Abstract
Microalbuminuria has been conclusively established as an independent cardiovascular risk factor, and there is evidence of an association between insulin resistance and microalbuminuria, the former preceding the latter in prospective studies. It has been demonstrated that even the slightest degree of metabolic acidosis produces insulin resistance in healthy humans. Many recent epidemiological studies link metabolic acidosis indicators with insulin resistance and systemic hypertension. The strongly acidogenic diet consumed in developed countries produces a lifetime acidotic state, exacerbated by excess body weight and aging, which may result in insulin resistance, metabolic syndrome, and type 2 diabetes, contributing to cardiovascular risk, along with genetic causes, lack of physical exercise, and other factors. Elevated fruits and vegetables consumption has been associated with lower diabetes incidence. Diseases featuring severe atheromatosis and elevated cardiovascular risk, such as diabetes mellitus and chronic kidney failure, are typically characterized by a chronic state of metabolic acidosis. Diabetic patients consume particularly acidogenic diets, and deficiency of insulin action generates ketone bodies, creating a baseline state of metabolic acidosisworsened by inadequate metabolic control, which creates a vicious circle by inducing insulin resistance. Even very slight levels of chronic kidney insufficiency are associated with increased cardiovascular risk, which may be explained at least in part by deficient acid excretory capacity of the kidney and consequent metabolic acidosis-induced insulin resistance.
Introduction
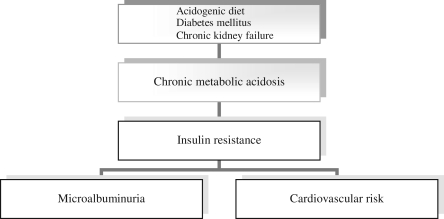
Cardiovascular diseases continue to be important mortality causes in Western-type societies despite aggressive dietary and pharmacologic therapy for hypercholesterolemia. Obesity and the metabolic syndrome are very frequently present in the industrialized world, and insulin resistance has been currently positioned at the hub of the cardiovascular risk. In healthy humans, metabolic acidosis induces insulin resistance, which in turn increases the cardiovascular risk and produces microalbuminuria. A life-span state of low-grade metabolic acidosis is usually present in normal subjects due to the modern acidogenic diet in Western civilization, and this situation may induce insulin resistance and increase the cardiovascular risk. Dietary acid load may be an important variable in predicting cardiovascular risk. Furthermore, conditions typically featuring a chronic state of metabolic acidosis, such as diabetes mellitus and chronic kidney failure, are associated with a chronic state of insulin resistance and very elevated cardiovascular risk. (Fig. 1).
FIG. 1.
Metabolic acidosis, microalbuminuria, and cardiovascular risk.
Microalbuminuria Is a Marker of Elevated Cardiovascular Risk
Microalbuminuria is currently defined as urinary albumin excretion of 30–300 mg/day (20–200 μg/min) or a urinary albumin-to-creatinine ratio in a spot urine sample of 30–300 mg/g (3.4–34 mg/mmol) in at least two of three early-morning urine samples. At present, a urinary albumin excretion rate below these limits is defined as normal, whereas values above them are referred to as proteinuria. The prevalence of microalbuminuria in U.S. and European general population surveys ranges from 6% to 10%. Similar prevalence figures have been reported in adolescents (8.9%) and patients with type 1 diabetes (9.7%). However, the prevalence of microalbuminuria is much higher in essential hypertension (35–40%) and type 2 diabetes (20–36%).1,2
In 1984, microalbuminuria was shown to be an independent cardiovascular risk factor in type 2 diabetes.3,4 In 1997, a systematic overview of the literature, including 11 European cohort studies, firmly established microalbuminuria as a strong predictor of cardiovascular morbidity and mortality in type 2 diabetes independently of conventional cardiovascular risk factors.2 In 1987, microalbuminuria was linked with cardiovascular events in type 1 diabetes, and the next year microalbuminuria was reported to be an independent cardiovascular risk factor in nondiabetic subjects.5–7 Since then, microalbuminuria has been consistently confirmed as an independent risk factor for cardiovascular morbidity and mortality in every population investigated.
