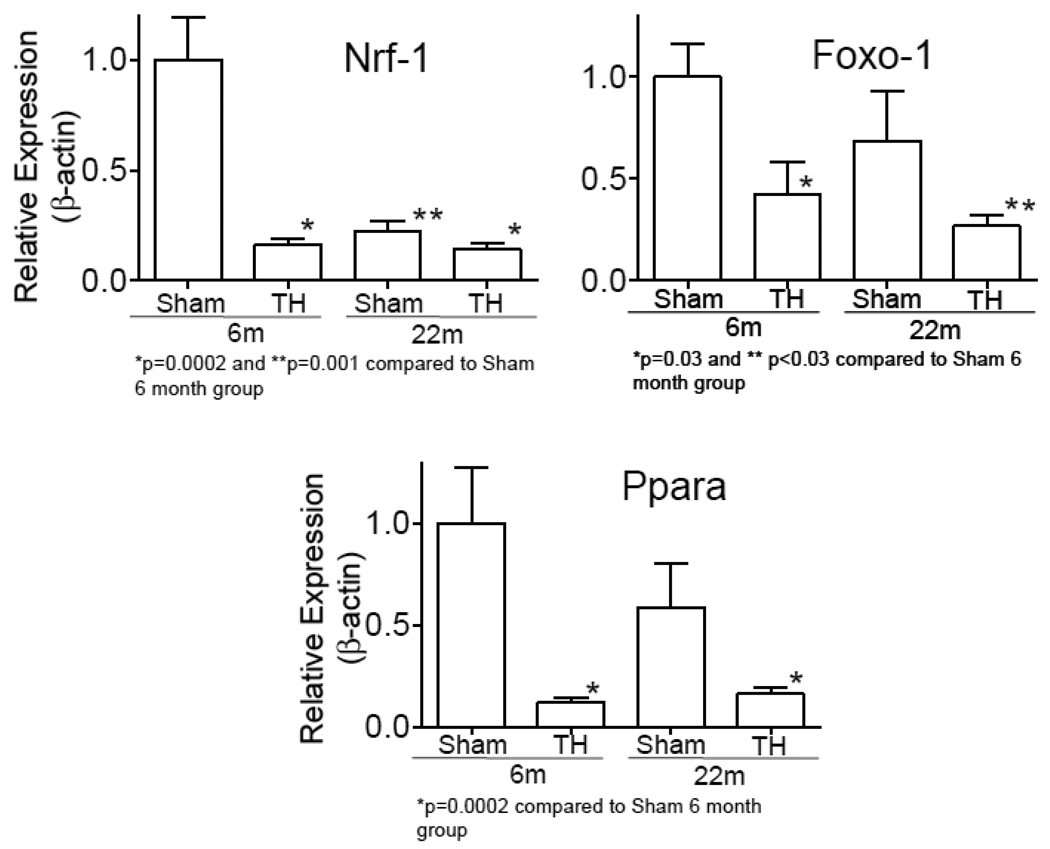
3.4. Expression of mitochondrial biogenesis genes with aging and hemorrhage injury
Real-time RT-PCR confirmed a significant change in the expression of Ppara in six and 22 month old animals following T-H. The expression of Ppara in 22 month-old sham animals also was lower as compared to the expression at 6 months sham group (Fig. 5). This was further followed up by determining the expression of Nrf-1 and Foxo-1, transcription factors downstream to Ppara, and found that the expression of these genes were also significantly declined following T-H. Though the decrease following T-H at 22 month was not significant as compared to their expression levels at 22 months, the expression following T-H in the aged group was significantly lower as compared to 6 month sham.
Fig.5. Expression of mitochondrial biogenesis transcription factors following T-H and in relation to age.
Nrf-1, FoxO1 and Ppara mRNA were PCR amplified by realtime RT-PCR using Taqman primers. Values were normalized to β-actin and represented as Mean±SEM.
4. DISCUSSION
Our recent mitochondrial gene expression studies have demonstrated the alteration of several critical genes involved in mitochondrial structure and function after T-H in young and aged rats [10]. We have found a decreased number of genes to be altered following T-H at 22 months as compared to at 6 months and we speculated that this lack of response of the rat to hemorrhagic injury could be due to cellular senescence [10]. We have now confirmed the decline in mitochondrial function not only with age, but also following T-H, as indicated by a significant increase in the level of cytosolic cytochrome c (Fig. 1). Cytochrome c, a component of the electron transport chain found loosely bound to the inner mitochondrial membrane is released from the mitochondria following pro-apoptotic signals. Our previous reports have demonstrated that T-H leads to apoptosis of cells in several organs [8,20,40]. The increased age has not only led to elevated mitochondrial dysfunction, but has also significantly reduced overall mitochondrial content of left ventricular cells, which will also markedly influence cardiac energetics (Fig 2). These findings are consistent with the report of progressive decline in muscle mitochondrial DNA abundance and protein synthesis with age [41,42]. However, surprisingly, we also observed a marked decrease in the mitochondrial DNA copy number following T-H in both age groups (Fig 2). As the tissue was harvested only two hours following hemorrhage and resuscitation, such rapid deterioration in mitochondria was not expected.
