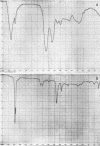
Abstract
Kawakami, Masaya (Gunma University, Maebashi, Japan), and Susumu Mitsuhashi. Experimental salmonellosis. IV. Lipid content of toxin L obtained from Salmonella enteritidis. J. Bacteriol. 89:193–197. 1965.—An improved method is described for the chromatographic purification of toxin L from virulent Salmonella enteritidis. Toxin L is heat-labile, and its toxicity is reduced by trypsin and by phospholipase A treatment. Chemical studies of chromatographically and ultracentrifugally homogeneous toxin preparations show that toxin L is a phosphorus-containing protein. The infrared-absorption spectrum of a petroleum ether extract of toxin L hydrolysate indicates that this material possesses a lipid component.
Full text
PDF
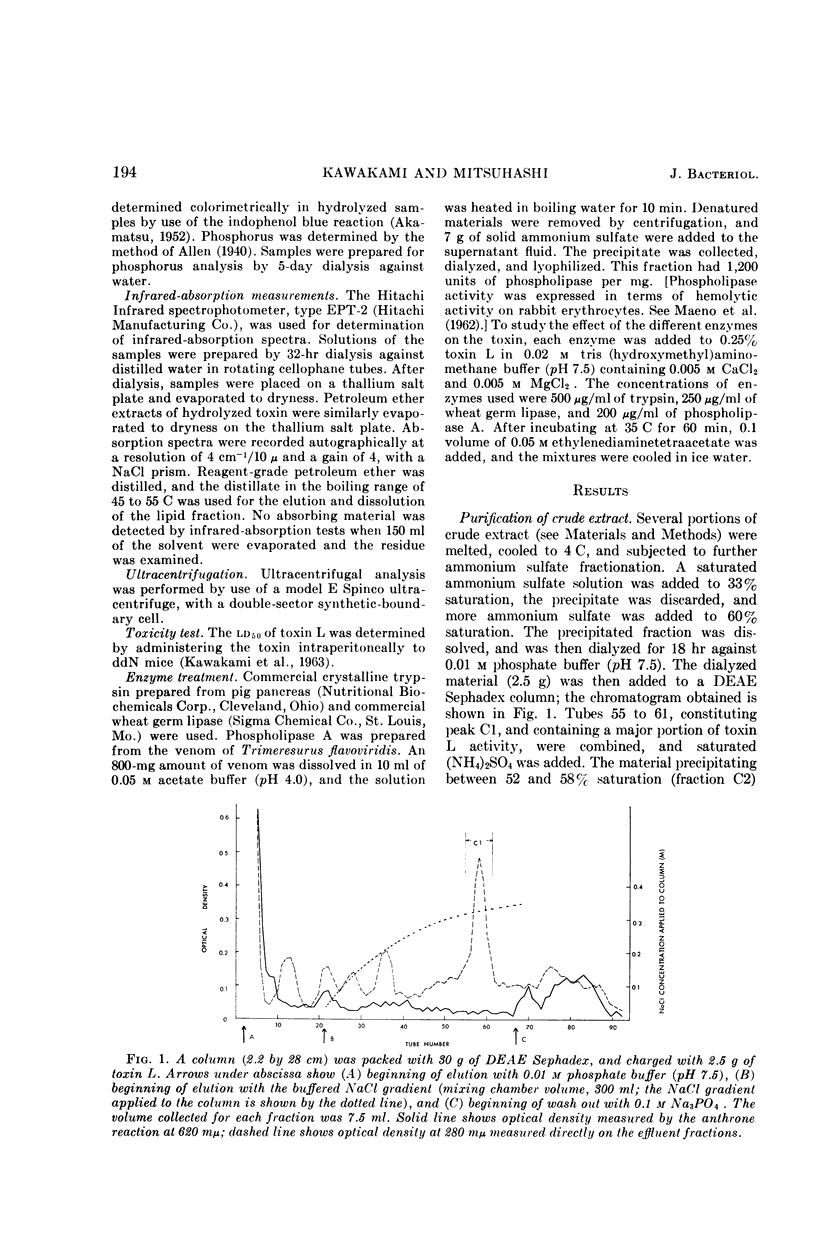
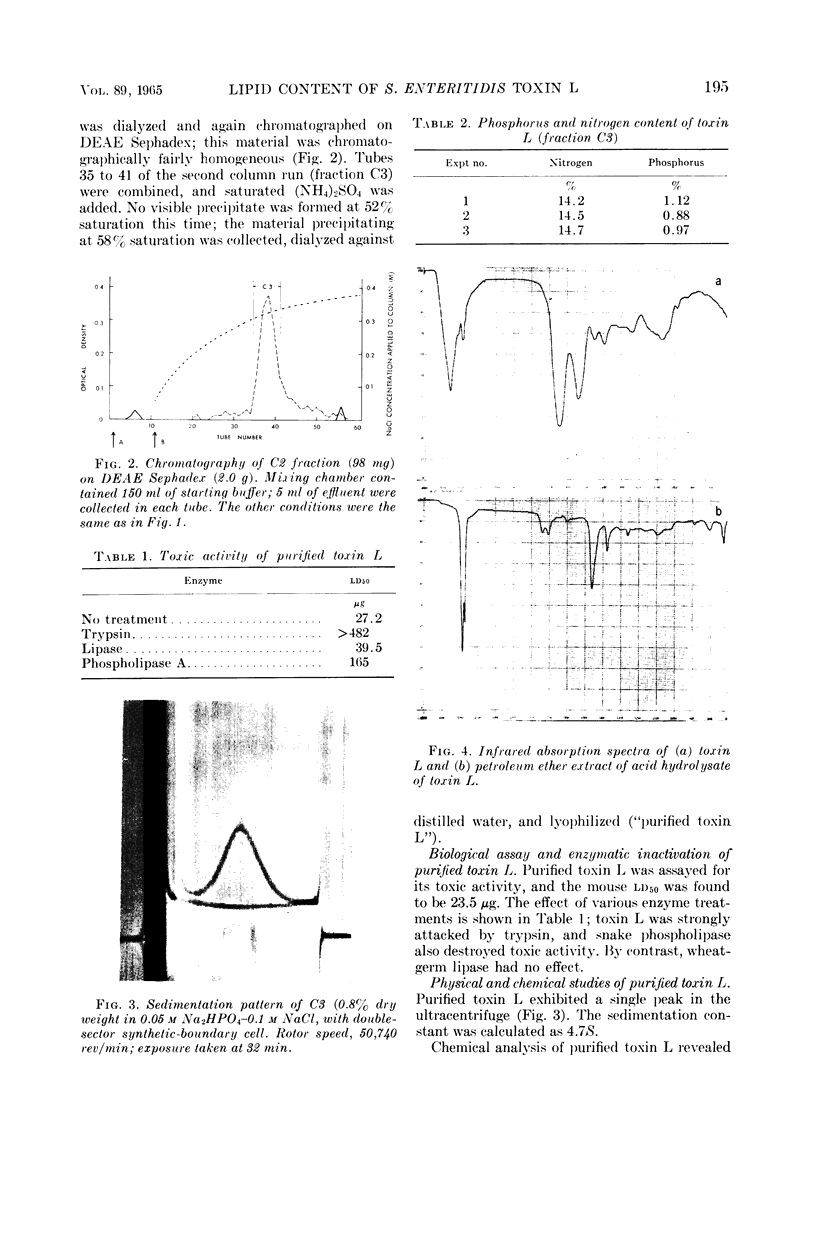


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLUFF L. E. Immunochemical study of a bacterial endotoxin: Shigella flexneri type Z. J Exp Med. 1954 Oct 1;100(4):391–404. doi: 10.1084/jem.100.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAKAMI M., OSAWA N., MITSUHASHI S. EXPERIMENTAL SALMONELLOSIS. III. NEW TOXIC FRACTION (L) OBTAINED FROM SALMONELLA ENTERITIDIS AND ITS IMMUNOLOGICAL PROPERTIES. J Bacteriol. 