The degree of albuminuria at which the risk for cardiovascular events is increased has been investigated in a number of studies, which have revealed that a very low albumin concentration in urine is a predictor of cardiovascular disease. In participants of the Heart Outcomes Prevention Evaluation study, microalbuminuria was detected in 32.6% of patients with diabetes and 14.8% of those without diabetes at baseline. Patients with a urinary albumin-to-creatinine ratio greater than 0.6 mg/mmol (5 mg/g) display an increased relative risk of cardiovascular events and death. For every 0.4 mg/mmol increase in the albumin-to-creatinine ratio level, the adjusted hazard of major cardiovascular events increased by approximately 6%.8 In nondiabetic hypertensive patients with left ventricular hypertrophy, participants of the Losartan Intervention for Endpoint Reduction study, death increased continuously as albuminuria increased, with no specific threshold for augmented risk.9 In participants of the Third Copenhagen City Heart Study, the risk for both coronary heart disease and death was significantly increased if the urinary albumin excretion rate was above 4.8 μg/min (approximately 6 mg/L or albumin-to-creatinine ratio of 0.7 mg/mmol).10 In a recent meta-analysis, a urinary albumin-to-creatinine ratio greater than 0.6 mg/mmol (5 mg/g) was independently associated with increased all-cause and cardiovascular mortality in the general population.11
Insulin Resistance Induces Microalbuminuria
Accumulating evidence during the last two decades discloses a definite relationship between insulin resistance and microalbuminuria. The first connection between the two conditions was probably reported in 1989, when decreased high-density lipoprotein cholesterol (HDL-c) and increased triglyceride blood concentration was found in albuminuric patients with type 1 diabetes.12,13 The next year, the association between these lipid abnormalities associated with insulin resistance and albuminuria was noted also in type 2 diabetes.14 A direct association between hyperinsulinemia (and impaired glucose tolerance) and microalbuminuria was observed for the first time in population-based surveys in nondiabetic individuals. Additionally, subjects with microalbuminuria had increased triglyceride and decreased HDL-c blood concentration relative to subjects without microalbuminuria.15
Since then, many cross-sectional analyses have found a close association between insulin resistance and microalbuminuria in different populations, including type 1 diabetes, type 2 diabetes, essential hypertension, and several groups of the general population, including elderly persons and children.16–20 Prospective studies determine that insulin resistance precedes the onset of microalbuminuria in type 1 diabetes, type 2 diabetes, and nondiabetic individuals.17,21–23 Associations between surrogates of insulin resistance and microalbuminuria have also been reported, strengthening the presence of a connection between the two conditions. Prospective studies reveal that waist circumference and the waist-to-hip ratio independently predict the subsequent development of microalbuminuria in type 1 diabetes and in nondiabetics participants of the Data from an Epidemiological Study on the Insulin Resistance syndrome (DESIR) cohort.24,25 A strong association between the metabolic syndrome and microalbuminuria has been found in participants of prospective studies, including the Copenhagen City Heart Study and the DESIR cohort.23,25 Urinary albumin excretion rate and waist-to-hip ratio are independently associated with adiponectin levels.26
Metabolic Acidosis Produces Insulin Resistance
The observation that glucose intolerance develops in healthy subjects following ammonium chloride–induced metabolic acidosis was first made in 1924. Fifty five years later, it was demonstrated that even a very mild degree of metabolic acidosis results in decreased sensitivity to insulin and subsequent impairment of glucose tolerance in healthy individuals.27 Other situations leading to metabolic acidosis, such as elevations of basal lactate in healthy persons, the presence of ketone bodies in diabetic subjects, and chronic kidney disease-related metabolic acidosis, are associated with the development of insulin resistance as well.28–31
Recent observational studies confirm an association between signs of metabolic acidosis (low serum bicarbonate, high serum anion gap, hypocitraturia, and low urine pH) and insulin resistance in different populations. Lower bicarbonate and higher anion gaps are independently associated with insulin resistance in cross-sectional analysis of participants in the 1999–2000 and 2001–2002 National Health and Nutrition Examination Surveys (NHANES).32 Insulin resistance estimated by the homeostasis model assessment (HOMA-IR) is negatively correlated with urinary citrate excretion in cross-sectional analysis of nondiabetic patients with calcium nephrolithiasis. Patients of the highest HOMA-IR tertile show lower urine citrate concentrations than patients of the lowest HOMA-IR tertile.33 A significant inverse relationship between 24-h urine pH and the degree of insulin resistance has been found in healthy volunteers, uric acid stone formers, and in patients with gout,34–36 and the incidence of either diabetes or glucose intolerance is much higher in persons with lower urinary pH than in normal volunteers.37
Epidemiologic studies have also established an association between metabolic acidosis and systemic hypertension, which frequently is a component of the metabolic syndrome associated with insulin resistance. Participants of the 1999–2000 and 2001–2002 NHANES in the highest quintile of anion gap have systolic blood pressure (SBP) values higher than participants in the lowest quintile. Plasma bicarbonate is inversely related to blood pressure, and participants in the highest quintile of bicarbonate had SBP values lower than participants in the lowest quintile.38 A cross-sectional direct association between the serum anion gap and blood pressure is also present among nondiabetic patients followed at a multispecialty group practice, the Harvard Vanguard Medical Associates, in which it is estimated that every 1 mEq/L higher serum anion gap is associated with a 0.27 mmHg higher SBP and 0.20 mmHg higher diastolic arterial blood pressure (DBP).39 In healthy participants in the Nurses Health Studies I and II and the Health Professionals Follow-up Study, lower urinary citrate excretion is independently associated with prevalent hypertension.40 In healthy Japanese subjects, a cross-sectional study shows that the dietary acid load was positively and independently associated with SBP and DBP.41
Metabolic Acidosis-Induced Insulin Resistance May Be Important in Determining Cardiovascular Risk
A lifetime acidotic state is present in healthy individuals of Western countries whose diet is strongly acidogenic. This diet-induced, low-grade metabolic acidosis state may generate insulin resistance and metabolic syndrome, contributing to the diabetes pandemic and elevated cardiovascular morbidity and mortality of the developed world, along with genetic causes, sedentary life, and other factors.