One of the genes that demonstrated the most upregulation following T-H at both 6 and 22 months, in our gene expression profiling studies, was the pleotropic transcription factor c-myc [10]. An inflammatory response following T-H was previously confirmed and it is not known whether c-myc plays a direct role in this effect. Nevertheless, a c-myc-IL-6 collaboration has been postulated in conditions such as multiple myeloma, where an IL-6 dependency was observed for c-myc induced plasma cell neoplasia [43]. Our experiments as shown (Fig. 3) also demonstrate a significantly elevated IL-6 in the heart following T-H. Though this is similar to the previous finding by our group [44], we now find that the aged animals express significantly more IL-6 in the heart than 6 month old ones, following T-H.
Our previous report indicated c-myc upregulation following T-H injury, but the nature of IL-6 and c-myc collaboration remains unknown. Additionally, c-myc has been shown to be negatively regulated by Sirt1 [38]. Sirt1 or sirtuin (silent mating type information regulation 2 homolog) 1, is a member of the sirtuin family of proteins, and deacetylates histones and several other proteins involved in regulation of cellular processes. A decreased Sirt1 activity has been directly correlated with aging and mitochondrial function. Methods such as caloric restriction were found to be efficient in promoting mitochondrial function and longevity. Several reports demonstrate that the beneficial effect of caloric restriction or action of polyphenols in improving mitochondrial function may be mediated by augmenting the activity or elevating the expression level of a histone deacetelyse enzyme, Sirt1 [45–47]. The activity of Sirt1 has also been shown to be inversely related to inflammation [48]. As seen in Fig 1, there is a significant decline in mitochondrial function following T-H injury and the age factor caused a further decline in mitochondrial function as evidenced by the increased level of cytoplasmic cytochrome c. This was further confirmed by a markedly declining activity of complex I enzyme with aging and injury (data not shown). Based upon the report that there is a negative regulation of c-myc expression by Sirt1 [38], we investigated whether there was a change in Sirt1 expression following T-H and our results clearly indicate a significant down regulation of Sirt-1 expression following T-H (Fig. 4). This is the first report that implicates a possible role for Sirt1 in hemorrhage mediated mitochondrial dysfunction. This may be consistent with the hypothesis that c-myc is negatively regulated by Sirt1. Following T-H, c-myc was significantly increased in both age groups (6.3 and 3.8-fold), though more at 6 months [10]. In pathological stress c-myc regulates the increased metabolic energy demand by promoting glycolysis, rather than mitochondrial biogenesis, [49]. HIF, the transcript of which was also significantly increased in both age groups [10], and which is a hypoxic sensor [50,51], specifically blocks access of glycolytic end products to mitochondria [9,36,37]. It is also suggested that c-Myc promotes cell cycle and simultaneously primes activation of the Bcl-2 family controlled mitochondria apoptosis pathway [52].
Our results also show decreased levels of Nrf-1 and Foxo1, transcription factors that can positively modulate mitochondrial function (Fig. 5). Foxo1-mediated transcription of antioxidant genes such as MnSOD genes are facilitated by activation of Foxo1 through deacetylation by Sirt1. It has been reported that Foxo1 plays an essential role in mediating Sirt1-induced upregulation of MnSOD gene in cardiomyocytes [53]. This was further confirmed by the observed upregulation of cardioprotective molecules such as MnSOD and Bcl-xL in the heart of mice transgenically expressing Sirt1 and subjected to ischemia/reperfusion [53]. A similar down modulation was observed for the transcription factor, nuclear respiratory factor-1 (Nrf-1), which is a target of Pgc-1α and a direct modulator of mitochondrial biogenesis. Our previous studies have clearly demonstrated a decreased mRNA and protein expression of Pgc-1α in 6-month old rats following T-H [10]. Pgc-1α activates both Ppara and Nrf-1 to promote mitochondrial biogenesis; and as Pgc-1α activation depends on deacetylation by Sirt1, the declined Sirt1 expression following T-H will have direct effect on Pgc1-α acitivity and mitochondrial biogenesis.