1963 Oct;86:872–879. doi: 10.1128/jb.86.4.872-879.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDERITZ O., WESTPHAL O., EICHENBEGER E., NETER E. Uber die Komplexbildung von bakteriellen Lipopolysacchariden mit Proteinen und Lipoiden. Biochem Z. 1958;330(1):21–33. [PubMed] [Google Scholar]
- MAENO H., MITSUHASHI S., OKONOGI T., HOSHI S., HOMMA M. Studies on Habu snake venom. (V). Myolysis caused by phospholipase A in Habu snake venom. Jpn J Exp Med. 1962 Feb;32:55–64. [PubMed] [Google Scholar]
- MESROBEANU L., MESROBEANU I., MITRICA N., CROITORESCO I., MARX A. STADE ACTUEL DES RECHERCHES SUR LES NEUROTOXINES DES GERMES GRAM-N'EGATIFS. Arch Roum Pathol Exp Microbiol. 1963 Sep;23:775–782. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- Morgan W. T., Partridge S. M. Studies in immunochemistry: The use of phenol and of alkali in the degradation of antigenic material isolated from Bact. dysenteriae (Shiga). Biochem J. 1941 Nov;35(10-11):1140–1163. doi: 10.1042/bj0351140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- TAL C., GOEBEL W. F. On the nature of the toxic component of the somatic antigen of Shigella paradysenteriae type Z (Flexner). J Exp Med. 1950 Jul 1;92(1):25–34. doi: 10.1084/jem.92.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]