Clinical conditions typically associated with severe atheromatosis and high cardiovascular risk, such as diabetes mellitus and chronic kidney failure, are also characteristically associated with a chronic state of metabolic acidosis and consequently the development of insulin resistance, suggesting that metabolic acidosis may be one important factor originally determining the high cardiovascular risk in these conditions.
Diet-Induced Metabolic Acidosis Produces Insulin Resistance
Contemporary human diet in industrialized countries is deficient in fruits and vegetables and contains excessive animal products, being profoundly acidogenic. Fruits and vegetables are abundant in potassium and magnesium salts of metabolizable anions, including citrate and malate, which consume hydrogen ions when metabolized, having an alkalinizing effect.
By contrast, the accompanying anions for potassium and magnesium in animal products and cereals are mainly nonmetabolizable anions, such phosphate and chloride, making animal products and cereals more acidogenic than fruits and vegetables. Moreover, plant proteins are usually rich in glutamate, an anionic amino acid whose metabolism also consumes hydrogen ions, whereas animal proteins and cereals are particularly abundant in sulfur-containing amino acids (methionine, homocysteine, and cysteine), whose oxidation generates sulfate, a nonmetabolizable anion that constitutes a major determinant of the daily acid load. The content of these amino acids is from two- to five-fold higher in meat and eggs than in grains and legumes.42,43 The modern Western diet based on animal products generates an acid load not compensated by the shortage of fruit and vegetables, causing a life-span state of low-grade metabolic acidosis whose magnitude increases progressively with age, probably due to the decline in kidney function occurring with aging.44 The baseline acidotic state is intensified in obese and overweight persons in whom the intake of fruits and vegetables usually does not compensate for the consumption of acidogenic nutrients.
The ratio of dietary protein to potassium shows an independent positive correlation with body mass index and waist circumference in healthy Japanese individuals.41 The diet-induced metabolic acidosis state may generate insulin resistance and ultimately metabolic syndrome and type 2 diabetes. Fruit and vegetable consumption improves insulin sensitivity, and it has been shown that amelioration of insulin sensitivity may delay or prevent the onset of type 2 diabetes.43,45 In normal-weight subjects, glucose, insulin, and HOMA-IR values are significantly lower in vegetarians than in subjects on a Western-type diet. Vegetarian individuals maintain HOMA-IR values of approximately 1 in all age decades, whereas there is a significant increase of HOMA-IR values in nonvegetarians already in the age decade 31–40 years.46 A vegan diet is associated with increased insulin sensitivity in overweight women as well.47
High dietary potassium and magnesium intake, which is predominantly achieved by the consumption of fresh fruits and vegetables, has been associated with lower risk of developing insulin resistance and type 2 diabetes mellitus, and a deficiency in dietary magnesium has been associated with insulin resistance.48–50 In the Atherosclerosis Risk in the Communities study, a graded inverse relationship between serum magnesium levels and incident type 2 diabetes was observed.51 Essential hypertension is usually a component of the metabolic syndrome caused by insulin resistance. The Dietary Approaches to Stop Hypertension (DASH) diet is rich in fruits and vegetables and low in animal protein, but with plant protein from legumes and nuts. The DASH diet substantially reduces blood pressure and is usually recommended for the prevention and treatment of systemic hypertension. Adherence to the DASH diet is associated with a lower risk of coronary heart disease and stroke in prospective studies.52
Diabetes Mellitus–Induced Metabolic Acidosis Causes Insulin Resistance
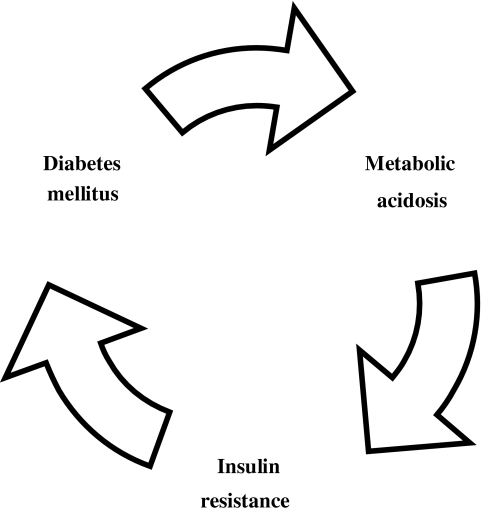