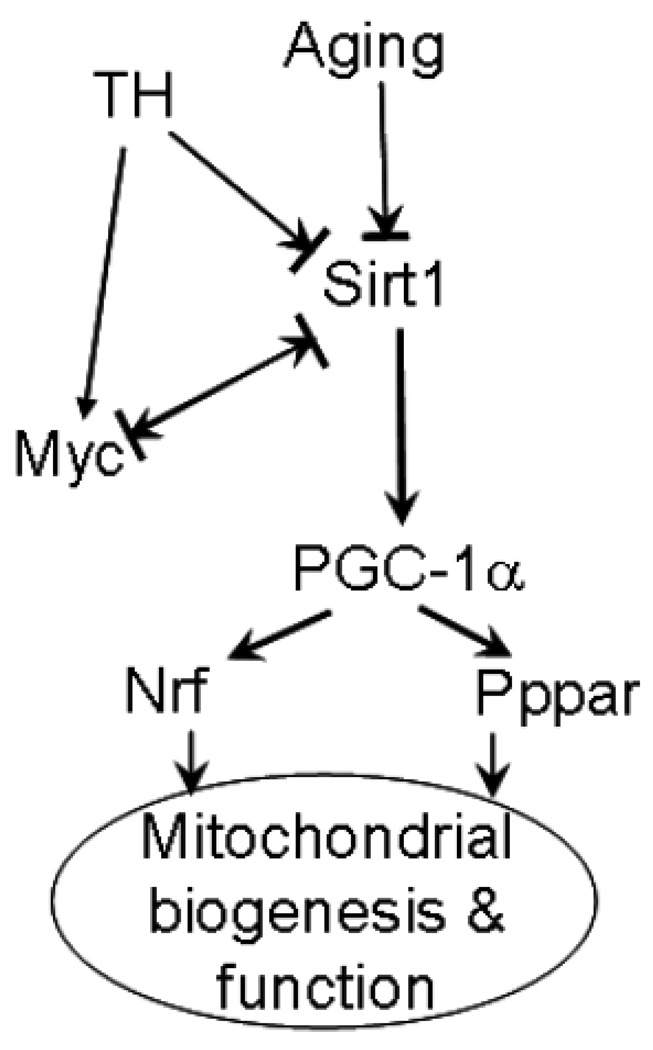
Following hemorrhage, we observed an increase in c-Myc and decrease in Sirt1, Pgc-1α, Foxo1 and Nrf-1 with a concomitant decrease in mitochondrial function. The observed changes in these factors allow us to speculate a role for c-myc-Sirt1 negative regulation in mitochondrial dysfunction following aging and T-H (Fig 6). The Sirt1-regulated mitochondrial function may also be relevant in other health and disease conditions such as myocardial ischemia/reperfusion syndrome. Considering that older patients with less severe hemorrhage have an increased risk of developing myocardial infarction and that in developed countries with aging populations, the prevalence of heart failure is increasing [54,55], identification of novel pathways such as Sirt1-modulated mitochondial regulation may have translational potential in injury management. The individual roles of c-myc and Sirt1 or their mutual regulation in T-H induced mitochondrial dysfunction and consequent cellular injury may be identified by using specific small molecule inhibitors or activators of these key proteins. Such small molecule modulators may also have therapeutic significance in injury resolution.
Fig 6. Sirt-Myc regulation following aging and injury.
A possible pathway of mitochondrial biogenesis regulation in aging and hemorrhage.
Acknowledgements
The study was supported by NIH grants AG 031440(RR), GM 39519 (IC), and UAB HSF GEF Scholar Award (RR). The microarray experiments were carried out in the Heflin Center for Genomic Science by Dr. Michael Crowley and supported by the UAB Comprehensive Cancer Center Core Grant 5P30 CA13148-37.
Abbreviations
- T-H
Trauma-hemorrhage
- Ppar
peroxisome proliferator activated receptor
- Pgc
peroxisome proliferative activated receptor, gamma, coactivator
- Sirt
silent mating type information regulation 2 homolog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hipkiss AR. Aging, Proteotoxicity, Mitochondria, Glycation, NAD and Carnosine: Possible Inter-Relationships and Resolution of the Oxygen Paradox. Front Aging Neurosci. 2010;2:10. doi: 10.3389/fnagi.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linford NJ, Pletcher SD. Aging: fruit flies break the chain to a longer life. Curr. Biol. 2009;19:R895–R898. doi: 10.1016/j.cub.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Muller WE, Eckert A, Kurz C, Eckert GP, Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease--therapeutic aspects. Mol. Neurobiol. 2010;41:159. doi: 10.1007/s12035-010-8141-5. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock. 2004;22:364. doi: 10.1097/01.shk.0000142552.77473.7d. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37:1018. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Bridenbaugh EA, Akintola AD, Catania JM, Vaidya VS, Bonventre JV, Dearman AC, Sampson HW, Zawieja DC, Burghardt RC, Parrish AR. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am. J. Physiol Renal Physiol. 2007;293:F1272–F1281. doi: 10.1152/ajprenal.00138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jian B, Hsieh CH, Chen J, Choudhry M, Bland K, Chaudry I, Raju R. Activation of endoplasmic reticulum stress response following trauma-hemorrhage. Biochim Biophys Acta. 2008;1782:621. doi: 10.1016/j.bbadis.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jian B, Wang D, Chen D, Voss J, Chaudry I, Raju R. Hypoxia induced alteration of mitochondrial genes in cardiomyocytes- role of Bnip3 and Pdk1. Shock. 2010;34:169. doi: 10.1097/SHK.0b013e3181cffe7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian B, Yang S, Chen D, Zou L, Chatham JC, Chaudry I, Raju R. Aging influences cardiac mitochondrial gene expression and cardiovascular function following hemorrhage injury. Molecular Medicine. 2010 doi: 10.2119/molmed.2010.00195. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayala A, Ertel W, Chaudry IH. Trauma-induced suppression of antigen presentation and expression of major histocompatibility class II antigen complex in leukocytes. Shock. 1996;5:79. doi: 10.1097/00024382-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ba ZF, Wang P, Koo DJ, Cioffi WG, Bland KI, Chaudry IH. Alterations in tissue oxygen consumption and extraction after trauma and hemorrhagic shock. Crit Care Med. 2000;28:2837. doi: 10.1097/00003246-200008000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann. Surg. 2007;245:635. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Raju R, Bland KI, Chaudry IH. Estrogen: a novel therapeutic adjunct for the treatment of trauma-hemorrhage-induced immunological alterations. Mol. Med. 2008;14:213. doi: 10.2119/2008-00001.Raju. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rushing GD, Britt LD. Reperfusion injury after hemorrhage: a collective review. Ann. Surg. 2008;247:929. doi: 10.1097/SLA.0b013e31816757f7. [DOI] [PubMed] [Google Scholar]
- 17.Childs EW, Tharakan B, Hunter FA, Isong M, Liggins ND. Mitochondrial complex III is involved in proapoptotic BAK-induced microvascular endothelial cell hyperpermeability. Shock. 2008;29:636. doi: 10.1097/SHK.0b013e318157f524. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann. Surg. 2000;232:673. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarlagadda S, Rajendran P, Miss JC, Banki NM, Kopelnik A, Wu AH, Ko N, Gelb AW, Lawton MT, Smith WS, Young WL, Zaroff JG. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit. Care. 2006;5:102. doi: 10.1385/NCC:5:2:102. [DOI] [PubMed] [Google Scholar]
- 20.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann. Surg. 2007;245:971. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayeed MM, Zhu M, Maitra SR. Alterations in cellular calcium and magnesium during circulatory/septic shock. Magnesium. 1989;8:179. [PubMed] [Google Scholar]
- 22.Johnson RA, Durante W, Craig T, Peyton KJ, Myers JG, Stewart RM, Johnson FK. Vascular arginase contributes to arteriolar endothelial dysfunction in a rat model of hemorrhagic shock. J. Trauma. 2010;69:384. doi: 10.1097/TA.0b013e3181e771a3. [DOI] [PubMed] [Google Scholar]
- 23.CDC. Injury Prevention & Control: Data & Statistics. 2007 [Google Scholar]
- 24.NeSmith EG, Weinrich SP, Andrews JO, Medeiros RS, Hawkins ML, Weinrich M. Systemic inflammatory response syndrome score and race as predictors of length of stay in the intensive care unit. Am. J. Crit Care. 2009;18:339. doi: 10.4037/ajcc2009267. [DOI] [PubMed] [Google Scholar]
- 25.Nasa P, Juneja D, Singh O, Dang R, Arora V. Severe Sepsis and its Impact on Outcome in Elderly and Very Elderly Patients Admitted in Intensive Care Unit. J. Intensive Care Med. 2011 doi: 10.1177/0885066610397116. [DOI] [PubMed] [Google Scholar]