Patients with diabetes mellitus are particularly prone to suffer a life-span state of undetected metabolic acidosis because their usual diet is especially acidogenic, being restricted in carbohydrates and fat and therefore abundant in animal protein. Furthermore, they tend to generate ketone bodies, especially if they are not properly controlled, and the insulin-mediated suppression of ketogenesis is deficient. A significant increase of the plasma fasting levels of ketone bodies in patients with type 2 diabetes has been observed, being blood ketone bodies concentration directly correlated with plasma glucose and free fatty acid concentrations.53 Reduced sensitivity to exogenous insulin in diabetes with ketoacidosis followed by an improvement in insulin sensitivity after the correction of the acidosis was noted for the first time in 1963.29 Marked insulin resistance was also observed in untreated ketotic diabetics, which ameliorates after insulin therapy, whereas carefully controlled diabetic patients have normal sensitivity to insulin.30 The ability of ketone bodies to alter the acid–base balance is underlined by the fact that even a very modest degree of metabolic acidosis induces a marked reduction in β-hydroxybutyrate production rate in healthy individuals by decreasing free fatty acid availability.54 Inadequate metabolic control of the disease with the formation of ketone bodies intensifies the baseline acidotic state and generates a vicious circle by inducing resistance to the insulin action, either exogenous or endogenous (Fig. 2). Before diabetes is diagnosed, there usually is a long period of time during which apparently healthy subjects are subjected to the effect of a sustained acidotic state that is able to increase their cardiovascular risk.
FIG. 2.
Vicious circle created by inadequate metabolic control in diabetes.
Chronic Kidney Failure–Induced Metabolic Acidosis Causes Insulin Resistance
There is evidence that even a mild degree of kidney insufficiency increases the cardiovascular risk,11 although the mechanisms linking kidney dysfunction with cardiovascular disorders have not been completely elucidated. Chronic kidney failure typically features a state of metabolic acidosis, which has been known to be associated with insulin resistance since 1973.31 The reduced ability of the kidney to eliminate the daily acid load in chronic kidney failure may contribute to the elevated cardiovascular risk in this condition by creating an insulin resistant state.
Metabolic acidosis brings about significant functional changes in the kidney, including an increase in renal plasma flow (RPF) and glomerular filtration rate (GFR), probably as adaptive mechanisms to eliminate the excess acid load.55–58 Clinical situations imposing an excessive acid load on the kidney (such as high animal protein consumption, obesity, and diabetes) display similar renal hemodynamic changes. It has been long known that high dietary protein intake is associated with an increase in RPF and GFR in healthy subjects and patients with chronic kidney disease.59,60 Animal and vegetable proteins differ profoundly in their effect upon kidney hemodynamics: Unlike animal proteins, vegetable proteins do not induce renal vasodilatation or glomerular hyperfiltration.42,59,60 Additionally, vegetarian diets reduce the urinary albumin excretion rate in healthy individuals, patients with chronic kidney disease, and diabetic patients compared with animal protein diets.60–63 After a meat load, healthy persons maintain acid–base parameters in the normal range, whereas patients with chronic kidney failure disclose a slight metabolic acidosis, indicating that the acid load imposed to the kidney by the meat ingested exceeds its excretory capacity.61
Excess body weight induces functional changes in the kidney similar to those related to high animal protein intake, namely elevated GFR, RPF, and urinary albumin excretion, affecting to both overweight and obese individuals. Weight loss is associated with an improvement of these alterations and a reduction of the urinary excretion of albumin.64,65 Protein intake (assessed from urinary excretion of urea) is higher in overweight than in lean subjects, and the GFR is positively correlated with the urinary excretion of urea, suggesting that the excessive animal dietary protein consumption is the initiating event causing the functional changes in the kidney.64 Insulin resistance is positively correlated with GFR and RPF in overweight and obese subjects.64,66
Similar kidney hemodynamic alterations to those associated with animal protein ingestion and excess body weight are observed in type 1 and type 2 diabetes.67,68 Both the consumption of vegetable proteins and the careful metabolic control of the disease contribute to ameliorate these modifications, presumably via improvement of the acidotic state associated with high animal protein dietary intake and uncontrolled diabetes. The differing effect of vegetable and animal proteins on kidney hemodynamics observed in healthy subjects and patients with chronic kidney failure is also apparent in diabetic patients, which show lower GFR and RPF during the consumption of vegetable protein diets compared to animal protein diets.69 Strict metabolic control induces a significant reduction of both GFR and RPF.63 In addition, intensive metabolic control and vegan diets reduce urinary albumin excretion in diabetic patients.62,63 The potential significance of these hemodynamic changes rests in their possible association with more severe kidney dysfunction later in the course of the disease. Faster decline in kidney function has been associated with baseline hyperfiltration in prospective studies and meta-analysis using study level data.70,71
Indirect support for the importance of metabolic acidosis in determining cardiovascular risk may be provided by prospective studies revealing that elevated urinary sodium excretion predicts cardiovascular morbidity and mortality.72 It is known that metabolic acidosis induces a marked increase in the urinary excretion of sodium,56,57 and the high cardiovascular risk linked to high urinary sodium excretion may be explained at least in part by the occurrence of clinical conditions related to a chronic state of metabolic acidosis. The urinary excretion of sodium has been shown to be higher in obese than in lean subjects and it tends to increase after meat ingestion compared with vegetable protein intake.60 Elevated urinary sodium excretion is also independently associated with an increase in urinary albumin excretion, with this relationship being more pronounced in subjects with higher body mass index.73
Finally, besides being a consequence of insulin resistance, microalbuminuria might be an indirect marker of the presence of metabolic acidosis. The causes and mechanisms producing microalbuminuria are currently unclear. The hypothesis that the glomerular capillary pressure might determine the degree of microalbuminuria has not been supported by studies in which the urinary excretion of albumin underwent no change after the infusion of exogenous angiotensin II, indicating that factors other than glomerular transcapillary hydraulic pressure determine the degree of urinary albumin loss.74 The isoelectric pH (pI) of albumin is the pH value at which the ionizable groups of the protein cancel each other, and its net charge is nil. The pI value for human serum albumin is 5.9. At a pH less than 5.9, the albumin molecules become positively charged, whereas at a pH above 5.9, the imidazolium groups of histidines are partially titrated and albumin turns into an anion. In plasma, serum albumin acquires a slight negative charge. In urine, the concentration of hydrogen ions varies according to the acid load that the kidney is required to eliminate. When urinary pH is less than 5.9, albumin attains a positive charge, facilitating hydrogen ions excretion. When the acid load imposed to the kidney is high and the urine pH is consequently low, small amounts of albumin may be allowed in the urine to assist in the elimination of acid.
Ackowledgments
There is no financial support for this work
Author Disclosure Statement
There are no conflicts of interest.