- 26.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulme R, Lepage E, Le GR. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 27.Lukaszewicz AC, Payen D. The future is predetermined in severe sepsis, so what are the implications? Crit Care Med. 2010;38:S512–S517. doi: 10.1097/CCM.0b013e3181f23dc4. [DOI] [PubMed] [Google Scholar]
- 28.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 29.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell Sci. 2010;123:2533. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gucek M, Murphy E. What can we learn about cardioprotection from the cardiac mitochondrial proteome? Cardiovasc. Res. 2010;88:211. doi: 10.1093/cvr/cvq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Zhu J, Glass PS, Brink PR, Rampil IJ, Rebecchi MJ. Age-associated changes in cardiac gene expression after preconditioning. Anesthesiology. 2009;111:1052. doi: 10.1097/ALN.0b013e3181bbcb2a. [DOI] [PubMed] [Google Scholar]
- 32.Zingarelli B, Hake PW, O'Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor gamma. Crit Care Med. 2009;37:1978. doi: 10.1097/CCM.0b013e31819feb4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasawa M, Hayakawa M, Sugiyama S, Hattori K, Ito T, Ozawa T. Age-associated damage in mitochondrial function in rat hearts. Exp. Gerontol. 1993;28:269. doi: 10.1016/0531-5565(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 34.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med. 2008;36:2174. doi: 10.1097/CCM.0b013e31817d1a92. [DOI] [PubMed] [Google Scholar]
- 35.Zingarelli B, Chima R, O'Connor M, Piraino G, Denenberg A, Hake PW. Liver apoptosis is age-dependent and is reduced by activation of peroxisome proliferator activated receptor-{gamma} in hemorrhagic shock. Am. J. Physiol Gastrointest. Liver Physiol. 2009 doi: 10.1152/ajpgi.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grumont R, Lock P, Mollinari M, Shannon FM, Moore A, Gerondakis S. The mitogen-induced increase in T cell size involves PKC and NFAT activation of Rel/NF-kappaB-dependent c-myc expression. Immunity. 2004;21:19. doi: 10.1016/j.immuni.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Borrello MG, Degl'Innocenti D, Pierotti MA. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2008;267:262. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 38.Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J. Cell Biol. 2009;185:203. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Band M, Joel A, Hernandez A, Avivi A. Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J. 2009;23:2327. doi: 10.1096/fj.08-122978. [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki T, Choudhry MA, Suzuki T, Schwacha MG, Bland KI, Chaudry IH. 17beta-Estradiol's salutary effects on splenic dendritic cell functions following trauma-hemorrhage are mediated via estrogen receptor-alpha. Mol. Immunol. 2008;45:376. doi: 10.1016/j.molimm.2007.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459:277. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech. Ageing Dev. 2010;131:451. doi: 10.1016/j.mad.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutsch S, Neppalli VT, Shin DM, DuBois W, Morse HC, III, Goldschmidt H, Janz S. IL-6 and MYC collaborate in plasma cell tumor formation in mice. Blood. 2010;115:1746. doi: 10.1182/blood-2009-08-237941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW, III, Bland KI, Chaudry IH. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am. J. Physiol Heart Circ. Physiol. 2004;287:H2183–H2191. doi: 10.1152/ajpheart.00624.2003. [DOI] [PubMed] [Google Scholar]
- 45.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch. Biochem. Biophys. 2010 doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, Pallardo FV. Mitochondrial biogenesis in exercise and in ageing. Adv. Drug Deliv. Rev. 2009;61:1369. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol. Aspects Med. 2007;28:692. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9793. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J. Clin. Invest. 2005;115:547. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jian B, Nagineni CN, Meleth S, Grizzle W, Bland KI, Chaudry IH, Raju R. Anosmin-1 involved in neuronal migration is hypoxia inducible and cancer regulated. Cell Cycle. 2009;8:3770. doi: 10.4161/cc.8.22.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza GL. Regulation of vascularization by hypoxia-inducible factor 1. Ann. N. Y. Acad. Sci. 2009;1177:2. doi: 10.1111/j.1749-6632.2009.05032.x. [DOI] [PubMed] [Google Scholar]
- 52.Nieminen AI, Partanen JI, Klefstrom J. c-Myc blazing a trail of death: coupling of the mitochondrial and death receptor apoptosis pathways by c-Myc. Cell Cycle. 2007;6:2464. doi: 10.4161/cc.6.20.4917. [DOI] [PubMed] [Google Scholar]
- 53.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipschitz D. Medical and functional consequences of anemia in the elderly. J. Am. Geriatr. Soc. 2003;51:S10–S13. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- 55.Crisostomo PR, Wang M, Markel TA, Lahm T, Abarbanell AM, Herrmann JL, Meldrum DR. Stem cell mechanisms and paracrine effects: potential in cardiac surgery. Shock. 2007;28:375. doi: 10.1097/shk.0b013e318058a817. [DOI] [PubMed] [Google Scholar]