References
- 1.Bianchi S. Bigazzi R. Valtriani C. Chiapponi I. Sgherri G. Baldari G. Natali A. Ferrannini E. Campese VM. Elevated serum insulin levels in patients with essential hypertension and microalbuminuria. Hypertension. 1994;23(6 Pt 1):681–687. doi: 10.1161/01.hyp.23.6.681. [DOI] [PubMed] [Google Scholar]
- 2.Dinneen SF. Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 3.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 4.Jarrett RJ. Viberti GC. Argyropoulos A. Hill RD. Mahmud U. Murrells TJ. Microalbuminuria predicts mortality in non-insulin-dependent diabetics. Diabet Med. 1984;1:17–19. doi: 10.1111/j.1464-5491.1984.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 5.Borch-Johnsen K. Kreiner S. Proteinuria: Value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J. 1987;27:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen T. Borch-Johnsen K. Kofoed-Enevoldsen A. Deckert T. Coronary heart disease in young type 1 (insulin-dependent) diabetic patients with and without diabetic nephropathy: Incidence and risk factors. Diabetologia. 1987;30:144–148. doi: 10.1007/BF00274218. [DOI] [PubMed] [Google Scholar]
- 7.Yudkin JS. Forrest RD. Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet. 1988;3:530–530. doi: 10.1016/s0140-6736(88)92657-8. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC. Mann JF. Yi Q. Zinman B. Dinneen SF. Hoogwerf B. Halle JP. Young J. Rashkow A. Joyce C. Nawaz S. Yusuf S for the HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 9.Wachtell K. Ibsen H. Olsen MH. Borch-Johnsen K. Lindholm LH. Mogensen CE. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 10.Klausen K. Borch-Johnsen K. Feldt-Rasmussen B. Jensen G. Clausen P. Scharling H. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;6:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 11.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts GF. Naumova R. Slavin BM. Morris RW. Houlston R. Kubal C. Shaw KM. Serum lipids and lipoproteins in insulin-dependent diabetic patients with persistent microalbuminuria. Diabet Med. 1989;6:25–30. doi: 10.1111/j.1464-5491.1989.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones SL. Close CF. Mattock MB. Jarrett RJ. Keen H. Viberti GC. Plasma lipid and coagulation factor concentrations in insulin dependent diabetics with microalbuminuria. BMJ. 1989;298:487–490. doi: 10.1136/bmj.298.6672.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niskanen L. Uusitupa M. Sarlund H. Siitonen O. Voutilainen E. Penttilä I. Microalbuminuria predicts the development of serum lipoprotein abnormalities favouring atherogenesis in newly diagnosed type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990;33:237–243. doi: 10.1007/BF00404802. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM. Stern MP. Gruber MK. Hazuda HP. Mitchell BD. Patterson JK. Microalbuminuria. Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis. 1990;10:727–731. doi: 10.1161/01.atv.10.5.727. [DOI] [PubMed] [Google Scholar]
- 16.Yip J. Mattock MB. Morocutti A. Sethi M. Trevisan R. Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 17.Nosadini R. Solini A. Velussi M. Muollo B. Frigato F. Sambataro M. Impaired insulin-induced glucose uptake by extrahepatic tissue is hallmark of NIDDM patients who have or will develop hypertension and microalbuminuria. Diabetes. 1994;43:491–499. doi: 10.2337/diab.43.3.491. [DOI] [PubMed] [Google Scholar]
- 18.Parvanova AI. Trevisan R. Iliev IP. Dimitrov BD. Vedovato M. Tiengo A. Remuzzi G. Ruggenenti P. Insulin resistance and microalbuminuria: A cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 19.Mykkänen L. Zaccaro DJ. Wagenknecht LE. Robbins DC. Gabriel M. Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 20.Rademacher ER. Sinaiko AR. Albuminuria in children. Curr Opin Nephrol Hypertens. 2009;18:246–251. doi: 10.1097/MNH.0b013e3283294b98. [DOI] [PubMed] [Google Scholar]
- 21.Ekstrand AV. Groop PH. Grönhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 22.Niskanen L. Laakso M. Insulin resistance is related to albuminuria in patients with type II (non-insulin-dependent) diabetes mellitus. Metabolism. 1993;42:1541–1545. doi: 10.1016/0026-0495(93)90148-h. [DOI] [PubMed] [Google Scholar]
- 23.Klausen KP. Parving HH. Scharling H. Jensen JS. The association between metabolic syndrome, microalbuminuria and impaired renal function in the general population: impact on cardiovascular disease and mortality. J Intern Med. 2007;262:470–478. doi: 10.1111/j.1365-2796.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 24.de Boer IH. Sibley SD. Kestenbaum B. Sampson JN. Young B. Cleary PA. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007;18:235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnet F. Marre M. Halimi JM. Stengel B. Lange C. Laville M. DESIR Study Group. Waist circumference and the metabolic syndrome predict the development of elevated albuminuria in non-diabetic subjects: The DESIR Study. J Hypertens. 2006;24:1157–1163. doi: 10.1097/01.hjh.0000226206.03560.ac. [DOI] [PubMed] [Google Scholar]
- 26.Saraheimo M. Forsblom C. Fagerudd J. Teppo AM. Pettersson-Fernholm K. Frystyk J. FinnDiane study group. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005;28:1410–1414. doi: 10.2337/diacare.28.6.1410. [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo RA. Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol. 1979;236:E328–E334. doi: 10.1152/ajpendo.1979.236.4.E328. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy J. Newby FD. Gebhart SS. DiGirolamo M. Insulin resistance in obesity is associated with elevated basal lactate levels and diminished lactate appearance following intravenous glucose and insulin. Metabolism. 1992;41:22–27. doi: 10.1016/0026-0495(92)90185-d. [DOI] [PubMed] [Google Scholar]
- 29.Walker BG. Phear DN. Martin FIR. Baird CW. Inhibition of insulin by acidosis. Lancet. 1963;9:964–965. doi: 10.1016/s0140-6736(63)90670-6. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg HN. Investigation of insulin sensitivity in treated subjects with ketosis-prone diabetes mellitus. Diabetes. 1977;26:278–283. doi: 10.2337/diab.26.4.278. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA. Andres R. Edgar P. Walker WG. Carbohydrate metabolism in uremia: A review. Medicine. 1973;52:469–481. doi: 10.1097/00005792-197309000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Farwell WR. Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 33.Cupisti A. Meola M. D'Alessandro C. Bernabini G. Pasquali E. Carpi A. Barsotti G. Insulin resistance and low urinary citrate excretion in calcium stone formers. Biomed Pharmacother. 2007;61:86–90. doi: 10.1016/j.biopha.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Abate N. Chandalia M. Cabo-Chan AV., Jr. Moe OW. Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi S. Inokuchi T. Kobayashi T. Ka T. Tsutsumi Z. Moriwaki Y. Relationship between insulin resistance and low urinary pH in patients with gout, and effects of PPAR-alpha agonists on urine pH. Horm Metab Res. 2007;39:511–514. doi: 10.1055/s-2007-982517. [DOI] [PubMed] [Google Scholar]
- 36.Maalouf NM. Cameron MA. Moe OW. Adams-Huet B. Sakhaee K. Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 37.Sakhaee K. Adams-Huet B. Moe OW. Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 38.Taylor EN. Forman JP. Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50:320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 39.Forman JP. Rifas-Shiman SL. Taylor EN. Lane K. Gillman MW. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens. 2008;22:122–125. doi: 10.1038/sj.jhh.1002286. [DOI] [PubMed] [Google Scholar]
- 40.Taylor EN. Mount DB. Forman JP. Curhan GC. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Murakami K. Sasaki S. Takahashi Y. Uenishi K. Japan Dietetic Students Study for Nutrition and Biomarkers Group. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr. 2008;100:642–651. doi: 10.1017/S0007114508901288. [DOI] [PubMed] [Google Scholar]
- 42.Bresslau NA. Brinkley L. Hill KD. Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 43.Demigne C. Sabboh H. Remesy C. Meneton P. Protective effects of high dietary potassium: nutritional and metabolic aspects. J Nutr. 2004;134:2903–2906. doi: 10.1093/jn/134.11.2903. [DOI] [PubMed] [Google Scholar]
- 44.Frassetto LA. Morris RC., Jr Sebastian A. Effect of age on blood acid-base composition in adult humans: Role of age-related renal functional decline. Am J Physiol. 1996;271:F1114–F1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan TA. Xiang AH. Peters RK. Kjos SL. Marroquin A. Goico J. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 46.Valachovicová M. Krajcovicová-Kudlacková M. Blazicek P. Babinská K. No evidence of insulin resistance in normal weight vegetarians. A case control study. Eur J Nutr. 2006;45:52–54. doi: 10.1007/s00394-005-0563-x. [DOI] [PubMed] [Google Scholar]
- 47.Barnard ND. Scialli AR. Turner-McGrievy G. Lanou AJ. Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;188:991–997. doi: 10.1016/j.amjmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Colditz GA. Manson JE. Stampler MJ. Rosner B. Willet WC. Spelzer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992;55:1018–1023. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 49.Humphries S. Kushner H. Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am J Hypertens. 1999;12:747–756. doi: 10.1016/s0895-7061(99)00041-2. [DOI] [PubMed] [Google Scholar]
- 50.Huerta MG. Roemmich JN. Kington ML. Bovbjerg VE. Weltman AL. Holmes VF. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28:1175–1181. doi: 10.2337/diacare.28.5.1175. [DOI] [PubMed] [Google Scholar]
- 51.Kao WH. Folsom AR. Nieto FJ. Mo JP. Watson RL. Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159:2151–2159. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 52.Fung TT. Chiuve SE. McCullough ML. Rexrode KM. Logroscino G. Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 53.Avogaro A. Crepaldi C. Miola M. Maran A. Pengo V. Tiengo A. Del Prato S. High blood ketone body concentration in type 2 non-insulin dependent diabetic patients. J Endocrinol Invest. 1996;19:99–105. doi: 10.1007/BF03349844. [DOI] [PubMed] [Google Scholar]
- 54.Hood VL. Keller U. Haymond MW. Küry D. Systemic pH modifies ketone body production rates and lipolysis in humans. Am J Physiol. 1990;259:E327–E334. doi: 10.1152/ajpendo.1990.259.3.E327. [DOI] [PubMed] [Google Scholar]
- 55.Sartorius OW. Roemmelt JC. Pitts RF. Calhoon D. Miner P. The renal regulation of acid-base balance in man. The nature of the renal compensations in ammonium chloride acidosis. J Clin Invest. 1949;28:423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sulyok E. Guignard JP. Effect of ammonium-chloride-induced metabolic acidosis on renal electrolyte handling in human neonates. Pediatr Nephrol. 1990;4:415–420. doi: 10.1007/BF00862528. [DOI] [PubMed] [Google Scholar]
- 57.Györke ZS. Sulyok E. Guignard JP. Ammonium chloride metabolic acidosis and the activity of renin-angiotensin-aldosterone system in children. Eur J Pediatr. 1991;150:547–549. doi: 10.1007/BF02072203. [DOI] [PubMed] [Google Scholar]
- 58.Garibotto G. Verzola D. Sofia A. Saffioti S. Menesi F. Vigo E. Mechanisms of renal ammonia production and protein turnover. Metab Brain Dis. 2009;24:159–167. doi: 10.1007/s11011-008-9121-6. [DOI] [PubMed] [Google Scholar]
- 59.Bosch JP. Saccaggi A. Lauer A. Ronco C. Belledonne M. Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75:943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 60.Kontessis P. Jones S. Dodds R. Trevisan R. Nosadini R. Fioretto P. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136–144. doi: 10.1038/ki.1990.178. [DOI] [PubMed] [Google Scholar]
- 61.de Santo NG. Capasso G. Malnic G. Anastasio P. Spitali L. D'Angelo A. Effect of an acute oral protein load on renal acidification in healthy humans and in patients with chronic renal failure. J Am Soc Nephrol. 1997;8:784–792. doi: 10.1681/ASN.V85784. [DOI] [PubMed] [Google Scholar]
- 62.Barnard ND. Cohen J. Jenkins DJA. Turner-McGrievy G. Gloede L. Jaster B. Seidl K. Green AA. Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783. doi: 10.2337/dc06-0606. [DOI] [PubMed] [Google Scholar]
- 63.Azadbakht L. Atabak S. Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–654. doi: 10.2337/dc07-2065. [DOI] [PubMed] [Google Scholar]
- 64.Ribstein J. du Cailar G. Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26:610–615. doi: 10.1161/01.hyp.26.4.610. [DOI] [PubMed] [Google Scholar]
- 65.Chagnac A. Weinstein T. Herman M. Hirsh J. Gafter U. Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 66.Pruijm M. Wuerzner G. Maillard M. Bovet P. Renaud C. Bochud M. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant. 2010;25:2225–2231. doi: 10.1093/ndt/gfq008. [DOI] [PubMed] [Google Scholar]
- 67.Christiansen JS. Parving HH. The relationship between kidney size and function in short-term diabetic patients. Diabetologia. 1982;22:494. doi: 10.1007/BF00282601. [DOI] [PubMed] [Google Scholar]
- 68.Inomata S. Renal hypertrophy as a prognostic index for the progression of diabetic renal disease in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1993;7:28–33. doi: 10.1016/1056-8727(93)90020-y. [DOI] [PubMed] [Google Scholar]
- 69.Kontessis PA. Bossinakou I. Sarika L. Iliopoulou E. Papantoniou A. Trevisan R. Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type I diabetic patients. Diabetes Care. 1995;18:1233. doi: 10.2337/diacare.18.9.1233. [DOI] [PubMed] [Google Scholar]
- 70.Rigalleau V. Garcia M. Lasseur C. Laurent F. Montaudon M. Raffaitin C. Barthe N. Beauvieux M-C. Vendrely B. Chauveau P. Combe C. Gin H. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol. 2010;11:3. doi: 10.1186/1471-2369-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magee GM. Bilous RW. Cardwell CR. Hunter SJ. Kee F. Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 72.Tuomilehto J. Jousilahti P. Rastenyte D. Moltchanov V. Tanskanen A. Pietinen P. Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet. 2001;17;357:848–851. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 73.Verhave JC. Hillege HL. Burgerhof JG. Janssen WM. Gansevoort RT. Navis GJ. PREVEND Study Group. Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004;256:324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 74.Spooren PF. Gans RO. Adèr HJ. Vermes I. Donker AJ. Urinary albumin excretion rate during angiotensin II infusion in microalbuminuric patients with insulin and non-insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:281–285. doi: 10.1093/ndt/12.2.281. [DOI] [PubMed] [Google Scholar]